Abstract
Chronic use of opioid analgesics has been hindered by the development of opioid addiction and tolerance. We have reported that curcumin, a natural flavonoid from the rhizome of Curcuma longa, attenuated opioid tolerance, although the underlying mechanism remains unclear. In this study, we tested the hypothesis that curcumin may inhibit Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα), a protein kinase that has been previously proposed to be critical for opioid tolerance and dependence. In this study, we used state-of-the-art polymeric formulation technology to produce poly(lactic-co-glycolic acid) (PLGA)-curcumin nanoparticles (nanocurcumin) to overcome the drug’s poor solubility and bioavailability, which has made it extremely difficult for studying in vivo pharmacological actions of curcumin. We found that PLGA-curcumin nanoparticles reduced the dose requirement by 11- to 33-fold. Pretreatment with PLGA-curcumin (by mouth) prevented the development of opioid tolerance and dependence in a dose-dependent manner, with ED50 values of 3.9 and 3.2 mg/kg, respectively. PLGA-curcumin dose-dependently attenuated already-established opioid tolerance (ED50 = 12.6 mg/kg p.o.) and dependence (ED50 = 3.1 mg/kg p.o.). Curcumin or PLGA-curcumin did not produce antinociception by itself or affect morphine (1–10 mg/kg) antinociception. Moreover, we found that the behavioral effects of curcumin on opioid tolerance and dependence correlated with its inhibition of morphine-induced CaMKIIα activation in the brain. These results suggest that curcumin may attenuate opioid tolerance and dependence by suppressing CaMKIIα activity.
Introduction
Opioid analgesics such as morphine have been used widely for treating moderate-to-severe pain. Although opioids are highly efficacious for treating acute pain, their chronic use has been hindered by the development of opioid tolerance and dependence, the underlying mechanisms of which are not fully understood (Tang et al., 2006a; Wang et al., 2006).
Previous work by our laboratory and others demonstrated that Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) is critically important for the development and maintenance of opioid tolerance, opioid dependence, and opioid-induced hyperalgesia (Chen et al., 2010). CaMKIIα is a multifunctional serine/threonine protein kinase that is abundantly expressed in the central nervous system. CaMKIIα activity in the spinal cord and brain was found to be elevated after prolonged treatment with morphine (Wang et al., 2003; Liang et al., 2004; Tang et al., 2006b). Spinal and supraspinal inhibition of CaMKIIα were further demonstrated to be effective in preventing and reversing opioid tolerance and dependence in rodent models (Wang et al., 2003; Tang et al., 2006b).
Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a natural flavonoid component found in the rhizome of Curcuma longa (Zingiberaceae or ginger family). A number of pharmacological effects have been reported for curcumin, including antioxidant, anti-inflammatory, chemotherapeutic, and possibly even antinociceptive effects (Asher and Spelman, 2013; Marchiani et al., 2014). Several recent publications suggest that long-term treatment with curcumin is effective in attenuating opioid tolerance and dependence, although the underlying mechanism is not clear (Matsushita and Ueda, 2009; Lin et al., 2011; Liang et al., 2013). Interestingly, curcumin has been recently found to inhibit the Ca2+-dependent and -independent kinase activities of CaMKII based on cell-free assays (Mayadevi et al., 2012). We hypothesize that curcumin may attenuate opioid tolerance and dependence by inhibiting CaMKIIα in the central nervous system.
Despite the various reported pharmacologic actions, curcumin is not widely used as a therapeutic agent, likely due to its relatively low solubility and bioavailability (Anand et al., 2007) and lack of understanding of its mechanism of action. With the requirement of high doses in pharmacologic studies and poor solubility, it is difficult to independently confirm pharmacologic actions and ascertain the exact dose producing these effects. We have recently developed several polymeric nanoparticles encapsulating curcumin, including poly(lactic-co-glycolic acid) (PLGA)-curcumin (Shen et al., 2013). In this study, we have more thoroughly characterized PLGA-curcumin in two rodent models of opioid tolerance and dependence.
Materials and Methods
Morphine sulfate and naloxone HCL were purchased from Hospira (Lake Forest, IL). Curcumin, PLGA (acid terminated; poly(lactic acid):poly(glycolic acid) 50:50, w/w; Mol. Wt. 7000–17,000), tetrahydrofuran, trehalose, leucine, and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animals.
All experiments were performed after approval by the University of Illinois at Chicago Institutional Animal Care and Use Committee, and complied with policies and recommendations of the International Association for the Study of Pain and National Institutes of Health guidelines. Male ICR mice weighing 21–25 g were obtained from Harlan Laboratories (Indianapolis, IN), housed in standard conditions with a light/dark cycle (lights on at 5:00 AM and off at 7:00 PM), and provided with food and water ad libitum prior to experimental procedures.
Production and Characterization of PLGA-Curcumin Nanoparticles.
PLGA-curcumin nanoparticles were generated by using a multi-inlet vortex mixer as previously described (Shen et al., 2013). One stream was 0.2% weight percent PLGA and 0.2% weight percent curcumin dissolved in tetrahydrofuran. The other three inlet streams were deionized water as an antisolvent to precipitate the drug compound and PLGA. The volumetric flow rate of streams 1 and 2 was 6 ml/min, and it was 54 ml/min for streams 3 and 4. Freeze drying of the nanoparticle suspensions was carried out in a freeze dryer (FreeZone 1 Liter Console Freeze Dry Systems; Labconco, Kansas City, MO) at a vacuum pressure and −47°C. Trehalose and leucine were used to prevent nanoparticle permanent aggregation during the freeze-drying process. PLGA-curcumin nanoparticles were homogeneously resuspended using bath sonication for 10 minutes before use.
Drug loading, encapsulation efficiency of curcumin in nanoparticles, and nanoparticle size and size distributions were measured as previously described (Shen et al., 2013).
Antinociception Tests.
The tail-flick test was performed to assess basal nociception and morphine-induced antinociception as previously described (Tang et al., 2006b; Yang et al., 2011). In brief, the distal one-third of mouse tail was immersed into a 52°C water bath, and the latency to a quick tail-flick response was recorded. Morphine-induced antinociception was determined 30 minutes after an injection of morphine (10 mg/kg s.c.) and expressed as the percentage of maximal possible effect (MPE) according to the following formula:
 |
A cutoff time of 12 seconds was applied to prevent tissue damage.
Acute Opioid Tolerance and Dependence.
Mice were made acutely tolerant to and dependent on opioids by the treatment of a large dose of morphine sulfate (100 mg/kg s.c.). Morphine tolerance and dependence developed and peaked around 4–6 hours (Shukla et al., 2006; Tang et al., 2006b; Yang et al., 2011). An equal volume of saline was given to control mice. To assess tolerance to opioids, mice received a test dose of morphine sulfate (10 mg/kg s.c.) 4.5 hours later, and the antinociceptive effect was measured. Tolerance to morphine was demonstrated by a significant reduction of antinociceptive effect. Dependence to opioids was revealed by naloxone-precipitated withdraw jumping. Mice were treated with naloxone (10 mg/kg i.p.) 5 hours after the injection of morphine sulfate (100 mg/kg s.c.) and were then placed in glass cylinders. Mice were observed for a 15-minute period with the number of vertical jumps recorded. To prevent the development of morphine tolerance and dependence, PLGA-curcumin (2–20 mg/kg p.o.) was given 15 minutes before the induction dose of morphine sulfate (100 mg/kg s.c.). To reverse the established acute morphine tolerance and dependence, PLGA-curcumin (2–20 mg/kg p.o.) was given 15 minutes before the test dose of morphine sulfate (10 mg/kg s.c.) or naloxone.
Chronic Opioid Tolerance and Dependence.
Mice were treated with morphine sulfate (10 mg/kg s.c.) twice a day for 5 consecutive days to induce opioid tolerance and dependence (Herz and Teschemacher, 1973; Yang et al., 2011). Control mice received saline. Morphine tolerance and naloxone-precipitated withdrawal were evaluated as described earlier. On the experiment day, PLGA-curcumin (2–20 mg/kg) was given by gastric gavage (p.o.) 15 minutes before the test dose of morphine or naloxone.
Liquid Chromatography/Mass Spectrometry Analysis of Curcumin in Brain Tissue.
Mice were sacrificed 5 minutes after the administration of PLGA-curcumin (20 mg/kg p.o.) or saline, and brain tissues were collected immediately. Brain tissues were homogenized with saline and extracted using liquid-liquid extraction. In brief, ethyl acetate was added to the homogenized tissue sample and mixed vigorously for 10 minutes. The mixtures were then centrifuged at 35,000 rpm for 10 minutes, and supernatants were collected. Vacufuge (Eppendorf, Hauppauge, NY) was used to concentrate the samples. Naive brain tissue spiked with a known amount of curcumin was used as the standard. Curcumin in tissue samples was quantified by negative ion tandem mass spectroscopy, using a Triple Quad LC Mass Spectrometer (6410QQQ; Agilent Technologies, Santa Clara, CA). Identification of curcumin was performed by multiple reactions monitoring using suitable transitions (367 > 216.9 m/z).
Immunoblotting Analysis.
Prefrontal cortex sections were quickly dissected on ice and frozen on dry ice for western blotting analysis as described previously (Luo et al., 2008; Chen et al., 2010). Tissues were homogenized in ice-cold radioimmunoprecipitation assay buffer in the presence of protease inhibitors and phosphatase inhibitors and centrifuged. Protein content in the supernatant was determined by a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). Samples (20 μg of protein) were separated by 12% SDS-PAGE and electrotransferred onto a polyvinylidene difluoride membrane. The membrane was preblocked using 5% bovine serum albumin (Sigma-Aldrich) in 20 mM Tris-buffered saline (pH 7.6) containing 0.1% Tween 20. Antibodies including a rabbit anti–(T286)phosphorylated CaMKIIα (pCaMKIIα) antibody (1:1000; Santa Cruz Biotechnology, Dallas, TX), a mouse anti–β-actin antibody (1:10,000; Thermo Fisher Scientific, Waltham, MA), and horseradish peroxidase–conjugated donkey anti-rabbit (for pCaMKIIα) or anti-mouse (for β-actin) secondary antibodies (1:10,000; Santa Cruz Biotechnology) were used. The specificities of the anti–(T286)pCaMKIIα antibody were characterized in transgenic mice (CaMKIIαT286A) lacking the phosphorylation site (Chen et al., 2010). An enhanced chemiluminescence detection system (Thermo Fisher Scientific) was applied for detection. Enhanced chemiluminescence signals were captured by a ChemiDoc system and analyzed with the Quantity One program (Bio-Rad, Hercules, CA). Ratios of the optical densities of pCaMKIIα to those of β-actin were calculated for each sample.
Statistics.
All data are presented as the mean ± S.E.M. Comparisons between treatment groups were analyzed using a one-way analysis of variance followed by Dunnett’s post-hoc test. Statistical significance was established at the 95% confidence limit.
Results
Unformulated Curcumin Prevented Acute Opioid Tolerance and Dependence.
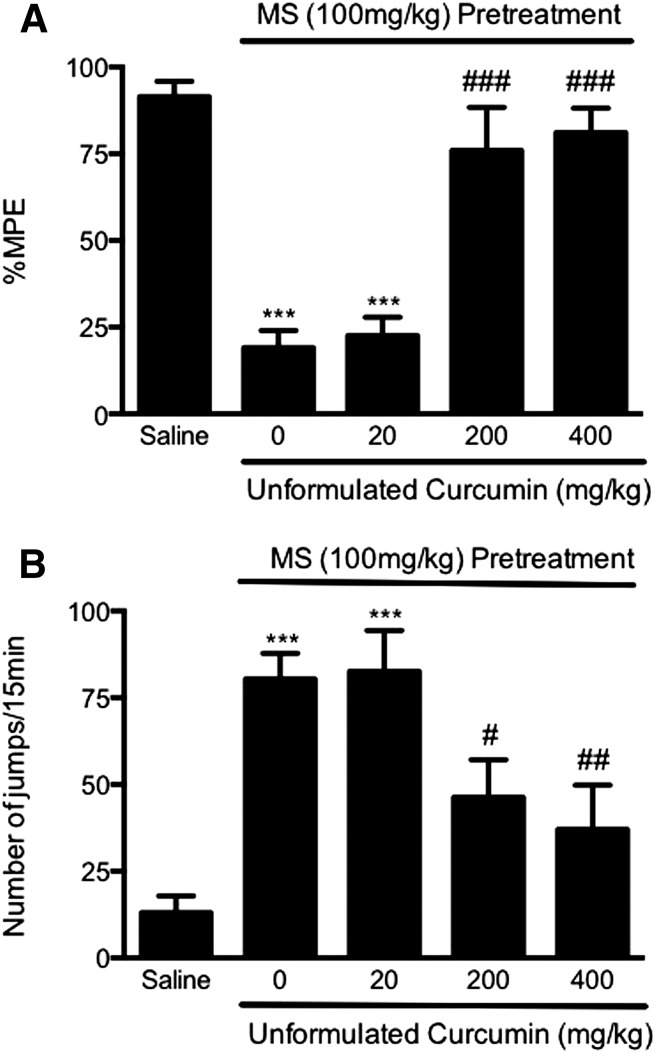
First, an acute model of opioid tolerance and dependence was used to investigate whether curcumin could prevent the development of opioid tolerance and dependence. To induce acute opioid tolerance, mice received a large dose of morphine sulfate (100 mg/kg s.c.), and 4.5 hours later, showed significantly reduced antinociception to morphine (10 mg/kg) (19.1 ± 4.9% MPE, P < 0.001) compared with MPE in the control mice pretreated with saline (91.5 ± 4.4% MPE) (Fig. 1A). Mice were treated with unformulated curcumin (20–400 mg/kg p.o.) 15 minutes before the induction dose of morphine. Mice treated with curcumin (20 mg/kg p.o.) developed morphine antinociceptive tolerance (22.6 ± 5.2% MPE versus 91.5 ± 4.4% MPE in the saline group, P < 0.001) and displayed a significant number of naloxone-precipitated withdrawal jumps (82.7 ± 11.7 versus 13.0 ± 4.9 in the saline group, P < 0.001) (Fig. 1). In mice treated with curcumin (200 or 400 mg/kg p.o.), morphine (100 mg/kg) did not produce antinociceptive tolerance (75.9 ± 12.4% and 81.1 ± 7.0% MPE, not significant from the saline-treated group, P < 0.001 versus morphine alone) (Fig. 1). In those mice, naloxone-precipitated withdrawal jumping was significantly reduced (46.3 ± 10.8 and 37.0 ± 12.8 versus 80.4 ± 7.4 in the morphine group, P < 0.05 and P < 0.01, respectively), suggesting that curcumin at high doses prevented the development of acute morphine tolerance and dependence (Fig. 1). The ED50 of curcumin is estimated to be 44.2 mg/kg (tolerance) and 109.0 mg/kg (dependence) (Fig. 3).
Fig. 1.
Prevention of acute opioid tolerance (A) and dependence (B) by curcumin at high doses. Separated groups of six mice were pretreated with curcumin (20, 200, 400 mg/kg p.o.) or saline before the treatment with morphine sulfate (100 mg/kg s.c.) or saline to induce acute opioid tolerance and dependence. Curcumin (200, 400 mg/kg) significantly attenuated opioid antinociceptive tolerance (A) and physical dependence (B), whereas it was not effective at 20 mg/kg. Data are expressed as the mean ± S.E.M. ***P < 0.001 compared with the saline group; #P < 0.05; ##P < 0.01; ###P < 0.001 compared with the morphine (MS) group.
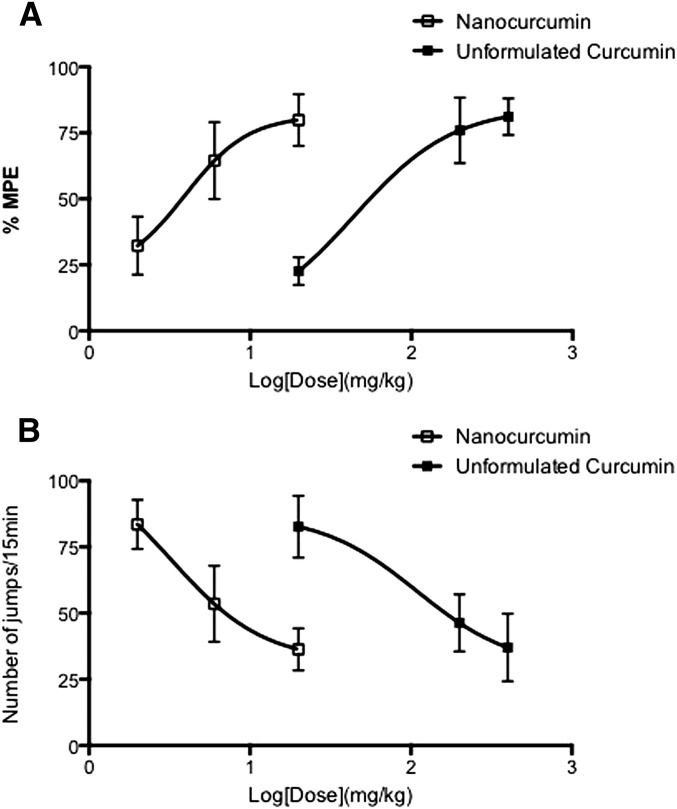
Fig. 3.
Dose-response curve of unformulated curcumin and PLGA-curcumin nanoparticles. Dose-response curves for the effects of unformulated curcumin and PLGA-curcumin nanoparticles on the acute morphine tolerance (A) and dependence (B) were plotted on a log-dose scale. ED50 values were calculated based on the dose-response curve. PLGA-curcumin nanoparticles left shifted the dose-response curve and showed higher potency than unconjugated curcumin in preventing both acute morphine tolerance and dependence.
PLGA-Curcumin Nanoparticles Prevented Acute Opioid Tolerance.
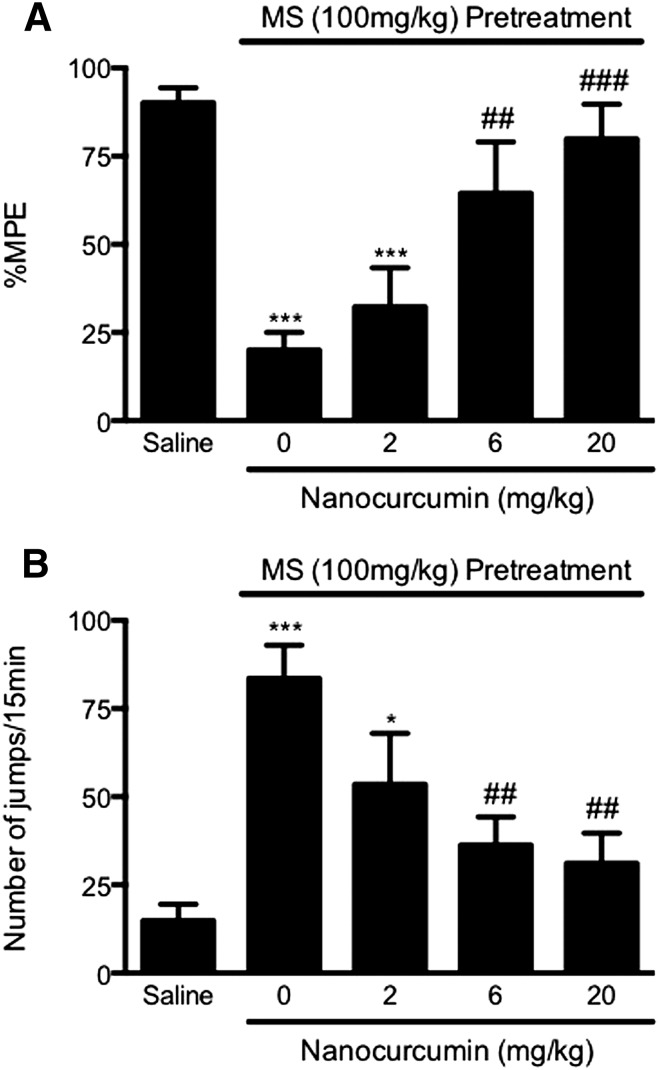
The major problem in working with curcumin was its poor solubility and bioavailability; therefore, the drug at very high doses was required in pharmacologic experiments. We found that PLGA-curcumin nanoparticles significantly improved the solubility of the compound. In this study, we compared the relative potency of unformulated versus PLGA-curcumin nanoparticles in attenuating the development of opioid tolerance and dependence. Mice received PLGA-curcumin nanoparticles at three different doses (2, 6, and 20 mg/kg p.o.) 15 minutes before the induction dose of morphine. In mice receiving the highest dose of PLGA-curcumin nanoparticles (20 mg/kg p.o.) and morphine (100 mg/kg s.c.), opioid tolerance was almost absent with largely intact morphine-induced antinociception (79.9 ± 9.8% MPE, P < 0.001 versus the morphine-alone group; not significant versus the saline group) (Fig. 2A). PLGA-curcumin nanoparticles at 6 mg/kg also significantly attenuated morphine antinociceptive tolerance (64.5 ± 14.6% MPE, P < 0.05 versus the morphine-alone group; not significant from the saline group) (Fig. 2A). Pretreatment with PLGA-curcumin nanoparticles at a lower dose (2 mg/kg p.o.) was ineffective in modulating opioid tolerance (32.3 ± 11.1% MPE, not significant versus the morphine-alone group) (Fig. 2A). These data suggest that curcumin encapsulated in PLGA nanoparticles prevented morphine tolerance, and the effect is dose-dependent with an estimated ED50 of 3.9 mg/kg (p.o.), which is 11 times lower than the ED50 of unformulated curcumin (Fig. 3A).
Fig. 2.
Prevention of acute opioid tolerance (A) and dependence (B) by PLGA-curcumin nanoparticles. Separate groups of eight mice were pretreated with PLGA-curcumin nanoparticles (2–20 mg/kg p.o.) or saline for 15 minutes. Acute opioid tolerance and dependence were established by treatment with morphine sulfate (100 mg/kg s.c.) or saline for 4.5 hours. PLGA-curcumin nanoparticles (6, 20 mg/kg p.o.) significantly prevented the development of opioid tolerance (A) and dependence (B) in a dose-dependent manner. Data are expressed as the mean ± S.E.M. *P < 0.05; ***P < 0.001 compared with the saline group; #P < 0.05; ##P < 0.01; ###P < 0.001 compared with the morphine (MS) group.
PLGA-Curcumin Nanoparticles Prevented Acute Opioid Dependence.
In these experiments, mice pretreated with morphine (100 mg/kg) or saline were challenged with naloxone (10 mg/kg i.p.) 5 hours later. Morphine-pretreated mice exhibited a significantly higher number of naloxone-precipitated withdrawal jumps (83.6 ± 9.4) compared with mice that received saline (14.9 ± 4.6, P < 0.001), which is indicative of the development of acute dependence on morphine (Fig. 2B). In mice copretreated with PLGA-curcumin nanoparticles (2, 6, 20 mg/kg p.o.) and morphine, the numbers of naloxone-precipitated withdrawal jumps were reduced to 53.5 ± 14.4, 36.3 ± 7.9, and 31.2 ± 8.5, respectively (Fig. 2B). These data indicated that curcumin encapsulated in PLGA nanoparticles dose-dependently blocked the development of acute physical dependence, with an estimated ED50 value of 3.2 mg/kg, which is 33 times lower than the ED50 of unformulated curcumin (Fig. 3B). Therefore, nanoparticles exhibited significantly improved curcumin solubility and higher potency than the unformulated curcumin.
Effect of PLGA-Curcumin Nanoparticles on Basal Nociception, Morphine Antinociception, and Locomotor Activity.
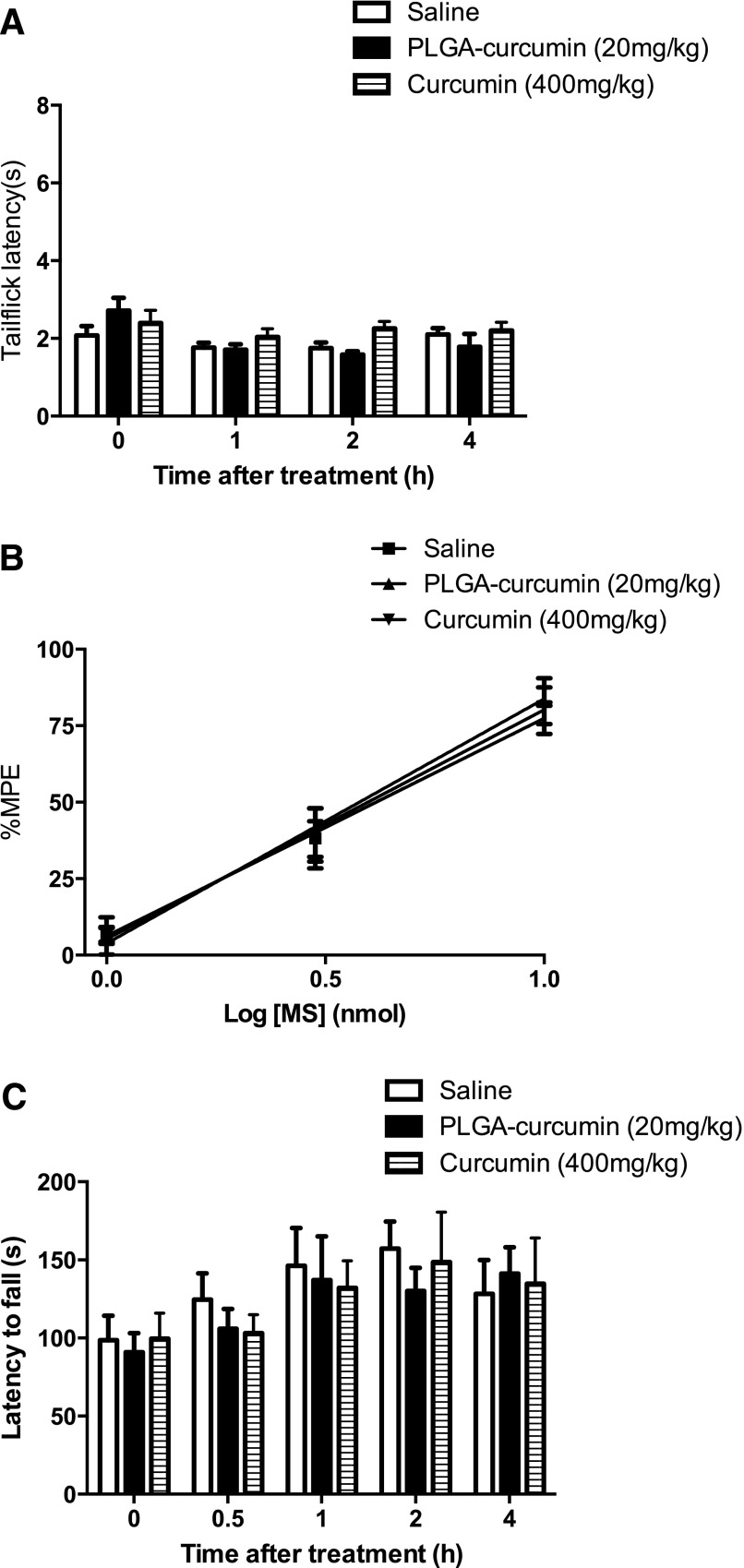
There are several potential confounding factors in interpreting the results presented in the previous sections, because free or nanoencapsulated curcumin may 1) produce antinociception, 2) interfere with morphine antinociception, and 3) impair locomotor activity. Therefore, we directly determined whether free or nanoencapsulated curcumin produced antinociception or interfered with morphine antinociception. Separate groups of mice received either nanocurcumin (20 mg/kg p.o.) or free curcumin (400 mg/kg p.o.), followed by morphine (0, 1, 3, or 10 mg/kg s.c.). Mice treated with nanocurcumin (20 mg/kg p.o.) or free curcumin (400 mg/kg p.o.) showed no difference in basal tail-flick withdrawal latencies compared with the saline-treated mice (P > 0.05) (Fig. 4A). Neither free nor nanocurcumin altered antinociception produced by morphine (1, 3, or 10 mg/kg s.c.) (P > 0.05) (Fig. 4B).
Fig. 4.
Effect of PLGA-curcumin nanoparticles on basal nociception, morphine antinociception, and locomotor activity. (A) Separate groups of six mice received (p.o.) PLGA-curcumin nanoparticles (20 mg/kg), curcumin (400 mg/kg), or saline. The tail-flick test was performed 1, 2, and 4 hours after drug administration. Neither PLGA-curcumin nanoparticles (20 mg/kg) nor curcumin (400 mg/kg) changed the basal nociception (A) in the mice. (B) Separate groups of six mice received (p.o.) PLGA-curcumin nanoparticles (20 mg/kg), curcumin (400 mg/kg), or saline, followed by morphine sulfate (1–10 mg/kg s.c.) 15 minutes later. PLGA-curcumin nanoparticles or curcumin did not interfere with antinociceptive effects produced by morphine. (C) Effects of PLGA-curcumin nanoparticles and curcumin on locomotor activity were assessed by the rotarod test in separate groups of six mice. PLGA-curcumin nanoparticles (20 mg/kg p.o.) or curcumin (400 mg/kg p.o.) did not affect the locomotor activities. MS, morphine.
We further tested the effect of curcumin and nanocurcumin on locomotor activity in a rotarod test (Chen et al., 2010). Mice were placed on an accelerating rotarod, and the latencies to fall off were recorded 0.5, 1, 2, and 4 hours after the administration of nanocurcumin (20 mg/kg p.o.) or free curcumin (400 mg/kg p.o.). The locomotor coordination of the mice treated with PLGA-curcumin nanoparticles or unformulated curcumin was indistinguishable from the saline-treated mice (P > 0.05, n = 6) (Fig. 4C).
Therefore, nanocurcumin (20 mg/kg p.o.) or curcumin (400 mg/kg p.o.) did not produce nociception, interfere with morphine antinociception, or impair locomotor activity.
PLGA-Curcumin Nanoparticles Reversed Acute Opioid Tolerance and Dependence.
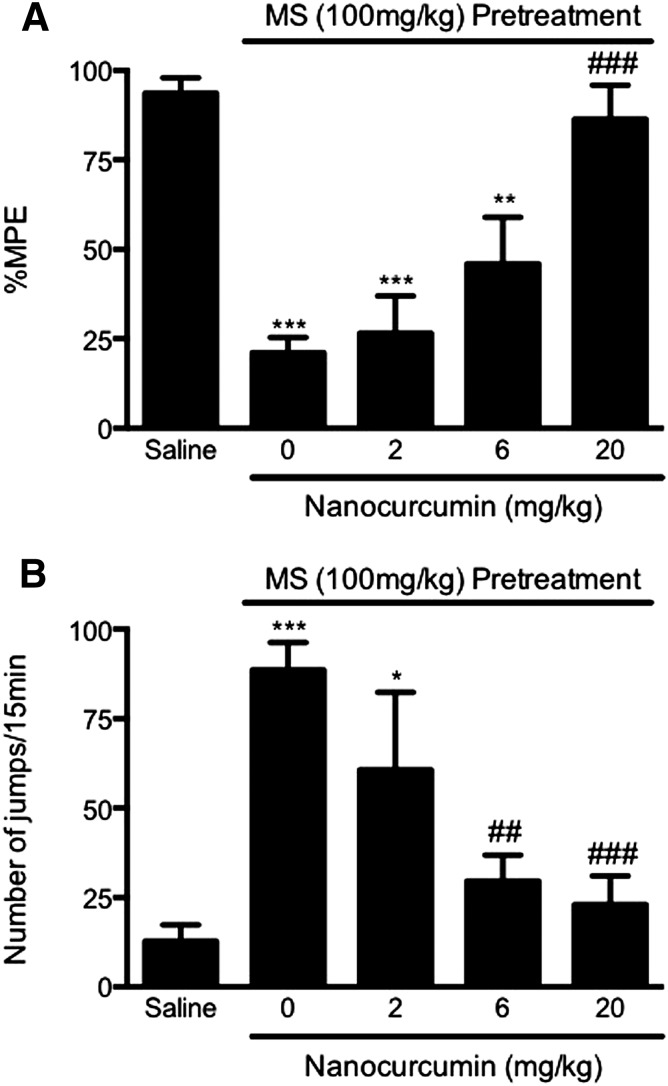
We further examined whether PLGA-curcumin nanoparticles can reverse the established opioid tolerance and dependence in the acute model. Mice were given nanocurcumin (2, 6, 20 mg/kg p.o.) 15 minutes before the test dose of morphine or naloxone. Nanocurcumin (20 mg/kg p.o.) completely restored the morphine antinociception (86.3 ± 9.5% MPE, P < 0.001) in morphine-pretreated mice, compared with the tolerance control group (21.1 ± 4.3% MPE) (Fig. 5A). Nanocurcumin at a lower dose (6 mg/kg p.o.) produced a partial effect (45.9 ± 13.0% MPE). No effect was found in mice treated with the lowest dose (2 mg/kg) of nanocurcumin (26.5 ± 10.4% MPE, P > 0.05) (Fig. 5A).
Fig. 5.
Reversal of acute opioid tolerance (A) and dependence (B) by PLGA-curcumin nanoparticles. Groups of eight mice received morphine sulfate (100 mg/kg s.c.) to induce acute opioid tolerance and dependence. PLGA-curcumin nanoparticles (2–20 mg/kg p.o.) or saline was administered 15 minutes before a test dose of morphine (10 mg/kg s.c.) or naloxone (10 mg/kg i.p.). PLGA-curcumin nanoparticles dose-dependently reversed the acute opioid tolerance (A) and opioid dependence (B). Data are expressed as the mean ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the saline group; ##P < 0.01; ###P < 0.001 compared with the morphine (MS) group.
PLGA-curcumin particles (20 and 6 mg/kg p.o.) also significantly attenuated the established physical dependence on morphine, as revealed by naloxone-precipitated withdrawal jumping, resulting in 23.0 ± 8.0 (P < 0.001) and 29.7 ± 7.2 (P < 0.01) jumps (Fig. 5B). In comparison, naloxone produced 88.6 ± 7.6 jumps in the mice treated with morphine alone (Fig. 5B). Nanocurcumin at the lowest dose (2 mg/kg p.o.) did not have a significant effect (60.7 ± 21.7 jumps) (Fig. 5B). The ED50 of PLGA-curcumin nanoparticles is estimated as 12.6 and 3.1 mg/kg for reversing the established acute morphine tolerance and dependence, respectively.
Liquid Chromatography/Mass Spectrometry Analysis of Curcumin in Brain Tissue after PLGA-Curcumin Administration.
To investigate whether PLGA-curcumin can be taken up in the brain, we directly determined the level of curcumin in brain tissues after PLGA-curcumin treatment using liquid chromatography/mass spectrometry (LC/MS). Mice were treated with PLGA-curcumin (20 mg/kg p.o.) or saline, and brain tissues were collected 5 minutes later. Brain samples from mice treated with PLGA-curcumin contained a significant amount of curcumin (367 > 216.9 m/z transition at ∼9.8-minute acquisition time) (Fig. 6C) compared with no curcumin peak in the naive brain tissue (Fig. 6A). The naïve brain tissues spiked with a known amount of standard curcumin (8 ng/g) had an identical peak (Fig. 6B). This suggests that curcumin infiltrated the blood-brain barrier into the brain within 5 minutes of PLGA-curcumin administration.
Fig. 6.
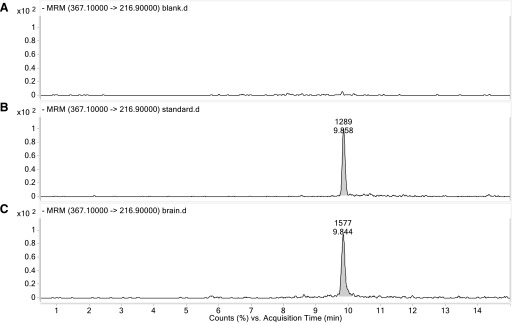
LC/MS analysis of curcumin in brain tissue after PLGA-curcumin administration. Mice were treated with PLGA-curcumin (20 mg/kg p.o.) or saline, and brain tissues were collected 5 minutes later. Blank brain tissue has no peak (A), whereas standard curcumin added in blank brain tissue peaked around 9.8 minutes of acquisition time (B). Brain samples from mice treated with PLGA-curcumin have a peak of curcumin at 9.8 minutes (C). MRM, multiple reaction monitoring.
PLGA-Curcumin Nanoparticles Reduced CaMKIIα Activation in Acute Opioid Tolerance and Dependence.
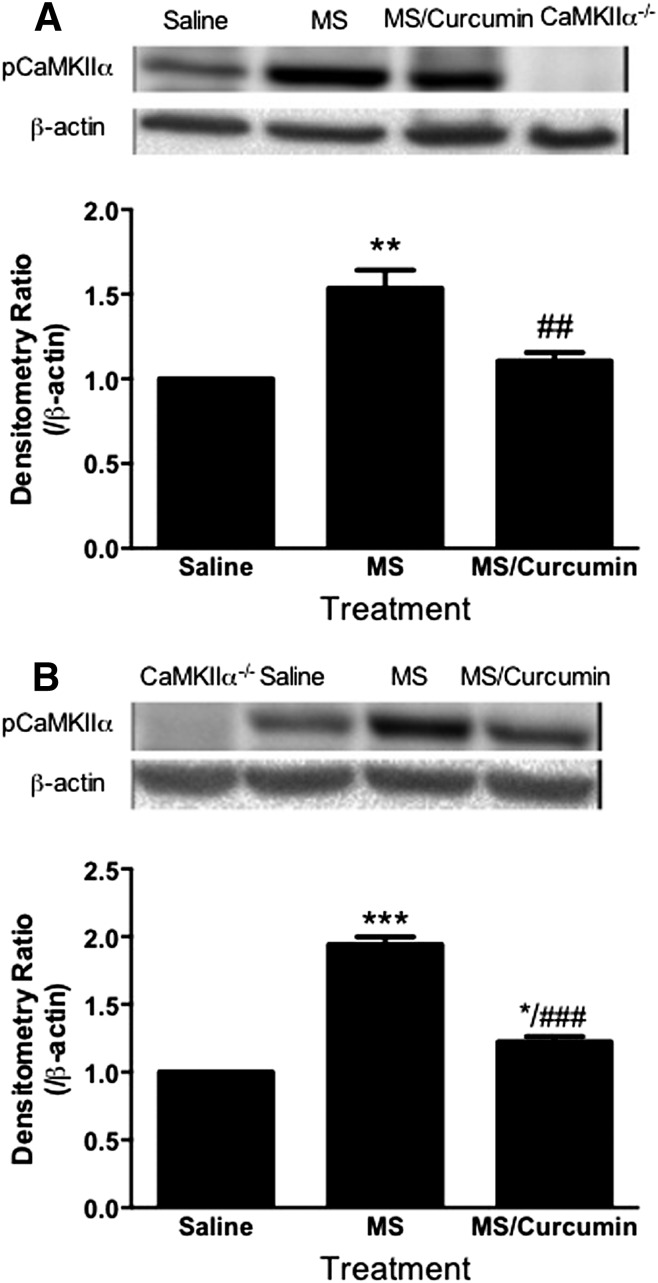
To investigate the hypothesis that curcumin can inhibit CaMKIIα, which may mediate the effect seen in opioid tolerance and dependence, we determined the CaMKIIα activity in the prefrontal cortex using western blotting analysis. Activation of CaMKIIα was determined by the amount of pCaMKIIα. The pCaMKIIα immunoreactivity was significantly elevated in the acute opioid tolerance and dependence state. Either pretreatment or acute treatment with nanocurcumin (20 mg/kg p.o.) significantly reduced pCaMKIIα in mice that are dependent on/tolerant to morphine (Fig. 7). These data suggest that PLGA-curcumin nanoparticles may attenuate acute opioid tolerance and dependence by inhibiting CaMKIIα activity.
Fig. 7.
Pretreatment (A) or acute treatment (B) with PLGA-curcumin nanoparticles inhibited supraspinal CaMKIIα activity. Prefrontal cortex samples were taken to determine CaMKIIα activity. (A) For preventing acute opioid tolerance and dependence, mice were pretreated with PLGA-curcumin nanoparticles (20 mg/kg p.o.) or saline 15 minutes before the large dose of morphine (100 mg/kg s.c.). Mice were sacrificed and prefrontal cortex samples were collected 4.5 hours later. Supraspinal CaMKIIα activity was significantly enhanced in morphine-treated mice. PLGA-curcumin nanoparticles (20 mg/kg p.o.) effectively prevented the elevation of CaMKIIα activity. (B) For reversing acute opioid tolerance and dependence, mice were treated with a large dose of morphine (100 mg/kg s.c.). After 4.5 hours, mice received PLGA-curcumin nanoparticles (20 mg/kg p.o.) or saline 15 minutes before the tissue collection. PLGA-curcumin nanoparticles (20 mg/kg p.o.) significantly reduced the activation of CaMKIIα in acute opioid-tolerant and -dependent mice. Densitometry ratio (arbitrary unit) over actin was first calculated, and was further normalized to that of control (fixed to 1). Final data are expressed as the mean ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the saline group; ##P < 0.01; ###P < 0.001 compared with the morphine (MS) group. CaMKIIα−/−, negative control using brain tissues from CaMKIIαT286A mice (Chen et al., 2010).
PLGA-Curcumin Nanoparticles Attenuate Opioid Tolerance and Dependence in a Chronic Model.
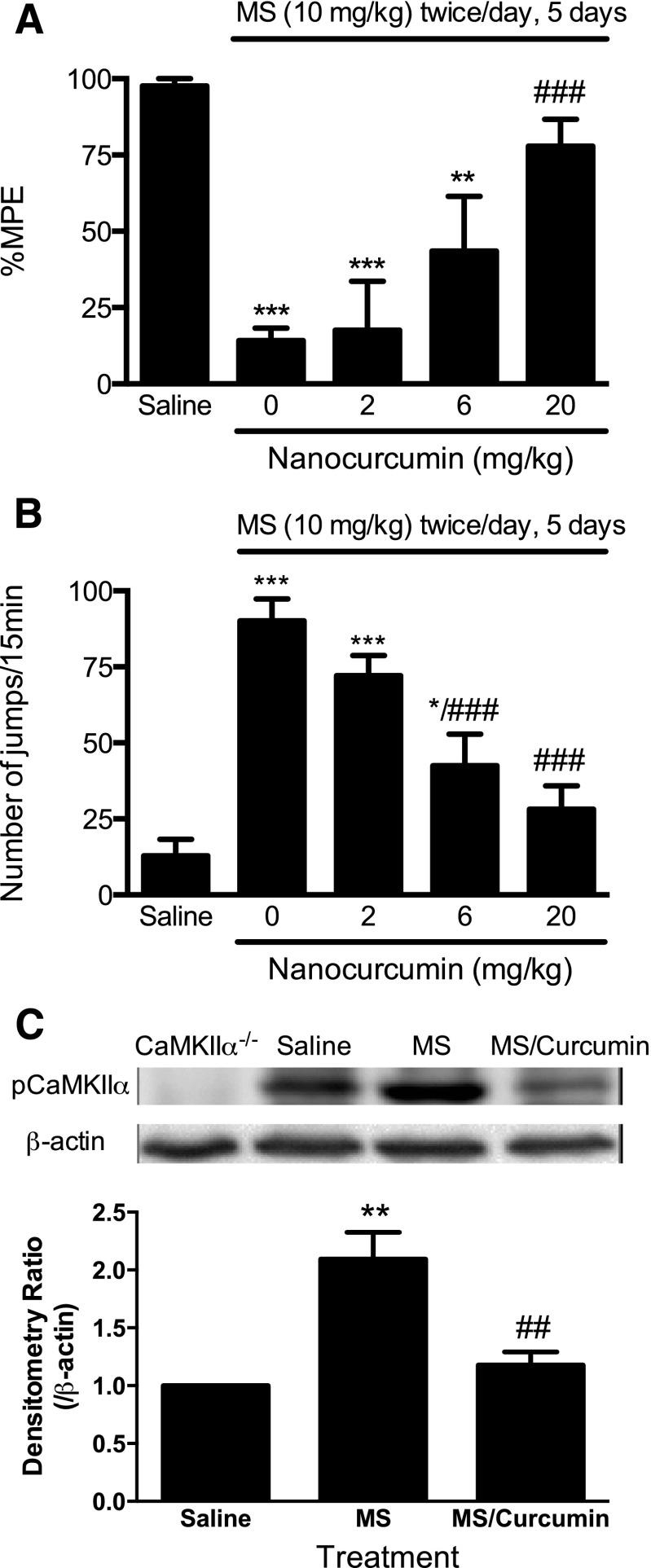
To eliminate the possibility that the promising effect of PLGA-curcumin nanoparticles was limited to acute morphine tolerance and dependence, we next studied the effects of PLGA-curcumin nanoparticles in a chronic model of opioid tolerance and dependence. Mice developed antinociceptive tolerance to and physical dependence on opioids within days after receiving twice-daily injections of morphine (10 mg/kg per injection s.c.) (Herz and Teschemacher, 1973; Yang et al., 2011). On day 6, morphine (10 mg/kg s.c.) antinociception was significantly reduced in mice chronically treated with morphine (14.2 ± 4.2% MPE, P < 0.001) compared with saline-treated mice (97.6 ± 2.4% MPE) (Fig. 8A). Administration of PLGA-curcumin nanoparticles (20 mg/kg p.o.) significantly attenuated the established chronic morphine tolerance (77.9 ± 8.8% MPE, P < 0.001 versus the morphine group), whereas nanocurcumin at lower doses (6 and 2 mg/kg p.o.) showed marginal effects on morphine tolerance (43.5 ± 17.9% and 17.6 ± 16.0% MPE, P > 0.05 from morphine alone) (Fig. 8A).
Fig. 8.
Reversal of chronic opioid tolerance (A), dependence (B), and supraspinal CaMKIIα activation (C) by PLGA-curcumin nanoparticles. Separate groups of six mice were treated with morphine sulfate (10 mg/kg s.c.) twice a day for 5 consecutive days to induce chronic opioid tolerance and dependence. Mice received PLGA-curcumin nanoparticles (2–20 mg/kg p.o.) or saline 15 minutes before a test dose of morphine (10 mg/kg s.c.) or immediately before naloxone (10 mg/kg i.p.) on day 6. Established chronic opioid tolerance (A) and dependence (B) were significantly reversed by PLGA-curcumin in a dose-dependent manner. (C) Chronic morphine exposure significantly increased the phosphorylation of CaMKIIα, which was attenuated by acute treatment with PLGA-curcumin nanoparticles (20 mg/kg p.o.). Densitometry ratio (arbitrary unit) over actin was first calculated, and was further normalized to that of control (fixed to 1). Final data are expressed as the mean ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the saline group; ##P < 0.01; ###P < 0.001 compared with the morphine (MS) group.
Nanocurcumin (20 and 6 mg/kg p.o.) significantly blocked naloxone-precipitated withdrawal jumping (28.2 ± 7.7, P < 0.001 and 42.5 ± 10.4, P < 0.01 compared with the morphine group), although at the lowest dose (2 mg/kg) it was not effective (72.2 ± 6.5, P > 0.05) (Fig. 8B). Therefore, PLGA-curcumin nanoparticles dose-dependently attenuated chronic morphine tolerance and dependence, with estimated ED50 values of 7.2 and 4.0 mg/kg, respectively.
PLGA-Curcumin Nanoparticles Attenuated Morphine-Induced Activation of CaMKIIα in the Chronic Model of Opioid Tolerance and Dependence.
To correlate the effect of PLGA-curcumin nanoparticles in chronic opioid tolerance and dependence with the activity of CaMKIIα, we tested the pCaMKIIα immunoreactivity in these mice. Chronic treatment with morphine significantly enhanced the activity of CaMKIIα in the prefrontal cortex (Fig. 8C). PLGA-curcumin nanoparticles (20 mg/kg p.o.) significantly reduced morphine-induced pCaMKIIα immunoreactivity in these mice (Fig. 8C), suggesting that the pharmacological effect of PLGA-curcumin nanoparticles in attenuating chronic opioid tolerance and dependence correlated with its ability to inhibit CaMKIIα activity. Since CaMKIIα has been implicated as a critical regulator of opioid tolerance and dependence, our data suggest that PLGA-curcumin nanoparticles attenuated opioid tolerance and dependence by inhibiting CaMKIIα.
Discussion
In the current study, we investigated the effects of nanocurcumin at three different doses (2, 6, and 20 mg/kg p.o.) in two mouse models of morphine tolerance and dependence, comparing the effects with free curcumin at three much higher doses (20, 200, and 400 mg/kg p.o.). Nanoencapsulation of curcumin lowered the ED50 approximately 11 (tolerance) to 33 (dependence) times.
We demonstrated that PLGA-curcumin nanoparticles not only prevented but also reversed opioid antinociceptive tolerance and physical dependence in mice. Since curcumin (up to 400 mg/kg) or nanocurcumin (up to 20 mg/kg) alone did not produce antinociception, interfere with morphine antinociception, or impair locomotor activity, we concluded that the action of free or nanoencapsulated curcumin was due to its interactions with endogenous mechanisms that are important for promoting and maintaining opioid tolerance and dependence.
We further demonstrated that the behavioral effect of PLGA-curcumin nanoparticles correlated with its inhibition of CaMKIIα activity in the central nervous system. These data are consistent with the findings from our previous studies that have implicated a role for CaMKIIα in the development and maintenance of opioid tolerance and dependence (Wang et al., 2003; Tang et al., 2006b). CaMKIIα is colocalized with the μ-opioid receptor in the anatomic areas that are critical for pain processing, such as the superficial layer of the spinal dorsal horn and the dorsal root ganglia (Bruggemann et al., 2000). It has been reported that constitutively active CaMKIIα increased agonist-induced desensitization of the μ-opioid receptor in cellular studies (Koch et al., 1997), and phosphorylation of the μ-opioid receptor by colocalized CaMKIIα may contribute to the development of opioid tolerance and dependence (Bruggemann et al., 2000).
CaMKIIα may interact with the N-methyl-d-aspartate (NMDA) receptors in the initiation process of opioid tolerance and dependence (Trujillo and Akil, 1991; Gutstein and Trujillo, 1993). Ca2+ influx through the activation of the NMDA receptor may lead to CaMKIIα autophosphorylation at Thr286 and resultant full activation of the kinase (Strack et al., 2000). In turn, activated CaMKIIα can phosphorylate and activate the NMDA receptor, forming a positive feed-forward loop between CaMKIIα and the NMDA receptor (Kitamura et al., 1993; McGlade-McCulloh et al., 1993).
Although the beneficial actions of inhibiting the NMDA receptor (Trujillo and Akil, 1991; Gutstein and Trujillo, 1993) or CaMKIIα (Wang et al., 2003; Tang et al., 2006b; Chen et al., 2009) in opioid tolerance, dependence, and in chronic pain have been unequivocally demonstrated and replicated by independent studies, little progress has been made in translating these findings to clinical use. In many attempts, drug toxicity was cited as a culprit for lack of success. We have taken an approach in drug repurposing whereby clinically used drugs were screened and studied for inhibiting CaMKIIα. The goal is to identify clinically used drugs with CaMKIIα inhibitory activity for alleviating problems associated with opioids, such as opioid tolerance, opioid dependence, and opioid-induced hyperalgesia (Chen et al., 2010). Here, we took another approach to identify the pharmacologic mechanisms and efficacy of a traditionally used, orally effective, relatively safe botanical ingredient that is not found in the U.S. pharmacopoeia. Moreover, we presented a method to improve compound druggability by formulating polymeric nanoparticles.
In this study, we have demonstrated that one-time administration of PLGA-curcumin (20 mg/kg p.o.) is effective in preventing acute morphine tolerance and dependence, and in reversing both established acute and chronic morphine tolerance and dependence. It has been reported in the literature that chronic treatment with curcumin at a dose of 100 mg/kg p.o. or 50 mg/kg i.p. effectively attenuated chronic morphine tolerance and dependence (Matsushita and Ueda, 2009; Liang et al., 2013). However, another group reported that chronic curcumin treatments at low doses (25 mg/kg i.p.) attenuated morphine tolerance, but high doses of curcumin (400 mg/kg i.p.) aggravated morphine tolerance (Lin et al., 2011). Since curcumin has a poor solubility and bioavailability, the actual doses of curcumin exhibiting the effect may be difficult to obtain precisely in those studies. We used curcumin encapsulated in PLGA, which greatly improves the solubility and bioavailability of curcumin, and we found that significantly lower doses of curcumin were needed to generate the comparable pharmacologic effects as evidenced by the dramatic left shift in dose-response curves.
This is our first study aiming to establish the mechanism of curcumin and efficacy of PLGA-curcumin. We correlated curcumin’s effect on morphine tolerance and dependence with the significantly reduced supraspinal CaMKIIα phosphorylation. Furthermore, using the LC/MS analysis, we demonstrated that curcumin, administered in PLGA-curcumin constructs, was able to infiltrate the blood-brain barrier and become available in the brain within 5 minutes after its administration. In addition to the brain, curcumin may also inhibit CaMKIIα in the spinal cord. Our preliminary data indicated that curcumin attenuated opioid-induced hyperalgesia by modulating spinal CaMKIIα activation (data not shown). Therefore, the brain may not be the only site of action for curcumin in attenuating CaMKIIα activity and opioid tolerance and dependence.
Although the current study was not designed to determine the pharmacokinetic profiles of PLGA-curcumin beyond the one-point LC/MS analysis, it has been reported that the t1/2 of curcumin is around 1.45 hours in rodents (Anand et al., 2007). In our experiments, acute action was tested within an hour after PLGA-curcumin, and we found it to be highly efficacious, so t1/2 does not appear to be a problem. In the pretreatment, PLGA-curcumin or curcumin was given immediately before morphine, and we recorded a powerful effect of these interventions in preventing the development of opioid tolerance/dependence; therefore, the presence of curcumin during the early time was sufficient to block tolerance to and dependence on morphine.
Curcumin is commonly used in traditional Asian cuisine, and its reported bioactivity profile includes antioxidant, anti-inflammatory, chemotherapeutic, and neuroprotective actions. It has been suggested that curcumin abolished morphine analgesic tolerance along with the morphine-induced upregulation of brain-derived neurotrophic factor transcription (Matsushita and Ueda, 2009). Liang et al. (2013) reported that daily administration of curcumin reduced opioid-induced hyperalgesia, tolerance, and physical dependence, possibly by inhibiting histone acetyltransferase. Other studies found that curcumin inhibited histone deacetylase (Liu et al., 2005; Chen et al., 2007, 2013; Lee et al., 2011). It has also been suggested that curcumin blocked corticosterone-induced phosphorylation of CaMKII in cultured hippocampal neurons (Xu et al., 2009) and inhibited CaMKII autophosphorylation in vitro (Mayadevi et al., 2012), although such a mechanism has not been studied in vivo. Since we have previously found a critical role of CaMKIIα in opioid tolerance and dependence, we tested the hypothesis that curcumin’s inhibitory action on CaMKIIα may be a mechanism attenuating the initiation or maintenance of opioid tolerance and dependence. Indeed, we found that curcumin was highly efficacious in inhibiting CaMKIIα in mice that were made tolerant to and dependent on morphine
In addition to opioid tolerance and dependence, curcumin has been reported to attenuate hyperalgesia in mice with chronic pain, including nerve injury–induced neuropathic pain (Zhao et al., 2012) and diabetic neuropathic pain (Sharma et al., 2006; Banafshe et al., 2014). Although neuropathic pain and opioid tolerance and dependence are distinct central nervous system processes, they could potentially share a common contributor, such as synaptic long-term potentiation, for which CaMKIIα is required (Wang and Wang, 2003; Lisman et al., 2012). Indeed, CaMKIIα has been found to be essential and required for chronic inflammatory pain (Luo et al., 2008), nerve injury–induced neuropathic pain (Chen et al., 2009), and opioid-induced hyperalgesia (Chen et al., 2010).
In summary, our study demonstrated that PLGA-curcumin nanoparticles, at relatively low doses, prevented and reversed opioid antinociceptive tolerance and physical dependence, correlating with its inhibitory actions on supraspinal CaMKIIα. PLGA-curcumin is significantly more potent than unformulated curcumin. Moreover, its high solubility allows us to obtain the precise doses that are required to produce these pharmacologic effects. These data provide a plausible molecular mechanism for the action of curcumin in in vivo preclinical models of opioid tolerance and dependence. The rational design, formulation, and characterization of stable PLGA-curcumin nanoparticles not only provide a pharmacologic reagent, but more importantly can be further developed for blocking opioid dependence and for improving chronic pain therapies by attenuating tolerance to opioid drugs.
Abbreviations
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- LC/MS
liquid chromatography/mass spectrometry
- MPE
maximal possible effect
- NMDA
N-methyl-D-aspartate
- pCaMKII
phosphorylated CaMKII
- PLGA
poly(lactic-co-glycolic acid)
Authorship Contributions
Participated in research design: Hu, Liu, Wang.
Conducted experiments: Hu, Huang, Szymusiak.
Performed data analysis: Hu, Wang.
Wrote or contributed to the writing of the manuscript: Hu, Liu, Wang.
Footnotes
This work was supported in part by the National Institutes of Health National Center for Complementary and Alternative Medicine [Grant K07-AT003647]. Nanoparticle formation was supported by a University of Illinois at Chicago Proof of Concept award. Mechanistic CaMKII study received funds from the National Science Foundation of China (81328009). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of National Center for Complementary and Alternative Medicine or the National Institutes of Health (NIH). The final peer-reviewed manuscript is subject to the NIH Public Access Policy.
References
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818. [DOI] [PubMed] [Google Scholar]
- Asher GN, Spelman K. (2013) Clinical utility of curcumin extract. Altern Ther Health Med 19:20–22. [PubMed] [Google Scholar]
- Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. (2014) Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 723:202–206. [DOI] [PubMed] [Google Scholar]
- Brüggemann I, Schulz S, Wiborny D, Höllt V. (2000) Colocalization of the mu-opioid receptor and calcium/calmodulin-dependent kinase II in distinct pain-processing brain regions. Brain Res Mol Brain Res 85:239–250. [DOI] [PubMed] [Google Scholar]
- Chen Y, Luo F, Yang C, Kirkmire CM, Wang ZJ. (2009) Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J Pharmacol Exp Ther 330:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Shu W, Chen W, Wu Q, Liu H, Cui G. (2007) Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin Pharmacol Toxicol 101:427–433. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang C, Wang ZJ. (2010) Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci 30:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CQ, Yu K, Yan QX, Xing CY, Chen Y, Yan Z, Shi YF, Zhao KW, Gao SM. (2013) Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class I histone deacetylases. Carcinogenesis 34:1442–1449. [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Trujillo KA. (1993) MK-801 inhibits the development of morphine tolerance at spinal sites. Brain Res 626:332–334. [DOI] [PubMed] [Google Scholar]
- Herz A, Teschemacher H. (1973) Development of tolerance to the antinociceptive effect of morphine after intraventricular injection. Experientia 29:64–65. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Miyazaki A, Yamanaka Y, Nomura Y. (1993) Stimulatory effects of protein kinase C and calmodulin kinase II on N-methyl-D-aspartate receptor/channels in the postsynaptic density of rat brain. J Neurochem 61:100–109. [DOI] [PubMed] [Google Scholar]
- Koch T, Kroslak T, Mayer P, Raulf E, Höllt V. (1997) Site mutation in the rat mu-opioid receptor demonstrates the involvement of calcium/calmodulin-dependent protein kinase II in agonist-mediated desensitization. J Neurochem 69:1767–1770. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Krauthauser C, Maduskuie V, Fawcett PT, Olson JM, Rajasekaran SA. (2011) Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer 11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Li X, Clark JD. (2004) Increased expression of Ca2+/calmodulin-dependent protein kinase II alpha during chronic morphine exposure. Neuroscience 123:769–775. [DOI] [PubMed] [Google Scholar]
- Liang DY, Li X, Clark JD. (2013) Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain 14:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JA, Chen JH, Lee YW, Lin CS, Hsieh MH, Chang CC, Wong CS, Chen JJ, Yen GC, Lin FY, et al. (2011) Biphasic effect of curcumin on morphine tolerance: a preliminary evidence from cytokine/chemokine protein array analysis. Evid-Based Complement Alternat Med 2011:452153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. (2012) Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci 13:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HL, Chen Y, Cui GH, Zhou JF. (2005) Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharmacol Sin 26:603–609. [DOI] [PubMed] [Google Scholar]
- Luo F, Yang C, Chen Y, Shukla P, Tang L, Wang LX, Wang ZJ. (2008) Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther 325:267–275. [DOI] [PubMed] [Google Scholar]
- Marchiani A, Rozzo C, Fadda A, Delogu G, Ruzza P. (2014) Curcumin and curcumin-like molecules: from spice to drugs. Curr Med Chem 21:204–222. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Ueda H. (2009) Curcumin blocks chronic morphine analgesic tolerance and brain-derived neurotrophic factor upregulation. Neuroreport 20:63–68. [DOI] [PubMed] [Google Scholar]
- Mayadevi M, Sherin DR, Keerthi VS, Rajasekharan KN, Omkumar RV. (2012) Curcumin is an inhibitor of calcium/calmodulin dependent protein kinase II. Bioorg Med Chem 20:6040–6047. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR. (1993) Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature 362:640–642. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Agrewala JN, Chopra K. (2006) Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol 536:256–261. [DOI] [PubMed] [Google Scholar]
- Shen H, Hu X, Szymusiak M, Wang ZJ, Liu Y. (2013) Orally administered nanocurcumin to attenuate morphine tolerance: comparison between negatively charged PLGA and partially and fully PEGylated nanoparticles. Mol Pharm 10:4546–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PK, Tang L, Wang ZJ. (2006) Phosphorylation of neurogranin, protein kinase C, and Ca2+/calmodulin dependent protein kinase II in opioid tolerance and dependence. Neurosci Lett 404:266–269. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. (2000) Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem 275:23798–23806. [DOI] [PubMed] [Google Scholar]
- Tang L, Shukla PK, Wang ZJ. (2006a) Attenuation of opioid tolerance by antisense oligodeoxynucleotides targeting neurogranin. Eur J Pharmacol 542:106–107. [DOI] [PubMed] [Google Scholar]
- Tang L, Shukla PK, Wang LX, Wang ZJ. (2006b) Reversal of morphine antinociceptive tolerance and dependence by the acute supraspinal inhibition of Ca(2+)/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther 317:901–909. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. (1991) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251:85–87. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Tang L, Xin L. (2003) Reversal of morphine antinociceptive tolerance by acute spinal inhibition of Ca(2+)/calmodulin-dependent protein kinase II. Eur J Pharmacol 465:199–200. [DOI] [PubMed] [Google Scholar]
- Wang LX, Wang ZJ. (2003) Animal and cellular models of chronic pain. Adv Drug Deliv Rev 55:949–965. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Wang LX. (2006) Phosphorylation: a molecular switch in opioid tolerance. Life Sci 79:1681–1691. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lin D, Li S, Li G, Shyamala SG, Barish PA, Vernon MM, Pan J, Ogle WO. (2009) Curcumin reverses impaired cognition and neuronal plasticity induced by chronic stress. Neuropharmacology 57:463–471. [DOI] [PubMed] [Google Scholar]
- Yang C, Chen Y, Tang L, Wang ZJ. (2011) Haloperidol disrupts opioid-antinociceptive tolerance and physical dependence. J Pharmacol Exp Ther 338:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Xu Y, Zhao Q, Chen CR, Liu AM, Huang ZL. (2012) Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: descending monoamine system and opioid receptors are differentially involved. Neuropharmacology 62:843–854. [DOI] [PubMed] [Google Scholar]