Abstract
Children and adolescents who sustain a distal forearm fracture (DFF) owing to mild, but not moderate, trauma have reduced bone strength and cortical thinning at the distal radius and tibia. Whether these skeletal deficits track into adulthood is unknown. Therefore, we studied 75 women and 75 men (age range, 20 to 40 years) with a childhood (age <18 years) DFF and 150 sex-matched controls with no history of fracture using high-resolution peripheral quantitative computed tomography (HRpQCT) to examine bone strength (ie, failure load) by micro–finite element (µFE) analysis, as well as cortical and trabecular bone parameters at the distal radius and tibia. Level of trauma (mild versus moderate) was assigned using a validated classification scheme, blind to imaging results. When compared to sex-matched, nonfracture controls, women and men with a mild trauma childhood DFF (eg, fall from standing height) had significant reductions in failure load (p < 0.05) of the distal radius, whereas women and men with a moderate trauma childhood DFF (eg, fall while riding a bicycle) had values similar to controls. Consistent findings were observed at the distal tibia. Furthermore, women and men with a mild trauma childhood DFF had significant deficits in distal radius cortical area (p < 0.05), and significantly lower dual-energy X-ray absorptiometry (DXA)-derived bone density at the radius, hip, and total body regions compared to controls (all p < 0.05). By contrast, women and men with a moderate trauma childhood DFF had bone density, structure, and strength that did not differ significantly from controls. These findings in young adults are consistent with our observations in children/adolescents with DFF, and they suggest that a mild trauma childhood DFF may presage suboptimal peak bone density, structure, and strength in young adulthood. Children and adolescents who suffer mild trauma DFFs may need to be targeted for lifestyle interventions to help achieve improved skeletal health.
Keywords: BONE STRENGTH; BONE STRUCTURE; HRPQCT; DISTAL FOREARM FRACTURE; YOUNG ADULTS; BONE ANALYSIS/QUANTITATION; GENERAL POPULATION STUDIES, EPIDEMIOLOGY; BONE DENSITOMETRY
Introduction
Personal fracture history is one of the strongest predictors of future fractures,(1,2) yet current practice guidelines ignore fractures that occur during childhood.(3–5) Distal forearm fractures (DFFs) are the most common fracture type suffered by young individuals,(6–8) and the incidence of these fractures appears to be rising.(9) Although most research has focused on determinants of fractures with aging,(10–14) DFF risk is bimodal with an earlier peak during puberty.(6–8) It remains unclear whether childhood fractures are related, in part, to transient reductions in bone strength during rapid growth or to skeletal deficits that will track into adulthood. Moreover, as hypothesized by Parfitt,(15) childhood fractures may be the price of risk-taking behaviors that optimize bone strength during growth. Because achieving optimal bone strength early in life likely predicts lower fracture risk later in life,(16) it is critical to identify events during childhood that foreshadow suboptimal peak bone strength in adulthood.
In a recent study, using high-resolution peripheral quantitative computed tomography (HRpQCT) imaging, we reported that children and adolescents with a DFF owing to mild (eg, fall from standing height), but not moderate (eg, fall while riding a bicycle), trauma have cortical thinning and deficits in bone microstructure at the distal radius and tibia compared to sex-matched, controls with no fracture history.(17) In addition, using micro–finite element (µFE) analysis of distal radius HRpQCT images, we found that boys and girls with a mild trauma DFF had significantly reduced bone strength (ie, failure load) compared to nonfracture controls, and had higher (“worse”) fall load-to-strength ratios (“factor-of-risk”(10)). Furthermore, whereas girls with a moderate trauma DFF had a similar factor-of-risk, boys with a moderate trauma DFF had a lower (“better”) factor-of-risk compared to their nonfracture controls.(17) These findings suggest that DFFs during growth have two distinct etiologies: those due to underlying skeletal fragility leading to fractures following mild trauma versus those due to more significant trauma in the setting of normal bone strength.(17)
Our findings in children and adolescents with a DFF due to mild trauma could have important clinical ramifications for future fracture risk if the skeletal deficits we observed track into adulthood. Consistent with this possibility, several observational studies have demonstrated that bone density, size, and shape tend to track throughout life.(18–20) Furthermore, although a number of studies have found that volumetric bone mineral density (vBMD) and bone structure are worse in young adults,(21–23) premenopausal women,(24,25) postmenopausal women,(10–13) and older men(14) with prior fracture, to our knowledge, no study has considered the severity of the associated trauma to test whether individuals who fracture in the setting of mild versus moderate trauma have altered bone morphology compared to nonfracture controls. If additional studies further validate that the skeletal deficits in children and adolescents with a mild trauma DFF persist into adult life, then individuals with a history of such fractures may need to be more aggressively monitored for subsequent skeletal complications.
Therefore, in a cross-sectional study of otherwise healthy young adults (age 20 to 40 years), we examined whether women and men who sustained a DFF during childhood (at age <18 years) owing to either mild or moderate trauma have alterations in bone density, microstructure, and/or strength compared to sex-matched controls with no history of fracture. In addition, we assessed lifestyle factors, body composition, and biochemical parameters to explore the determinants of bone strength and microstructure in young adults with and without a childhood DFF.
Subjects and Methods
Subjects
This study was approved by the Mayo Clinic Institutional Review Board, and all participants provided written informed consent. Between April 2010 and February 2013, we recruited 75 women and 75 men between the ages of 20 and 40 years, who sustained a DFF at an age <18 years and were residents of Olmsted County or surrounding counties from southeast Minnesota. We also recruited, from the same underlying population, 150 sex-matched (1:1 ratio) nonfracture controls with a similar age distribution. Subjects with a DFF during childhood were identified using computerized diagnostic and procedure indices generated from the Rochester Epidemiology Project (REP),(26) which is a unique medical records linkage system that provides comprehensive (inpatient and outpatient) community medical records for residents in Olmsted County; REP is now expanding its coverage area of research to the surrounding counties from southeast Minnesota. Review of medical records for research was carried out in accordance with Minnesota privacy law.(27) Nonfracture control subjects from Olmsted County and surrounding counties from southeast Minnesota were recruited by flyers and local newspaper and website advertisements. This community is highly characteristic of the United States white population but underrepresented with respect to persons of African or Asian ancestry.(26) Reflecting the ethnic composition of the population, 98% of the sample was white.
Potential subjects were rigorously screened for coexisting disease and excluded if they had any medical conditions associated with altered skeletal structure or function, such as osteogenesis imperfecta, osteomalacia, Paget’s disease, anorexia nervosa, history of organ transplant, chronic renal or liver disease, type 1 or type 2 diabetes mellitus, hypoparathyroidism or hyperparathyroidism, thyroid disorders, chronic gastrointestinal disorders, autoimmune rheumatologic diseases, neurologic disorders, or malignancy. Potential subjects were also excluded if they had ever taken any medication, except for combined oral contraceptives (COCs), known to affect bone metabolism, such as anabolic steroids, anticonvulsants, aromatase inhibitors, bisphosphonates, calcitonin, glucocorticoids, parathyroid hormone, sodium fluoride, or thyroid hormone replacement. Furthermore, females were excluded if they were pregnant, nursing, or taking progestin-only birth control. A screening interview was performed and clinical details in the medical records were reviewed to determine if potential subjects met study criteria.
Subjects with a DFF during childhood were eligible for the study if the DFF resulted from mild or moderate trauma, based on review of the clinical details available from various data sources (eg, emergency room records, radiographic reports, primary care or other provider notes, orthopedic consultation notes, and surgical reports). We excluded potential candidates with a DFF during childhood that was a result of severe trauma (eg, falls >3m, motor vehicle accidents, open fractures, crush injuries), with a DFF considered to be pathologic (ie, caused by a specific bone lesion), or with a history of bilateral DFFs. Following enrollment, DFF due to mild (eg, fall from standing height) versus moderate (eg, fall while riding a bicycle) trauma was ascertained, blind to the bone imaging results, using Landin’s modified criteria(6,28) (Supporting Table 1), based on the mechanism and circumstances of the injury as determined from clinical records and supplemented by an interview with the subject (additional details are provided in the Supporting Information). For subjects who had more than one DFF on the same side (n = 13), the lowest trauma level was used. Controls had no history of fracture.
Study protocol
All subjects were interviewed by trained study personnel for their medical history, medication use (including COCs), smoking status, and alcohol consumption habits using a standard protocol developed for use in our studies,(10,29–32) supplemented by review of each subject’s medical record. Weight was obtained using an electronic scale (Model 5002; Tronic, Inc., White Plains, NY, USA), and height was measured using a customized stadiometer (Mayo Section of Engineering). Body mass index (BMI) was defined as weight (kg) divided by height (m) squared. Physical activity was assessed using a validated questionnaire.(33) Fasting morning blood was obtained, and serum was stored at −80°C for batch analyses of circulating biochemical and hormonal parameters. Additional details regarding the physical activity assessments and assay methods for the various biochemical parameters are provided in the online supplement. Bone biomechanical strength of the distal radius and tibia was determined by µFE analysis of HRpQCT images. Cortical and trabecular bone macrostructure and microstructure of the distal radius and tibia were assessed by HRpQCT, although data from three radius scans (3 DFF subjects; 0 controls) were excluded because of motion artifact. Areal BMD (aBMD) of the hip, radius, lumbar spine (L1–L4), and total body was measured by dual-energy X-ray absorptiometry (DXA) using standard methods.(32) All procedures were performed in the outpatient Clinical Research Unit at the Mayo Clinic (Rochester, MN, USA).
HRpQCT imaging
The HRpQCT device (Xtreme-CT; Scanco Medical AG, Brüttisellen, Switzerland) and in vivo image processing and analysis protocols used in our laboratory have been described.(30–32) In subjects with a DFF during childhood, the nonfractured distal radius was scanned, and the distal radius of the same side was scanned in the respective sex-matched, nonfracture control (1 control subject could not hold the matched arm still for the entire duration of the scan so the opposite radius was scanned). In all subjects, the nondominant distal tibia was scanned, unless there was a recent injury (eg, sprained ankle) or prior fracture to that leg, in which case the contralateral tibia was scanned. A single dorsal-palmar projection image of the distal radius/tibia was acquired to define the scan region. Each 9.02-mm scan consisted of a three-dimensional stack of 110 high-resolution CT slices and was fixed starting at 9.5 mm and 22.5 mm (for the radius and tibia, respectively) proximal from the mid-joint line, and extending proximally. Total scan time was 2.8 minutes, with an isotropic voxel size and slice thickness of 82 µm, an effective energy of 40 keV, a field of view of 125.9 mm, and an image matrix of 1536 × 1536 pixels. A single operator performed all HRpQCT scans. Short-term precision (coefficients of variation [CVs]) of the HRpQCT device in our laboratory has been reported,(30) based on repeat measures on 20 volunteers on the same day after repositioning.
Trabecular bone volume fraction (bone volume/tissue volume [BV/TV]), trabecular number (Tb.N; 1/mm), trabecular thickness (Tb.Th; mm) and trabecular separation (Tb.Sp; mm) were derived as described.(30–32) For the cortical parameters, we used the extended cortical analysis available from the manufacturer to obtain cortical area (Ct.A; mm2), cortical thickness (Ct.Th; mm), cortical volumetric BMD (Ct.vBMD; mg/cm3), cortical tissue mineral density (Ct.TMD; mg/cm3), endocortical circumference (EC; mm), and periosteal circumference (PC; mm). Furthermore, we derived cortical porosity (Ct.Po; %) using a validated approach described in detail by Burghardt and colleagues(34) that we have used previously.(31,32) The validity of these approaches have been rigorously tested, and excellent correlations (r ≥ 0.96) have been shown between HRpQCT and the “gold standard” ex vivo µCT technique.(35)
µFE analysis
To evaluate biomechanical bone strength, linear µFE models of the distal radius and tibia were created directly from the HRpQCT images using software provided by the manufacturer (µFE analysis solver v.1.15; Scanco Medical AG), as described.(32) Bone strength (ie, failure load [N, Newtons]) was derived by scaling the resulting load from a test simulating 1% compression, such that 2% of all elements had an effective strain >7000 microstrain.(36) Failure loads calculated from such µFE models have been shown to correlate highly (r = 0.87) with compressive loads producing a DFF in cadaveric forearms.(36) The fall load applied to the wrist was estimated from predicted impact forces on the upper extremity during loading conditions for a forward fall on the outstretched hand.(37) We assessed the ratio of fall load to failure load at the distal radius, as determined by µFE analysis, as an estimate of the fall load-to-strength ratio, or factor-of-risk (Φ), for the distal radius, as described.(10)
DXA imaging
Regional aBMD (g/cm2) of the radius (ultradistal [UD] and total), lumbar spine (L1–L4), total body, nondominant femoral neck (FN), trochanter, and total hip regions was measured using DXA. Total body lean mass (TBLM), total body fat mass (TBFM), and percent body fat were obtained from DXA whole-body scans.
Statistical analyses
Descriptive, anthropometric, body composition, and physical activity characteristics were compared between the control and DFF groups (mild trauma, moderate trauma, and all DFF subjects) using one-way ANOVA. Further comparisons of the bone parameters between the control and DFF groups were made using an analysis of covariance (ANCOVA) model adjusted for age, height and weight. Last, comparisons of the biochemical parameters were made using an ANCOVA model, adjusted for age. For all parameters, Dunnett’s test was used to account for multiple comparisons when contrasting the mild trauma or moderate trauma DFF groups with the sex-matched control group. Results from the models are summarized as adjusted mean ± SEM. For consistency, unadjusted results are also expressed as mean ± SEM. Separate analyses were performed for females and males because of known skeletal differences between sexes.(29) To address the primary objective, we assessed bone strength (ie, failure load) using µFE analysis of HRpQCT images of the distal radius and tibia. Secondary outcomes included the cortical and trabecular bone parameters of the distal radius and tibia obtained by HRpQCT, as well as the regional DXA-derived BMD measurements and biochemical parameters. Bone strength and fall load-to-strength ratio were age, height, and weight standardized by fitting a linear regression model using all subjects for each gender separately, extracting the residuals, then adding to that the overall mean. The adjusted variables were summarized in box-plots. Our sample size was chosen to detect clinically meaningful differences in HRpQCT parameters based on our prior study in postmenopausal women with and without a DFF.(10) All testing was performed at a significance level of p < 0.05 (two-tailed). Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Fracture history
According to Landin’s modified criteria(6,28) (Supporting Table 1), of the 150 fracture subjects, 79 had a childhood DFF due to mild trauma (42 women, 37 men), whereas the other 71 had a DFF due to moderate trauma (33 women, 38 men); 52% of the fractures occurred at the left forearm. Altogether these 150 women and men suffered a total of 258 fractures, 91% of which occurred before 18 years of age. The childhood DFF was the only fracture suffered by 56% of subjects (46 women, 38 men), whereas 27% (21 women, 20 men) had a total of two fractures and 17% (8 women, 17 men) had a total of three to five fractures before age 18 years. Thirteen subjects suffered a second childhood DFF on the same side, and two of them suffered a third DFF at age ≥18 years. The remaining 93 fractures occurred at the following sites: hand/fingers (37%), other arm/shoulder (16%), ankle (16%), clavicle (13%), feet/toes (9%), face (3%), proximal femur (3%), and vertebrae (3%).
Descriptive characteristics
In this cohort of healthy, young adult women and men, the DFF and control groups were similar in age (Table 1). Furthermore, age of menarche did not differ among female groups. By contrast, relative to controls, a higher proportion of subjects with a childhood DFF were current smokers and reported daily consumption of alcohol. The DFF subjects and sex-matched controls did not differ in height, but the male DFF subjects who had suffered a moderate trauma DFF tended to be heavier and on average had higher BMIs than controls. Further, relative to sex-matched controls, women with a DFF due to moderate trauma tended to have lower percent body fat, whereas percent body fat tended to be higher in males with a moderate trauma DFF during childhood. In addition, compared to sex-matched controls, females with a childhood DFF owing to moderate trauma had significantly higher lean mass, whereas males with a mild trauma DFF tended to have lower lean mass. Finally, current physical activity level did not differ among any of the groups.
Table 1.
Clinical Characteristics of Subjects With a Childhood DFF (Mild Trauma, Moderate Trauma, All DFF Subjects) and Nonfracture Controls
| Controls | Mild trauma | Moderate trauma | All DFF subjects | pa | pb | pc | |
|---|---|---|---|---|---|---|---|
| n | |||||||
| Females | 75 | 42 | 33 | 75 | |||
| Males | 75 | 37 | 38 | 75 | |||
| DFF side (% left) | |||||||
| Females | NA | 43 | 48 | 45 | |||
| Males | NA | 59 | 58 | 59 | |||
| DFF age (year) | |||||||
| Females | NA | 8.4 ± 0.6 | 9.4 ± 0.5 | 8.8 ± 0.4 | |||
| Males | NA | 9.7 ± 0.5 | 11.6 ± 0.5 | 10.6 ± 0.4 | |||
| Current age (years) | |||||||
| Females | 29.7 ± 0.6 | 28.9 ± 0.9 | 30.2 ± 1.0 | 29.4 ± 0.6 | 0.702 | 0.889 | 0.804 |
| Males | 30.1 ± 0.6 | 29.9 ± 0.8 | 29.7 ± 0.8 | 29.8 ± 0.6 | 0.969 | 0.911 | 0.718 |
| Menarche age (year) | |||||||
| Females | 12.8 ± 0.1 | 13.0 ± 0.2 | 12.9 ± 0.2 | 12.9 ± 0.1 | 0.560 | 0.858 | 0.372 |
| Ever used COCs, n (%) | |||||||
| Females | 61 (81.3) | 31 (73.8) | 29 (87.9) | 60 (80.0) | 0.556 | 0.633 | 0.836 |
| Current use of COCs, n (%) | |||||||
| Females | 35 (46.7) | 19 (45.2) | 19 (57.6) | 38 (50.7) | 0.985 | 0.494 | 0.624 |
| Current smoker, n (%) | |||||||
| Females | 3 (4.0) | 7 (16.7) | 3 (9.1) | 10 (13.3) | 0.053 | 0.468 | 0.055 |
| Males | 8 (10.7) | 5 (13.5) | 11 (28.9) | 16 (21.3) | 0.872 | 0.034 | 0.080 |
| Current daily alcohol consumption, n (%) | |||||||
| Females | 5 (6.7) | 7 (16.7) | 7 (21.2) | 14 (18.7) | 0.168 | 0.063 | 0.034 |
| Males | 22 (29.3) | 18 (48.6) | 8 (21.1) | 26 (34.7) | 0.089 | 0.562 | 0.484 |
| Height (cm) | |||||||
| Females | 166 ± 1 | 167 ± 1 | 166 ± 1 | 167 ± 1 | 0.466 | 0.992 | 0.512 |
| Males | 181 ± 1 | 180 ± 1 | 180 ± 1 | 180 ± 1 | 0.829 | 0.690 | 0.432 |
| Weight (kg) | |||||||
| Females | 71.1 ± 2.0 | 76.5 ± 2.7 | 69.1 ± 3.0 | 73.3 ± 2.0 | 0.194 | 0.815 | 0.450 |
| Males | 89.2 ± 1.9 | 89.1 ± 2.7 | 98.1 ± 2.6 | 93.7 ± 1.9 | 0.999 | 0.014 | 0.099 |
| BMI (kg/m2) | |||||||
| Females | 25.9 ± 0.7 | 27.3 ± 0.9 | 25.1 ± 1.0 | 26.3 ± 0.7 | 0.390 | 0.784 | 0.647 |
| Males | 27.2 ± 0.5 | 27.4 ± 0.8 | 30.2 ± 0.8 | 28.8 ± 0.6 | 0.978 | 0.005 | 0.049 |
| Percent fat (%) | |||||||
| Females | 42.4 ± 1.2 | 43.2 ± 1.6 | 37.7 ± 1.8 | 40.8 ± 1.2 | 0.890 | 0.064 | 0.359 |
| Males | 29.2 ± 1.2 | 32.8 ± 1.7 | 33.3 ± 1.7 | 33.0 ± 1.2 | 0.152 | 0.090 | 0.023 |
| TBFM (kg) | |||||||
| Females | 31.0 ± 1.7 | 34.7 ± 2.3 | 26.9 ± 2.6 | 31.3 ± 1.7 | 0.339 | 0.331 | 0.906 |
| Males | 26.9 ± 1.6 | 29.7 ± 2.3 | 34.0 ± 2.2 | 31.9 ± 1.6 | 0.500 | 0.020 | 0.027 |
| TBLM (kg) | |||||||
| Females | 36.7 ± 0.5 | 38.2 ± 0.7 | 39.0 ± 0.8 | 38.6 ± 0.5 | 0.192 | 0.036 | 0.017 |
| Males | 58.1 ± 0.8 | 55.3 ± 1.1 | 59.4 ± 1.1 | 57.4 ± 0.8 | 0.084 | 0.536 | 0.539 |
| PA (kcal/week × 103) | |||||||
| Females | 29.3 ± 1.2 | 31.2 ± 1.6 | 31.4 ± 1.8 | 31.3 ± 1.2 | 0.555 | 0.545 | 0.242 |
| Males | 43.8 ± 1.6 | 41.9 ± 2.2 | 45.2 ± 2.2 | 43.6 ± 1.6 | 0.741 | 0.831 | 0.934 |
Values are presented as mean ± SEM and p values unless otherwise specified. Significant p values are in bold.
DFF = distal forearm fracture; NA = not applicable; COC = combined oral contraceptive; BMI = body mass index; TBFM = total body fat mass; TBLM = total body lean mass; PA = physical activity.
p = controls versus mild trauma subjects, using Dunnett’s adjustment for multiple comparisons.
p = controls versus moderate trauma subjects, using Dunnett’s adjustment for multiple comparisons.
p = controls versus all DFF subjects.
µFE analysis-derived bone strength and fall load-to-strength ratios of the distal radius
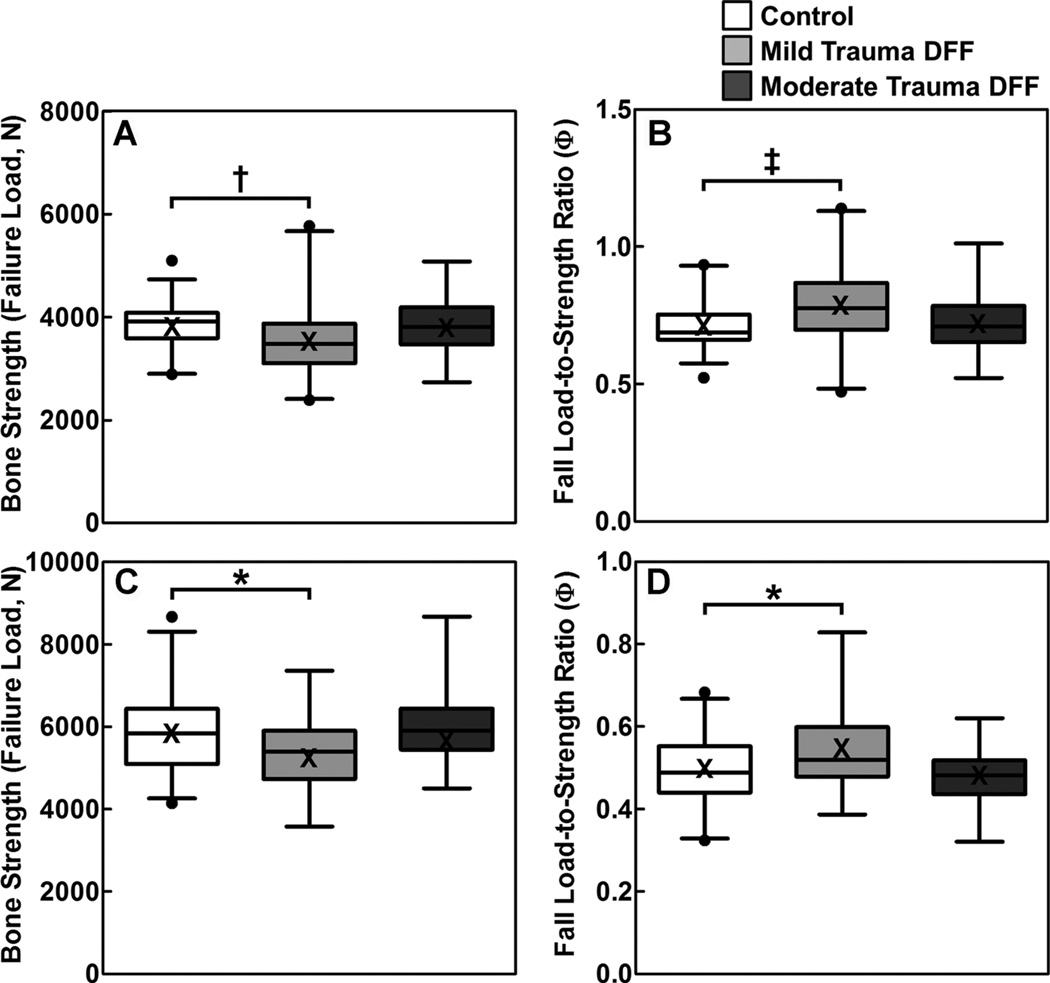
Figure 1 shows the µFE analysis-derived bone strength (ie, failure load) and fall load-to-strength ratios of the distal radius for the nonfracture controls and DFF subjects, by sex, stratified by mild or moderate trauma. As is evident in Fig. 1, women with a mild trauma DFF during childhood had a 9.1% reduction in failure load compared to female nonfracture controls (3493 ± 81 versus 3844 ± 60 N, respectively; p = 0.001), and 11.2% higher (“worse”) fall load-to-strength ratios (0.794 ± 0.016 versus 0.714 ± 0.012, respectively; p < 0.001). Similarly, men with a mild trauma DFF during childhood had an 8.2% reduction in failure load compared to male nonfracture controls (5353 ± 154 versus 5832 ± 109 N, respectively; p = 0.024), and 9.3% higher (“worse”) fall load-to-strength ratios (0.543 ± 0.014 versus 0.497 ± 0.010, respectively; p = 0.013) at the distal radius. By contrast, women and men with a moderate trauma DFF during childhood had similar values as sex-matched, nonfracture controls for these parameters (Tables 2 and 3).
Fig. 1.
Box plots (median with 25 to 75 percentile) and whiskers (2.5 to 97.5 percentile) for female (A) bone strength (failure load [N]) and (B) fall load-to-strength ratio (factor-of-risk [Φ]), and male (C) bone strength and (D) fall load-to-strength ratio at the distal radius, adjusted for age, height, and weight, in nonfracture controls and the mild-trauma and moderate-trauma DFF groups. Means are presented as an X in the box plots and are similar to the median values. *p < 0.05; †p < 0.01; ‡p < 0.001 compared with the sex-matched, nonfracture control group, using Dunnett’s adjustment for multiple comparisons. DFF = distal forearm fracture.
Table 2.
Strength, Cortical, and Trabecular Parameters of the Distal Radius and DXA Regional Areal BMD for Female Subjects With a Childhood DFF (Mild Trauma, Moderate Trauma, All DFF Subjects) and Nonfracture Controls
| Female controls (n = 75) |
Female DFF subjects | ||||||
|---|---|---|---|---|---|---|---|
| Mild trauma (n = 42) |
Moderate trauma (n = 32) |
All DFF subjects (n = 74) |
pa | pb | pc | ||
| Distal radius micro–finite element strength parameters | |||||||
| Failure load (N) | 3844 ± 60 | 3493 ± 81 | 3860 ± 92 | 3654 ± 62 | 0.001 | 0.986 | 0.033 |
| Fall load-to-strength ratio | 0.714 ± 0.012 | 0.794 ± 0.016 | 0.718 ± 0.018 | 0.761 ± 0.012 | <0.001 | 0.975 | 0.009 |
| Distal radius cortical parameters | |||||||
| Ct.A (mm2) | 59.3 ± 0.8 | 53.3 ± 1.1 | 61.2 ± 1.3 | 56.8 ± 0.9 | <0.001 | 0.363 | 0.051 |
| Ct.Th (mm) | 1.03 ± 0.02 | 0.96 ± 0.02 | 1.08 ± 0.02 | 1.01 ± 0.02 | 0.013 | 0.166 | 0.413 |
| EC (mm) | 47.5 ± 0.5 | 47.4 ± 0.7 | 46.2 ± 0.8 | 46.8 ± 0.5 | 0.982 | 0.270 | 0.354 |
| PC (mm) | 65.1 ± 0.5 | 64.7 ± 0.7 | 64.4 ± 0.8 | 64.6 ± 0.5 | 0.904 | 0.744 | 0.522 |
| Ct.vBMD (mg/cm3) | 989 ± 3 | 973 ± 4 | 997 ± 5 | 983 ± 3 | 0.003 | 0.299 | 0.199 |
| Ct.TMD (mg/cm3) | 999 ± 3 | 984 ± 4 | 1006 ± 4 | 994 ± 3 | 0.002 | 0.239 | 0.206 |
| Ct.Po (%) | 0.67 ± 0.05 | 0.72 ± 0.07 | 0.67 ± 0.07 | 0.70 ± 0.05 | 0.798 | 0.999 | 0.717 |
| Distal radius trabecular parameters | |||||||
| BV/TV | 0.133 ± 0.003 | 0.128 ± 0.005 | 0.132 ± 0.005 | 0.130 ± 0.003 | 0.613 | 0.980 | 0.501 |
| Tb.N (1/mm) | 1.86 ± 0.03 | 1.84 ± 0.04 | 1.84 ± 0.04 | 1.84 ± 0.03 | 0.857 | 0.936 | 0.617 |
| Tb.Th (mm) | 0.071 ± 0.001 | 0.069 ± 0.002 | 0.071 ± 0.002 | 0.070 ± 0.001 | 0.495 | 0.993 | 0.454 |
| Tb.Sp (mm) | 0.48 ± 0.01 | 0.49 ± 0.01 | 0.48 ± 0.02 | 0.48 ± 0.01 | 0.830 | 0.908 | 0.570 |
| DXA regional aBMD | |||||||
| UD radius (g/cm2) | 0.468 ± 0.005 | 0.431 ± 0.007 | 0.465 ± 0.008 | 0.446 ± 0.006 | <0.001 | 0.928 | 0.007 |
| Total radius (g/cm2) | 0.683 ± 0.005 | 0.649 ± 0.007 | 0.694 ± 0.008 | 0.669 ± 0.006 | <0.001 | 0.437 | 0.090 |
| Spine (g/cm2) | 1.240 ± 0.014 | 1.176 ± 0.019 | 1.247 ± 0.021 | 1.207 ± 0.014 | 0.012 | 0.946 | 0.106 |
| FN (g/cm2) | 1.068 ± 0.014 | 0.992 ± 0.018 | 1.081 ± 0.021 | 1.031 ± 0.014 | 0.002 | 0.849 | 0.066 |
| Trochanter (g/cm2) | 0.843 ± 0.012 | 0.773 ± 0.016 | 0.850 ± 0.018 | 0.807 ± 0.012 | 0.001 | 0.925 | 0.045 |
| Total hip (g/cm2) | 1.069 ± 0.014 | 0.995 ± 0.019 | 1.090 ± 0.022 | 1.037 ± 0.015 | 0.005 | 0.631 | 0.144 |
| Total body (g/cm2) | 1.203 ± 0.007 | 1.168 ± 0.010 | 1.208 ± 0.011 | 1.185 ± 0.007 | 0.008 | 0.936 | 0.092 |
Values are presented mean ± SEM (adjusted for age, height, weight) and p values. Significant p values are in bold.
DXA = dual-energy X-ray absorptiometry; BMD = bone mineral density; DFF = distal forearm fracture; Ct.A = cortical area; Ct.Th = cortical thickness; EC = endocortical circumference; PC = periosteal circumference; Ct.vBMD = cortical volumetric bone mineral density; Ct.TMD = cortical tissue mineral density; Ct.Po = cortical porosity; BV/TV = bone volume/total volume; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular separation; aBMD = areal BMD; UD = ultradistal; FN = femoral neck.
p = controls versus mild trauma subjects, using Dunnett’s adjustment for multiple comparisons.
p = controls versus moderate trauma subjects, using Dunnett’s adjustment for multiple comparisons.
p = controls versus all DFF subjects.
Table 3.
Strength, Cortical, and Trabecular Parameters of the Distal Radius and DXA Regional Areal BMD for Male Subjects With a Childhood DFF (Mild Trauma, Moderate Trauma, All DFF Subjects) and Nonfracture Controls
| Male DFF subjects | |||||||
|---|---|---|---|---|---|---|---|
| Male controls (n = 75) |
Mild trauma (n = 37) |
Moderate trauma (n = 36) |
All DFF subjects (n = 73) |
pa | pb | pc | |
| Distal radius micro–finite element strength parameters | |||||||
| Failure load (N) | 5832 ± 109 | 5353 ± 154 | 5995 ± 161 | 5661 ± 113 | 0.024 | 0.637 | 0.266 |
| Fall load-to-strength ratio | 0.497 ± 0.010 | 0.543 ± 0.014 | 0.482 ± 0.014 | 0.514 ± 0.0101 | 0.013 | 0.639 | 0.212 |
| Distal radius cortical parameters | |||||||
| Ct.A (mm2) | 76.3 ± 1.6 | 70.0 ± 2.2 | 82.5 ± 2.3 | 76.0 ± 1.7 | 0.041 | 0.061 | 0.834 |
| Ct.Th (mm) | 1.07 ± 0.02 | 1.01 ± 0.03 | 1.15 ± 0.03 | 1.07 ± 0.02 | 0.131 | 0.121 | 0.940 |
| EC (mm) | 58.7 ± 0.7 | 58.3 ± 1.0 | 57.7 ± 1.0 | 58.0 ± 0.7 | 0.933 | 0.698 | 0.521 |
| PC (mm) | 81.6 ± 0.7 | 80.0 ± 1.0 | 80.5 ± 1.1 | 80.3 ± 0.7 | 0.390 | 0.640 | 0.206 |
| Ct.vBMD (mg/cm3) | 941 ± 4 | 936 ± 5 | 954 ± 5 | 944 ± 4 | 0.708 | 0.096 | 0.496 |
| Ct.TMD (mg/cm3) | 961 ± 3 | 956 ± 5 | 974 ± 5 | 964 ± 3 | 0.505 | 0.080 | 0.593 |
| Ct.Po (%) | 1.54 ± 0.09 | 1.41 ± 0.12 | 1.47 ± 0.13 | 1.44 ± 0.09 | 0.641 | 0.878 | 0.430 |
| Distal radius trabecular parameters | |||||||
| BV/TV | 0.175 ± 0.003 | 0.161 ± 0.004 | 0.172 ± 0.005 | 0.166 ± 0.003 | 0.014 | 0.763 | 0.037 |
| Tb.N (1/mm) | 2.15 ± 0.02 | 2.04 ± 0.03 | 2.10 ± 0.03 | 2.07 ± 0.02 | 0.011 | 0.345 | 0.011 |
| Tb.Th (mm) | 0.082 ± 0.001 | 0.079 ± 0.002 | 0.082 ± 0.002 | 0.080 ± 0.001 | 0.392 | 0.985 | 0.493 |
| Tb.Sp (mm) | 0.39 ± 0.01 | 0.42 ± 0.01 | 0.40 ± 0.01 | 0.41 ± 0.01 | 0.003 | 0.378 | 0.006 |
| DXA regional aBMD | |||||||
| UD radius (g/cm2) | 0.572 ± 0.007 | 0.540 ± 0.010 | 0.587 ± 0.010 | 0.563 ± 0.008 | 0.024 | 0.444 | 0.368 |
| Total radius (g/cm2) | 0.806 ± 0.008 | 0.764 ± 0.011 | 0.821 ± 0.011 | 0.792 ± 0.008 | 0.003 | 0.468 | 0.182 |
| Spine (g/cm2) | 1.253 ± 0.016 | 1.197 ± 0.023 | 1.228 ± 0.023 | 1.212 ± 0.016 | 0.092 | 0.632 | 0.082 |
| FN (g/cm2) | 1.134 ± 0.017 | 1.045 ± 0.025 | 1.133 ± 0.025 | 1.088 ± 0.018 | 0.007 | 0.999 | 0.065 |
| Trochanter (g/cm2) | 0.969 ± 0.016 | 0.877 ± 0.022 | 0.934 ± 0.023 | 0.905 ± 0.016 | 0.002 | 0.363 | 0.005 |
| Total hip (g/cm2) | 1.162 ± 0.017 | 1.071 ± 0.023 | 1.144 ± 0.024 | 1.107 ± 0.017 | 0.003 | 0.767 | 0.020 |
| Total body (g/cm2) | 1.323 ± 0.010 | 1.265 ± 0.014 | 1.302 ± 0.014 | 1.283 ± 0.010 | 0.002 | 0.415 | 0.006 |
Values are presented mean ± SEM (adjusted for age, height, weight) and p values. Significant p values are in bold.
DXA = dual-energy X-ray absorptiometry; BMD = bone mineral density; DFF = distal forearm fracture; Ct.A = cortical area; Ct.Th = cortical thickness; EC = endocortical circumference; PC = periosteal circumference; Ct.vBMD = cortical volumetric bone mineral density; Ct.TMD = cortical tissue mineral density; Ct.Po = cortical porosity; BV/TV = bone volume/total volume; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular separation; aBMD = areal BMD; UD = ultradistal; FN = femoral neck.
p = controls versus mild trauma subjects, using Dunnett’s adjustment for multiple comparisons.
p = controls versus moderate trauma subjects, using Dunnett’s adjustment for multiple comparisons.
p = controls versus all DFF subjects.
Cortical and trabecular bone parameters of the distal radius by HRpQCT
Detailed macrostructural and microstructural analyses of the distal radius (Tables 2 and 3) revealed that, compared with controls, women and men with a mild trauma childhood DFF had significant deficits in cortical area and tended to have thinner cortices. Also at the radius, women with a mild trauma DFF had significantly lower cortical vBMD and cortical tissue mineral density compared to controls, whereas men with a mild trauma DFF in childhood had significantly lower trabecular bone volume fraction and trabecular number, and had higher trabecular separation compared to controls. By contrast, none of the radius bone parameters differed significantly between either the women and men with a moderate trauma childhood DFF and their sex-matched controls. Finally, endocortical/periosteal circumferences and cortical porosity at the radius did not differ among any of the groups (Tables 2 and 3).
DXA-derived regional BMD and distal tibia bone parameters by µFE analysis and HRpQCT
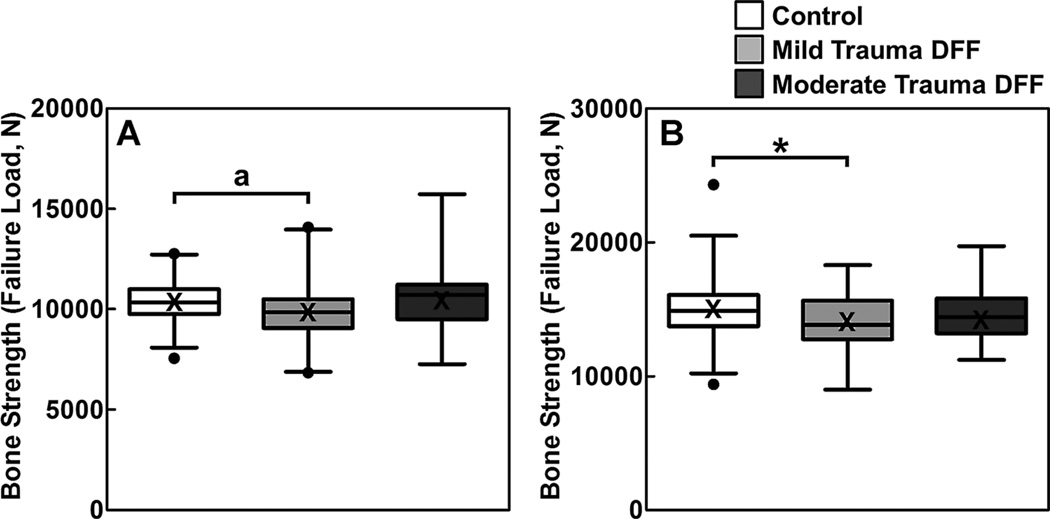
Skeletal deficits in the women and men with a childhood DFF owing to mild trauma were generalized, as these subjects had significantly lower (all p < 0.05) aBMD at the radius, hip, and total body regions compared to sex-matched controls (Tables 2 and 3). Further, compared to sex-matched controls, lumbar spine BMD was also significantly (p < 0.05) lower in the women with a mild trauma childhood DFF, whereas the deficit in this parameter among the men with a mild trauma childhood DFF approached statistical significance (p = 0.092). In addition, similar trends at the distal radius were observed at the distal tibia using µFE analysis and HRpQCT, although most of the bone micro-architectural parameters did not reach statistical significance (Fig. 2; Supporting Tables 2 and 3).
Fig. 2.
Box plots (median with 25 to 75 percentile) and whiskers (2.5 to 97.5 percentile) for bone strength (failure load [N]) at the distal tibia in (A) females and (B) males, adjusted for age, height, and weight, in nonfracture controls and the mild-trauma and moderate-trauma DFF groups. Means are presented as an X in the box plots and are similar to the median values. *p < 0.05; ap = 0.076 compared with the sex-matched, nonfracture control group, using Dunnett’s adjustment for multiple comparisons. DFF = distal forearm fracture.
Biochemical/hormonal parameters and COC use
In both the women and men, serum biochemical and hormonal parameters did not differ significantly between DFF subjects and sex-matched controls, either combined or separated by mild and moderate trauma DFFs (Supporting Table 4). Finally, there was no difference (p = 0.836) among the female groups (control, mild trauma DFF, and moderate trauma DFF) with respect to ever use of COCs (Table 1).
Discussion
In the present study, we found that young adult women and men with a childhood DFF due to mild trauma have reduced radial bone strength compared to sex-matched controls with no fracture history. In both women and men, this is primarily due to thinner radial cortices. In addition, we found that women with a mild trauma DFF during childhood have deficits in cortical microstructure, whereas men with a mild trauma DFF during childhood have less advantageous trabecular microstructure. Notably, the skeletal differences in the women and men with a mild trauma DFF during childhood were not confined to the distal radius because these subjects had generalized skeletal deficits as evidenced by their significantly reduced DXA-derived aBMD at both appendicular and axial skeletal sites, as well as the trend for reduced bone strength at the distal tibia. By contrast, both women and men with a moderate trauma DFF during childhood had similar bone strength and microstructure compared to sex-matched, nonfracture controls. Importantly, these observations are consistent with our previous findings in children and adolescents,(17) suggesting that a DFF due to mild, but not moderate, trauma during childhood may be an indicator of skeletal fragility.
Recent studies in young adult men(23) and premenopausal women(24,25) have reported significant deficits in bone strength and microstructure in subjects with prevalent fractures using µFE analysis and HRpQCT imaging of the distal radius and tibia. Similar to our results, Rudang and colleagues(23) showed that reductions in bone strength in young men with prevalent fractures were attributable to thinner cortices and deficits in trabecular microstructure, whereas in a study of young men by Taes and colleagues,(21) who used standard pQCT, a childhood fracture was associated with thinner radial cortices. Finally, in a 27-year prospective study of men and women with a childhood fracture, Buttazzoni and colleagues(22) recently found that men had low BMD and smaller bone size in young adulthood, and that similar trends were present in women. Thus, taken together, the available data suggest that young adults with prevalent fractures may have suboptimal peak bone structure leading to reduced bone strength.
The present study expands on our prior work by showing for the first time that trauma severity of a childhood DFF may help identify young adults who are likely to have skeletal deficits in bone strength and structure. Furthermore, we found that women and men with a mild trauma DFF during childhood had significantly lower DXA-derived BMD at both peripheral and central skeletal sites, suggesting that the structural alterations we observed in these subjects at appendicular sites may also be present in the axial skeleton. This possibility is supported by recent findings by Liu and colleagues(38) demonstrating moderate to strong correlations among bone parameters assessed by DXA, HRpQCT, and central QCT at peripheral and central sites.
We are unaware of previous studies in young adults that considered the severity of the associated trauma when comparing subjects with and without prior fracture. Our data complement our previous findings in children and adolescents(17) by showing that women and men who suffer a childhood DFF in the setting of mild, but not moderate, trauma have significant reductions in bone microstructure (eg, cortical vBMD, cortical tissue mineral density, trabecular bone volume fraction, trabecular number) compared to sex-matched controls. Interestingly, we did not observe any differences in cortical porosity at the distal radius or tibia between the DFF and nonfracture control groups. Our cortical porosity findings in young women and men are consistent with previous HRpQCT studies in boys and girls,(17) young men,(23) and premenopausal women.(25) Cortical porosity increases with aging, therefore it might be more a hallmark of fracture risk with aging,(39,40) but not necessarily during young adulthood.
Although several studies in children have found that bone mass and density are lower in those with fractures (reviewed in Clark and colleagues(41)) and that children with fractures gain less bone during growth and have a higher recurrence of fracture than fracture-free children,(42–45) not all studies have found this to be the case.(46,47) One explanation may be the lack of trauma severity classification in these studies, acknowledging that the actual forces involved in each instance cannot be quantified. Indeed, based on our observations, the severity of trauma leading to distal forearm fracture in childhood/adolescence, which is not usually accounted for in current clinical practice, may help identify individuals who are at high likelihood for impaired skeletal strength in adulthood, and who would be at further increased risk for fragility fractures later in life. Longitudinal follow-up would be required to better address this issue. We did examine a population-based cohort of 1776 children and adolescents with a DFF occurring in 1935 to 1992 who had ascertainment of future incident fractures during adulthood confirmed through medical records review.(48) In that study, we found an increased risk for a subsequent fragility fracture among the males when older, but no increased risk for future fractures among the females. However, trauma severity of the childhood DFF could not be classified in that cohort using the Landin criteria because relevant information was not always available in the medical records and additional interview with study subjects regarding fracture circumstance, as was performed in the present study, was not feasible.
Consistent with previous studies in boys and girls(17) and young men,(21) our data did not reveal any differences among the DFF and control groups in physical activity or in any of the biochemical parameters assessed. We acknowledge that our study may not have been sufficiently powered to detect statistically significant differences in these parameters. Notably, however, we did find that smoking and alcohol consumption tended to be more common in the DFF subjects relative to nonfracture controls. Another factor that could influence skeletal health and fracture risk is medication use. Although we excluded potential subjects if they had taken medications known to affect bone metabolism, we did not exclude women on COCs. Although some studies have reported negative associations between COC use and bone turnover or BMD,(49,50) others have not.(51–53) A recent systematic review of published studies from January 1975 through January 2012 concluded that COCs do not seem to exert any significant effects on bone.(54) Regardless, we found no significant differences in COC use among the women (control, mild trauma DFF, and moderate trauma DFF). Additional potential determinants of increased fracture risk during childhood include body weight at fracture, physical activity during childhood, and risk-taking behaviors(16)—factors we did not assess—certainly warrant attention in future studies.
Interestingly, both the women and men with a mild trauma childhood DFF tended to have higher adiposity than sex-matched controls. In addition, relative to sex-matched controls, women and men with a moderate trauma childhood DFF tended to have higher lean mass. Given that lean mass and bone are closely tied throughout the lifespan(55–59) and that obese children are overrepresented among DFF cases,(60,61) our findings suggest the possibility that behavioral traits established early in life may extend into adulthood. Future work will be necessary to further elucidate the roles of key biochemical parameters, lifestyle factors, and aspects of body composition that determine peak bone density, structure, and strength in women and men with childhood DFFs due to mild versus moderate trauma.
One of the major strengths of this study was the supplementation of medical record data by interview to better ascertain trauma severity. Additional study strengths include our focus on subjects with a childhood DFF, rather than a mix of fracture types, and the use of µFE analysis and HRpQCT because these techniques are perhaps currently state-of-the-art in terms of assessing bone strength and microstructure in vivo.
Our study also had a number of limitations. First, because correlation does not prove causality, our cross-sectional findings need to be confirmed prospectively. Second, it is possible that genetic factors might partly explain the underlying skeletal alterations in the subjects with a mild trauma childhood DFF, and we did not assess these factors as part of our study. A third issue is that our findings are based predominantly on white subjects and may not be generalizable to other races and ethnic groups. Last, a potential concern with the HRpQCT imaging is that despite permitting much higher resolution, the measures of bone microstructure are, in fact, estimates of the true values. For this reason, we used the bone strength measures derived from the µFE models as our primary outcome, which correlate well with biomechanically measured bone strength ex vivo(36) and should be less influenced by the resolution of the technique. Nonetheless, the cortical and trabecular microstructural bone variables do provide potential structural explanations for the alterations in bone strength that we observed.
In conclusion, a mild trauma DFF during childhood presages suboptimal peak bone density, structure, and strength in young adulthood. By contrast, young adult women and men with a DFF due to moderate trauma during childhood have similar bone strength compared to sex-matched controls with no history of fracture. Further work is needed to determine the underlying causes for these observations. Nevertheless, children and adolescents who suffer mild trauma fractures may benefit from lifestyle interventions that encourage achievement of maximal skeletal health.
Supplementary Material
Acknowledgments
This work was supported by NIH grants: P01 AG004875 (SKh) from the National Institute of Aging (NIA); R01 AR027065 (SKh/SA) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS); UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS) (to the Mayo Clinic Center for Clinical and Translational Science [CCaTS]); T32 DK007352 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Training Program in Endocrinology, Diabetes, and Metabolism (to JNF). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. We thank the women and men for their participation in this study. We also thank Susan Demaray for sample processing, James Peterson for data management, Margaret Holets for performing the HRpQCT scans, and the Mayo Immunochemical Core Laboratory for performing the biochemical assays.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
SA has served on a scientific advisory board for Merck & Co. SKh has served on scientific advisory boards for Amgen and Bone Therapeutics. All other authors state that they have no conflicts of interest.
Authors’ roles: Data collection: JNF and LKM. Data analysis: SJA and EJA. Data interpretation: JNF and SA. Drafting manuscript: JNF and SA. Revising manuscript content: JNF, SKh, SJA, EJA, SKi, LKM, LJM, and SA. Approving final version of manuscript: JNF, SKh, SJA, EJA, SKi, LKM, LJM, and SA. SA had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–727. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35(2):375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Lewiecki EM. Review of guidelines for bone mineral density testing and treatment of osteoporosis. Curr Osteoporos Rep. 2005;3(3):75–83. doi: 10.1007/s11914-005-0014-x. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2014. Available from: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop Scand Suppl. 1983;202:1–109. [PubMed] [Google Scholar]
- 7.Kramhoft M, Bodtker S. Epidemiology of distal forearm fractures in Danish children. Acta Orthop Scand. 1988;59:557–559. doi: 10.3109/17453678809148784. [DOI] [PubMed] [Google Scholar]
- 8.Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth. J Bone Joint Surg. 1989;71-A:1225–1231. [PubMed] [Google Scholar]
- 9.Khosla S, Melton LJ, III, Dekutoski MB, et al. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA. 2003;290(11):1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ, 3rd, Riggs BL, van Lenthe GH, et al. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res. 2007;22(9):1442–1448. doi: 10.1359/jbmr.070514. [DOI] [PubMed] [Google Scholar]
- 11.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: The OFELY Study. J Bone Miner Res. 2007;22(3):425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 12.Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010;25(12):2296–2305. doi: 10.1002/jbmr.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu XS, Stein EM, Zhou B, et al. Individual trabecula segmentation (ITS)-based morphological analyses and microfinite element analysis of HR-pQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J Bone Miner Res. 2012;27(2):263–272. doi: 10.1002/jbmr.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szulc P, Boutroy S, Vilayphiou N, et al. Cross-sectional analysis of the association between fragility fractures and bone microarchitecture in older men: the STRAMBO study. J Bone Miner Res. 2011;26(6):1358–1367. doi: 10.1002/jbmr.319. [DOI] [PubMed] [Google Scholar]
- 15.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4(6):382–398. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 16.Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 17.Farr JN, Amin S, Melton LJ, III, et al. Bone strength and structural deficits in children and adolescents with a distal forearm fracture due to mild trauma. J Bone Miner Res. 2014;29:590–599. doi: 10.1002/jbmr.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari S, Rizzoli R, Slosman D, Bonjour JP. Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab. 1998;83:358–361. doi: 10.1210/jcem.83.2.4583. [DOI] [PubMed] [Google Scholar]
- 19.Loro ML, Sayre J, Roe TF, et al. Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab. 2000;85(10):3908–3918. doi: 10.1210/jcem.85.10.6887. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Cheng S, Alen M, Seeman E. Bone’s structural diversity in adult females is established before puberty. J Clin Endocrinol Metab. 2009;94(5):1555–1561. doi: 10.1210/jc.2008-2339. [DOI] [PubMed] [Google Scholar]
- 21.Taes Y, Lapauw B, Griet V, et al. Prevalent fractures are related to cortical bone geometry in young healthy men at age of peak bone mass. J Bone Miner Res. 2010;25(6):1433–1440. doi: 10.1002/jbmr.17. [DOI] [PubMed] [Google Scholar]
- 22.Buttazzoni C, Rosengren BE, Tveit M, et al. Does a childhood fracture predict low bone mass in young adulthood? A 27-year prospective controlled study. J Bone Miner Res. 2013;28(2):351–359. doi: 10.1002/jbmr.1743. [DOI] [PubMed] [Google Scholar]
- 23.Rudang R, Darelid A, Nilsson M, et al. X-ray-verified fractures are associated with finite element analysis-derived bone strength and trabecular microstructure in young adult men. J Bone Miner Res. 2013;28(11):2305–2316. doi: 10.1002/jbmr.1974. [DOI] [PubMed] [Google Scholar]
- 24.Rozental TD, Deschamps LN, Taylor A, et al. Premenopausal women with a distal radial fracture have deteriorated trabecular bone density and morphology compared with controls without a fracture. J Bone Joint Surg Am. 2013;95(7):633–642. doi: 10.2106/JBJS.L.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevalley T, Bonjour JP, van Rietbergen B, Ferrari S, Rizzoli R. Fracture history of healthy premenopausal women is associated with a reduction of cortical microstructural components at the distal radius. Bone. 2013;55(2):377–383. doi: 10.1016/j.bone.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melton LJ., III Thethreattomedical-recordsresearch. N Engl J Med. 1997;337(20):1466–1470. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 28.Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2008;23(2):173–179. doi: 10.1359/jbmr.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riggs BL, Melton LJ, III, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 30.Khosla S, Riggs BL, Atkinson EJ, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21(1):124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicks KM, Amin S, Atkinson EJ, et al. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res. 2012;27(3):637–644. doi: 10.1002/jbmr.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farr JN, Charkoudian N, Barnes JN, et al. Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab. 2012;97(11):4219–4227. doi: 10.1210/jc.2012-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 34.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–528. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29(10):1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Pistoia W, van Rietbergen B, Lochmuller EM, et al. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842–848. doi: 10.1016/s8756-3282(02)00736-6. [DOI] [PubMed] [Google Scholar]
- 37.Chiu J, Robinovitch SN. Prediction of upper extremity impact forces during falls on the outstretched hand. J Biomech. 1998;31(12):1169–1176. doi: 10.1016/s0021-9290(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 38.Liu XS, Cohen A, Shane E, et al. Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res. 2010;25(10):2229–2238. doi: 10.1002/jbmr.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender related differences in the geometric properties and biomechanical significance of intra-cortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25(5):983–993. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bala Y, Zebaze R, Ghasem-Zadeh A, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014;29:1356–1362. doi: 10.1002/jbmr.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones IE, Taylor RW, Williams SM, Manning PJ, Goulding A. Four-year gain in bone mineral in girls with and without past forearm fractures: a DXA study. J Bone Miner Res. 2002;17(6):1065–1072. doi: 10.1359/jbmr.2002.17.6.1065. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistentbone fragility? J BoneMiner Res. 2006;21(4):501–507. doi: 10.1359/jbmr.051215. [DOI] [PubMed] [Google Scholar]
- 44.Cheng S, Xu L, Nicholson PHF, et al. Low volumetric BMD is linked to upper-limb fracture in pubertal girls and persists into adulthood: a seven-year cohort study. Bone. 2009;45:480–486. doi: 10.1016/j.bone.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Goulding A, Jones L, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15(10):2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 46.Pye SR, Tobias J, Silman AJ, et al. Childhood fractures do not predict future fractures: results from the European prospective osteoporosis study. J Bone Miner Res. 2009;24:1314–1318. doi: 10.1359/jbmr.090220. [DOI] [PubMed] [Google Scholar]
- 47.Beccard R, Land C, Semler O, et al. Do bone mineral density, bone geometry and the functional muscle-bone unit explain bone fractures in healthy children and adolescents? Horm Res Paediatr. 2010;74(5):312–318. doi: 10.1159/000313380. [DOI] [PubMed] [Google Scholar]
- 48.Amin S, Melton LJ, 3rd, Achenbach SJ, et al. A distal forearm fracture in childhood is associated with an increased risk for future fragility fractures in adult men, but not women. J Bone Miner Res. 2013;28(8):1751–1759. doi: 10.1002/jbmr.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholes D, Ichikawa L, LaCroix AZ, et al. Oral contraceptive use and bone density in adolescent and young adult women. Contraception. 2010;81(1):35–40. doi: 10.1016/j.contraception.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scholes D, Hubbard RA, Ichikawa LE, et al. Oral contraceptive use and bone density change in adolescent and young adult women: a prospective study of age, hormone dose, and discontinuation. J Clin Endocrinol Metab. 2011;96(9):E1380–E1387. doi: 10.1210/jc.2010-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed SD, Scholes D, LaCroix AZ, et al. Longitudinal changes in bone density in relation to oral contraceptive use. Contraception. 2003;68(3):177–182. doi: 10.1016/s0010-7824(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 52.Massaro M, Di Carlo C, Gargano V, et al. Effects of the contraceptive patch and the vaginal ring on bone metabolism and bone mineral density: a prospective, controlled, randomized study. Contraception. 2010;81(3):209–214. doi: 10.1016/j.contraception.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Gai L, Jia Y, Zhang M, et al. Effect of two kinds of different combined oral contraceptives use on bone mineral density in adolescent women. Contraception. 2012;86(4):332–336. doi: 10.1016/j.contraception.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Nappi C, Bifulco G, Tommaselli GA, Gargano V, Di Carlo C. Hormonal contraception and bone metabolism: a systematic review. Contraception. 2012;86(6):606–621. doi: 10.1016/j.contraception.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Goulding A, Taylor RW, Grant AM, et al. Relationships of appendicular LMI and total body LMI to bone mass and physical activity levels in a birth cohort of New Zealand five-year olds. Bone. 2009;45(3):455–459. doi: 10.1016/j.bone.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unite in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95(4):1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wetzsteon RJ, Zemel BS, Shults J, et al. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48(5):1103–1108. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lebrasseur NK, Achenbach SJ, Melton LJ, 3rd, Amin S, Khosla S. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res. 2012;27(10):2159–2169. doi: 10.1002/jbmr.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker JF, Davis M, Alexander R, et al. Associations between body composition and bone density and structure in men and women across the adult age spectrum. Bone. 2013;53(1):34–41. doi: 10.1016/j.bone.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goulding A, Cannan R, Williams SM, et al. Bone mineral density in girls with forearm fractures. J Bone Miner Res. 1998;13(1):143–148. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 61.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20(12):2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.