Abstract
We previously developed SKI-178 (N′-[(1E)-1-(3,4-dimethoxyphenyl)ethylidene]-3-(4-methoxxyphenyl)-1H-pyrazole-5-carbohydrazide) as a novel sphingosine kinase-1 (SphK1) selective inhibitor and, herein, sought to determine the mechanism-of-action of SKI-178–induced cell death. Using human acute myeloid leukemia (AML) cell lines as a model, we present evidence that SKI-178 induces prolonged mitosis followed by apoptotic cell death through the intrinsic apoptotic cascade. Further examination of the mechanism of action of SKI-178 implicated c-Jun NH2-terminal kinase (JNK) and cyclin-dependent protein kinase 1 (CDK1) as critical factors required for SKI-178–induced apoptosis. In cell cycle synchronized human AML cell lines, we demonstrate that entry into mitosis is required for apoptotic induction by SKI-178 and that CDK1, not JNK, is required for SKI-178–induced apoptosis. We further demonstrate that the sustained activation of CDK1 during prolonged mitosis, mediated by SKI-178, leads to the simultaneous phosphorylation of the prosurvival Bcl-2 family members, Bcl-2 and Bcl-xl, as well as the phosphorylation and subsequent degradation of Mcl-1. Moreover, multidrug resistance mediated by multidrug-resistant protein1 and/or prosurvival Bcl-2 family member overexpression did not affect the sensitivity of AML cells to SKI-178. Taken together, these findings highlight the therapeutic potential of SKI-178 targeting SphK1 as a novel therapeutic agent for the treatment of AML, including multidrug-resistant/recurrent AML subtypes.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by a multitude of genetic mutations and chromosomal abnormalities (Kumar, 2011). Although extensive efforts are being made to develop new therapeutics, the standard of care for AML has remained relatively unchanged for more than four decades, and long-term survival remains poor (Sweet and Lancet, 2014). Bioactive sphingolipids have emerged as important regulators of numerous biologic processes, including cell survival, cell proliferation, inflammation, and carcinogenesis (Adan-Gokbulut et al., 2013; Heffernan-Stroud and Obeid, 2013). Among these sphingolipids, ceramide, sphingosine, and sphingosine-1-phosphate (S1P) play key roles in determining cell fate by promoting opposing effects on cell survival and proliferation. Ceramide and sphingosine exert antiproliferative, proapoptotic effects (Hannun and Luberto, 2000; Taha et al., 2006), whereas S1P promotes proliferation, cell survival, and inhibition of apoptosis (Goetzl et al., 1999; Kwon et al., 2001; Spiegel and Milstien, 2003). Sphingosine kinase-1 (SphK1), an oncogenic lipid kinase responsible for converting sphingosine to S1P, plays a fundamental role in this process as it regulates the levels of all three above-mentioned bioactive sphingolipids (Spiegel and Milstien, 2003).
Elevated expression of SphK1 is associated with numerous cancer types (Li et al., 2007; Sobue et al., 2008; Paugh et al., 2009; Knapp et al., 2010; Malavaud et al., 2010; Guan et al., 2011), and several lines of evidence link SphK1 deregulation with the progression of various hematologic malignancies (Van Brocklyn et al., 2005; Bonhoure et al., 2006; Bayerl et al., 2008; Paugh et al., 2008; Pitson et al., 2011). For example, overexpression of SphK1 has been shown to be an oncogenic event in acute erythroid leukemia progression (Le Scolan et al., 2005). Furthermore, SphK1 is also shown to mediate chemotherapy sensitivity in AML cells. In fact, a study by Sobue et al. (2008) identified the cellular ceramide:S1P ratio as a critical biosensor for predicting AML cell sensitivity to daunorubicin. Specifically, they demonstrated that in AML cells, a low ceramide:S1P ratio, associated with high SphK1 activity, is correlated with a robust intrinsic chemoresistance to daunorubicin. They further demonstrated that increasing the ceramide:S1P ratio [using SphK inhibitors (SKI-II) or SphK1 small interfering RNAs (siRNAs) to reduce SphK1 activity and upregulate intracellular ceramide levels] sensitizes AML cells to daunorubicin. Another study demonstrated synergism between SKI-II treatment (resulting in increased ceramide accumulation) and imatinib mesylate in K562 chronic myelogenous leukemia cells, which results in increased apoptosis (Ricci et al., 2009). Additionally, Bonhoure et al. (2006) demonstrated that targeting SphK1 induces apoptosis of multidrug-resistant HL-60 cells (HL-60/VCR). Together, these findings implicate SphK1 as a novel chemotherapeutic target to overcome chemoresistance in hematologic malignancies.
We developed SKI-178 (N′-[(1E)-1-(3,4-dimethoxyphenyl)ethylidene]-3-(4-methoxxyphenyl)-1H-pyrazole-5-carbohydrazide) as a novel small-molecule, isotype-specific, nonlipid substrate inhibitor of SphK1 that is cytotoxic toward a broad panel of cancer types, including AML (Hengst et al., 2010). Given the association of SphK1 with development and progression of leukemias in general, we believe that SKI-178 could represent a novel, effective therapeutic strategy for the treatment of AML. As a first step in this process we sought to elucidate the molecular mechanism by which SKI-178 induces apoptosis in human AML cell lines. Herein, we present evidence that SKI-178 induces prolonged mitosis, leading to induction of apoptotic cell death in HL-60 cells. Detailed analysis of this mitosis-dependent mechanism revealed that SKI-178–induced sustained activation of cyclin-dependent protein kinase 1 (CDK1) activity is responsible for the apoptotic effect. To ensure that these findings were not limited to HL-60 cells, SKI-178–induced apoptotic cell death was further extended to multiple AML cell lines, including multidrug resistance types. Taken together, we provide evidence that SKI-178 induces apoptosis in a CDK1-dependent manner and is not a substrate for multidrug-resistant protein 1 (MDR1), making it a promising chemotherapeutic candidate for the treatment of AML, including those known to be drug resistant.
Materials and Methods
Reagents.
Reagents were purchased as follows: SKI-178 (ChemBridge Corporation, San Diego, CA), vincristine (Thermo Fisher Scientific, Waltham, MA), colchicine (Enzo Life Sciences, Farmingdale, NY), RO3306 [(5Z)-5-quinolin-6-ylmethylene-2-[(thiophen-2-ylmethyl)-amino]-thiazol-4-one; Sigma-Aldrich, St. Louis, MO], antibodies against total Bcl-2, pBcl-2(Ser70), Mcl-1, caspase-3, caspase-9, cleaved caspase-3 (active), cleaved caspase-9 (active), cleaved caspase-7 (active), pHistone H3 (Ser10), pCDK1 (Tyr15), p-c-Jun NH2-terminal kinase (JNK) (Thr183/Tyr185), poly(ADP ribose) polymerase, and actin (Cell Signaling Technology, Danvers, MA), Sphk1 (Abgent, San Diego, CA), pBcl-xl (Ser62) (Abcam, Cambridge, MA), and glyceraldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology, Santa Cruz, CA).
Cell Lines and Culture Conditions.
Human AML-derived cell lines, HL-60 (CCL-240), U937 (CRL-1593.2), and THP-1 (TIB-202), as well as the human pancreatic cell line MIA PaCa-2 (CRL-1420) were obtained from the American Type Culture Collection (Manassas, VA). HL-60/VCR cells were a gift from H.-G.W. (Penn State College of Medicine, Hershey, PA). HL-60 and HL-60/VCR were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; Denville Scientific, South Plainfield, NJ). U937 and THP-1 cell lines were maintained in RPMI (Thermo Fisher Scientific) supplemented with 10% FBS. All cell lines were maintained at 37°C with 5% CO2 in a humidified incubator
Cell Viability Assays.
Cells were treated with increasing concentrations of SKI-178, vincristine, or colchicine in 96-well plates for 48 hours. Cell viability was measured using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell metabolism assay (American Type Culture Collection) according to manufacturer recommendations. Briefly, following treatment, 10 µl of MTT was added to each well and incubated for 4 hours at 37°C with 5% CO2 in a humidified incubator. After the 4-hour incubation, stop solution was added, kept overnight at room temperature, and absorbance was recorded at 570 nM. The results were normalized to vehicle [dimethyl sulfoxide (DMSO)] control. IC50 values based on cell viability were determined using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA) based on three separate experiments (n = 3).
Sphingosine Kinase Activity Assay and Thin-Layer Chromatography.
Briefly, whole cell lysates were prepared from 1.0 × 106 cells in buffers selective for SphK1 (20 mM Tris pH 7.4, 1 mM β-mercaptoethanol, 1 mM EDTA, 1.0% Triton X-100, 1 mM Na3VO4, 15 mM NaF, 0.5 mM 4-deoxypyridoxine) or SphK2 (20 mM Tris pH 7.4, 1 mM β-mercaptoethanol, 1 mM EDTA, 1 M KCl, 1 mM Na3VO4, 15 mM NaF, 0.5 mM 4-deoxypyridoxine). Fifty micrograms of total protein lysates was combined with 50 µM d-erythro-sphingosine, 200 µM ATP, and 2 µCi [γ-32P]ATP in a 100 µl final reaction volume and incubated for 1 hour at 37°C with shaking. Kinase reactions were terminated by the addition of 10 µl 6N HCl, and the radiolabeled lipids were extracted by the addition of 400 µl of chloroform/MeOH (100:200 v/v) and 125 µl chloroform and 125 µl 1 M KCl. The organic phase containing lipids was dried down under nitrogen stream. Samples, along with separate S1P standards generated by sphingosine kinase activity assay using purified recombinant SphK1 as an enzyme source (Sigma-Aldrich), were then resuspended in 30 µl chloroform and applied to a Silica Gel thin-layer chromatography plate (Whatman, Florham Park, NJ) and the lipids were separated using a butanol:water:acetic acid (3:1:1 v:v:v) solvent system. The plates were analyzed by X-ray film exposure, and the region of the thin-layer chromatography plate corresponding to the Rf value (0.32) of S1P was examined.
Sphingolipid Analyses.
Sphingolipids from treated HL-60 cells were analyzed by liquid chromatography-electrospray ionization-tandem mass spectrometry essentially as described previously (Fox et al., 2011) with minor modifications as described herein. Total lipids were extracted from HL-60 cell lysates equivalent to 500 μg of total protein. Extracted lipids were then separated on an Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA) according to chromatography conditions described previously (Fox et al., 2011), but with 0.1% formic acid in the mobile phases at a flow rate of 0.4 ml/min on a Phenomenex Kinetex C8 (2.6 μm) 2 mm internal diameter × 5 cm column maintained at 60°C (Phenomenex, Torrance, CA). The eluate was analyzed with an inline AB Sciex 4000 Q Trap mass spectrometer equipped with a turbo ion spray source (Applied Biosystems, Framingham, MA). The peak areas for the different sphingolipid subspecies were compared with that of the internal standards. All data reported are based on monoisotopic mass and are represented as picomoles per milligram total cellular protein.
Western Blot Analysis.
This was performed as previously described (Francy et al., 2007) with some modifications. Briefly, whole cell lysates were harvested in 100 μl 1× radioimmunoprecipitation assay buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM sodium orthovandate, 30 mM sodium fluoride, and complete protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany)]. Lysates were centrifuged (≥10,000g) for 15 minutes at 4°C to remove cell debris. Total protein concentrations were quantified using the BCA assay from Pierce (Thermo Fisher Scientific). Equal amounts of denatured total protein were resolved by NuPAGE 4–12% Bis-Tris gel electrophoresis (Life Technologies, Carlsbad, CA) and transferred to polyvinylidene difluoride membranes (Life Technologies). Membranes were blocked for 1 hour at room temperature in 5% milk/Tris-buffered saline/Tween 20, incubated overnight at 4°C with primary antibodies (1:1000), and immunodetection was done with corresponding secondary IgG horseradish peroxidase–linked antibodies (1:5000) using the ECL chemiluminescence reagents (Thermo Fisher Scientific).
Analysis of Apoptosis.
HL-60 cells were treated with either vehicle (DMSO) for 48 hours or SKI-178 (5 μM) for 16, 24, or 48 hours. Stages of apoptosis were assayed using the MUSE Annexin V & Dead Cell kit combined with laser-based fluorescence detection using a MUSE cell analyzer (EMD Millipore, Billerica, MA) according to manufacturer recommendations. Final analysis of the data were done using FlowJo 3.2 software (Tree Star, Inc., San Carlos, CA). Histograms are representative of three independent experiments (n = 3).
Cell Cycle Synchronization.
Cells were synchronized according to the previously established double-thymidine block-and-release protocol (Bostock et al., 1971). Briefly, cells were synchronized at the G1/S phase border by culturing cells in DMEM + 10% FBS containing 2 mM thymidine (Sigma-Aldrich) for 19 hours. Cells were then released from the G1/S phase block by washing twice with phosphate-buffered saline (PBS) and resuspending them in thymidine-free culture medium for 9 hours. Cells were again treated with 2 mM thymidine in DMEM + 10% FBS for an additional 16 hours. After the second block, cell were washed twice with PBS and resuspended in thymidine-free culture medium containing appropriate treatment or control.
Cell Cycle Analysis.
The cell cycle distribution of HL-60 cells after SKI-178 or DMSO treatment was determined by flow cytometry of propidium iodide (PI)–stained cells. Briefly, cells were treated with SKI-178 (5 μM) or DMSO (vehicle) for 4, 8, or 16 hours. Cells were washed with PBS, fixed in cold 70% ethanol, and incubated at −20°C for at least 30 minutes. Once all time points were collected, fixed cells were washed twice with PBS and resuspended in 1 ml PBS containing 0.1% (v/v) Triton X-100, 100 μg/ml PI (Invitrogen, Carlsbad, CA), and 200 μg/ml ribonuclease A (Qiagen, Venlo, The Netherlands). Cells were stained with PI for at least 30 minutes and analyzed using the FACScan analyzer (Becton Dickinson, Franklin Lakes, NJ). Data were processed and analyzed using FlowJo V10 software (Tree Star, Inc.).
siRNA Transfection.
Duplexed Stealth siRNA (Life Technologies) was used to knockdown SphK1 expression in human MIA PaCa-2 cell line.
siRNA sequences:
siSphK1 #1: 5′-CCUACUUGGUAUAUGUGCCCGUGGU-3′.
siSphK1 #2: 5′-GAGGCUGAAAUCUCCUUCACGCUGA-3′.
Transfections of MIA Paca-2 cells were carried out by reverse-transfecting 0.5 × 106 cells with RNAiMAX (Life Technologies) containing 12.5 or 25 pmol of respective siRNAs according to manufacturer’s recommendations. Lysates for Western blotting or SphK activity assays were collected after a 72-hour incubation.
Results
SKI-178 Induces Cytotoxicity in a Range of AML Cell Lines, Including Multidrug-Resistant HL-60/VCR.
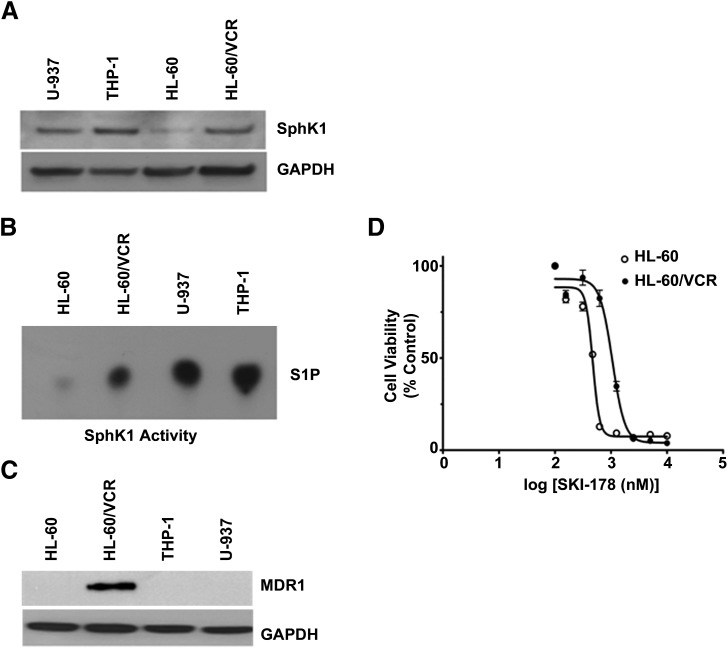
We previously demonstrated that SKI-178 is cytotoxic toward a broad panel of cancer cell lines (Hengst et al., 2010). The efficacy of SKI-178 toward the various leukemia cell lines tested in this previous study highlighted the potential utility of SKI-178 as a novel AML therapeutic strategy. To further evaluate the therapeutic potential and determine the mechanism of action of SKI-178, we chose to restrict the focus of our studies to human AML cell lines. Numerous studies have employed HL-60, HL-60/VCR, and U937 cell lines in the evaluation of SphK inhibitors and determination of the role of SphK1 in leukemia pathogenesis, indicating that these human AML cell lines are appropriate models for the determination of the mechanism of action of SKI-178 (Bonhoure et al., 2006; Paugh et al., 2008; Kennedy et al., 2011). As shown in Fig. 1A, we confirmed that SphK1 protein is expressed in each of these cell lines. Interestingly, we observed that THP-1 cells expressed the highest levels of SphK1 protein among the cell lines examined. Consistent with our previous studies (Hengst et al., 2010), we determined that SKI-178 is cytotoxic toward all AML cell lines examined, with IC50 values ranging from ∼500 nM to ∼1 μM (Table 1). Although not statistically significant, the range of IC50 values appears to correlate with the relative levels of SphK1 catalytic activity (Fig. 1B) as determined by thin-layer chromatography. For example, HL-60 cells have the lowest level of Sphk1 expression, as determined by Western blot analysis, of the four cell lines examined. They also have lower levels of SphK1 activity and lower IC50 values than HL-60/VCR, U937, and THP-1 cells.
Fig. 1.
SKI-178 induces cell death in a range of AML cell lines, including multidrug-resistant HL-60/VCR. (A and C) Whole cell lysates from the indicated AML cell lines were subjected to Western blot analysis to assess SphK1 (A) and MDR-1 (C) expression. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serves as an equal loading control. (B) Whole cell lysates from the indicated AML cell lines were prepared in buffer selective for SphK1 activity, and SphK1 catalytic activity was subsequently determined by thin-layer chromatography. (D) HL-60 and HL-60/VCR cells were exposed to increasing concentrations of SKI-178 over a 48-hour time period and cytotoxicity was assessed by MTT assays. Error bars indicate S.D. of triplicate counts of 2500 cells.
TABLE 1.
Cytotoxicity (IC50) of SKI-178 versus vincristine and colchicine
| AML Cell Line | SKI-178 | Vincristine | Colchicine |
|---|---|---|---|
| nM | |||
| HL-60 | 472 ± 2.95 | 2.3 ± 0.016 | 9.5 ± 0.034 |
| HL-60/VCR | 1057 ± 3.06 | 2280 ± 9.3 | 583 ± 2.6 |
| THP-1 | 686.7 ± 1.98 | NA | NA |
| U-937 | 885.4 ± 1.98 | NA | NA |
NA, not available.
A major obstacle for treatment of AML patients is the fact that many patients have leukemic cells that are either inherently refractory to certain chemotherapeutics or develop multidrug resistance in relapsed AML (Cortes et al., 2004; Funato et al., 2004). We therefore included in our panel the HL-60 AML cell line and its vincristine-resistant variant, HL-60/VCR, to determine whether SKI-178 would be effective against multidrug-resistant cells. HL-60/VCR cells are known to be highly resistant to numerous chemotherapeutics (Baran et al., 2006; Zhao et al., 2010). Figure 1C confirms that HL-60/VCR cells express high levels of MDR1 compared with all other AML cell lines tested. Vincristine and colchicine are chemotherapeutics known to be effluxed by multidrug-resistant proteins (Yang et al., 1999; Licht et al., 2000). We therefore confirmed drug resistance in HL-60/VCR cells by comparing IC50 values for vincristine and colchicine to the parental HL-60 cell line. As outlined in Table 1, the IC50 values of vincristine and colchicine were significantly higher in HL-60/VCR compared with HL-60 cells, verifying that HL-60/VCR cells are indeed multidrug resistant. This observation is consistent with previous findings that MDR1 overexpression in HL-60/VCR underlies its multidrug resistance (Marquardt et al., 1990; Baran et al., 2006). Interestingly, as shown in Fig. 1D and summarized in Table 1, the IC50 value for SKI-178 is only about 2-fold higher in HL-60/VCR compared with HL-60 cells. This is considerably lower than the 1000- and 60-fold differences for vincristine and colchicine, respectively, indicating that SKI-178 is not a substrate of drug efflux pump proteins (i.e., MDR1, MDR2).
SKI-178 Induces Apoptosis through the Intrinsic Apoptotic Signaling Pathway.
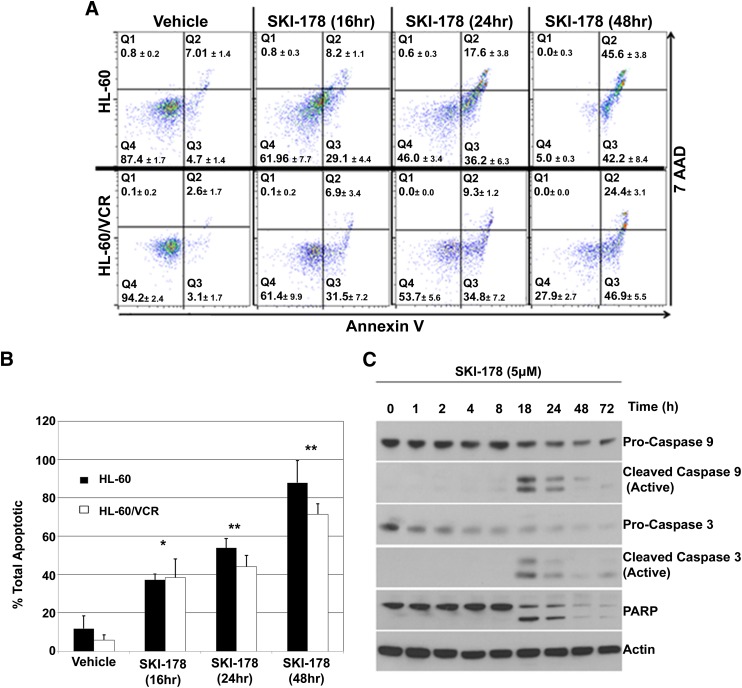
To confirm SKI-178–induced cytotoxicity is a result of apoptotic cell death, HL-60 and HL-60/VCR cells were treated with SKI-178 for indicated time periods, and the stages of apoptosis were quantified using Annexin V-PE as a marker for apoptosis and 7-amino-actinomycin D (7AAD) as a marker of necrosis. In this assay, cells in the early stages of apoptosis stain Annexin V–positive and 7AAD-negative (lower right quadrant, Q3), whereas cells in the later stages of apoptosis stain Annexin V–positive and 7AAD-positive (upper right quadrant, Q2). As shown in Fig. 2A, the majority of vehicle treated cells was viable and stain negative for both Annexin V and 7AAD (lower left quadrant, Q4). After 16 hours of SKI-178 treatment, we observed an increase in the number of early apoptotic cells (Q3). By 24 hours of SKI-178 treatment, almost 60% of HL-60 and almost 50% of HL-60/VCR cells were apoptotic (Q2 + Q3) (Fig. 2B) with an increasing percentage of these in the later stages of apoptosis (Q2) (Fig. 2A). By 48 hours, greater than 90% of HL-60 and almost 70% of HL-60/VCR cells were in either the early or late stages of apoptosis (Fig. 2B). The fact that cells progress through early apoptosis in a time-dependent manner before staining positive for 7AAD suggests that positive 7AAD staining is indicative of cells dying as a result of apoptosis and not necrosis.
Fig. 2.
SKI-178 induces apoptosis through the intrinsic apoptotic signaling pathway. (A) Cytometric analysis of Annexin V/7AAD-stained HL-60 and HL-60/VCR cells treated with SKI-178 (5 μM) for indicated time periods or vehicle (DMSO) control for 48 hours. Results shown are representative of three separate experiments. (B) Quantification of Annexin V–positive staining including both early (Q3) and late (Q2) stage apoptosis. Error bars indicate S.D. of triplicate counts of 5000 cells. Statistical significance was assessed by two-tailed paired Student’s t test. Asterisks indicate significance: *P ≤ 0.001; **P ≤ 0.0001. (C) HL-60 cells treated with SKI-178 (5 μM) for indicated time intervals. Western blot analysis was performed on whole cell lysates with proteins indicated. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. PARP, poly(ADP-ribose) polymerase.
Mitochondrion-centered intrinsic apoptosis is mediated by mitochondrial outer membrane permeabilization, resulting in apoptosome formation, activation of caspase-9, and subsequent activation of effector caspases-3 and -7. To confirm intrinsic apoptotic cell death, HL-60 cells were treated with 5 μM SKI-178 for indicated time points. The activation of proapoptotic signaling [i.e., poly(ADP ribose) polymerase cleavage] correlated with the cleavage and activation of caspases-9 and -3 (Fig. 2C), indicating an activation of the intrinsic apoptotic pathway. In contrast, we were unable to detect any changes in signaling pathways indicative of the extrinsic apoptotic pathway, such as caspase-8 activation (data not shown).
SKI-178 Alters Bioactive Sphingolipid Levels.
In previous studies we demonstrated selective SphK1 inhibition with SKI-178 in vitro as well as in whole cells using A549 lung cancer cells (Hengst et al., 2010). Hence, we used an established liquid chromatography–tandem mass spectrometry method (Sullards and Merrill, 2001; Seefelder et al., 2002; French et al., 2006) to examine the changes in intracellular levels of sphingolipid metabolites in HL-60 cells in response to SKI-178 treatment. Levels of S1P were significantly decreased after 24-hour SKI-178 (5 μM) treatment relative to the control vehicle (DMSO)-treated HL-60 cells (Table 2). Sphingosine levels were also decreased after 24 hours, most likely attributed to the reacylation of sphingosine to ceramide by the action of ceramide synthase enzymes. Interestingly, SKI-178 had a differential effect on the levels of long-chain ceramide species (LCC: C16–C18) compared with very long-chain ceramide (VLCC: C22–C24.1) species. The LCC species are known to exert proapoptotic effects, whereas VLCC species were recently suggested to promote cell growth (Koybasi et al., 2004; Grösch et al., 2012; Hartmann et al., 2012). According, we observed a significant increase in the levels of LCC species and a simultaneous decrease in the levels of VLCC species in cells treated with SKI-178 for 24 hours relative to vehicle control. Together, these results demonstrate that SKI-178 treatment alters levels of bioactive sphingolipids that favor antiproliferation and/or apoptosis.
TABLE 2.
Sphingolipid analysis of vehicle- and SKI-178 (5 μM)–treated HL-60 cells at 24 hours
| Sph | S1P | C16 | C18 | C22 | C24:1 | |
|---|---|---|---|---|---|---|
| pmol/mg total protein | ||||||
| Vehicle | 21.96 ± 1.98 | 6.98 ± 1.24 | 22.72 ± 2.07 | 0.22 ± 0.06 | 1.58 ± 0.43 | 4.22 ± 0.42 |
| SKI-178 | 15.12 ± 1.22 | 2.02 ± 0.02 | 49.84 ± 2.57 | 0.89 ± 0.08 | 0.3 ± 0.07 | 0.86 ± 0.05 |
SKI-178–Induced Apoptotic Cell Death Correlates with Prolonged Bcl-2 Phosphorylation.
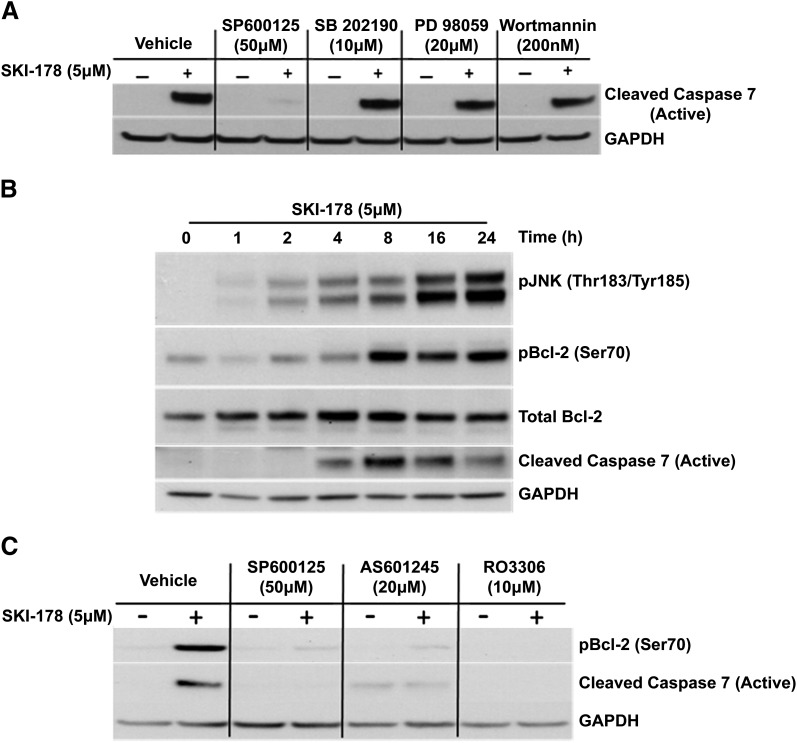
The overall balance between ceramide and S1P is known to play an important role in determining cell fate through differential effects on members of the mitogen-activated protein kinase (MAPK) family of proteins (Cuvillier et al., 1996). S1P is known to exert some of its mitogenic effects through the activation of both extracellular signal-regulated kinase (ERK), a prosurvival antiapoptotic MAPK, and the phosphoinositide 3-kinase (PI3K)/AKT prosurvival pathway (Wu et al., 1995; Bonnaud et al., 2010). Sphingosine and ceramide, on the other hand, are believed to induce cell death via activation of proapoptotic MAPKs, such as JNK and p38 mitogen-activated protein kinase (p38) (Westwick et al., 1995; Verheij et al., 1996; Jarvis et al., 1997; Yoon et al., 2009). Based on these previous studies, we examined the effect of inhibiting MAPK and PI3K/AKT signaling on SKI-178–induced cell death. Specifically, HL-60 cells were treated with SKI-178 alone, or in combination with SP600125 (1,9-pyrazoloanthrone), SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole], PD98059 [2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one], or Wortmannin, inhibitors of JNK, p38, mitogen-activated protein kinase kinase 1 (ERK1/2 inhibition), and PI3K (AKT inhibition), respectively. Interestingly, inhibiting either p38, ERK1/2, or AKT had little to no effect on SKI-178–mediated apoptotic induction, indicated by caspase-7 cleavage/activation (Fig. 3A), demonstrating these MAPK are not required for SKI-178–induced apoptosis. On the other hand, JNK inhibition with SP600125 profoundly inhibited SKI-178–mediated caspase-7 cleavage. To further evaluate the role of JNK activity in the apoptotic mechanism of SKI-178, we next examined the effects of SKI-178 treatment on JNK activation and correlated its activity to the induction of apoptotic signaling. As shown in Fig. 3B, JNK activity (indicated by phosphorylation at Thr183/Tyr185) increased in a time-dependent manner starting as early as 2 hours after 5 μM SKI-178 treatment and continued to increase for at least 24 hours. Between 8 and 24 hours of SKI-178 treatment, where JNK activity was at its highest, there was a concomitant increase in apoptotic cell death indicated by the cleavage of caspase-7. As an indication of JNK activity, we also examined the phosphorylation of Bcl-2 at Ser70, a known JNK substrate (Yamamoto et al., 1999; Deng et al., 2001). Bcl-2 phosphorylation at Ser70 increased with time in response to SKI-178 treatment, reaching maximal levels at 8 hours, which was consistent with the timing of caspase-7 activation (Fig. 3B).
Fig. 3.
SKI-178–induced Bcl-2 phosphorylation and caspase-7 activation are blocked by JNK and CDK1 inhibitors. (A) HL-60 cells treated for 24 hours with various MAPK inhibitors or vehicle (DMSO) alone or in combination with SKI-178 (5 μM). Western blot analysis was performed on whole cell lysate using antibody for cleaved caspase-7. (B) HL-60 cells treated with SKI-178 (5 μM) for indicated time intervals. Western blot analysis was performed on whole cell lysates with pJNK (Thr183/Tyr185) antibody or for the proteins indicated. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (C) HL-60 cells treated for 24 hours with vehicle (DMSO), SP600125, a JNK-specific inhibitor (AS601245), or a CDK1-specific inhibitor (RO3306) alone or in combination with SKI-178. Western blot analysis was performed on whole cell lysate using antibody for pBcl-2 (Ser70) and cleaved active caspase-7. GAPDH serves as a loading control. Results shown are representative of at least three independent experiments.
Numerous studies have implicated JNK in the phosphorylation of Bcl-2 at Ser70 in response to agents that induce mitotic arrest and subsequent apoptotic cell death (Fan et al., 2000; Kelkel et al., 2012). However, a recent study provided evidence that a known JNK inhibitor, SP600125, also inhibits CDK1 (Kim et al., 2010). Our findings in Fig. 3A, implicating JNK in the apoptotic mechanism of SKI-178, are based on the use of SP600125. To further clarify whether JNK or CDK1 was required for induction of apoptosis in response to SKI-178, we examined the effect of more selective inhibitors of JNK (AS601245 [(Z)-2-(benzo[d]thiazol-2(3H)-ylidene)-2-(2-((-(pyridine-3-yl)ethyl)amino)pyridine-4-yl)acetonitrile]) and CDK1 (RO3306) on SKI-178 mediated Bcl-2 phosphorylation. Interestingly, as shown in Fig. 3C, both the JNK- and CDK-specific inhibitors abrogated SKI-178–induced Bcl-2 phosphorylation and caspase-7 cleavage similar to SP600125. Together, these results indicated that SKI-178 induces phosphorylation of Bcl-2 at Ser70 through a JNK- and/or CDK1-dependent mechanism. Thus, we next focused our efforts on deciphering the roles of these kinases in Bcl-2 phosphorylation and its role in the apoptotic mechanism of action of SKI-178.
Prolonged G2/M Precedes Apoptosis in Response to SKI-178 Treatment.
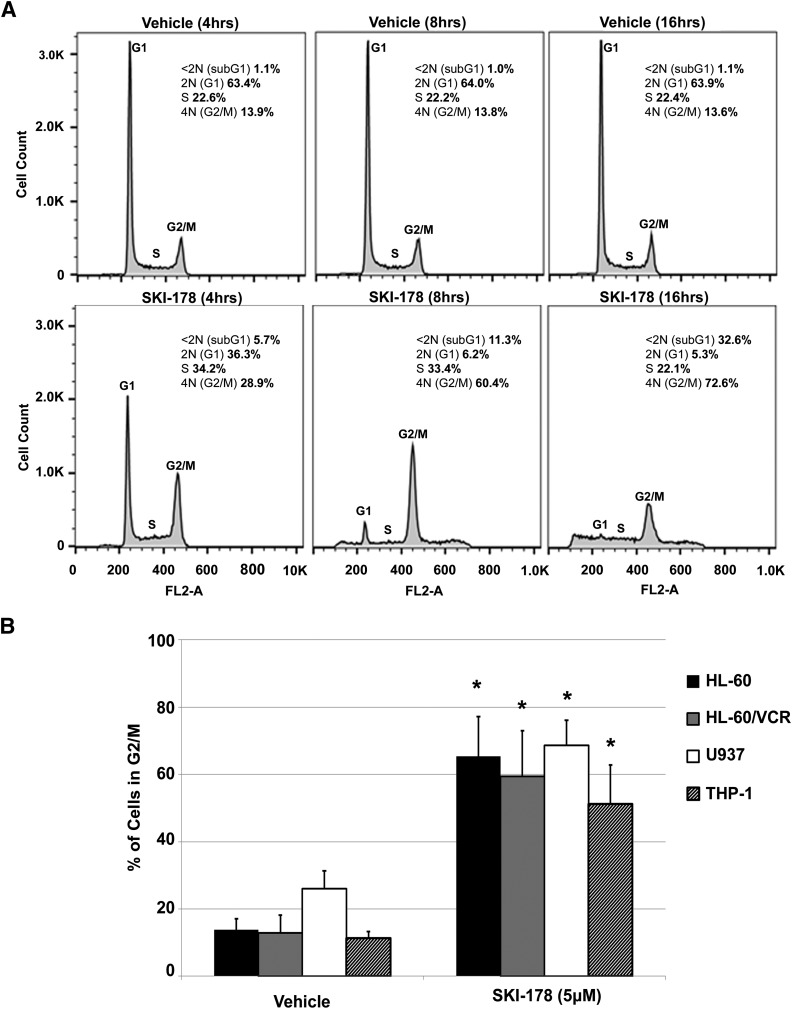
Bcl-2 is known to undergo transient cell cycle–dependent phosphorylation at multiple sites, including Ser70, during mitosis (Scatena et al., 1998; Barboule et al., 2005). In contrast, enhanced and prolonged levels of Bcl-2 phosphorylation in mitosis are commonly associated with apoptotic cell death (Haldar et al., 1998; Eichhorn et al., 2013). Therefore, we next examined the effects of SKI-178 on cell cycle progression to determine whether SKI-178–induced apoptosis is associated with mitotic progression. HL-60 cells were treated with SKI-178 or vehicle for 4, 8, and 16 hours, and DNA content was examined using propidium iodide staining followed by flow cytometry. As shown in Fig. 4A, the DNA content of vehicle-treated cells reflected a normal distribution of cells in G1, S, or G2/M at all three time points. In contrast, we observed a substantial increase in the number of cells in G2/M after only 4 hours of SKI-178 treatment. By 8 and 16 hours of treatment, the majority of cells were in G2/M phase. Importantly, we observed a significant increase in sub-G1 DNA content, indicative of DNA condensation/fragmentation during apoptotic cell death. It is important to note that DNA fragmentation in cells arrested in mitosis results in an incremental decrease in DNA (Sakurikar et al., 2014). This incremental degradation of DNA starting in cells with G2/M DNA content (i.e., 4N DNA) results in a broad sub-G1 peak that is not easily distinguished from G1 or S phase. The quantification of the sub-G1 peak includes only cells with strictly sub-G1 DNA content (<2N DNA) and therefore may be an underrepresentation of the number of cells undergoing apoptosis. Nonetheless, these results, in combination with Annexin V staining in Fig. 2, A and B, strongly indicate that SKI-178–induced G2/M arrest precedes apoptotic cell death. To verify that these effects are not cell type specific, three additional human AML cell lines, including multidrug resistant HL-60/VCR, were treated with SKI-178 for 16 hours. Analysis of their DNA content also revealed substantial increase in cells with G2/M DNA content (Fig. 4B). These results indicate that SKI-178 induces apoptosis through a sustained/prolonged cell cycle at mitosis.
Fig. 4.
Prolonged mitosis precedes SKI-178–induced apoptotic cell death. (A) Flow cytometry analysis of cell cycle distribution in HL-60 cells treated with SKI-178 or vehicle (DMSO) control for indicated time intervals. Histograms are representative of three separate experiments. (B) Percentage of AML cell lines in G2/M after 16 hours of treatment with SKI-178 (5 μM) or vehicle (DMSO). Error bars indicate S.D. of triplicate counts of 25,000 cells. Statistical significance was assessed by two-tailed paired student’s t test. Asterisks indicate significance: *P ≤ 0.01.
SKI-178 Induces Sustained Bcl-2 Phosphorylation during Mitosis.
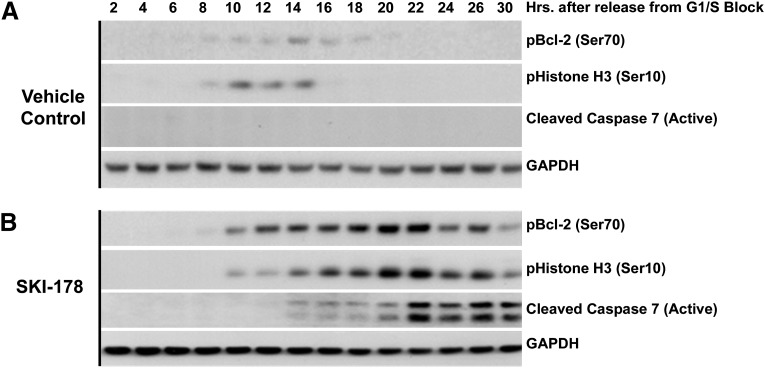
The results presented in Fig. 4, A and B, strongly suggest SKI-178–induced apoptosis may be the result of prolonged mitosis. Because analysis of DNA content does not distinguish between G2 and M phase, we employed a cell synchronization method to further examine the relationship between cell cycle and apoptosis in response to SKI-178. To this end, HL-60 cells were synchronized at the G1/S phase transition using a double thymidine block method (Bostock et al., 1971) and released into either 5 µM SKI-178 or vehicle DMSO control for a period of up to 30 hours. Whole cell lysates were prepared from cells collected every 2 hours and subjected to Western blot analysis for pBcl-2 (Ser70) as well as pHistone H3 (Ser10), which is a known marker for mitosis (Hendzel et al., 1997). Consistent with previous studies, HL-60 cells released from G1/S blockade into vehicle containing medium have a slight but transient increase in the phosphorylation of Bcl-2, because the cells progress through mitosis, indicated by histone H3 phosphorylation 10–14 hours after release (Yamamoto et al., 1999). Cells released into 5 μM SKI-178 containing medium also entered mitosis about 10 hours after release, but unlike vehicle-treated cells, histone H3 remains phosphorylated for up to 20 hours (Fig. 5). Furthermore, histone H3 phosphorylation in SKI-178–treated cells is paralleled by a robust and sustained phosphorylation of Bcl-2 at Ser70. Importantly, although no caspase-7 cleavage was detected in vehicle-treated cells, SKI-178 treatment showed substantial cleavage (activation) of caspase-7 beginning about 4 hours after the onset of mitotic arrest (14 hours postrelease). These results indicate that SKI-178–induced apoptotic cell death is closely associated with prolonged mitosis.
Fig. 5.
SKI-178 induces sustained Bcl-2 phosphorylation during prolonged mitosis. (A and B) Bcl-2 phosphorylation dynamics during cell cycle progression of vehicle (DMSO) treated or SKI-178 (5 μM) treated cells. HL-60 cells were synchronized at G1/S phase transition using a double thymidine block. Cells were released from the block into media containing vehicle (DMSO) (A) or SKI-178 (5 μM) (B). Whole cell lysates were collected at indicated time points, and Western blot analysis was performed using antibodies for pBcl-2 (Ser70), pHistone H3 (Ser10), or cleaved active caspase-7. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serves as a loading control. Results shown are representative of at least three independent experiments.
CDK1 Inhibitor Completely Abrogates SKI-178–Induced Bcl-2 Phosphorylation and Caspase-7 Activation.
The results presented thus far clearly indicate that SKI-178 induces prolonged mitosis indicated by prolonged Bcl-2 and histone H3 phosphorylation. Additionally, our pharmacologic inhibitor studies indicate that JNK and/or CDK1 are required for SKI-178–induced apoptosis. However, elucidation of the role of JNK and CDK1 is complicated by the fact that inhibition of either kinase was previously shown to block cell cycle progression from G2 into mitosis. Hence, we next sought to clarify the roles of JNK and CDK1 in the apoptotic mechanism of SKI-178 associated with prolonged mitosis.
CDK1, in complex with cyclin B1, functions as a mitosis promoting factor initiating the transition into early prophase (Morgan, 1995). Inhibition of CDK1 activity before entry into mitosis blocks cell cycle progression at the G2/M boundary (Vassilev, 2006). Similarly, there are data to suggest that JNK is involved in progression from G2 into mitosis by regulating expression of Aurora B (Oktay et al., 2008). Thus it is possible that simultaneous addition of JNK/CDK1 inhibitors with SKI-178 in unsynchronized cells or in synchronized cells before entry into mitosis would abrogate SKI-178–induced apoptosis. However, this effect could be an artifact of the blockage of cell cycle before mitosis and would not accurately reflect the roles of these kinases in SKI-178–induced mitosis-dependent apoptotic cell death.
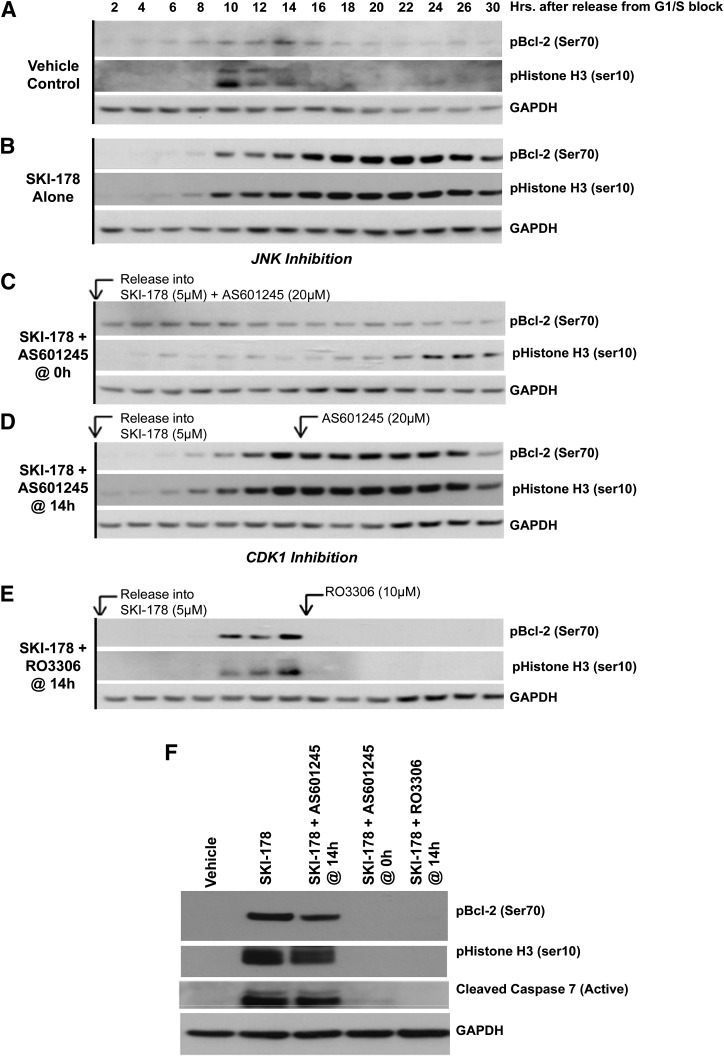
To overcome these limitations, HL-60 cells were synchronized in G1/S phase using a double thymidine block, released into fresh medium containing SKI-178 and then treated with JNK (AS601245) or CDK1 (RO3306) inhibitors at different time points as cells progress through the cell cycle. Whole cell lysates were prepared from cells collected at the indicated time points and subjected to Western blot analysis for pBcl-2 (Ser70) and pHistone H3 (Ser10). Our results show that cells released into either vehicle or SKI-178 alone (Fig. 6, A and B, respectively) responded as previously observed (Fig. 5) and entered into mitosis, as indicated by the presence histone H3 phosphorylation (Ser10), approximately 10 hours after release from G1/S blockade. Additionally, vehicle-treated control cells showed a slight but transient increase in Bcl-2 phosphorylation as the cell cycle progresses through mitosis 10–14 hours after release. In contrast, SKI-178–treated cells showed enhanced and prolonged Bcl-2 phosphorylation, prolonged mitosis indicated by sustained histone H3 phosphorylation beginning 10 hours after release (Fig. 6B), and apoptotic cell death indicated by caspase-7 cleavage (activation) at 26 hours (Fig. 6F).
Fig. 6.
Inhibition of CDK1 completely abrogates SKI-178–induced Bcl-2 phosphorylation and caspase-7 activation after mitotic arrest. HL-60 cells were synchronized at G1/S phase transition using a double thymidine block and released into either vehicle (A) or SKI-178 (5 μM) (B). Cells released into SKI-178 were subdivided into four additional treatments: (C) HL-60 cells released into SKI-178 and cotreated with AS601245 at the time of release; (D) HL-60 cells released into SKI-178 and cotreated with AS601245 14 hours after release; (E) HL-60 cells released into SKI-178 and cotreated with RO3306 14 hours after release. Whole cell lysates were collected at indicated time points and blot analysis was performed using antibodies for pBcl-2 (Ser70) or pHistone H3 (Ser10). (F) To directly compare pBcl-2 (Ser70), pHistone H3 (Ser10), and caspase cleavage between the various treatments, Western blot analysis was performed using indicated antibodies on the 26-hour time points from (A–E). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serves as a loading control. Results shown are representative of at least three independent experiments.
We next evaluated the role of JNK in SKI-178–induced apoptosis by treating synchronized HL-60 cells with AS601245 (selective JNK inhibitor) either at the time of release from G1/S blockade or after cells had entered mitosis. When HL-60 cells were simultaneously treated with SKI-178 and the JNK inhibitor (AS601245) at the time of release from G1/S blockade, we observed abrogation of pBcl-2 (Ser70) and pHistone H3 (Ser10) (Fig. 6C) and no caspase-7 cleavage (Active) (Fig. 6F). Conversely, when synchronized HL-60 cells were released from the G1/S blockade into medium containing SKI-178, allowed to enter into mitosis [∼10 hours indicated by pHistone H3 (Ser10)], and subsequently treated with AS601245 (addition at 14 hours), JNK inhibition did not have an effect on SKI-178–induced Bcl-2 or histone H3 phosphorylation (Fig. 6D). Similarly, AS601245 addition at 14 hours after release did not have an effect on caspase-7 cleavage (activation) (Fig. 6F). Together, these results indicate that JNK activity plays a critical role in cell cycle progression from G2 into mitosis and confirms that entry into mitosis is required for SKI-178–mediated apoptotic cell death.
The same experimental design was employed to evaluate the role of CDK1 on SKI-178–induced apoptosis. Given the established role of CDK1/cyclin B1 as the mitosis promoting factor (Vassilev, 2006), we did not evaluate the role of CDK1 inhibition before to entry into mitosis. As shown in Fig. 6E, inhibition of CDK1 with RO3306 after synchronized cells entered mitosis (14 hours after release) completely abrogated SKI-178–induced Bcl-2 phosphorylation, indicating that CDK1 is responsible for Bcl-2 phosphorylation. Similarly, RO3306 treatment also abrogated histone H3 phosphorylation. This is likely due to the fact that CDK1 regulates the activity of Aurora kinase A, the kinase responsible for histone H3 phosphorylation at Ser10 (Van Horn et al., 2010; Ding et al., 2011). In addition, RO3306 inhibition of CDK1 dramatically reduced SKI-178–induced apoptosis indicated by abrogation of caspase-7 cleavage (activation) (Fig. 6F). Together, these results indicate that CDK1, not JNK, is the kinase responsible for SKI-178–induced apoptotic cell death.
SKI-178 Induces Prolonged CDK1 Activation.
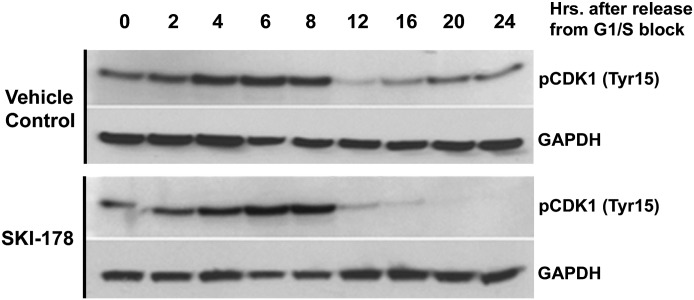
The results presented above clearly indicate that SKI-178 induces prolonged mitosis where CDK1 is active. Furthermore, inhibition of CDK1 during mitosis completely abrogates SKI-178–induced enhanced/sustained Bcl-2 phosphorylation and caspase-7 cleavage. These results suggest that SKI-178 induces sustained activation of CDK1 once cells enter into mitosis. Before mitosis, CDK1 is held inactive by phosphorylation at two residues, Thr14 and Tyr15 (Chow et al., 2011). Tyr15 phosphorylation, in particular, is an essential checkpoint preventing entry into and progression through mitosis (Fletcher et al., 2002; Welburn et al., 2007). To confirm sustained CDK1 activity, HL-60 cells were synchronized at G1/S and released into SKI-178 or vehicle control for a period of 24 hours. CDK1 activity was examined by the presence or absence of the inhibitory phosphorylation of CDK1 at Tyr15. HL-60 cells released into both SKI-178 and vehicle control showed sustained CDK1 inactivation [indicated by phosphorylation at pCDK1 (Tyr15)] up to 8 hours after release (Fig. 7). Between 8 and 12 hours, the inactivating phosphorylation of CDK1 [pCDK1 (Tyr15)] is rapidly reduced in both vehicle- and SKI-178–treated cells as cells enter mitosis. Vehicle-treated cells start to exit mitosis around 16 hours, indicated by the reappearance of CDK1 inhibitory phosphorylation. On the other hand, CDK1 remained active in SKI-178–treated cells (indicated by the lack of phosphorylation at Tyr15) once cells have entered into mitosis. These results are consistent with our previous finding that SKI-178 induces prolonged mitotic arrest and further indicate that sustained activation of CDK1 in response to SKI-178 is responsible for the phosphorylation and inactivation of antiapoptotic Bcl-2.
Fig. 7.
SKI-178 induces sustained CDK1 activation during mitosis. HL-60 cells were treated with either vehicle or SKI-178 (5 μM) for the indicated time points. Whole cell lysates were collected at indicated time points, and Western blot analysis was performed using antibodies for pCDK1 (Tyr15). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serves as a loading control. Results shown are representative of three independent experiments.
SKI-178–Induced CDK1 Activation Results in Mcl-1 Degradation.
Although our data suggest a strong correlation between Bcl-2 phosphorylation at Ser70 and induction of apoptotic cell death, the functional role of this phosphorylation is not yet clear. Although many studies have implicated this Bcl-2 phosphorylation in making cells more susceptible to apoptosis (Haldar et al., 1998; Eichhorn et al., 2013), there are recent studies that suggest it actually enhances its cytoprotective effects (Dai et al., 2013; Zhou et al., 2014). Nonetheless, Bcl-2 is not the only CDK1 substrate that displays extensive/prolonged phosphorylation during prolonged mitotic arrest. CDK1 has been shown to phosphorylate and inactivate other antiapoptotic Bcl-2 family members including Bcl-xl and Mcl-1. Bcl-xl phosphorylation during mitotic arrest inhibits its antiapoptotic activity by disrupting its interaction with Bax, leading to mitochondrial outer membrane permeabilization and cytochrome c release (Bah et al., 2014). Unlike Bcl-2 and Bcl-xl, Mcl-1 phosphorylation at Thr92 by CDK1 quickly targets it for proteasomal degradation (Harley et al., 2010).
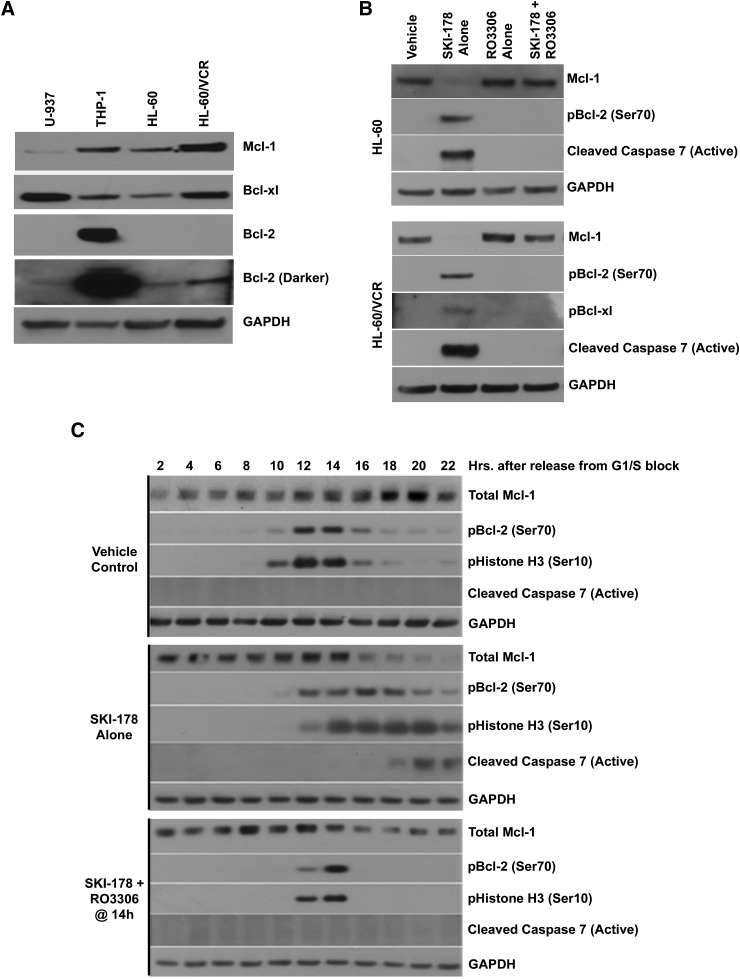
As demonstrated in Fig. 8A, all four AML cell lines, to varying degrees, express Bcl-2, Mcl-1, and Bcl-xl. Relative to HL-60 cells, HL-60/VCR cells express higher levels of all three antiapoptotic Bcl-2 family members. Interestingly, THP-1 cells express extensively higher levels of Bcl-2 relative to all other cell lines examined. Given that CDK1-dependent phosphorylation of Mcl-1 targets it for degradation, it is hypothesized that CDK1 inhibition would prevent Mcl-1 degradation in response to SKI-178. To test this hypothesis, HL-60 and HL-60/VCR cells were treated with SKI-178 alone or in combination with RO3306 for a 24-hour period, and the expression levels of pBcl-2 (Ser70), pBcl-xl (Ser62), and total Mcl-1 were examined by Western blot analysis. As expected, SKI-178 treatment led to a dramatic increase in Bcl-2 phosphorylation, Mcl-1 degradation, and caspase-7 cleavage (activation) in both HL-60 and HL-60/VCR cells (Fig. 8B). SKI-178 also induced phosphorylation of Bcl-xl in HL-60/VCR cells, whereas Bcl-xl phosphorylation in HL-60 was not detected (data not shown), likely due to antibody limitations because HL-60 express considerably lower levels of total Bcl-xl relative to HL-60/VCR cells (Fig. 8A).
Fig. 8.
SKI-178–induced CDK1 activation results in MCL-1 degradation. (A) Whole cell lysates from the indicated AML cell lines were subjected to Western blot analysis to assess expression of various antiapoptotic family members (Bcl-2, Bcl-xl, and Mcl-1). (B) HL-60 and HL-60/VCR cells treated for 24 hours with SKI-178, RO3306, or a combination of SKI-178 and RO3306. Western blot analysis was performed on whole cell lysates using indicated antibodies. (C) HL-60/VCR cells were synchronized at the G1/S phase transition using a double thymidine block and released into either vehicle or SKI-178. Cells released into SKI-178 were either maintained in SKI-178 alone or cotreated with RO3306 14 hours after release. Whole cell lysates were collected at indicated time points, and Western blot analysis was performed using indicated antibodies. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serves as a loading control.
As discussed previously with regard to Bcl-2 phosphorylation, inhibition of Mcl-1 degradation by RO3306 could occur indirectly by inhibiting cell cycle entry into mitosis where Mcl-1 phosphorylation/degradation occurs. To clarify this point, HL-60/VCR cells were synchronized as previously described, released into media containing SKI-178, and treated with RO3306 after cells had entered into mitosis (∼14 hours after release). HL-60/VCR were chosen based on their high expression of Mcl-1 relative to other cell lines (Fig. 8A) and to extend the cell cycle profiling seen in HL-60 to a multidrug-resistant cell line. The results seen here with HL-60/VCR (Fig. 8C) mimicked those previously observed in HL-60 cells. Specifically, cells released into either vehicle or SKI-178 alone entered into mitosis, as indicated by the presence histone H3 phosphorylation (Ser10), approximately 10–12 hours after release from G1/S blockade. Vehicle-treated cells showed a slight but transient increase in Bcl-2 phosphorylation as cells progress through mitosis 10–16 hours after release. Cells treated with SKI-178 alone showed sustained Bcl-2 phosphorylation, prolonged mitosis, and subsequent caspase-7 cleavage (active) starting around 6–8 hours after the onset of mitosis. As expected, SKI-178 also lead to almost complete Mcl-1 degradation beginning shortly after entry into mitosis but before the appearance of caspase-7 cleavage. As shown previously in HL-60 cells, inhibition of CDK1 in HL-60/VCR cells during mitosis completely blocked Bcl-2 phosphorylation and caspase-7 activation. Furthermore, inhibition of CDK1 prevented the complete degradation of Mcl-1 observed in cells treated with SKI-178 alone. Together, these results indicate that SKI-178 induces apoptotic cell death during prolonged apoptosis by activating intrinsic apoptosis signaling pathways (i.e., Bcl-2 phosphorylation and Mcl-1 degradation).
siRNA Knockdown of SphK1 Recapitulates the Effects of SKI-178.
To further demonstrate that the mechanism of action we observed with SKI-178 was attributable to its ability to inhibit SphK1 activity, we treated MIA PaCa-2 cells with siRNAs directed to SphK1. MIA PaCa-2 cells were used to overcome the difficulty of transfection of suspension cell lines, such as HL-60 cells, and to extend our observations to non-AML cell lines. In vitro SphK activity assays using isotype specific buffer conditions confirm the ability of SphK1 siRNAs to block SphK1 expression without affecting SphK2 activity (Supplemental Fig. 1A). Western blot analysis of siRNA-treated cells revealed that blockage of SphK1 expression activated JNK and induced phosphorylation of Bcl-2 (Ser70) relative to scrambled controls (Supplemental Fig. 1B), as was observed previously with SKI-178 treatment. Furthermore, we also treated MIA PaCa-2 cells that have been transfected with SphK1 siRNAs with low doses of SKI-178 (500 nM). As shown in Supplemental Fig. 1C, SphK1 knockdown (KD) increases the phosphorylation of JNK and Bcl-2 to the same extent as scrambled siRNA control cells treated with 500 nM SKI-178. Treatment of SphK1 KD cells with 500 nM SKI-178 further enhanced the activation of JNK, phosphorylation of Bcl-2, and induced substantial activation of caspase-7. Together, the fact that SphK1 siRNAs recapitulate the effects of SKI-178 and that SphK1 KD enhances the effects of SKI-178 strongly supports the mechanism of action of SKI-178 described herein is due to the target-specific effects of SKI-178 on SphK1 activity rather than “off-target” effects.
Discussion
In this study we use multiple human AML cell lines to elucidate the molecular mechanism of action of SKI-178, a potent, selective inhibitor of SphK1. Elevated expression of SphK1 has consistently been observed in various cancer types, including leukemia (Heffernan-Stroud and Obeid, 2013), and has become a promising target for novel therapeutic strategies (Orr Gandy and Obeid, 2013). We previously established SKI-178 as a potent selective inhibitor of SphK1 through in vitro activity assays with purified SphK1 protein (Hengst et al., 2010). Our results presented here support these findings by demonstrating that SKI-178 treatment of HL-60 cells induces alterations in intracellular levels of bioactive sphingolipid metabolites that are consistent with SphK1 inhibition. After 24 hours of treatment, levels of S1P are significantly decreased compared with control, whereas levels of proapoptotic long-chain ceramide species (C16 and C18) are increased. Interestingly, SKI-178 treatment concomitantly decreased levels of very long chain ceramide species, which recent studies suggest have antiapoptotic properties (Koybasi et al., 2004; Grösch et al., 2012; Hartmann et al., 2012). Ceramide is produced by the addition of a fatty acyl chain to a molecule of sphingosine by the activity of six different ceramide synthase enzymes (CerS1–6), each with different substrate specificities (Park et al., 2014). The majority of very long chain ceramide species are produced specifically by ceramide synthase 2 (CerS2) (Petrache et al., 2013). The decrease in very long chain ceramide species implies that CerS2 might be inhibited by SKI-178. Considering both SphK1 and ceramide synthase enzymes use sphingosine as a substrate and SKI-178 acts as a sphingosine competitive inhibitor, this is a distinct possibility. Another potential explanation is that CerS2 activity is regulated by sphingolipid levels. Indeed, Laviad et al. (2008) demonstrated that S1P inhibits CerS2 activity. Because SKI-178 is a SphK1-selective inhibitor and does not inhibit SphK2, it is possible that S1P levels remain high enough to restrict CerS2 activity such that very long chain ceramide levels remain unchanged or decrease over time through further metabolic conversion in response to SKI-178. Conversely, Beverly et al. (2013) recently demonstrated that induction of apoptotic signaling stimulates the formation of long-chain ceramide species. Thus SKI-178 may induce the formation of long-chain ceramides from the excess pool of sphingosine that is liberated by the inhibition of S1P formation. Regardless of how SKI-178 induces these opposing effects, simultaneous induction and inhibition of pro- and antiapoptotic ceramide species, respectively, presents a unique characteristic of SKI-178, making it a promising tool in the field of sphingolipid biology.
We next verified SKI-178–induced cytotoxicity is the result of mitochondria-dependent apoptosis based on Annexin V staining and caspases-9, -3, and -7 activation. Numerous MAPKs, such as ERK, p38, JNK, and Akt, have been identified as important regulators of this intrinsic apoptotic pathway (Xia et al., 1995; Kennedy et al., 1997; Cagnol and Chambard, 2010). Furthermore, activation of these MAPKs plays an important role in ceramide-induced apoptotic cell death (Oh et al., 2006; Kim et al., 2008). Therefore, as SKI-178 treatment induces accumulation of proapoptotic ceramide species, we proposed that activation of various MAPKs may be critical to the apoptotic mechanism of SKI-178. Of all the MAPKs tested, only JNK activation appeared to be essential for the apoptotic mechanism for SKI-178. SKI-178 treatment in combination with JNK inhibition completely abrogated SKI-178–induced caspase cleavage, whereas inhibition of p38, ERK, or Akt had little to no effect. In addition to JNK activation, SKI-178 induced a time-dependent increase in the phosphorylation of antiapoptotic Bcl-2 at Ser70. Although JNK has been linked to the phosphorylation of Bcl-2 in mitotically arrested cells (Yamamoto et al., 1999; Fan et al., 2000; Du et al., 2005), there is conflicting evidence in the literature implicating CDK1 as the kinase responsible for this effect (Terrano et al., 2010; Sakurikar et al., 2014). Our results concur with the latter findings, demonstrating that CDK1 is required for SKI-178–induced Bcl-2 phosphorylation. We, however, do find that JNK activity is important for cell cycle progression from G2 into mitosis and that JNK inhibition, before the onset of mitosis, abrogates SKI-178–mediated cell death by preventing entry into mitosis where SKI-178–mediated cell death occurs. This is consistent with previous finding that JNK activity peaks during late G2, and inactivation of JNK before mitosis inhibits the expression of Aurora kinase B and phosphorylation of histone H3 at Ser10 (Du et al., 2004; Oktay et al., 2008).
Our findings further suggest a link between SphK1 inhibition with SKI-178 and prolonged activation of CDK1 leading to apoptotic cell death. Although it is possible that SKI-178 induces prolonged mitosis as a polypharmacologic agent (i.e., “off-target” effects), several studies provide evidence to suggest that SphK1 and/or SphK2 plays an important role in cell cycle progression. For example, SphK1, SphK2, and the S1P5 receptor have been shown to colocalize to the centrosome of mammalian cells, where the authors proposed that the S1P5 receptor acts as a guanine nucleotide exchange factor stimulated by localized S1P production (Gillies et al., 2009). Thus, SphK1 inhibition by SKI-178 or other SphK inhibitors could alter the activity of the centrosome. In support of this, SphK inhibition and RNAi-mediated SphK1 downregulation has been shown to block mitotic exit, leading to cytokinesis failure in breast carcinoma cell lines (Kotelevets et al., 2012), and nonselective small molecule inhibitors of SphK1 and SphK2 induce substantial G2/M arrest in melanoma cell lines (Madhunapantula et al., 2012). Although outside the scope of this investigation, it is interesting to suggest that sphingolipid metabolism plays a role in regulation of the cell cycle.
Of all the stages of the cell cycle, mitosis is considered the most critical. During mitosis, condensed chromosomes are no longer protected by the nuclear membrane and DNA damage is not easily repairable (Heijink et al., 2013). This leaves cells extremely vulnerable and potentially more susceptible to apoptotic stimuli. Mitosis is tightly controlled by the spindle-assembly checkpoint that ensures proper chromosome replication and segregation. A failure to satisfy the spindle-assembly checkpoint often results in prolonged mitotic arrest and the induction of an intrinsic proapoptotic pathway responsible for clearing cells that fail to exit mitosis in a timely fashion (Topham and Taylor, 2013). This intrinsic phenomenon, normally used as a quality control mechanism to ensure proper chromosome segregation, has been widely exploited by many current chemotherapeutics to induce apoptotic cell death in rapidly growing cancer cells (Chan et al., 2012).
Several lines of evidence indicate that extensive phosphorylation of Bcl-2 at Ser70 and other sites by CDK1 inactivates its antiapoptotic function and is a key feature of apoptotic cell death in response to agents that induce prolonged mitotic arrest (Haldar et al., 1998; Barboule et al., 2005; Eichhorn et al., 2013). However, Dai et al. (2013) recently demonstrated that phosphorylation of Bcl-2 at Ser70 enhances the antiapoptotic function of Bcl-2 by promoting Bim and Bak binding. Interestingly, CDK1-mediated phosphorylation of Bcl-xl at Ser62 during mitotic arrest has more consistently been shown to play a proapoptotic role, at least in part by disrupting its ability to bind and inhibit proapoptotic Bax (Upreti et al., 2008; Bah et al., 2014). Furthermore, CDK1-mediated phosphorylation of Mcl-1 at Thr92 and its subsequent proteasomal degradation during prolonged mitotic arrest have also been shown to play a key role in linking mitotic arrest to the induction of apoptotic cell death (Harley et al., 2010). Our results clearly show that during SKI-178–induced prolonged mitosis, these antiapoptotic Bcl-2 family members undergo these posttranslational modifications, and that they are strictly dependent upon sustained CDK1 activity. The functional role of Bcl-2 phosphorylation at Ser70 remains controversial, but irrespective of whether it enhances its antiapoptotic effects or induces apoptosis, pBcl-2 (Ser70) is a clear maker of CDK1 activity that consistently correlates with induction of apoptotic cell death.
It is important to note that different cell lines depend more heavily on different antiapoptotic Bcl-2 family members for survival. Although many leukemic cells are highly dependent on Bcl-2 (Tothova et al., 2002), the other antiapoptotic Bcl-2 family members, Mcl-1 and Bcl-xl, have overlapping functions and can compensate for Bcl-2 inhibition (van Delft et al., 2006; Lin et al., 2007; Wei et al., 2012). Furthermore, alterations in the expression of Bcl-2 family members play a potential role in the mechanism of resistance to various chemotherapeutic agents. Resistance to ABT-737, a small molecule inhibitor of Bcl-2 currently undergoing clinical trials, is attributed to transcriptional upregulation of Mcl-1 (Yecies et al., 2010). Similarly, high levels of Bcl-xl expression confers resistance to Mcl-1 inhibitors (Wei et al., 2012). All four AML cell lines expressed Bcl-2, Bcl-xl, or Mcl-1 to varying degrees. THP-1, in particular, expresses extensively high levels of Bcl-2 relative to all other cell lines but remain sensitive to the cytotoxic effects of SKI-178.
Although our findings here focus more heavily on Bcl-2, it is well established in the literature that CDK1, in response to inducers of mitotic arrest, simultaneously phosphorylates Bcl-2, Bcl-xl, and Mcl-1 (Harley et al., 2010; Terrano et al., 2010; Chu et al., 2012). We demonstrate that in addition to Bcl-2, SKI-178–induced CDK1 activity also leads to the inhibitory phosphorylation of Bcl-xl (Ser62) and Mcl-1 phosphorylation, leading to subsequent degradation. The fact that SKI-178 leads to the prolonged and simultaneous inhibition of multiple antiapoptotic Bcl-2 proteins makes it an attractive potential therapeutic agent for the treatment of AML. In fact, many of the most effective chemotherapeutic agents currently in clinical use are inducers of mitotic arrest (Mukhtar et al., 2014). Unfortunately, a major problem in the treatment of leukemic cancers is the development of resistance to such chemotherapeutic agents (Holohan et al., 2013). Much of this resistance has been attributed to the overexpression of MDR1 (Broxterman and Schuurhuis, 1997; Cianfriglia, 2013). Taken together, our results demonstrate that SKI-178 induces apoptotic cell death in multiple leukemic cell lines, including multidrug-resistant cell lines. These results highlight the promising potential of developing SKI-178 as a therapeutic strategy, not only for AML but also for an array of cancer types including those with multidrug resistance. Further studies are underway to examine the in vivo efficacy of SKI-178 in animal models.
Supplementary Material
Abbreviations
- 7AAD
7-amino-actinomycin
- AML
acute myeloid leukemia
- AS601245
(Z)-2-(benzo[d]thiazol-2(3H)-ylidene)-2-(2-((-(pyridine-3-yl)ethyl)amino)pyridine-4-yl)acetonitrile
- CDK1
cyclin-dependent kinase 1
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- JNK
c-Jun NH2-terminal kinase
- KD
knockdown
- LCC
long-chain ceramide
- MAPK
mitogen-activated protein kinase
- MDR
multidrug-resistant protein
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
phosphate-buffered saline
- PD98059
2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one
- PI
propidium iodide
- PI3K
phosphoinositide 3-kinase
- RO3306
(5Z)-5-quinolin-6-ylmethylene-2-[(thiophen-2-ylmethyl)-amino]-thiazol-4-one
- S1P
sphingosine-1-phosphate
- SB202190
4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole
- siRNA
small interfering RNA
- SKI-178
N′-[(1E)-1-(3,4-dimethoxyphenyl)ethylidene]-3-(4-methoxxyphenyl)-1H-pyrazole-5-carbohydrazide
- SP600125
1,9-pyrazoloanthrone
- SphK1
sphingosine kinase-1
- VLCC
very long-chain ceramide
Authorship Contributions
Participated in research design: Dick, Hengst, Fox, Sung, Sharma, Amin, Loughran, Kester, Wang, Yun.
Conducted experiments: Dick, Hengst, Fox, Colledge, Kale.
Contributed new reagents or analytic tools: Fox.
Performed data analysis: Dick, Hengst, Yun.
Wrote or contributed to the writing of the manuscript: Dick, Hengst, Colledge, Kale, Yun.
Footnotes
Support for this study was provided by Penn State Hershey College of Medicine; Penn State Hershey Cancer Institute; Jake Gittlen Cancer Research Foundation; the National Institutes of Health National Cancer Institute [Grant P01-CA171983]; and the Pennsylvania Department of Health (SAP# 4100054865).
This work was previously presented as a poster at the following workshop: Dick T, Hengst J, Fox T, Colledge A, Kale V, Sung SS, Amin S, Loughran T, Kester M, Wang HG, and Yun J (2013) Novel sphingosine kinase 1 selective inhibitor, SKI-178, induces apoptotic cell death through prolonged activation of CDK1. 7th International Ceramide Conference; 2013 Oct 20–24; Montauk, NY.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Adan-Gokbulut A, Kartal-Yandim M, Iskender G, Baran Y. (2013) Novel agents targeting bioactive sphingolipids for the treatment of cancer. Curr Med Chem 20:108–122. [PubMed] [Google Scholar]
- Bah N, Maillet L, Ryan J, Dubreil S, Gautier F, Letai A, Juin P, Barillé-Nion S. (2014) Bcl-xL controls a switch between cell death modes during mitotic arrest. Cell Death Dis 5:e1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran Y, Gunduz U, Ural AU. (2006) Cross-resistance to cytosine arabinoside in human acute myeloid leukemia cells selected for resistance to vincristine. Exp Oncol 28:163–165. [PubMed] [Google Scholar]
- Barboule N, Truchet I, Valette A. (2005) Localization of phosphorylated forms of Bcl-2 in mitosis: co-localization with Ki-67 and nucleolin in nuclear structures and on mitotic chromosomes. Cell Cycle 4:590–596. [DOI] [PubMed] [Google Scholar]
- Bayerl MG, Bruggeman RD, Conroy EJ, Hengst JA, King TS, Jimenez M, Claxton DF, Yun JK. (2008) Sphingosine kinase 1 protein and mRNA are overexpressed in non-Hodgkin lymphomas and are attractive targets for novel pharmacological interventions. Leuk Lymphoma 49:948–954. [DOI] [PubMed] [Google Scholar]
- Beverly LJ, Howell LA, Hernandez-Corbacho M, Casson L, Chipuk JE, Siskind LJ. (2013) BAK activation is necessary and sufficient to drive ceramide synthase-dependent ceramide accumulation following inhibition of BCL2-like proteins. Biochem J 452:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoure E, Pchejetski D, Aouali N, Morjani H, Levade T, Kohama T, Cuvillier O. (2006) Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia 20:95–102. [DOI] [PubMed] [Google Scholar]
- Bonnaud S, Niaudet C, Legoux F, Corre I, Delpon G, Saulquin X, Fuks Z, Gaugler MH, Kolesnick R, Paris F. (2010) Sphingosine-1-phosphate activates the AKT pathway to protect small intestines from radiation-induced endothelial apoptosis. Cancer Res 70:9905–9915. [DOI] [PubMed] [Google Scholar]
- Bostock CJ, Prescott DM, Kirkpatrick JB. (1971) An evaluation of the double thymidine block for synchronizing mammalian cells at the G1-S border. Exp Cell Res 68:163–168. [DOI] [PubMed] [Google Scholar]
- Broxterman HJ, Schuurhuis GJ. (1997) Transport proteins in drug resistance: detection and prognostic significance in acute myeloid leukemia. J Intern Med Suppl 740:147–151. [PubMed] [Google Scholar]
- Cagnol S, Chambard JC. (2010) ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J 277:2–21. [DOI] [PubMed] [Google Scholar]
- Chan KS, Koh CG, Li HY. (2012) Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis 3:e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JP, Poon RY, Ma HT. (2011) Inhibitory phosphorylation of cyclin-dependent kinase 1 as a compensatory mechanism for mitosis exit. Mol Cell Biol 31:1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu R, Terrano DT, Chambers TC. (2012) Cdk1/cyclin B plays a key role in mitotic arrest-induced apoptosis by phosphorylation of Mcl-1, promoting its degradation and freeing Bak from sequestration. Biochem Pharmacol 83:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfriglia M. (2013) Targeting MDR1-P-glycoprotein (MDR1-Pgp) in immunochemotherapy of acute myeloid leukemia (AML). Ann Ist Super Sanita 49:190–208. [DOI] [PubMed] [Google Scholar]
- Cortes J, Thomas D, Koller C, Giles F, Estey E, Faderl S, Garcia-Manero G, McConkey D, Ruiz SL, Guerciolini R, et al. (2004) Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res 10:3371–3376. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. (1996) Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381:800–803. [DOI] [PubMed] [Google Scholar]
- Dai H, Ding H, Meng XW, Lee SH, Schneider PA, Kaufmann SH. (2013) Contribution of Bcl-2 phosphorylation to Bak binding and drug resistance. Cancer Res 73:6998–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May WS., Jr (2001) Novel role for JNK as a stress-activated Bcl2 kinase. J Biol Chem 276:23681–23688. [DOI] [PubMed] [Google Scholar]
- Ding J, Swain JE, Smith GD. (2011) Aurora kinase-A regulates microtubule organizing center (MTOC) localization, chromosome dynamics, and histone-H3 phosphorylation in mouse oocytes. Mol Reprod Dev 78:80–90. [DOI] [PubMed] [Google Scholar]
- Du L, Lyle CS, Chambers TC. (2005) Characterization of vinblastine-induced Bcl-xL and Bcl-2 phosphorylation: evidence for a novel protein kinase and a coordinated phosphorylation/dephosphorylation cycle associated with apoptosis induction. Oncogene 24:107–117. [DOI] [PubMed] [Google Scholar]
- Du L, Lyle CS, Obey TB, Gaarde WA, Muir JA, Bennett BL, Chambers TC. (2004) Inhibition of cell proliferation and cell cycle progression by specific inhibition of basal JNK activity: evidence that mitotic Bcl-2 phosphorylation is JNK-independent. J Biol Chem 279:11957–11966. [DOI] [PubMed] [Google Scholar]
- Eichhorn JM, Sakurikar N, Alford SE, Chu R, Chambers TC. (2013) Critical role of anti-apoptotic Bcl-2 protein phosphorylation in mitotic death. Cell Death Dis 4:e834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA, Chambers TC. (2000) Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem 275:29980–29985. [DOI] [PubMed] [Google Scholar]
- Fletcher L, Cheng Y, Muschel RJ. (2002) Abolishment of the Tyr-15 inhibitory phosphorylation site on cdc2 reduces the radiation-induced G(2) delay, revealing a potential checkpoint in early mitosis. Cancer Res 62:241–250. [PubMed] [Google Scholar]
- Fox TE, Bewley MC, Unrath KA, Pedersen MM, Anderson RE, Jung DY, Jefferson LS, Kim JK, Bronson SK, Flanagan JM, et al. (2011) Circulating sphingolipid biomarkers in models of type 1 diabetes. J Lipid Res 52:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francy JM, Nag A, Conroy EJ, Hengst JA, Yun JK. (2007) Sphingosine kinase 1 expression is regulated by signaling through PI3K, AKT2, and mTOR in human coronary artery smooth muscle cells. Biochim Biophys Acta 1769:253–265. [DOI] [PubMed] [Google Scholar]
- French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. (2006) Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther 318:596–603. [DOI] [PubMed] [Google Scholar]
- Funato T, Harigae H, Abe S, Sasaki T. (2004) Assessment of drug resistance in acute myeloid leukemia. Expert Rev Mol Diagn 4:705–713. [DOI] [PubMed] [Google Scholar]
- Gillies L, Lee SC, Long JS, Ktistakis N, Pyne NJ, Pyne S. (2009) The sphingosine 1-phosphate receptor 5 and sphingosine kinases 1 and 2 are localised in centrosomes: possible role in regulating cell division. Cell Signal 21:675–684. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Kong Y, Mei B. (1999) Lysophosphatidic acid and sphingosine 1-phosphate protection of T cells from apoptosis in association with suppression of Bax. J Immunol 162:2049–2056. [PubMed] [Google Scholar]
- Grösch S, Schiffmann S, Geisslinger G. (2012) Chain length-specific properties of ceramides. Prog Lipid Res 51:50–62. [DOI] [PubMed] [Google Scholar]
- Guan H, Liu L, Cai J, Liu J, Ye C, Li M, Li Y. (2011) Sphingosine kinase 1 is overexpressed and promotes proliferation in human thyroid cancer. Mol Endocrinol 25:1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S, Basu A, Croce CM. (1998) Serine-70 is one of the critical sites for drug-induced Bcl2 phosphorylation in cancer cells. Cancer Res 58:1609–1615. [PubMed] [Google Scholar]
- Hannun YA, Luberto C. (2000) Ceramide in the eukaryotic stress response. Trends Cell Biol 10:73–80. [DOI] [PubMed] [Google Scholar]
- Harley ME, Allan LA, Sanderson HS, Clarke PR. (2010) Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. EMBO J 29:2407–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann D, Lucks J, Fuchs S, Schiffmann S, Schreiber Y, Ferreirós N, Merkens J, Marschalek R, Geisslinger G, Grösch S. (2012) Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int J Biochem Cell Biol 44:620–628. [DOI] [PubMed] [Google Scholar]
- Heffernan-Stroud LA, Obeid LM. (2013) Sphingosine kinase 1 in cancer. Adv Cancer Res 117:201–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijink AM, Krajewska M, van Vugt MA. (2013) The DNA damage response during mitosis. Mutat Res 750:45–55. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360. [DOI] [PubMed] [Google Scholar]
- Hengst JA, Wang X, Sk UH, Sharma AK, Amin S, Yun JK. (2010) Development of a sphingosine kinase 1 specific small-molecule inhibitor. Bioorg Med Chem Lett 20:7498–7502. [DOI] [PubMed] [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. (2013) Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13:714–726. [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Fornari FA, Jr, Auer KL, Freemerman AJ, Szabo E, Birrer MJ, Johnson CR, Barbour SE, Dent P, Grant S. (1997) Coordinate regulation of stress- and mitogen-activated protein kinases in the apoptotic actions of ceramide and sphingosine. Mol Pharmacol 52:935–947. [DOI] [PubMed] [Google Scholar]
- Kelkel M, Cerella C, Mack F, Schneider T, Jacob C, Schumacher M, Dicato M, Diederich M. (2012) ROS-independent JNK activation and multisite phosphorylation of Bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis 33:2162–2171. [DOI] [PubMed] [Google Scholar]
- Kennedy AJ, Mathews TP, Kharel Y, Field SD, Moyer ML, East JE, Houck JD, Lynch KR, Macdonald TL. (2011) Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J Med Chem 54:3524–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordán J, Bellacosa A, Tsichlis PN, Hay N. (1997) The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11:701–713. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Oh JE, Kim SW, Chun YJ, Kim MY. (2008) Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer Lett 260:88–95. [DOI] [PubMed] [Google Scholar]
- Kim JA, Lee J, Margolis RL, Fotedar R. (2010) SP600125 suppresses Cdk1 and induces endoreplication directly from G2 phase, independent of JNK inhibition. Oncogene 29:1702–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp P, Baranowski M, Knapp M, Zabielski P, Błachnio-Zabielska AU, Górski J. (2010) Altered sphingolipid metabolism in human endometrial cancer. Prostaglandins Other Lipid Mediat 92:62–66. [DOI] [PubMed] [Google Scholar]
- Kotelevets N, Fabbro D, Huwiler A, Zangemeister-Wittke U. (2012) Targeting sphingosine kinase 1 in carcinoma cells decreases proliferation and survival by compromising PKC activity and cytokinesis. PLoS ONE 7:e39209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM, Hannun YA, et al. (2004) Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem 279:44311–44319. [DOI] [PubMed] [Google Scholar]
- Kumar CC. (2011) Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer 2:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YG, Min JK, Kim KM, Lee DJ, Billiar TR, Kim YM. (2001) Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J Biol Chem 276:10627–10633. [DOI] [PubMed] [Google Scholar]
- Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Jr, Futerman AH. (2008) Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem 283:5677–5684. [DOI] [PubMed] [Google Scholar]
- Le Scolan E, Pchejetski D, Banno Y, Denis N, Mayeux P, Vainchenker W, Levade T, Moreau-Gachelin F. (2005) Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood 106:1808–1816. [DOI] [PubMed] [Google Scholar]
- Li QF, Huang WR, Duan HF, Wang H, Wu CT, Wang LS. (2007) Sphingosine kinase-1 mediates BCR/ABL-induced upregulation of Mcl-1 in chronic myeloid leukemia cells. Oncogene 26:7904–7908. [DOI] [PubMed] [Google Scholar]
- Licht T, Goldenberg SK, Vieira WD, Gottesman MM, Pastan I. (2000) Drug selection of MDR1-transduced hematopoietic cells ex vivo increases transgene expression and chemoresistance in reconstituted bone marrow in mice. Gene Ther 7:348–358. [DOI] [PubMed] [Google Scholar]
- Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, Fesik SW, Shen Y. (2007) ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene 26:3972–3979. [DOI] [PubMed] [Google Scholar]
- Madhunapantula SV, Hengst J, Gowda R, Fox TE, Yun JK, Robertson GP. (2012) Targeting sphingosine kinase-1 to inhibit melanoma. Pigment Cell Melanoma Res 25:259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavaud B, Pchejetski D, Mazerolles C, de Paiva GR, Calvet C, Doumerc N, Pitson S, Rischmann P, Cuvillier O. (2010) Sphingosine kinase-1 activity and expression in human prostate cancer resection specimens. Eur J Cancer 46:3417–3424. [DOI] [PubMed] [Google Scholar]
- Marquardt D, McCrone S, Center MS. (1990) Mechanisms of multidrug resistance in HL60 cells: detection of resistance-associated proteins with antibodies against synthetic peptides that correspond to the deduced sequence of P-glycoprotein. Cancer Res 50:1426–1430. [PubMed] [Google Scholar]
- Morgan DO. (1995) Principles of CDK regulation. Nature 374:131–134. [DOI] [PubMed] [Google Scholar]
- Mukhtar E, Adhami VM, Mukhtar H. (2014) Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther 13:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh HL, Seok JY, Kwon CH, Kang SK, Kim YK. (2006) Role of MAPK in ceramide-induced cell death in primary cultured astrocytes from mouse embryonic brain. Neurotoxicology 27:31–38. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Oktem O, Oktay M, Giancotti FG. (2008) The c-Jun N-terminal kinase JNK functions upstream of Aurora B to promote entry into mitosis. Cell Cycle 7:533–541. [DOI] [PubMed] [Google Scholar]
- Orr Gandy KA, Obeid LM. (2013) Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim Biophys Acta 1831:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Park WJ, Futerman AH. (2014) Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta 1841:671–681. [DOI] [PubMed] [Google Scholar]
- Paugh BS, Bryan L, Paugh SW, Wilczynska KM, Alvarez SM, Singh SK, Kapitonov D, Rokita H, Wright S, Griswold-Prenner I, et al. (2009) Interleukin-1 regulates the expression of sphingosine kinase 1 in glioblastoma cells. J Biol Chem 284:3408–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S, et al. (2008) A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood 112:1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrache I, Kamocki K, Poirier C, Pewzner-Jung Y, Laviad EL, Schweitzer KS, Van Demark M, Justice MJ, Hubbard WC, Futerman AH. (2013) Ceramide synthases expression and role of ceramide synthase-2 in the lung: insight from human lung cells and mouse models. PLoS ONE 8:e62968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson SM, Powell JA, Bonder CS. (2011) Regulation of sphingosine kinase in hematological malignancies and other cancers. Anticancer Agents Med Chem 11:799–809. [DOI] [PubMed] [Google Scholar]
- Ricci C, Onida F, Servida F, Radaelli F, Saporiti G, Todoerti K, Deliliers GL, Ghidoni R. (2009) In vitro anti-leukaemia activity of sphingosine kinase inhibitor. Br J Haematol 144:350–357. [DOI] [PubMed] [Google Scholar]
- Sakurikar N, Eichhorn JM, Alford SE, Chambers TC. (2014) Identification of a mitotic death signature in cancer cell lines. Cancer Lett 343:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatena CD, Stewart ZA, Mays D, Tang LJ, Keefer CJ, Leach SD, Pietenpol JA. (1998) Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and Taxol-induced growth arrest. J Biol Chem 273:30777–30784. [DOI] [PubMed] [Google Scholar]
- Seefelder W, Schwerdt G, Freudinger R, Gekle M, Humpf HU. (2002) Liquid chromatography/electrospray ionisation-mass spectrometry method for the quantification of sphingosine and sphinganine in cell cultures exposed to fumonisins. J Chromatogr B Analyt Technol Biomed Life Sci 780:137–144. [DOI] [PubMed] [Google Scholar]
- Sobue S, Nemoto S, Murakami M, Ito H, Kimura A, Gao S, Furuhata A, Takagi A, Kojima T, Nakamura M, et al. (2008) Implications of sphingosine kinase 1 expression level for the cellular sphingolipid rheostat: relevance as a marker for daunorubicin sensitivity of leukemia cells. Int J Hematol 87:266–275. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4:397–407. [DOI] [PubMed] [Google Scholar]
- Sullards MC, Merrill AH., Jr (2001) Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci STKE 2001:1–11. [DOI] [PubMed] [Google Scholar]
- Sweet K, Lancet JE. (2014) Novel therapeutics in acute myeloid leukemia. Curr Hematol Malig Rep 9:109–117. [DOI] [PubMed] [Google Scholar]
- Taha TA, Mullen TD, Obeid LM. (2006) A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta 1758:2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrano DT, Upreti M, Chambers TC. (2010) Cyclin-dependent kinase 1-mediated Bcl-xL/Bcl-2 phosphorylation acts as a functional link coupling mitotic arrest and apoptosis. Mol Cell Biol 30:640–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham CH, Taylor SS. (2013) Mitosis and apoptosis: how is the balance set? Curr Opin Cell Biol 25:780–785. [DOI] [PubMed] [Google Scholar]
- Tóthová E, Fricova M, Stecová N, Kafková A, Elbertová A. (2002) High expression of Bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Neoplasma 49:141–144. [PubMed] [Google Scholar]
- Upreti M, Galitovskaya EN, Chu R, Tackett AJ, Terrano DT, Granell S, Chambers TC. (2008) Identification of the major phosphorylation site in Bcl-xL induced by microtubule inhibitors and analysis of its functional significance. J Biol Chem 283:35517–35525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. (2005) Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol 64:695–705. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn RD, Chu S, Fan L, Yin T, Du J, Beckmann R, Mader M, Zhu G, Toth J, Blanchard K, et al. (2010) Cdk1 activity is required for mitotic activation of aurora A during G2/M transition of human cells. J Biol Chem 285:21849–21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT. (2006) Cell cycle synchronization at the G2/M phase border by reversible inhibition of CDK1. Cell Cycle 5:2555–2556. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al. (1996) Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 380:75–79. [DOI] [PubMed] [Google Scholar]
- Wei G, Margolin AA, Haery L, Brown E, Cucolo L, Julian B, Shehata S, Kung AL, Beroukhim R, Golub TR. (2012) Chemical genomics identifies small-molecule MCL1 repressors and BCL-xL as a predictor of MCL1 dependency. Cancer Cell 21:547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Tucker JA, Johnson T, Lindert L, Morgan M, Willis A, Noble ME, Endicott JA. (2007) How tyrosine 15 phosphorylation inhibits the activity of cyclin-dependent kinase 2-cyclin A. J Biol Chem 282:3173–3181. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA. (1995) Ceramide activates the stress-activated protein kinases. J Biol Chem 270:22689–22692. [DOI] [PubMed] [Google Scholar]
- Wu J, Spiegel S, Sturgill TW. (1995) Sphingosine 1-phosphate rapidly activates the mitogen-activated protein kinase pathway by a G protein-dependent mechanism. J Biol Chem 270:11484–11488. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer SJ. (1999) BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol 19:8469–8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Yang GY, Medina DJ, Vassil AD, Liao J, Hait WN. (1999) Treatment of multidrug resistant (MDR1) murine leukemia with P-glycoprotein substrates accelerates the course of the disease. Biochem Biophys Res Commun 266:167–173. [DOI] [PubMed] [Google Scholar]
- Yecies D, Carlson NE, Deng J, Letai A. (2010) Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 115:3304–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CH, Kim MJ, Park MT, Byun JY, Choi YH, Yoo HS, Lee YM, Hyun JW, Lee SJ. (2009) Activation of p38 mitogen-activated protein kinase is required for death receptor-independent caspase-8 activation and cell death in response to sphingosine. Mol Cancer Res 7:361–370. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang L, Hu J, Ruan J. (2010) miR-138 might reverse multidrug resistance of leukemia cells. Leuk Res 34:1078–1082. [DOI] [PubMed] [Google Scholar]
- Zhou L, Cai X, Han X, Xu N, Chang DC. (2014) CDK1 switches mitotic arrest to apoptosis by phosphorylating Bcl-2/Bax family proteins during treatment with microtubule interfering agents. Cell Biol Int 38:737–746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.