Abstract
Bacterial sepsis is characterized by a rapid increase in the expression of inflammatory mediators to initiate the acute phase response in liver. Inflammatory mediator release is counterbalanced by the coordinated expression of anti-inflammatory molecules such as interleukin 1 receptor antagonist (IL1-Ra) through time. This study determined whether activation of pregnane X receptor (PXR, NR1I2) alters the lipopolysaccharide (LPS)-inducible gene expression program in primary cultures of hepatocytes (PCHs). Preactivation of PXR for 24 hours in PCHs isolated from wild-type mice suppressed the subsequent LPS-inducible expression of the key inflammatory mediators interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNFα) but not in PCHs isolated from Pxr-null (PXR-knockout [KO]) mice. Basal expression of key inflammatory cytokines was elevated in PCHs from PXR-KO mice. Stimulation of PCHs from PXR-KO mice with LPS alone produced enhanced levels of IL-1β when compared with wild-type mice. Experiments performed using PCHs from both humanized-PXR transgenic mice as well as human donors indicate that prolonged activation of PXR produces an increased secretion of IL1-Ra from cells through time. Our data reveal a working model that describes a pivotal role for PXR in both inhibiting as well as in resolving the inflammatory response in hepatocytes. Understanding the molecular details of how PXR is converted from a positive regulator of drug-metabolizing enzymes into a transcriptional suppressor of inflammation in liver will provide new pharmacologic strategies for modulating inflammatory-related diseases in the liver and intestine.
Pregnane X receptor (PXR, NR1I2) is a ligand-activated nuclear receptor (NR) superfamily member expressed at high levels within the enterohepatic system of mammals. The biologic function of PXR is mediated together with its obligate partner retinoid X receptor α (Kliewer et al., 1998; Lehmann et al., 1998). To date, the ligands identified for PXR have been numerous, and they are structurally diverse as naturally occurring steroids (Kliewer et al., 1998), antibiotics (Lehmann et al., 1998), bile acids (Staudinger et al., 2001a; Xie et al., 2001; Goodwin et al., 2003), anticancer agents (Desai et al., 2002; Nallani et al., 2004), and the active ingredients in several herbal remedies (Moore et al., 2000; Brobst et al., 2004; Ding and Staudinger, 2005). Ligand-activated PXR positively regulates the drug-inducible expression of genes encoding key drug transporters and drug metabolizing enzymes that function coordinately to increase the uptake, metabolism, excretion, and efflux of xenobiotics from the body. In this way, PXR activation is associated with increased metabolism and clearance of a myriad of potentially toxic compounds, and is classically thought of as a protective response.
Clinical treatment with PXR activators can also lead to the repression or attenuation of other biochemical pathways in liver and intestine including both energy metabolism and the inflammatory response (Moreau et al., 2008). For example, it was demonstrated nearly 45 years ago that treatment with rifampicin (Rif), a prototypical ligand of human PXR, leads to a compromised ability to mount an effective immune response in cell-based assays (Păunescu, 1970). In vivo studies in rodents suggest that PXR activation suppresses inflammation and the acute phase response (APR) by attenuating the activity of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signaling (Shah et al., 2007). More recent studies using rodents indicate that PXR activation regulates intestinal barrier function through an interaction with Toll-like receptor 4 (TLR4), the molecular target of lipopolysaccharide (LPS) stimulation (Venkatesh et al., 2014). It is now widely accepted that the activation of PXR is associated with general suppression of the immune response, particularly in the intestine (Cheng et al., 2012; Dou et al., 2012, 2014). Currently, little is known regarding the molecular mechanism of this phenomenon in the liver or in hepatocytes.
Recent investigations indicate that ligand-mediated activation of liver-enriched nuclear receptors (NRs), liver receptor homolog-1, and liver X receptor β (LXRβ) initiate anti-inflammatory mechanisms and pathways that suppress the hepatic APR (Venteclef et al., 2010). These investigations reveal that post-translational modification of these two NRs by small-ubiquitin-related modifier (SUMO) is required for suppression of the hepatic expression of the acute phase protein and marker of the APR haptoglobin. A theme emerges in which metabolic NRs are modified by SUMO proteins to suppress the inflammatory response, particularly in hepatocyte and in macrophage cell types (reviewed in Treuter and Venteclef, 2011). Previous work from our laboratory revealed that PXR is also the target of SUMO proteins to suppress tumor necrosis factor α (TNFα)–mediated production of interleukin 1β (IL-1β) in primary cultured hepatocytes (PCHs) (Hu et al., 2010). Although much is known about LPS administration as an experimental model of Gram-negative bacterial sepsis in vivo in rodents, very little is known about PXR-mediated modulation of the LPS-inducible gene expression program in PCHs across species. We therefore wanted to using both mouse and human PCHs as model systems to further characterize the ability of ligand-mediated activation of PXR to suppress a broad array of LPS-inducible hepatic inflammatory response genes.
Administration of LPS, a glycolipid constituent of the outer membrane of Gram-negative bacteria, to live animals or cultured cells initiates a signaling cascade in cells through TLR4 receptor multiprotein complexes that include CD14, Myd88, and MD-2 as coreceptor proteins (Buer and Balling, 2003). Here, we perform concentration- and time-response analyses of the LPS-inducible gene expression program in both mouse and human PCHs. Our data reveal that PCHs respond to stimulation with LPS to rapidly induce the expression of key inflammatory mediators, including IL-1β and interleukin 6 (IL-6). Using a commercial gene array platform, we show that 24-hours of pretreatment of mouse PCHs with a strong rodent PXR activator, pregnenolone 16α carbonitrile (PCN), suppresses subsequent LPS-inducible inflammatory responses in PCHs. The follow-up experiments using PCHs isolated from Pxr-null (PXR-knockout [KO]) mice demonstrate that the diminution of LPS-inducible gene expression by PCN requires functional PXR in hepatocytes. Finally, using PCHs derived from both humanized-PXR transgenic mice (hPXRtg) and human donors, we indicate that activation of PXR enhances the secretion of interleukin 1 receptor antagonist (IL1-Ra), a key negative regulator of IL1 signaling, from hepatocytes. Taken together, these data shed new light on the molecular mechanisms that comprise the interface between PXR activation and resolution of the APR in liver in mammals.
Materials and Methods
Isolation and Culturing of Primary Hepatocytes.
PXR knockout (PXR-KO) mice were generated as previously described elsewhere (Staudinger et al., 2001b). The hPXRtg mice were previously described elsewhere (Lichti-Kaiser and Staudinger, 2008). Hepatocytes were isolated from male congenic (C57BL6) wild-type and PXR-KO mice aged 6 to 10 weeks using a standard collagenase perfusion method as described previously elsewhere (Staudinger et al., 2003). The primary cultures of human hepatocytes used in this study were derived from samples collected and provided by the University of Kansas Medical Center (KUMC) Department of Pharmacology, Toxicology and Therapeutics Hepatocyte Core Laboratory, and the KU Liver Center which is sponsored by the Department of Pharmacology, Toxicology and Therapeutics Biospecimen Core Laboratory and the Liver Center at the University of Kansas Medical Center.
Fresh isolated human hepatocytes were plated at a cell density of 0.5 × 106 cells/well in 12-well plates previously coated with 0.2 mg/ml type I collagen. The isolated hepatocytes (>80% viability) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 100 nM dexamethasone, 100 nM insulin, 100 U/ml penicillin G, 100 µg/ml streptomycin, and 10% bovine calf serum and kept at 37°C in a humidified incubator with 95% air/5% CO2. The hepatocytes were allowed to attach to the plate for 4 hours, and the medium was then replaced with serum-free Williams E medium, as described previously elsewhere (Staudinger et al., 2003).
Total RNA Isolation, Reverse Transcription, and Real-Time Quantitative-Polymerase Chain Reaction Analysis.
Real-time quantitative polymerase chain reaction (RT-qPCR) was performed as described in Ding and Staudinger (2005), according to the manufacturer’s instructions (SABiosciences, Frederick, MD).
Analysis of Secreted IL1-Ra in PCHs.
A 200-μl aliquot of cell medium was removed and combined with 200-μl 2X Laemmli buffer (4% SDS, 20% glycerol, 120 mM Tris-Cl pH 6.8, 10% 2-mercaptoethanol, 0.004% Bromphenol Blue) with 50 mM dithiothreitol. After removal of the culture medium, the cells were harvested by scraping them into 1X phosphate-buffered saline, and they were pelleted briefly in a microfuge. After the removal of the phosphate-buffered saline, the cells were lysed in 1X Laemmli buffer, and equal amounts were resolved using 10% SDS-PAGE. Western blot analysis was performed as described previously elsewhere (Xu et al., 2009) using a monoclonal antibody that recognizes human and mouse IL1-Ra (NBP1-96673; Novus Biologicals, Oakville, Ontario, Canada). Western blot images were quantitated by densitometry scanning of the X-ray films with the UVP Biodoc-It 220 image analysis system (UVP, LLC, Upland, CA) and 1D Gel Analysis Software (TotalLab, Newcastle upon Tyne, United Kingdom).
Statistical Analysis.
Where appropriate the statistical differences within an experimental group were determined using a one-way analysis of variance followed by the Duncan’s multiple range post hoc test. Statistical differences between the experimental groups were determined using Student’s t test.
Results
LPS-Inducible Concentration- and Time-Response Analysis of Key Inflammatory Mediators in Mouse and Human PCHs.
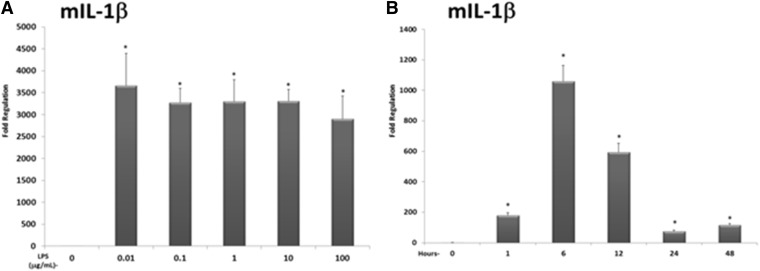
Our primary goal was to determine the extent to which preactivation of PXR alters the subsequent LPS-inducible gene expression program in a PCHs. Therefore, we first examined LPS-inducible IL-1β gene expression in both mouse and human PCHs, performing concentration- and time-response analyses using RT-qPCR. Treatment of PCHs isolated from wild-type male mice aged 6 to 10 weeks with increasing amounts of LPS (0.01, 0.1, 1, 10, and 100 μg/ml of medium) for 12 hours produced robust induction of IL-1β messenger RNA expression at all concentrations examined (Fig. 1A). Based upon these results, and to provide a relatively strong inflammatory stimulus for our subsequent studies of possible PXR-mediated effects, we reasoned that LPS treatment should be performed using a relatively high concentrations (10 μg/ml) for initiation of the inflammatory response.
Fig. 1.
Concentration- and time-dependent analysis of the expression of IL-1β in mouse PCHs. (A) PCHs isolated from wild-type (C57Bl6) mice were treated with either vehicle (0.1% saline in medium) or increasing concentrations of LPS (0.01, 0.1, 1.0, 10, or 100 μg/ml) for 12 hours. Total RNA was isolated, and the relative expression level of IL-1β was determined using RT-qPCR. (B) PCHs isolated from wild-type (C57Bl6) mice were treated with LPS (10 μg/ml) for increasing times (1, 6, 12, 24, and 48 hours). Total RNA was isolated, and the relative expression level of IL-1β was determined. All data are normalized to β-actin levels and are presented as fold regulation. Asterisks indicate a statistical difference from vehicle-treated samples (n = 3, and P < 0.05).
Although all the time points examined (1, 6, 12, 24, and 48 hours) exhibited significant increases in IL-1β gene expression levels in mouse PCHs, the 6- and 12-hour time points had the largest increases, showing an approximately 1,000- and 600-fold increase in IL-1β expression, respectively (Fig. 1B). When PCHs derived from human donors were used in identical analyses, a similar concentration- and time-dependent induction of IL-1β was observed (Fig. 2, A and B). Examination of the kinetics of LPS-inducible mouse and human IL-6 produced very similar results (Supplemental Figs. 1–4).
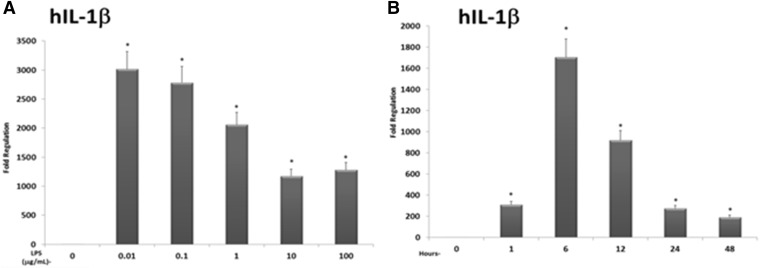
Fig. 2.
Concentration- and time-dependent analysis of the expression of IL-1β in human PCHs. (A) PCHs isolated from a human donor were treated with either vehicle (0.1% saline in medium) or increasing concentrations of LPS (0.01, 0.1, 1.0, 10, or 100 μg/ml) for 12 hours. Total RNA was isolated, and the relative expression level of IL-1β was determined. (B) PCHs isolated from a human donor were treated with LPS (10 μg/ml) for increasing times (1, 6, 12, 24, and 48 hours). Total RNA was isolated, and the relative expression level of IL-1β was determined. All data are normalized to β-actin levels and are presented as fold regulation. Asterisks indicate a statistical difference from vehicle-treated samples (n = 3, and P < 0.05).
Taken together, these data indicate that treatment with LPS induces the expression of key inflammatory mediators in both mouse and human PCHs, even at very low concentrations (10 ng/ml). Our data are in agreement with other previously published investigations regarding the production of inflammatory cytokines in PCHs in response to treatment with LPS (Panesar et al., 1999; Liu et al., 2002). A time-response analysis of CYP3A gene expression using PCN (10 μM) as a prototypical rodent PXR activator and Rif (10 μM) as a prototypical human PXR activator indicated that 24-hours of treatment produces maximal CYP3A gene expression that is sustained through the 48-hour time point (Supplemental Fig. 5). Taken together, these data reveal that pretreatment with PXR activators for 24 hours and subsequent cotreatment of 12 hours together with LPS is expected to be useful treatment regimen in a broad assessment of the effect of preactivation of PXR on the subsequent LPS-inducible gene expression program in PCHs.
Preactivation of NR Superfamily Members PXR, FXR, and LXRα Suppresses LPS-inducible IL-1β Gene Expression in Mouse PCHs.
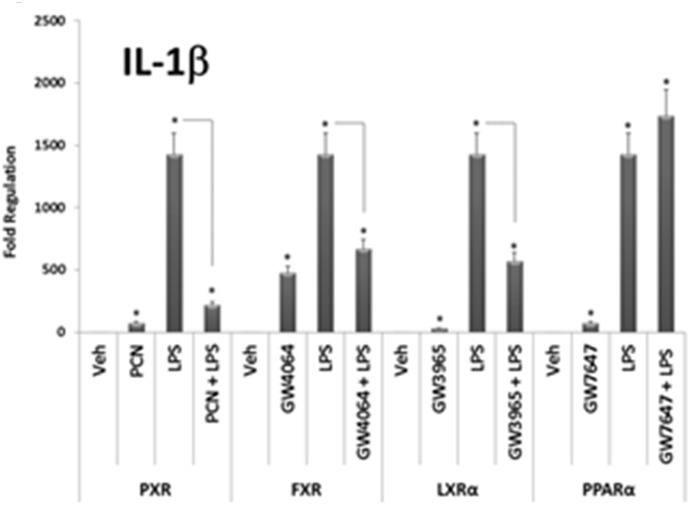
Together, IL-6 and IL-1β impart key aspects of the inflammatory response. IL-1β, in particular, is involved in regulating a variety of cellular activities including cell proliferation, differentiation, and apoptosis. Ligand-activation of NR superfamily members including PXR (Zhou et al., 2006), farnesoid x receptor (FXR, NR1H4) (Hollman et al., 2012), liver X receptor alpha (LXRα, NR1H3) (Ghisletti et al., 2007), and peroxisome proliferator antigen receptor α (PPARα, NR1C1) (Devchand et al., 1996) has been shown to suppress key aspects of the inflammatory response in several different cell types. Because expression of these four NR family members is highly enriched in hepatocytes, we sought to determine whether ligand-mediated activation of these receptors could alter LPS-inducible IL-1β gene expression in our PCHs (Fig. 3).
Fig. 3.
LPS induces the expression of the compensatory anti-inflammatory response gene IL1-Ra in primary cultures of mouse hepatocytes. Primary hepatocytes isolated from wild-type (C57Bl6) mice were treated with either vehicle (0.1% dimethylsulfoxide), PCN (10 μM), GW4064 [3-[(E)-2-[2-chloro-4-[[3-(2,6-dichlorophenyl)-5-propan-2-yl-1,2-oxazol-4-yl]methoxy]phenyl]ethenyl]benzoic acid] (1 μM), GW3965 [2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methyl-(2,2-diphenylethyl)amino]propoxy]phenyl]acetic acid] (1 μM), or GW7647 [2-[4-[2-[4-cyclohexylbutyl(cyclohexylcarbamoyl)amino]ethyl]phenyl]sulfanyl-2-methylpropanoic acid] (1 μM) for 24 hours. The medium was removed and replenished with medium containing the treatments for an additional 12 hours as indicated, respectively. Total RNA was isolated, and the relative expression level of IL-1β was determined. All data are normalized to β-actin levels and are presented as fold regulation. Asterisks indicate a statistical difference from vehicle-treated samples (n = 3, and P < 0.05). Dashed lines indicate a statistical difference between treatment with LPS alone for 12 hours when compared with pretreatment with cognate ligand for 24 hours and subsequent cotreatment together with LPS for an additional 12 hours.
As expected, treatment of mouse PCHs with LPS alone for 12 hours increased IL-1β expression dramatically. Preactivation of PXR, FXR, and LXRα for 24 hours with their cognate ligands suppressed subsequent LPS-inducible IL-1β gene expression. In contrast, preactivation of PPARα had no effect on subsequent LPS-inducible IL-1β gene expression. Treatment of PCHs with each cognate ligand alone for 24 hours produced comparatively small but statistically significant increases in the expression of IL-1β. Treatment with each ligand also induced expression of their prototypical target genes Cyp3a11, bile salt excretory protein, Cyp7a1, and Cyp4a14 for PXR, FXR, LXRα, and PPARα, respectively (data not shown). These data indicate that some, but not all, liver-enriched NR superfamily members may exhibit anti-inflammatory properties in PCHs, namely, PXR, FXR, and LXRα. It is also possible that these three liver-enriched NRs share a common molecular mechanism of repression of LPS-inducible inflammatory responses in PCHs, likely through alterations in posttranslational modifications such as SUMOylation (Treuter and Venteclef, 2011).
Unexpectedly, treatment with NR ligands alone induced statistically significant but relatively low levels of IL-1β expression, which was observed consistently throughout our studies and those performed by others (Vavassori et al., 2009). Although the molecular mechanism of this phenomenon remains elusive, it could include the presence of cryptic or low-affinity NR response elements within the IL-1β promoter.
Preactivation of PXR Suppresses Key LPS-Inducible NF-κB Target Genes.
An important transcriptional mediator of LPS signaling in cells is NF-κB (Sen and Baltimore, 1986). Treatment of primary cultures of mouse hepatocytes with LPS for 12 hours induced expression of 16 well-known NF-κB target genes (Table 1). Treatment with PCN alone for 24 hours suppressed the basal expression of numerous NF-κB target genes (Table 2). When compared with LPS treatment alone, treatment with PCN for 24 hours and subsequent cotreatment together with LPS for an additional 12 hours produced significantly lower expression levels of several notable LPS-inducible NF-κB target genes, including IL-1β, IL-6, TNFα, and IL1-Ra (Table 3). These data suggest that PCN diminishes the APR by inhibiting the LPS-inducible gene expression program of key inflammatory mediators.
TABLE 1.
Genes increased by LPS
PCHs isolated from wild-type mice were treated for 12 hours with either vehicle (0.9% saline) or LPS (10 μg/ml) (n = 4). Total RNA was isolated and RT-qPCR was performed. Data are expressed as fold induction ± S.D. where P ≤ 0.05.
| Gene Name | Fold Induction | S.D. |
|---|---|---|
| Cxcl3 | 393.9 | 80.8 |
| IL-1β | 314.4 | 77.4 |
| IL-6 | 128.9 | 43.2 |
| Csf3 | 63.4 | 20.4 |
| IL12β | 52.5 | 12.4 |
| Ccl5 | 50.5 | 11.5 |
| Ptgs2 | 50.5 | 10.6 |
| Ltb | 50.0 | 10.6 |
| IL1-Ra | 49.7 | 9.2 |
| IL-1α | 31.0 | 7.8 |
| Cxcl1 | 10.2 | 2.3 |
| TNFα | 10.0 | 4.6 |
| Sele | 6.3 | 2.9 |
| Cd74 | 6.3 | 2.1 |
| Vcam1 | 5.0 | 1.1 |
| Bcl2a1a | 3.9 | 1.3 |
TABLE 2.
Genes suppressed by PCN
PCHs isolated from wild-type mice were treated with either vehicle (0.1% dimethylsulfoxide) or PCN (10 μM) for 24 hours (n = 3). Total RNA was isolated and RT-qPCR was performed. in the PCN-treated group when compared with vehicle-treated cells (P ≤ 0.05).
| Gene Name | Fold Suppression | S.D. |
|---|---|---|
| Selp | 5.1 | 2.4 |
| C3 | 4.2 | 2.0 |
| Csf2 | 4.1 | 0.3 |
| TNFsf10 | 4.1 | 2.0 |
| Agt | 4.1 | 1.9 |
| Myd88 | 3.3 | 1.4 |
| Aldh3a2 | 3.3 | 1.6 |
| Csf2rb | 3.2 | 1.5 |
| F8 | 3.2 | 1.5 |
| Ifnb1 | 2.6 | 1.0 |
| Cfb | 2.6 | 1.0 |
| IL1-Ra | 2.5 | 0.8 |
| Ifnɣ | 2.2 | 0.8 |
| Trp53 | 2.1 | 0.8 |
| Akt1 | 2.1 | 0.8 |
| Ccl22 | 2.1 | 0.8 |
| Nqo1 | 2.1 | 0.8 |
| Cxcl3 | 2.1 | 0.6 |
| Mitf | 2.0 | 0.8 |
| Fas | 2.0 | 0.8 |
| Stat3 | 2.0 | 0.8 |
| TNFrsf1b | 2.0 | 0.8 |
| Rel | 2.0 | 0.8 |
| Stat5b | 2.0 | 0.8 |
| Xiap | 2.0 | 0.8 |
| Irf1 | 2.0 | 0.8 |
| Fasl | 2.0 | 0.7 |
TABLE 3.
Expression profiling of primary cultures of mouse hepatocytes cotreated with LPS and PCN when compared with LPS alone
PCHs from wild-type mice were pretreated with PCN or vehicle (0.1% dimethylsulfoxide) for 24 hours (n = 6). Cultures were divided into two experimental groups and were treated with LPS alone or were cotreated with PCN and LPS together for an additional 12 hours (n = 3). Total RNA was isolated, and RT-qPCR was performed. Data are expressed as fold suppression ± S.D. in the cotreated (PCN + LPS) group when compared with LPS alone (P ≤ 0.05).
| Gene Name | Fold Suppression | S.D. |
|---|---|---|
| Ptgs2 | 2.5 | 0.6 |
| Mmp9 | 2.5 | 0.8 |
| Cd83 | 2.5 | 0.8 |
| Cd74 | 2.5 | 0.6 |
| IL-6 | 2.0 | 0.4 |
| IL1-Ra | 2.0 | 0.5 |
| IL-1β | 1.6 | 0.2 |
| TNFα | 1.6 | 0.3 |
To further examine this PCN-mediated effect, we next chose to examine the LPS-inducible gene expression of IL-1β, IL-6, TNFα, and IL1-Ra in PCHs derived from wild-type and PXR-KO mice using independently designed RT-qPCR primer sets (Table 4). In wild-type PCHs, treatment with LPS alone for 12 hours induced the expression levels of all four genes examined; whereas preactivation of PXR with PCN for 24 hours and subsequent cotreatment together with LPS for an additional 12 hours produced approximately half that observed with LPS treatment alone (Table 5).
TABLE 4.
Primer sequences used for independent analysis of gene expression
Primer sequences were obtained from Primer Bank (http://pga.mgh.harvard.edu/primerbank/).
| Gene | Primer Sequences | |
|---|---|---|
| mIL-1β | Forward | 5′ gAA ATg CCA CCT TTT gAC AgT g 3′ |
| Reverse | 5′ CTg gAT gCT CTC ATC Agg ACA 3′ | |
| mIL-6 | Forward | 5′ CTg CAA gAg ACT TCC ATC Cag 3′ |
| Reverse | 5′ AgT ggT ATA gAC Agg TCT gTT gg 3′ | |
| mIL1-Ra | Forward | 5′ TAg ACA Tgg TgC CTA TTg ACC T 3′ |
| Reverse | 5′ TCg TgA CTA TAA ggg gCT CTT C 3′ | |
| mTNFα | Forward | 5′ CAg gCg gTg CCT ATg TCT C 3′ |
| Reverse | 5′ CgA TCA CCC CgA AgT TCA gAT g 3′ | |
| hIL-1β | Forward | 5′ ATg ATg gCT TAT TAC AgT ggC AA 3′ |
| Reverse | 5′ gTC ggA gAT TCg TAg CTg gA 3′ | |
| hIL-6 | Forward | 5′ ACT CAC CTC TTC AgA ACg AAT Tg 3′ |
| Reverse | 5′ CCA TCT TTg gAA ggT TCA ggT Tg 3′ | |
| hIL-1Ra | Forward | 5′ CAT TgA gCC TCA TgC TCT gTT 3′ |
| Reverse | 5′ CgC TgT CTg AgC ggA TgA A 3′ | |
| m/h β-actin | Forward | 5′ CAA gAT CAT TgC TCC TCC Tg 3′ |
| Reverse | 5′ TAA CAg TCC gCC TAg AAg CA 3′ |
TABLE 5.
Expression level of IL-1β, IL-6, TNFα, and IL1-Ra in wild-type and PXR-KO PCHs
PCHs isolated from wild-type (n = 6) or PXR-KO mice (n = 6) were pretreated for 24 hours with either vehicle (0.1% dimethylsulfoxide) or PCN (10 μM). The cultures were split into two experimental groups (n = 3) and then were treated with vehicle, PCN, LPS (10 μg/ml), or were cotreated with PCN and LPS together for an additional 12 hours. Total RNA was isolated, and RT-qPCR was performed using custom designed primer pairs (Supplemental Table 4). Data are expressed as fold induction ± S.D.
| Hepatocyte Cultures | Vehicle | LPS | PCN + LPS | PCN |
|---|---|---|---|---|
| Wild Type | ||||
| IL-1β | 1.0 ± 0.1 | 1723.2 ± 21.7a | 884.4 ± 96.2a,b | 96.11 ± 8.3a |
| IL-6 | 1.0 ± 0.1 | 379.9 ± 5.1a | 154.2 ± 17.9a,b | 15.1 ± 2.5a |
| TNFα | 1.0 ± 0.2 | 54.2 ± 8.7a | 24.5 ± 3.1a,b | 8.2 ± 1.2a |
| IL1-Ra | 1.0 ± 0.3 | 35.2 ± 4.2a | 15.3 ± 1.2a,b | 5.4 ± 0.5a |
| PXR-KO | ||||
| IL-1β | 204.4 ± 24.1a | 3351.2 ± 294.4a,c | 4094.8 ± 201.2a,c | 246.11 ± 38.3a |
| IL-6 | 25.9 ± 2.9a | 176.2 ± 5.1a | 199.22 ± 11.9a | 25.1 ± 4.6a |
| TNFα | 5.8 ± 0.8a | 34.2 ± 18.6a | 38.4 ± 25.3a | 8.1 ± 4.2a |
| IL1-Ra | 3.7 ± 0.6a | 21.9 ± 4.2a | 25.3 ± 3.7a | 5.2 ± 0.5a |
Statistically significant compared with the vehicle-treated wild-type experimental group (P ≤ 0.05).
For the wild-type [PCN + LPS] experimental group, indicates a statistical difference when compared with LPS-treatment alone.
For the PXR-KO experimental group, indicates that IL-1β expression was enhanced in both of the LPS alone and the [LPS + PCN] experimental groups when compared with IL-1β expression levels in wild-type hepatocyte cultures.
Remarkably, treatment with PCN alone for 36 hours produced comparatively small but statistically significant increases in the levels of all four genes examined in PCHs isolated from wild-type mice, but this effect was completely absent in the PXR-KO PCHs. All four genes examined were expressed at slightly higher levels in the vehicle-treated PXR-KO PCHs when compared with vehicle-treated wild-type PCHs. These data suggest a repressive role for PXR in regulating basal expression levels of key inflammatory mediators, and are also consistent with previous reports indicating that deletion of PXR in mice produces elevated levels of cytokine expression (Zhou et al., 2006; Wallace et al., 2010).
Treatment of PXR-KO PCHs with LPS alone for 12 hours significantly increased the expression levels of all four genes examined. Contrary to the results obtained with wild-type hepatocyte PCHs, pretreatment of PXR-KO PCHs with PCN for 24 hours and subsequent cotreatment together with LPS for an additional 12 hours did not suppress subsequent LPS-inducible expression of any of the genes examined. These data reveal that PXR is required for PCN-mediated decreases in LPS-inducible target gene expression.
Of note, the fold-induction of IL-6, TNFα, and IL1-Ra after 12 hours of LPS treatment was less robust in the PXR-KO cultures when compared with the 12-hour LPS-treated wild-type hepatocyte cultures. In stark contrast, LPS-inducible IL-1β expression levels were enhanced in PXR-KO PCHs when compared with those observed in wild-type PCHs (Table 5, note c). These data reveal that the absence of PXR produces a condition in which the expression of inflammatory mediators is heightened, and suggest that PXR is required for the effective resolution of the IL-1β inflammatory response through time. Closer inspection of the biologic function of the gene products identified in our gene expression analysis indicates that one gene product in particular, IL1-Ra, is intimately associated with resolution of the IL1-signaling pathway and is expressed at high levels in hepatocytes.
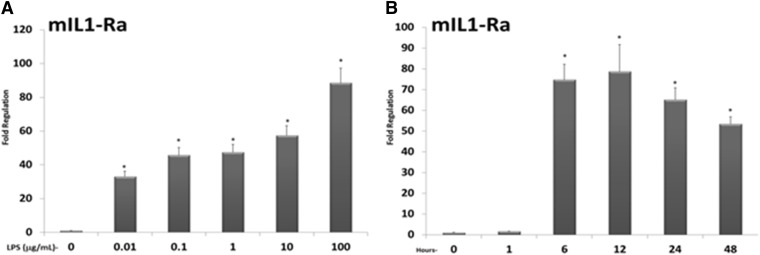
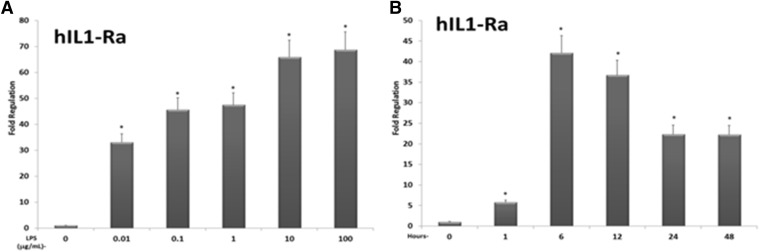
The expression of IL1-Ra messenger RNA, which is detected primarily in hepatocytes, is induced by several inflammatory mediators and encodes a secreted antagonist of IL1-signaling (Arend and Gabay, 2000; Arend and Guthridge, 2000). We therefore more closely examined the concentration- and time-dependent LPS-inducible expression of IL1-Ra messenger RNA in wild-type PCHs (Fig. 4A). Treatment with increasing concentrations of LPS (0.01, 0.1, 1, 10, and 100 μg/ml) for 12 hours produced significant increases in the expression level of IL1-Ra. We next treated wild-type cultures with high concentration LPS (10 μg/ml) for increasing amounts of time (1, 6, 12, 24, and 48 hours) to examine time-dependent LPS-inducible IL1-Ra expression levels (Fig. 4B). Significant levels of LPS-inducible IL1-Ra gene expression were observed at the 6-, 12-, 24-, and 48-hour time points. When compared with the time- and concentration-response analysis of IL-1β and IL-6, the detectable induction of IL1-Ra was delayed by several hours but remained relatively high all the way through the 48-hour time point (Fig. 4B vs Fig. 1B). Similar results were obtained using PCHs derived from a human donor to examine the concentration- and time-dependent induction of IL1-Ra messenger RNA (Fig. 5, A and B). These data indicate that the kinetics of LPS-inducible IL1-Ra gene expression are distinct from those observed for the inflammatory mediators IL-1β and IL-6, with expression levels of IL1-Ra increasing at later time points (6–12 hours) and exhibiting a longer period of sustained expression through the 48-hour time point.
Fig. 4.
LPS induces the expression of the compensatory anti-inflammatory response gene IL1-Ra in mouse PCHs. (A) PCHs isolated from wild-type (C57Bl6) mice were treated with either vehicle (0.1% saline in medium) or increasing concentrations of LPS (0.01, 0.1, 1.0, 10, or 100 μg/ml) for 12 hours. Total RNA was isolated and the relative expression level of IL1-Ra was determined. (B) PCHs isolated from wild-type (C57Bl6) mice were treated with LPS (10 μg/ml) for increasing times (1, 6, 12, 24, and 48 hours). Total RNA was isolated and the relative expression level of IL1-Ra was determined. All data are normalized to β-actin levels and are presented as fold regulation. Asterisks indicate a statistical difference from vehicle-treated samples (n = 3, and P < 0.05).
Fig. 5.
LPS induces the expression of the compensatory anti-inflammatory response gene IL1-Ra in human PCHs. (A) PCHs isolated from a human donor were treated with either vehicle (0.1% saline in medium) or increasing concentrations of LPS (0.01, 0.1, 1.0, 10, or 100 μg/ml) for 12 hours. Total RNA was isolated, and the relative expression level of IL1-Ra was determined. (B) PCHs isolated from a human donor were treated with LPS (10 μg/ml) for increasing times (1, 6, 12, 24, and 48 hours). Total RNA was isolated, and the relative expression level of IL1-Ra was determined. All data are normalized to β-actin levels and are presented as fold regulation. Asterisks indicate a statistical difference from vehicle-treated samples (n = 3, and P < 0.05).
There are two major isoforms of the IL1-Ra protein that arise from a single gene called IL1RN, and their expression is differentially regulated at the level of transcription by alternative promoters (Butcher et al., 1994). One form of IL1-Ra is a heavily glycosylated and secreted isoform (sIL1-Ra); the other is an intracellular isoform (icIL1-Ra). The primary function of sIL1-Ra is to competitively inhibit IL1-binding to cell surface receptors (Arend et al., 1990). The expression and secretion of sIL1-Ra is highly inducible in hepatocytes by inflammatory stimuli, whereas the expression of icIL1-Ra is not inducible and its biologic function remains largely unknown (Arend and Guthridge, 2000).
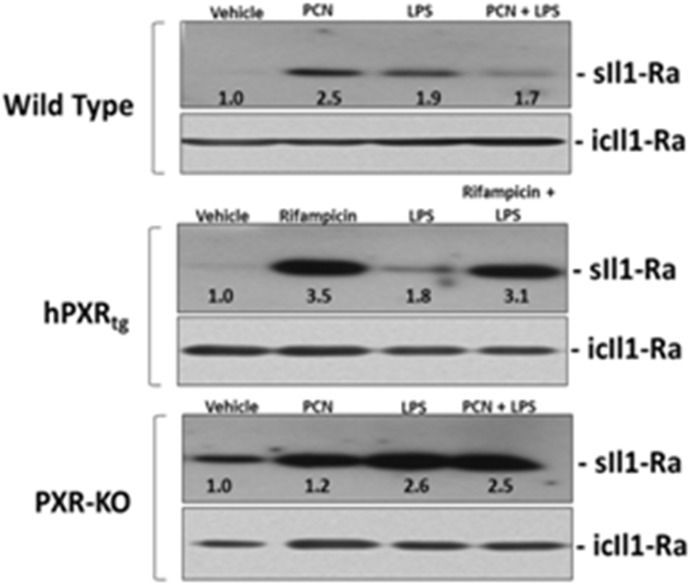
The observation that LPS-inducible IL-1β messenger RNA expression was enhanced in the PXR-KO PCHs when compared with LPS-treated wild-type PCHs (Table 5) prompted us to examine the PXR- and LPS-inducible level of sIL1-Ra and icIL1-Ra proteins in both medium and whole-cell lysate, respectively (Fig. 6). When treated with PCN or Rif alone for 36 hours, the level of sIL1-Ra protein increased in the medium approximately 2.5- and 3.5-fold in PCHs from wild-type and hPXRtg mice, respectively. When treated with LPS for 12 hours, the level of sIL1-Ra protein increased in the medium approximately 1.9- and 1.8-fold from wild-type and hPXRtg, respectively. When treated with PCN or Rif for 24 hours and then subsequently cotreated with LPS for an additional 12 hours, the level of sIL1-Ra protein increased in the medium approximately 1.7- and 3.1-fold from wild-type and hPXRtg, respectively. When PCHs from PXR-KO mice were used in identical experiments, PCN had no effect on induction of sIL1-Ra protein in the medium. In contrast, 12 hours of LPS treatment produced an enhanced effect in PXR-KO PCHs when compared with PXR-positive cultures. In addition, cotreatment with PCN and LPS together failed to diminish sIL1-Ra levels in the medium from the PXR-KO PCHs.
Fig. 6.
Analysis of the secreted form of IL1-Ra protein in culture medium from PCHs isolated from wild-type, hPXRtg, and PXR-KO Mice. PCHs were isolated from the indicated genotype and were treated with vehicle (0.1% dimethylsulfoxide) or 10 μM PCN for 24 hours. Cell cultures were then divided into four experimental groups and were treated for an additional 12 hours with either vehicle, PCN alone, 10 μg/ml LPS alone, or PCN and LPS together. Western blot analysis of the secreted form (sIL1-Ra) and intracellular form (icIL1-Ra) of IL1-Ra was performed. Western blot analysis of the secreted form (sIL1-Ra) and intracellular form (icIL1-Ra) of IL1-Ra was performed. Western Blot images were quantitated by densitometric scanning of the X-ray films with the UVP Biodoc-It 220 image analysis system and 1D Gel Analysis Software, and the numbers represent the densitometric image intensity of sIL1-Ra divided by the image intensity of icIL1-Ra.
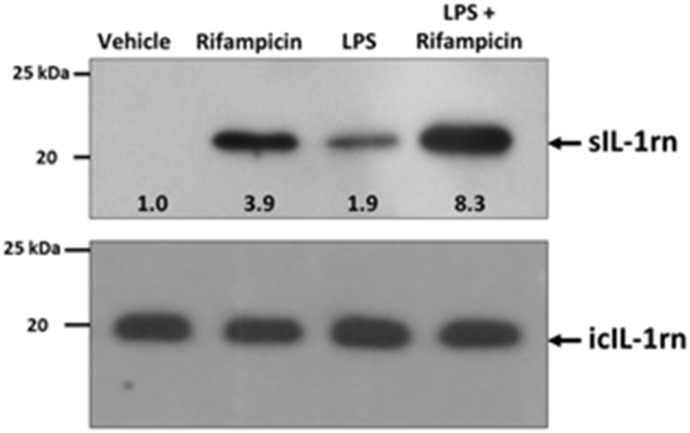
Taken together, our studies indicate that PXR activation has both an early negative regulatory role in the LPS-inducible expression of key inflammatory mediators such as IL-1β and a likely positive role in regulating ligand-inducible expression of the secreted form of the IL1-Ra protein at later time points. To more closely examine the potential positive role of PXR activation in regulating sIL1-Ra protein levels across species, we chose to perform a longer time-course study using primary cultures of human hepatocytes (Fig. 7). Treatment of PCHs from a human donor with Rif for 72 hours produced an approximately 3.9-fold increase in sIL1-Ra levels in the culture medium, whereas 24 hours of treatment with LPS increased levels by approximately 1.9-fold. Treatment of human hepatocyte cultures with Rif for 48 hours, followed by cotreatment with Rif and LPS together for an additional 24 hours produced an approximate 8.3-fold increase in sIL1-Ra in the culture medium. These data indicate that long-term preactivation of PXR in both rodent and human PCHs has a strong positive effect upon the secretion of sIL1-Ra from cells, an important and systemic negative regulator of IL1 signaling and key participant in the compensatory anti-inflammatory response in mammals.
Fig. 7.
Analysis of the secreted form of IL1-Ra protein in culture medium from human PCHs. PCHs from a human donor were treated with vehicle (0.1% dimethylsulfoxide) or 10 μM Rif for 48 hours. The cultures were then divided into four experimental groups and were treated for an additional 24 hours with either vehicle, Rif alone, 10 μg/ml LPS alone, or Rif and LPS together. Western Blot images were quantitated by densitometric scanning of the X-ray films with the UVP Biodoc-It 220 image analysis system and 1D Gel Analysis Software, and the numbers represent the densitometric image intensity of sIL1-Ra divided by the image intensity of icIL1-Ra.
Discussion
The liver is a crucial organ that plays a central role in acute phase protein synthesis during bacterial sepsis. The resulting cytokine stimulation rapidly up-regulates expression of acute phase proteins, and simultaneously down-regulates key drug metabolism pathways in liver. Although proinflammatory cytokines initiate the APR through different cell surface receptors, they share a high level of redundancy with respect to the signal transduction pathways (e.g., Iκ-B kinase, p38, and JNK) by which they exert their influence in the nucleus (reviewed in Heinrich et al., 2003; Wajant et al., 2003; Lu et al., 2008; Weber et al., 2010). Initiation of the acute inflammatory response is mainly achieved through signal-dependent activation of NF-κB and activator protein 1 transcription factors through promoter response elements that regulate expression of genes encoding important proinflammatory cytokines.
Treatment of experimental models with LPS stimulates pattern recognition receptors, mainly TLR4, to induce the rapid expression and release of proinflammatory cytokines such as TNFα. Secreted TNFα further exerts its inflammatory function through binding to TNF receptor type 1 (TNFR1) and TNFR2 on various hepatic cell types, including hepatocytes. Separate receptor types recognize either IL-6 or IL-1β, respectively, to further initiate and amplify the acute inflammatory response in feed-forward loops. Importantly, treatment of liver with LPS leads to release of proinflammatory cytokines IL1β and TNFα from both nonparenchymal cells and hepatocytes. Stimulation with IL-1β induces TNFα secretion from rat hepatocytes, and stimulation of hepatocytes with either IL-1β or TNFα produces IL-6 secretion (Panesar et al., 1999; Yoshigai et al., 2014). Hence, there are multiple levels of interconnection and amplification that occur rapidly between and among inflammatory cytokines after bacterial sepsis.
From a historical perspective, hepatocytes were initially viewed as passive recipients of immune messages from nonparenchymal cells, including Kupffer cells (Volpes et al., 1992). However, more recent investigations have indicated that this is in fact not the case. Hepatocytes mount a robust response to challenge with either LPS or IL-1β to produce key inflammatory cytokines, including IL-6, TNFα, and IL-1β (Liu et al., 2002; Panesar et al., 1999; Spencer et al., 2013; Takano et al., 2012; Yoshigai et al., 2014). It is also now well known that hepatocytes express all the necessary machinery to respond to bacterial sepsis, including TLR4, CD14, Myd88, and MD-2 (Liu et al., 2002). A recent study has indicated that hepatocyte-specific knockout of the TLR4 receptor in mice significantly attenuates the systemic serum levels of the inflammatory mediators TNFα, IL-6, and IL-1β in response to a high-fat diet (Jia et al., 2014). Hepatocytes are therefore not merely passive recipients of immune signals from nonparenchymal cells but instead can be viewed as active participants in mediating an immune response to a variety of signals that include sepsis and morbid obesity.
Much attention has recently been given to the notion that PXR activation by Rif and its analogs may be beneficial in the treatment of inflammatory liver and bowel diseases (Wallace et al., 2010; Kakizaki et al., 2011; Cheng et al., 2012; Jonker et al., 2012). Additional research indicates a key role for PXR in maintaining the barrier function of the gut (Dou et al., 2012, 2014; Venkatesh et al., 2014). Thus, detailed knowledge of the molecular mechanisms governing PXR-mediated suppression of the APR in these tissues is vital but is currently lacking. It is well known that SUMOylation modifies the transactivation capacity of a myriad of transcription factors, and in most cases correlates with transcriptional suppression (reviewed in Gill, 2005). For example, the SUMO-modification of liver-enriched NR family members is implicated in suppression of NR-function and in modulation of the APR (Ghisletti et al., 2007; Venteclef et al., 2010; Treuter and Venteclef, 2011; Zhou et al., 2012; Balasubramaniyan et al., 2013). While several liver-enriched NRs are the molecular target of the SUMO signaling pathway, not all of them are. For example, constitutive androstane receptor, a close relative of PXR, is not SUMOylated (unpublished observation). We have previously shown that PXR is SUMOylated in response to TNFα treatment in both human and mouse PCHs (Hu et al., 2010). These observations suggest that shared molecular mechanisms exist to govern the conversion of the primary metabolic function of the liver during the nonseptic or noninflamed state to one that is involved in resolving the APR during sepsis or injury, which likely involves SUMOylation.
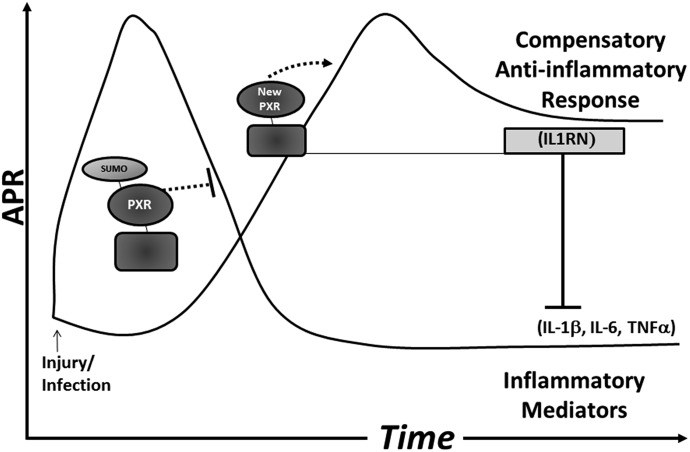
Here, we present a working model that defines a novel pathway for the feedback inhibition and resolution of the inflammatory response in hepatocytes through time (Fig. 8). After injury or infection, we propose that low stoichiometric amounts of SUMO-modified PXR function to directly suppress the proinflammatory mediators IL-1β, IL-6, and TNFα at the level of transcription, while the remainder of PXR protein is likely ubiquitinated and subsequently degraded by the 26S proteasome in a signal-dependent manner through time. Indeed, significantly lower levels of PXR protein are detected in endotoxin-treated mice (Teng and Piquette-Miller, 2005).
Fig. 8.
Model of the mechanism of PXR-mediated interaction with the inflammatory response in hepatocytes.
As the APR ensues through time, newly synthesized PXR protein becomes available for up-regulating ligand-dependent expression of novel or alternative PXR-target genes, including the negative regulator of IL1 signaling sIL1-Ra, possibly through cryptic or low-affinity PXR-response elements. Of note, a recent investigation indicates that peroxisome proliferator-activated receptor γ coactivator 1α, a strong PXR-coactivator protein, controls expression of IL1-Ra in liver (Buler et al., 2012). In this way, PXR activation gains a novel repressive anti-inflammatory function and plays an active role in the resolution of the inflammatory response through time. Future efforts should seek to determine whether this mechanism by which PXR is converted from a positive regulator of drug-metabolizing enzymes into a transcriptional suppressor of inflammation in the liver will provide new pharmacologic strategies for modulating inflammatory-related diseases in the liver and intestine.
In our current study, LPS was used to initiate the acute inflammatory response in an effort to determine the extent to which PXR-mediated suppression of the inflammatory response was dependent upon cell surface receptor types such as TLR4 receptor versus TNFR. Data presented here indicate that PXR activation negatively regulates the LPS-inducible gene expression program in hepatocytes similar to that observed with TNFα or phorbol ester stimulation (Zhou et al., 2006; Hu et al., 2010). Taken together, these data indicate that the negative regulatory role for PXR in inflammation is not specific to cell surface receptor type, and likely operates at the level of the promoters for key inflammatory cytokines TNFα, IL1β, and IL-6. In a symmetrical manner, stimulation of the inflammatory response is well known to suppress drug metabolism pathways in liver through the rapid and selective down-regulation of specific CYP enzymes. Several possible mechanisms have been proposed for this phenomenon including the reduction of PXR mRNA levels during sepsis (Beigneux et al., 2002). Another line of thought has postulated the disruption the association between PXR-RXRα heterodimer complex by a signal-dependent interaction of NF-κB with RXRα, thereby sequestering the active form of PXR that regulates drug-inducible gene expression (Gu et al., 2006). While plausible and not mutually exclusive, the precise signals and molecular mechanisms for inflammation-inducible repression of drug metabolism deserve further scrutiny.
Supplementary Material
Abbreviations
- APR
acute phase response
- FXR
farnesoid X receptor (NR1H4)
- GW3965
2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methyl-(2, 2-diphenylethyl)amino]propoxy]phenyl]acetic acid
- GW4064
3-[(E)-2-[2-chloro-4-[[3-(2,6-dichlorophenyl)-5-propan-2-yl-1,2-oxazol-4-yl]methoxy]phenyl]ethenyl]benzoic acid
- GW7647
2-[4-[2-[4-cyclohexylbutyl(cyclohexylcarbamoyl)amino]ethyl]phenyl]sulfanyl-2-methylpropanoic acid
- hPXRtg
humanized-PXR transgenic mice
- IL
interleukin
- IL1-Ra
interleukin 1 receptor antagonist
- KO
knockout
- LXRα
liver X receptor α (NR1H3)
- LXRβ
liver X receptor beta (NR1H2)
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- NR
nuclear receptor
- PCH
primary cultured hepatocyte
- PCN
pregnenolone 16α-carbonitrile
- PPARα
peroxisome proliferator antigen receptor α (NR1C1)
- PXR
pregnane X receptor (NR1I2)
- Rif
rifampicin
- RT-qPCR
real-time quantitative polymerase chain reaction
- SUMO
small-ubiquitin-related modifier
- tg
transgenic
- TLR4
Toll-like receptor 4
- TNFα
tumor necrosis factor α.
- TNFR
tumor necrosis factor receptor
Authorship Contributions
Participated in research design: Sun, Cui, Woody, Staudinger.
Conducted experiments: Sun, Cui, Woody.
Performed data analysis: Sun, Cui, Woody, Staudinger.
Wrote or contributed to the writing of the manuscript: Sun, Woody, Staudinger.
Footnotes
This work was supported by the National Institutes of Health National Institute Diabetes and Digestive and Kidney Diseases (NIDDK) [Grant R01 DK090558]. The human hepatocytes used in this study were derived from samples collected and provided by the University of Kansas Medical Center (KUMC) Department of Pharmacology, Toxicology and Therapeutics Hepatocyte Core Laboratory and the KU Liver Center.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Arend WP, Gabay C. (2000) Physiologic role of interleukin-1 receptor antagonist. Arthritis Res 2:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Guthridge CJ. (2000) Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis 59 (Suppl 1):i60–i64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Welgus HG, Thompson RC, Eisenberg SP. (1990) Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest 85:1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniyan N, Luo Y, Sun AQ, Suchy FJ. (2013) SUMOylation of the farnesoid X receptor (FXR) regulates the expression of FXR target genes. J Biol Chem 288:13850–13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. (2002) Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun 293:145–149. [DOI] [PubMed] [Google Scholar]
- Brobst DE, Ding X, Creech KL, Goodwin B, Kelley B, Staudinger JL. (2004) Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J Pharmacol Exp Ther 310:528–535. [DOI] [PubMed] [Google Scholar]
- Buer J, Balling R. (2003) Mice, microbes and models of infection. Nat Rev Genet 4:195–205. [DOI] [PubMed] [Google Scholar]
- Buler M, Aatsinki SM, Skoumal R, Komka Z, Tóth M, Kerkelä R, Georgiadi A, Kersten S, Hakkola J. (2012) Energy-sensing factors coactivator peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) and AMP-activated protein kinase control expression of inflammatory mediators in liver: induction of interleukin 1 receptor antagonist. J Biol Chem 287:1847–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher C, Steinkasserer A, Tejura S, Lennard AC. (1994) Comparison of two promoters controlling expression of secreted or intracellular IL-1 receptor antagonist. J Immunol 153:701–711. [PubMed] [Google Scholar]
- Cheng J, Shah YM, Gonzalez FJ. (2012) Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci 33:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, Buckley AR. (2002) Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4-hydroxytamoxifen. Drug Metab Dispos 30:608–612. [DOI] [PubMed] [Google Scholar]
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. (1996) The PPARalpha-leukotriene B4 pathway to inflammation control. Nature 384:39–43. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. (2005) Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther 312:849–856. [DOI] [PubMed] [Google Scholar]
- Dou W, Mukherjee S, Li H, Venkatesh M, Wang H, Kortagere S, Peleg A, Chilimuri SS, Wang ZT, Feng Y, et al. (2012) Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS ONE 7:e36075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L, Ren G, Wang Z, Mani S. (2014) Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem 25:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. (2007) Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell 25:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. (2005) Something about SUMO inhibits transcription. Curr Opin Genet Dev 15:536–541. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Gauthier KC, Umetani M, Watson MA, Lochansky MI, Collins JL, Leitersdorf E, Mangelsdorf DJ, Kliewer SA, Repa JJ. (2003) Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc Natl Acad Sci USA 100:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. (2006) Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem 281:17882–17889. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman DA, Milona A, van Erpecum KJ, van Mil SW. (2012) Anti-inflammatory and metabolic actions of FXR: insights into molecular mechanisms. Biochim Biophys Acta 1821:1443–1452. [DOI] [PubMed] [Google Scholar]
- Hu G, Xu C, Staudinger JL. (2010) Pregnane X receptor is SUMOylated to repress the inflammatory response. J Pharmacol Exp Ther 335:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, et al. (2014) Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun 5:3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Liddle C, Downes M. (2012) FXR and PXR: potential therapeutic targets in cholestasis. J Steroid Biochem Mol Biol 130:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizaki S, Takizawa D, Tojima H, Horiguchi N, Yamazaki Y, Mori M. (2011) Nuclear receptors CAR and PXR; therapeutic targets for cholestatic liver disease. Front Biosci (Landmark Ed) 16:2988–3005. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, et al. (1998) An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92:73–82. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. (1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti-Kaiser K, Staudinger JL. (2008) The traditional Chinese herbal remedy tian xian activates pregnane X receptor and induces CYP3A gene expression in hepatocytes. Drug Metab Dispos 36:1538–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. (2002) Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun 70:3433–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. (2008) LPS/TLR4 signal transduction pathway. Cytokine 42:145–151. [DOI] [PubMed] [Google Scholar]
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. (2000) St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA 97:7500–7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau A, Vilarem MJ, Maurel P, Pascussi JM. (2008) Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm 5:35–41. [DOI] [PubMed] [Google Scholar]
- Nallani SC, Goodwin B, Buckley AR, Buckley DJ, Desai PB. (2004) Differences in the induction of cytochrome P450 3A4 by taxane anticancer drugs, docetaxel and paclitaxel, assessed employing primary human hepatocytes. Cancer Chemother Pharmacol 54:219–229. [DOI] [PubMed] [Google Scholar]
- Panesar N, Tolman K, Mazuski JE. (1999) Endotoxin stimulates hepatocyte interleukin-6 production. J Surg Res 85:251–258. [DOI] [PubMed] [Google Scholar]
- Păunescu E. (1970) In vivo and in vitro suppression of humoral and cellular immunological response by rifampicin. Nature 228:1188–1190. [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D. (1986) Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47:921–928. [DOI] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. (2007) Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol 292:G1114–G1122. [DOI] [PubMed] [Google Scholar]
- Spencer NY, Zhou W, Li Q, Zhang Y, Luo M, Yan Z, Lynch TJ, Abbott D, Banfi B, Engelhardt JF. (2013) Hepatocytes produce TNF-α following hypoxia-reoxygenation and liver ischemia-reperfusion in a NADPH oxidase- and c-Src-dependent manner. Am J Physiol Gastrointest Liver Physiol 305:G84–G94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. (2001a) Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos 29:1467–1472. [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. (2001b) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98:3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Madan A, Carol KM, Parkinson A. (2003) Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos 31:523–527. [DOI] [PubMed] [Google Scholar]
- Takano M, Sugano N, Mochizuki S, Koshi RN, Narukawa TS, Sawamoto Y, Ito K. (2012) Hepatocytes produce tumor necrosis factor-α and interleukin-6 in response to Porphyromonas gingivalis. J Periodontal Res 47:89–94. [DOI] [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. (2005) The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther 312:841–848. [DOI] [PubMed] [Google Scholar]
- Treuter E, Venteclef N. (2011) Transcriptional control of metabolic and inflammatory pathways by nuclear receptor SUMOylation. Biochim Biophys Acta 1812:909–918. [DOI] [PubMed] [Google Scholar]
- Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. (2009) The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 183:6251–6261. [DOI] [PubMed] [Google Scholar]
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al. (2014) Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41:296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteclef N, Jakobsson T, Ehrlund A, Damdimopoulos A, Mikkonen L, Ellis E, Nilsson LM, Parini P, Jänne OA, Gustafsson JA, et al. (2010) GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev 24:381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpes R, van den Oord JJ, Desmet VJ. (1992) Can hepatocytes serve as ‘activated’ immunomodulating cells in the immune response? J Hepatol 16:228–240. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. (2003) Tumor necrosis factor signaling. Cell Death Differ 10:45–65. [DOI] [PubMed] [Google Scholar]
- Wallace K, Cowie DE, Konstantinou DK, Hill SJ, Tjelle TE, Axon A, Koruth M, White SA, Carlsen H, Mann DA, et al. (2010) The PXR is a drug target for chronic inflammatory liver disease. J Steroid Biochem Mol Biol 120:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M. (2010) Interleukin-1 (IL-1) pathway. Sci Signal 3:cm1. [DOI] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. (2001) An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA 98:3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang X, Staudinger JL. (2009) Regulation of tissue-specific carboxylesterase expression by pregnane x receptor and constitutive androstane receptor. Drug Metab Dispos 37:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshigai E, Hara T, Inaba H, Hashimoto I, Tanaka Y, Kaibori M, Kimura T, Okumura T, Kwon AH, Nishizawa M. (2014) Interleukin-1β induces tumor necrosis factor-α secretion from rat hepatocytes. Hepatol Res 44:571–583. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, et al. (2006) Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116:2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Hannoun Z, Jaffray E, Medine CN, Black JR, Greenhough S, Zhu L, Ross JA, Forbes S, Wilmut I, et al. (2012) SUMOylation of HNF4α regulates protein stability and hepatocyte function. J Cell Sci 125:3630–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.