Abstract
The COX-2/PGE2 pathway has been implicated in the occurrence and progression of cancer. The underlying mechanisms facilitating the production of COX-2 and its mediator, PGE2, in cancer survival remain unknown. Herein, we investigated PGE2-induced COX-2 expression and signaling in HL-60 cells following menadione treatment. Treatment with PGE2 activated anti-apoptotic proteins such as Bcl-2 and Bcl-xL while reducing pro-apoptotic proteins, thereby enhancing cell survival. PGE2 not only induced COX-2 expression, but also prevented casapse-3, PARP, and lamin B cleavage. Silencing and inhibition of COX-2 with siRNA transfection or treatment with indomethacin led to a pronounced reduction of the extracellular levels of PGE2, and restored the menadione-induced cell death. In addition, pretreatment of cells with the MEK inhibitor PD98059 and the PKA inhibitor H89 abrogated the PGE2-induced expression of COX-2, suggesting involvement of the MAPK and PKA pathways. These results demonstrate that PGE2 signaling acts in an autocrine manner, and specific inhibition of PGE2 will provide a novel approach for the treatment of leukemia. [BMB Reports 2015; 48(2): 109-114]
Keywords: Apoptosis, Autocrine signaling, Cyclooxygenase-2, Leukemia, Prostaglandine E2
INTRODUCTION
Prostaglandins (PGs) are arachidonate metabolites produced by the enzymatic action of cyclooxygenase (COX), as a rate-limiting enzyme. The COX enzyme is known to exist in two isoforms, COX-1 and COX-2. Studies have shown that COX-1 is constitutively expressed in various tissues, whereas COX-2 is induced by diverse stimuli, including growth factors, cytokines, and tumor promoters (1). COX enzymes convert arachidonic acid to a transitional PG, known as PGH2, which is then converted by specific PG synthases to PGE2 (2). PGE2 exerts diverse actions and stimulates key downstream signal transduction pathways by binding to its prostanoid receptors. These receptors (EP1, EP2, EP3, and EP4) differentially bind with PGE2 to activate various signaling pathways. EP1 is known to activate intracellular Ca2+ signaling, whereas EP2 and EP4 are coupled to Gαs and stimulate adenylyl cyclase and phosphoinositide 3-kinase. EP3 binds with Gαi to inhibit adenylyl cyclase (3). The tumor-promoting activity of PGE2 is mediated by a vascular endothelial growth factor (VEGF) and cyclic adenosine monophosphate (cAMP)-dependent mechanism, which causes activation of cancer cell proliferation and has anti-apoptotic effects in several tissues (4).
Acute myeloid leukemia (AML) is characterized by genetic alteration causing myeloblast accumulation in circulation and in the bone marrow (5). In 2014, it is estimated that a total of about 18,860 (11,530 men and 7,330 women) new cases will be diagnosed, while 10,460 patients (6,010 men and 4,450 women) are expected to die from AML (6). Treatment of AML has been achieved by the improvement of anti-tumor drugs. Among cytotoxic drugs, menadione has been used in anti-cancer chemotherapy, inducing cell death through the activation of diverse apoptotic signaling pathways in leukemia cell lines (7). Menadione functions as a precursor in vitamin K production. It generates intracellular reactive oxygen species (ROS) through redox cycling, concurrently inducing cell death in a concentration- and time-dependent manner (8).
We previously reported that PGE2-EP2 signaling inhibits menadione-induced apoptosis in human promyelocytic leukemia (HL-60) cells (7). However, the exact mechanism of action by which PGE2 mediates the inhibition of apoptosis has not yet been determined. Herein, cells were first treated with PGE2, which caused increased expression of COX-2, Bcl-2 and Bcl-xL, as well as preventing casapse-3, poly (ADP-ribose) polymerase (PARP) and lamin B cleavage. Silencing of COX-2 with siRNA transfection and/or treatment with the MEK inhibitor PD98059 or protein kinase A (PKA) inhibitor H89 prevented the survival effects of PGE2 while enhancing menadione-induced cell death. Furthermore, a similar enhancing effect on the menadione-induced cell death as that observed with COX-2-siRNA was obtained after treatment with indomethacin. Improved understanding of the autocrine mechanism of PGE2 might provide novel therapeutic options to inhibit COX-2 function and thereby induce cell death and apoptosis in leukemia.
RESULTS
PGE2 prevents menadione-induced apoptosis in HL-60 cells
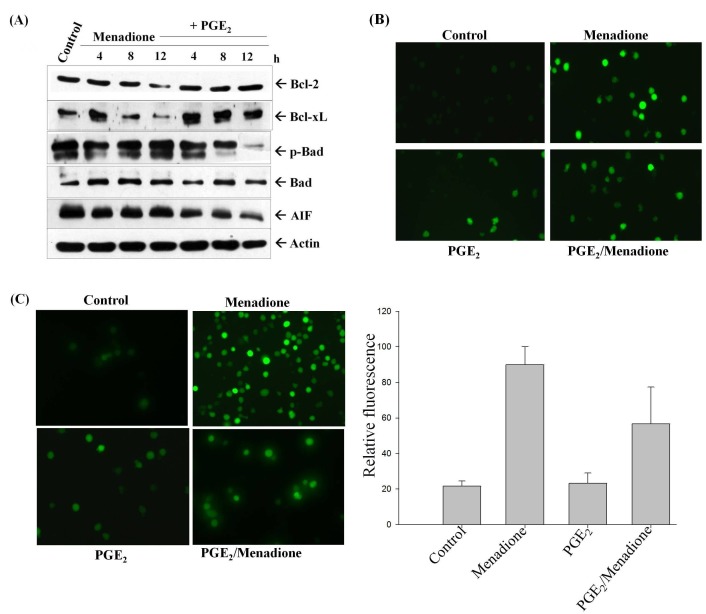
We previously reported that the PGE2-Ras signaling pathway inhibits menadione-induced apoptosis in HL-60 cells (7). In order to examine the effect of exogenously added PGE2 on apoptotic proteins herein, cells were treated with 1 μM of PGE2 and 10 μM of menadione for the indicated periods of time. As shown in Fig. 1A, the addition of menadione caused decrease of the expression of anti-apoptotic proteins such as Bcl-2 and Bcl-xL, whereas the levels remained constant when PGE2 treatment was carried out, and reduced expression of the pro-apoptotic protein, apoptosis inducing factor (AIF), along with reduced phosphorylation of Bad were observed. Bad is a known pro-apoptotic protein of the Bcl-2 family which enhances cells apoptosis by binding to and inactivating anti-apoptotic proteins such as Bcl-2 and Bcl-xL. Studies have shown that apoptosis can be blocked during cell survival by inhibiting the phosphorylation of Bad, consequently disrupting its binding with Bcl-2 protein (11).
Fig. 1. PGE2 inhibits menadione-induced apoptosis. (A) Cells were treated with 1 μM PGE2 and 10 μM menadione, and total protein was isolated from the cells and analyzed by Western blotting with anti-Bcl-2, Bcl-xL, Bad, p-Bad and AIF antibodies. Actin was used as a reference in all experiments. (B) PGE2 inhibits menadioneinduced generation of ROS. HL-60 cells were incubated with DHR 123 after exposure to 1 μM PGE2 and/or 10 μM menadione. Images of DHR-loaded cells were obtained under a fluorescence microscope. (C) HL-60 cells were treated with PGE2 and/or menadione, and then incubated with DCFHDA for 30 min. Analysis was carried out by fluorescence microscopy with an emission wavelength of 520 nm and excitation wavelength of 480 nm.
To confirm the role of ROS generation in menadione-induced apoptosis in HL-60 cells, the levels of intracellular ROS were measured using DCFHDA. The levels of mitochondrial ROS were evaluated by using a fluorescence microscope with the oxidant-sensitive probe DHR 123 (Fig. 1B). Menadione treatment induced DCF-detectable ROS production in HL-60 cells, whereas ROS generation was reduced by pretreatment with PGE2 for 2 h (Fig. 1C). Menadione-induced apoptosis was found to be directly mediated by ROS generation. Treatment with PGE2 blocked menadione-induced apoptosis, at least in part, through the blocking of ROS production. PGE2 accelerated the exclusion of menadione and prevented its accumulation inside of mitochondria to induce cell death in HL-60 cells. These data support our hypothesis that PGE2 provides protection from the apoptotic activity of menadione by enhancing the expression of anti-apoptotic proteins, as well as by reducing the steady-state level of intracellular oxidants.
Indomethacin inhibits PGE2-induced anti-apoptotic effects
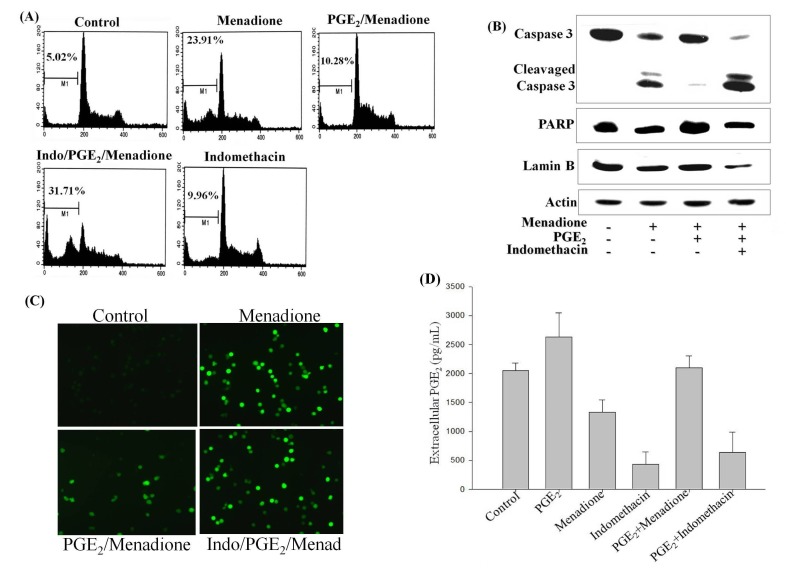
Since PGE2 is well known to promote cell survival and cancer progression, examination of its underlying molecular mechanism was carried out by blocking COX-2 with indomethacin, an inhibitor of PGE2 synthesis. Cells were pretreated with 20 μM indomethacin for 2 h and then exposed to menadione with or without PGE2 treatment. The ratio of apoptotic cell death was markedly elevated in menadione-treated HL-60 cells compared to the control, whereas addition of PGE2 for 2 h inhibited the menadione-induced cell death (Fig. 2A). Indomethacin treatment was also observed to effectively suppress the protective effects of PGE2, enhancing the menadione-induced cell death (Fig. 2A). Next, the same module of treatments was examined using Western blotting. As shown in Fig. 2B, treatment with menadione activated casapse-3, cleaved lamin B and PARP, and induced apoptosis, all of which were reversed by initial treatment with PGE2. However, incubation of the cells with indomethacin blocked the protective effects of PGE2, potentiating the menadione-induced apoptosis (Fig. 2B).
Fig. 2. COX-2 inhibitor indomethacin abolishes the anti-apoptotic effect of PGE2 and enhances menadione-induced apoptosis. (A) HL-60 cells were exposed to 20 μM indomethacin for 10 min prior to treatment with PGE2 and/or menadione. After incubation for 24 h, cell were fixed with 70% ethanol and stained with PI, and DNA content was analyzed by flow cytometry. The sub-G1 region (presented as M1) includes cells undergoing apoptosis. (B) Cell extracts were obtained from cells at 24 h and then subjected to Western blot analysis with antibodies against cleaved casapse-3, PARP, and lamin B. Actin was run as an internal control. (C) HL-60 cells were preincubated with indomethacin (Indo) for 10 min. Cells were then treated with PGE2 and/or menadione (Mena), and then incubated with DCFHDA, a cell-permeable dichlorofluorescein, for 30 min. Accumulation of the probe in the cells was measured based on an increase in fluorescence at 530 nm when the sample was excited at 485 nm. (D) Growth medium of HL-60 cells were collected and assayed for extracellular PGE2 levels using an EIA kit by monitoring the absorbance between 405-420 nm. The values presented are the means ± SD from measurements of three independent wells performed in a single experiment.
Given that PGE2 reversed the menadione-induced generation of ROS, cells were pretreated with indomethacin before exposure to PGE2 and menadione, and ROS production was examined. As shown in Fig. 2C, indomethacin potently inhibited the anti-apoptotic effects of PGE2 by enhancing the menadione-induced ROS generation. An integrated test was further performed to measure the extracellular levels of PGE2 in regards to the treatments carried out with menadione, PGE2, and/or COX-2 inhibition with indomethacin. The extracellular PGE2 reached the highest level upon PGE2 exposure. Whereas menadione treatment caused reduction in the extracellular PGE2 level compared to the control and PGE2 -treated cells, HL-60 cells exhibited higher extracellular PGE2 levels upon initial treatment with PGE2 before exposure to menadione. This suggests that PGE2 prevented the inhibitory effects of menadione on PGE2 production. However, treatment with indomethacin markedly reduced the extracellular PGE2 levels with or without PGE2 treatment (Fig. 2D). This result confirms the central role of COX-2 inhibition by indomethacin, which leads to pronounced reduction of PGE2 production. Collectively, these data suggest that autocrine signaling by PGE2 mediated via COX-2 expression prevents menadione-induced apoptosis of HL-60 cells.
PGE2 activates COX-2 expression via MAPK and PKA pathways
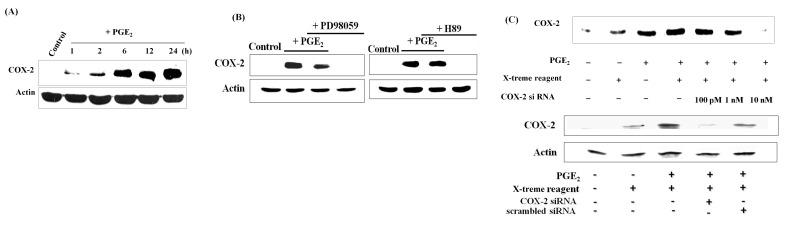
Several pharmacological and epidemiological studies have shown that induction of COX-2 correlates with tumorigenesis and poor prognosis (12). Therefore, the effect of PGE2 on COX-2 expression was next evaluated. As shown in Fig. 3A, time-dependent activation of COX-2 expression was observed, which suggests that PGE2 caused up-regulation of COX-2, at least in part, by autocrine signaling and provision of a positive feedback mechanism. As the MAPK pathway was shown to mediate the anti-apoptotic activity of PGE2, and we previously confirmed the involvement of PKA (7), inhibition of MEK and PKA was carried out with PD98059 and H89 inhibitors, respectively. Pretreatment of cells with PD98059 or H89 prevented the PGE2-induced expression of COX-2, suggesting roles of the MAPK and PKA pathways (Fig. 3B). Small interfering RNA (siRNA) was then utilized for functional gene silencing to study COX-2 genetic loss-of-function in HL-60 cells upon PGE2 treatment. Transfection of cells with 10 nM COX-2 siRNA diminished the PGE2-induced expression of COX-2 (Fig. 3C). These results suggest that PGE2 mediates COX-2 expression via the MAPK and PKA pathways, and may be processed in an autocrine manner.
Fig. 3. COX-2 siRNA demolishes PGE2- induced expression of COX-2. (A) Cells were exposed to different doses of PGE2 for 12 h and to 1 μM PGE2 for the indicated times. PGE2 induced COX-2 expression in a dose- and time- dependent manner. (B) PGE2 induced COX-2 expression via the MEK and PKA pathways. Cells were pretreated with 10 μM PD98059 or 1 μM H89 for 2 h, and then exposed to PGE2, which failed to induce COX-2 expression. (C) Cells were stably transfected with different concentrations of COX-2 siRNA and then exposed to PGE2. Transfection with COX-2 siRNA prevented PGE2-induced expression of COX-2 at a dose of 10 nM. Scrambled siRNA displayed similar effects as the control upon PGE2 treatment.
Silencing of COX-2 enhances menadione-induced apoptosis in HL-60 cells
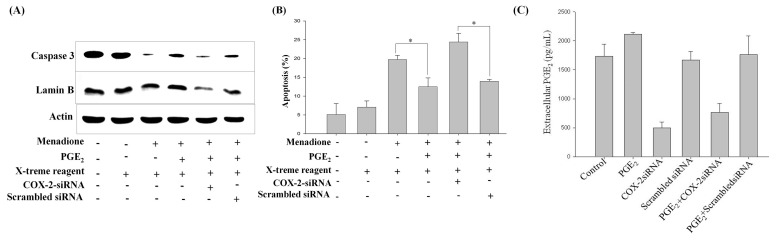
To investigate the underlying mechanism of the PGE2-mediated inhibition of menadione-induced apoptosis, cells were transfected with COX-2 siRNA, followed by treatment with PGE2 and menadione. Menadione treatment activated caspase-3 and lamin B cleavage, which was reversed by the exogenous addition of PGE2 prior to menadione treatment. However, the knock-out of COX-2 with siRNA demolished the protective effect of PGE2, enhancing menadione-induced caspase-3 and lamin B cleavage (Fig. 4A). Actin expression was used as an internal control, as it remained consistent upon siRNA transfection as well as upon treatment with PGE2 and menadione. Furthermore, the silencing effect of COX-2 was examined with regards to menadione-induced cell death. Menadione-induced apoptosis, as evidenced by increased accumulation of cells in the sub-G1 phase of the cell cycle, was suppressed by the addition of PGE2 (Fig. 4B). However, silencing of COX-2 with siRNA perturbed the inhibition of menadione-induced cell death by PGE2. To further illustrate the contribution of COX-2 to the anti-apoptotic effect of PGE2, COX-2 was knocked out by treating HL-60 cells with COX-2 siRNA, after which PGE2 assay was performed to measure the extracellular level of PGE2 in regards to COX-2 silencing and PGE2 treatment. As shown in Fig. 4C, silencing of COX-2 with siRNA limited the extracellular level of PGE2 as compared to the control and PGE2-treated HL-60 cells. This reduction of the extracellular PGE2 level was mainly due to COX-2 silencing, as transfection with scrambled siRNA did not display similar effects, and PGE2 treatment elevated the extracellular PGE2 level (Fig. 4C). To sum up, these results indicated COX-2 to be the central player in PGE2 -mediated anti-apoptotic effects.
Fig. 4. COX-2 siRNA-transfection of cells inhibits the anti-apoptotic effects of PGE2. (A) Cells were stably transfected with 10 nM COX-2 siRNA and then exposed to PGE2 for 48 h. Total proteins were then isolated from the cells and analyzed by Western blotting with anti-caspase-3, -lamin B, and -actin antibodies. Actin expression remained consistent, and was used as a reference. (B) HL-60 cells were harvested at 24 h and stained with PI, after which DNA content was analyzed by flow cytometry. Sub-G1 fractions of COX-2 siRNA-transfected cells (%) were plotted against PGE2 and menadione treatments for 24 h. (C) Cells were transfected with COX-2 siRNA before exposure to PGE2 for 24 h and supernatant medium was collected to assay the extracellular PGE2 levels using an EIA kit. The values presented are the means ± SD from the measurements of three independent wells performed in a single experiment.
DISCUSSION
Constitutive COX-2 expression and PGE2 production have been reported in the progression of several cancers, including colon, prostate, breast, and lung cancers (13). Studies have also correlated the up-regulation of COX-2 with cell proliferation in pre-malignant and malignant cancer, as well as with increased angiogenesis, chemotherapeutic failure, and immune suppression (14). PGE2, production of which is catalyzed by COX-2 enzymatic activity, has been shown to mediate cell proliferation and have anti-apoptotic effects in several cancers. PGE2 regulates several cellular markers and mediates different signal transduction pathways by interacting with their prostanoid receptors (4). More recently, over-expression of COX-2 was identified in leukemic and lymphoblastic models, suggesting that chemotherapy utilizing inhibitors of COX-2 might provide an effective therapeutic approach to overcome the malfunction of COX-2 (15). The underlying mechanism for the anti-apoptotic role of the COX-2/PGE2 pathway remains to be elucidated; at the same time, it is clear that this pathway facilitates an important role in cancer progression by suppressing cell death mechanisms, consequently reducing the sensitivity of cancer cells to anti-cancer agents.
Our results showed that PGE2 induces the expression of Bcl-2 and Bcl-xL, while reversing menadione-induced cell death, possibly via the MEK pathway (16). High levels of Bcl-2 and Bcl-xL expression have been detected in leukemia, suggesting that inhibition of these proteins may be a starting point to allow tumor cell apoptosis due to chemotherapy (17). Bad promotes cellular apoptosis by inactivating anti-apoptotic proteins such as Bcl-2 and Bcl-xL. Cell survival signaling pathways inhibit the phosphorylation of Bad at Ser155, thereby disrupting physical interactions between Bad and Bcl-2 proteins and consequently blocking apoptosis. Furthermore, ROS generation is involved in the apoptosis of a variety of cancer cells, triggered by various anti-cancer drugs. PGE2 inhibits menadione-induced generation of ROS, as evidencede by DCFHDA and DHR 123 fluorescence (18). Taken together, our results showed that PGE2 protects HL-60 cells from apoptosis by inhibiting the generation of ROS, as well as by modulating the expressions of Bcl-2 family proteins.
Indomethacin binds to COX enzymes and selectively inhibits the activities of both COX-1 and COX-2. Studies have shown indomethacin to induce apoptosis in various tumors by blocking the migration and proliferation of cancer cells (19). Therefore, COX-2-mediated signaling downstream of PGE2 was blocked by the specific COX-2 inhibitor indomethacin, ultimately enhancing menadione-induced cell death. In addition, accumulation of PARP and lamin B cleavage were detected in indomethacin-treated cells (Fig. 2B). Moreover, blockage of the MEK pathway by the specific inhibitor PD98059 reduced PGE2-induced expression of COX-2. Herein, it can be suggested that PGE2 promotes cell survival in a MEK-dependent manner, as specific inhibition attenuated the PGE2-induced cell proliferation (20). When using the PKA-specific inhibitor H89, PGE2 also failed to induce COX-2 over-expression. Therefore, it can be assumed that PGE2 interacts with the EP2 receptor and activates PKA through elevation of intracellular cAMP levels (7).
Recently, the functional role of COX-2 silencing was elucidated in cell proliferation and apoptosis (21). The results obtain herein demonstrated that COX-2 siRNA could significantly inhibit the PGE2-induced expression of COX-2, while also enhancing menadione-induced cell death (Fig. 4B). Noticeably, COX-2 silencing decreased the level of extracellular PGE2, which was elevated by PGE2 exposure (Fig. 3C). The decrement in extracellular PGE2 levels by COX-2 silencing further validates the important role of the COX-2 pathway in PGE2-mediated signaling in leukemia.
Treatment with PGE2 was also observed to increase the level of extracellular PGE2, reversing the menadione-induced reduction of PGE2 levels. Importantly, COX-2 inhibition by indomethacin blocked up-regulation of PGE2 upon incubation with PGE2 (Fig. 2D). These results exploit the function of COX-2, triggering the production of PGE2 to protect the HL-60 cells from death (7). The results of this study support a novel mechanism by which the COX-2/PGE2 pathway enhances the growth of leukemia cancer cells through autoregulation of PGE2. Therefore, gaining a deep understanding of PGE2 signaling is critical for the elucidation of cancer progression, and may lead to the development of novel and chemoprotective therapeutic candidates for the treatment of leukemia.
In summary, the results uncovered a critical role of the COX-2/PGE2 signaling pathway, which inhibits menadione-induced apoptosis in HL-60 cells, thereby constituting an autocrine signaling loop which enhances cell proliferation and growth. PGE2 induces anti-apoptotic effects by modulating the activities of apoptotic proteins in HL-60 cells. Furthermore, PGE2-regulated suppression of ROS has reflective implications for the prevention and treatment of leukemia. Thus, reinforcement of apoptosis-inducing strategies based on selective targeting of PGE2 signaling could constitute a useful therapeutic approach against various types of human cancers.
MATERIALS AND METHODS
Detailed experimental procedures are described in online Supplementary information.
Acknowledgments
This research was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Korea government (MEST) (NRF-2009-0078234), and by the Basic Science Research Program through the NRF, funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1 A2063612).
References
- 1.Poon R, Smits R, Li C, et al. Cyclooxygenase-two (COX-2) modulates proliferation in aggressive fibromatosis (desmoid tumor). Oncogene. (2001);20:451–460. doi: 10.1038/sj.onc.1204107. [DOI] [PubMed] [Google Scholar]
- 2.Venza I, Giordano L, Piraino G, Medici N, Ceci G, Teti D. Prostaglandin E2 signalling pathway in human T lymphocytes from healthy and conjunctiva basal cell carcinoma-bearing subjects. Immunol Cell Biol. (2001);79:482–489. doi: 10.1046/j.1440-1711.2001.01034.x. [DOI] [PubMed] [Google Scholar]
- 3.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. (2012);188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. (2010);10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rücker FG, Russ AC, Cocciardi S, et al. Altered miRNA and gene expression in acute myeloid leukemia with complex karyotype identify networks of prognostic relevance. Leukemia. (2013);27:353–361. doi: 10.1038/leu.2012.208. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clinic. (2014);64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 7.Yeo HS, Shehzad A, Lee YS. Prostaglandin E2 blocks menadione-induced apoptosis through the Ras/Raf/Erk signaling pathway in promonocytic leukemia cell lines. Mol Cells. (2012);33:371–378. doi: 10.1007/s10059-012-2293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loor G, Kondapalli J, Schriewer JM, Chandel NS, Vanden Hoek TL, Schumacker PT. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Rad Biol Med. (2010);49:1925–1936. doi: 10.1016/j.freeradbiomed.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kil IS, Jung KH, Nam WS, Park JW. Attenuated mitochondrial NADP+-dependent isocitrate dehydrogenase activity enhances EGCG-induced apoptosis. Biochimie. (2011);93:1808–1815. doi: 10.1016/j.biochi.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Basnakian AG, James SJ. A rapid and sensitive assay for the detection of DNA fragmentation during early phases of apoptosis. Nucleic Acids Res. (1994);22:2714–2715. doi: 10.1093/nar/22.13.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem. (2005);275:25046–25051. doi: 10.1074/jbc.M002526200. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Zeng LH, Taniguchi T, Xie QM. Activation of PKA and phosphorylation of sodium-dependent vitamin C transporter 2 by prostaglandin E2 promote osteoblast-like differentiation in MC3T3-E1 cells. Cell Death Differ. (2007);14:1792–1801. doi: 10.1038/sj.cdd.4402190. [DOI] [PubMed] [Google Scholar]
- 13.Harris RE. Cyclooxygenase-2 (COX-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. (2009);17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. (2002);190:279–286. doi: 10.1002/jcp.10068. [DOI] [PubMed] [Google Scholar]
- 15.Sobolewski C, Cerella C, Dicato M, Diederich M. Cox-2 inhibitors induce early c-Myc downregulation and lead to expression of differentiation markers in leukemia cells. Cell Cycle. (2011);10:2978–2993. doi: 10.4161/cc.10.17.16460. [DOI] [PubMed] [Google Scholar]
- 16.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. (1998);58:362–366. [PubMed] [Google Scholar]
- 17.Fadeel B, Hassan Z, Hellstr#246;m-Lindberg E, Henter JI, Orrenius S, Zhivotovsky B. Cleavage of Bcl-2 is an early event in chemotherapy-induced apoptosis of human myeloid leukemia cells. Leukemia. (1999);13:719–728. doi: 10.1038/sj.leu.2401411. [DOI] [PubMed] [Google Scholar]
- 18.Oh JM, Kim SH, Cho EA, Song YS, Kim WH, Juhnn YS. Human papillomavirus type 16 E5 protein inhibits hydrogen-peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis. (2010);31:402–410. doi: 10.1093/carcin/bgp318. [DOI] [PubMed] [Google Scholar]
- 19.Blobaum AL, Uddin MJ, Felts AS, Crews BC, Rouzer CA, Marnett LJ. The 2'-trifluoromethyl analogue of indomethacin is a potent and selective COX-2 inhibitor. ACS Med Chem Lett. (2013);9:486–490. doi: 10.1021/ml400066a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Wu WK, Li ZJ, Li HT, Wu YC, Cho CH. Prostaglandin E(2) promotes cell proliferation via protein kinase C/extracellular signal regulated kinase pathway-dependent induction of c-Myc expression in human esophageal squamous cell carcinoma cells. Int J Cancer. (2009);125:2540–2546. doi: 10.1002/ijc.24607. [DOI] [PubMed] [Google Scholar]
- 21.Zhong Y, Xia Z, Liu J, Lin Y, Zan H. The effects of cyclooxygenase-2 gene silencing by siRNA on cell proliferation, cell apoptosis, cell cycle and tumorigenicity of Capan-2 human pancreatic cancer cells. Oncol Rep. (2012);27:1003–1010. doi: 10.3892/or.2011.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]