Abstract
Cells express several antioxidant enzymes to scavenge reactive oxygen species (ROS) responsible for oxidative damages and various human diseases. Therefore, antioxidant enzymes are considered biomedicine candidates. Among them, extracellular superoxide dismutase (SOD3) had showed prominent efficacy against asthma and inflammation. Despite its advantages as a biomedicine, the difficulty in obtaining large quantity of active recombinant human SOD3 (rhSOD3) has limited its clinical applications. We found that a significant fraction of overexpressed rhSOD3 was composed of the inactive apo-enzyme and its potency against inflammation depended on the rate of metal incorporation. Also, purified rhSOD3 was unstable and lost its activity very quickly. Here, we suggest an ideal preparative method to express, purify, and store highly active rhSOD3. The enzymatic activity of rhSOD3 was maximized by incorporating metal ions into rhSOD3 after purification. Also, albumin or polyethylene glycol prevented rapid inactivation or degradation of rhSOD3 during preparative procedures and long-term storage. [BMB Reports 2015; 48(2): 91-96]
Keywords: Extracellular superoxide dismutase, Inflammation, Metalloenzyme, Reactive oxygen species, Refolding
INTRODUCTION
Reactive oxygen species (ROS), such as hydrogen peroxide and superoxide radicals, are generated either by enzymatic reactions that transfer electrons in the mitochondria and the endoplasmic reticulum, or by external stimuli such as irradiation. ROS can induce cellular oxidative damages by modifying proteins and nucleic acids. Therefore, cells have developed diverse systems to reduce ROS. First, ROS-generating systems are tightly regulated, so as not to produce high levels of ROS. Second, cells have antioxidant systems to scavenge ROS, including small scavengers such as glutathione, vitamin C, and vitamin E, in addition to antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and peroxidase.
Despite cellular regulatory systems, ROS can accumulate upon prolonged stimulation. It has been shown that ROS is closely related to numerous chronic human diseases, including inflammation (1, 2). Therefore, many antioxidant molecules and cellular antioxidant enzymes have been considered as medicines (3-6). Among them, SOD scavenges superoxide radicals, which directly modify proteins or generate its derivatives, such as H2O2 and ONOO- (7-9). Mammalian cells express SOD1, SOD2, and SOD3 in cytosol, mitochondria, and the extracellular region, respectively (9). Mice lacking SOD showed organ failure or increased sensitivity to oxygen toxicity and inflammatory responses and exogenous SOD has alleviated inflammatory responses (10-15), suggesting that SOD family members are critical for preventing ROS-derived diseases.
SOD3 is a strong biomedicine candidate. First, exogenous SOD3 can accomplish its functions without cell penetration, since it is originally an extracellular anti-oxidant enzyme. Second, an earlier study suggested that SOD3 also inhibited inflammatory responses via non-enzymatic functions (13), providing additional impetus for the use of SOD3. Third, transgenic mice over-expressing SOD3 displayed no abnormal phenotypes. Instead, they were more resistant to inflammatory responses (10, 16, 17), while SOD1 transgenic mice showed neuronal abnormalities (18). Last, SOD3 exhibited a longer half-life (∼20 h) in the blood, whereas SOD1 had a very short half-life (∼20 min), possibly due to its rapid renal clearance (19, 20).
One obstacle to developing recombinant human SOD3 (rhSOD3) into a novel biomedicine is the high costs for producing large quantities of active protein. Despite many efforts, rhSOD3 expressed from bacteria or yeast has showed very low activity compared to rhSOD3 from mammalian cells (Table 1), possibly due to lack of proper folding and maturation machineries. Therefore, mammalian cells are the most suitable over-expression system for producing active rhSOD3. SOD3 is a secretory protein that matures in the ER and Golgi apparatus and undergoes post-translational modifications, including glycosylation, disulfides, and proteolysis. In addition, a proteolytic variant, 209E, which is missing the C-terminal heparin-binding domain, can be observed in normal tissue (21-23). Considering its diverse post-translational modifications, the difficulty in producing rhSOD3 using a bacterial system is explainable.
Table 1. Activities of recombinant human SOD3.
| Protein | Host | Prep method | Unit/mg | Source | Reference |
|---|---|---|---|---|---|
|
| |||||
| SOD3 | Bacteria | Ni column | 50-510 | Inclusion body | (34,36) |
| Gel filtration | |||||
| SOD3 | Bacteria | Ni column | 120 | Soluble lysate | (29) |
| Heparin column | |||||
| SOD3 | Yeast | Not purified | 760 | Culture media | (35) |
| SOD3 | Mammalian | Antibody affinity | > 50,000 | Culture media | (33) |
| CHO | Ion exchange | ||||
| Heparin column | |||||
| SOD3 | Mammalian | Ni column* | 30,000 | Culture media | In this study |
| 293T-EBNA | Suggested method** | 60,000 | |||
| C195S | Mammalian | Suggested method** | 80,000 | Culture media | In this study (no data) |
| 293T-EBNA | |||||
| C219S | Mammalian | Suggested method** | 140,000 | Culture media | In this study (no data) |
| 293T-EBNA | |||||
| 209E | Mammalian | Suggested method** | 120,000 | Culture media | In this study (no data) |
| 293T-EBNA | |||||
The enzyme activity was determined after nickel purification (*) or after incubating purified rhSOD3 with 0.1% BSA and 100 μM CuSO4/ZnCl2 for 24hrs (**).
During the course of this study, we noticed that even the rhSOD3 expressed from mammalian cells were not fully active. Moreover, purified rhSOD3 lost its activity very quickly. The activity of rhSOD3 was maximized by incorporating metal ions post-translationally and rhSOD3 was stabilized by serum albumin (BSA) or polyethylene glycol (PEG). This study will provide invaluable information for the preparation of potent rhSOD3 in advance of preclinical or clinical applications.
RESULTS AND DISCUSSION
Purification of rhSOD3
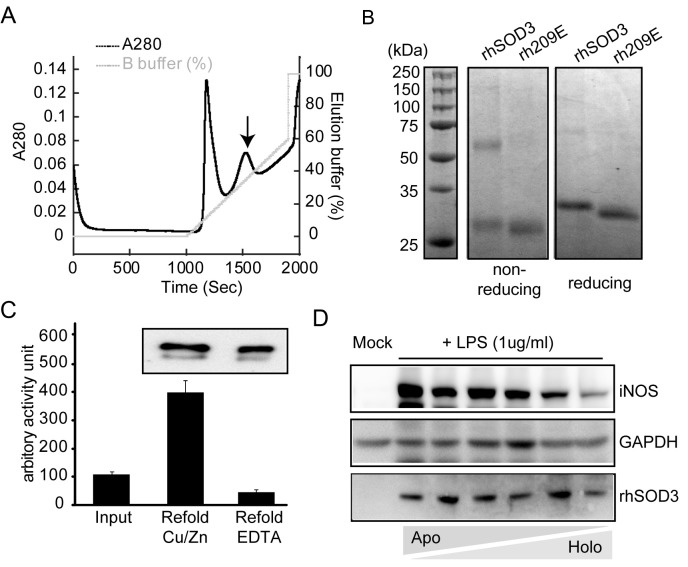
rhSOD3 tagged with C-terminal His6 was purified directly from culture media by single-step purification on a nickel column (Fig. 1A). Purified rhSOD3 showed the correct monomer size, around 27 kDa and half of the rhSOD3 formed a dimer with an intermolecular disulfide bond, whereas recombinant 209E showed no dimeric bands in non-reducing SDS-PAGE (Fig. 1B), as noticed elsewhere (21, 22).
Fig. 1. Purification of rhSOD3 and post-translational metal incorporation. (A) Culture media containing rhSOD3 were filtered and loaded onto a nickel column, followed by washing and gradient elution. Arrow indicates the peak corresponding fraction of pure rhSOD3. (B) Elution fractions of rhSOD3 and recombinant 209E were analyzed by non-reducing and reducing SDS- PAGE with blue staining. (C) Generation of holo- and apo-enzyme from purified rhSOD3. Purified rhSOD3 corresponding to 100 units of the initial activity were denatured and refolded in the presence of either Cu/Zn or EDTA. Enzyme activities and protein amounts after refolding were compared (Western blot analysis in the enclosed figure). (D) Anti-inflammatory effect by enzymatically active rhSOD3. Prevention of iNOS induction by LPS correlated to percentage of holo-enzyme.
Catalytic activity of rhSOD3
SOD3 is a metalloenzyme that uses copper and zinc ions as cofactors for catalysis. If the basal amount of metal ions in the culture media is insufficient to accommodate over-expressed rhSOD3, metal-free rhSOD3 can be secreted into the media. Cells employ the copper chaperones CCS1 and ATOX1 to assist with copper incorporation into SOD1 and SOD3, respectively (24, 25). However, SOD3 has been shown to bind metal cofactors under in vitro conditions without chaperone proteins (24). Therefore, we examined if exogenous copper/zinc ions could enhance the catalytic activity of purified rhSOD3. Right after elution from the nickel column, rhSOD3 was dialyzed into either PBS or PBS containing 50 μM Cu/Zn ions, followed by additional dialysis into PBS to remove the unbound metal ions. rhSOD3 dialyzed into the buffer containing Cu/Zn ions was much more active compared to when dialyzed into plain PBS (data not shown). This result suggested that some over-expressed rhSOD3 was secreted from cells without metal incorporation or the rhSOD3 lost metal ions after secretion, resulting in the formation of apo-enzyme.
In order to estimate the approximate percentage of apo-enzyme in the purified sample, we denatured purified rhSOD3 and allowed it to refold in the presence of either a metal chelator, EDTA, to maximize the apo-enzyme percentage or in the presence of Cu/Zn ions to maximize the holo-enzyme percentage. Refolded rhSOD3 in the presence of Cu/Zn ions showed nearly 4-fold higher activity compared to the initial activity of purified rhSOD3 (Fig. 1C). However, refolded rhSOD3 in the presence of EDTA showed the least activity. rhSOD3 did not show any visible aggregation during refolding in any condition (data not shown) and protein amounts were quite similar in both conditions (Fig. 1C enclosed figure). These results suggested that around 75% of the purified rhSOD3 was apo-enzyme or unfolded enzyme which can recover its activity by post-translational metal incorporation and refolding.
Anti-inflammatory efficacy of rhSOD3 is correlated to activity
Although rhSOD3 demonstrated non-enzymatic activity against asthma (12), its enzymatic activity must be critical for anti-inflammation considering the impact of ROS on inflammation. In order to examine the anti-inflammatory effect of rhSOD3 depending on the rate of metal incorporation, Raw 264.7 cells activated by LPS were treated with different ratios of holo-enzyme to apo-enzyme. LPS-activated cells showed significant induction of iNOS, one of the inflammatory markers (26). The inhibitory efficacy of rhSOD3 on iNOS induction decreased as the percentage of holo-enzyme was reduced (Fig. 1D). This correlation between the enzyme activity of rhSOD3 and its efficacy emphasizes the importance of preparing catalytically active rhSOD3 for future applications.
Effects of metal ions on rhSOD3 expression from cells
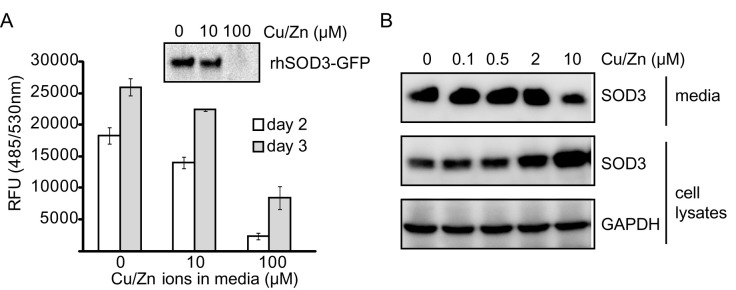
We noticed that over-expressed SOD3 was secreted without metal ions, suggesting that the amount of metal ions available was insufficient. Therefore, we also investigated whether supplementation of Cu/Zn ions increased the expression level of active rhSOD3. Unexpectedly, exogenous supplementation of Cu/Zn ions significantly reduced the secretion of rhSOD3. The extracellular fluorescence signal of rhSOD3-GFP decreased with a higher concentration of Cu/Zn ions in the media and Western blot analysis also supported this result (Fig. 2A and 2B). Instead, rhSOD3 seemed to accumulate inside cells that had higher metal concentrations (Fig. 2B), suggesting that Cu/Zn ions might inhibit secretion or induce internalization of SOD3. Therefore, we concluded that supplementation of Cu/Zn ions in culture media was unsuitable for enhancing the expression of active rhSOD3.
Fig. 2. Supplementation with metal ions decreased secretion of rhSOD3 from cells. 293T cells stably expressing rhSOD3 or rhSOD3-EGFP were cultured in DMEM and supplemented with various concentration of CuSO4/ ZnCl2 for 3 days. (A) GFP fluorescence signals in the 293T-rhSOD3-EGFP culture media were measured. Amounts of rhSOD3-EGFP in culture media were compared by Western blot analysis (enclosed). (B) Amounts of intracellular and extracellular rhSOD3 in 293T-rhSOD3 cells were compared by Western blot analysis.
Stabilization of active rhSOD3
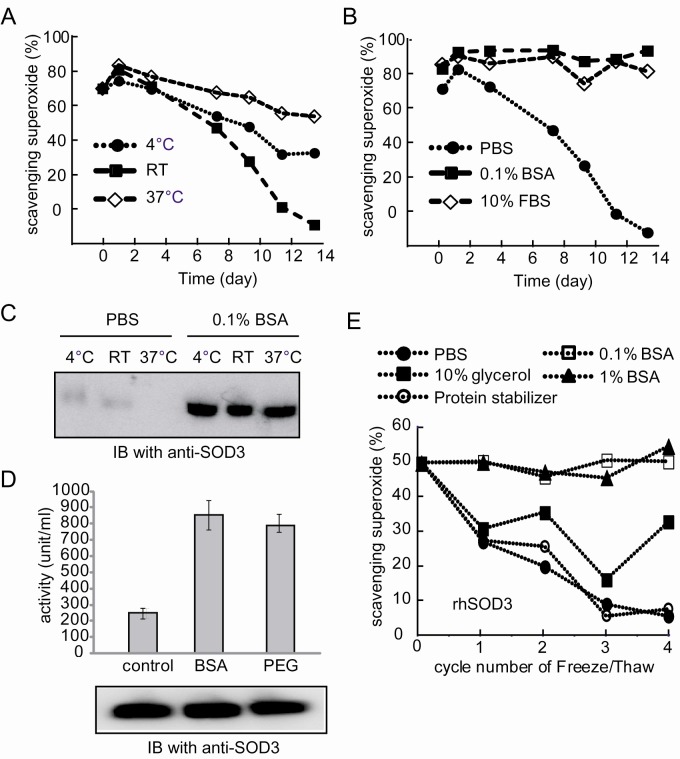
We noticed that rhSOD3 was very unstable even after optimal purification procedures. Purified rhSOD3 lost almost half of its initial activity in PBS buffer at 37℃ within 7 days (Fig. 3A). Activity loss was also detected at low temperatures. Therefore, it was necessary to optimize the preparation and storage conditions to stabilize active rhSOD3. First, purified rhSOD3 was supplemented with 0.1% BSA or 10% FBS. Both 0.1% BSA and 10% FBS maintained the catalytic activity of rhSOD3 for up to 13 days at 37℃ (Fig. 3B), suggesting that BSA is sufficient to stabilize rhSOD3. Previous studies showed that Cu/Zn SOD can be fragmented, following copper release and/or non-enzymatic glycation (27, 28). Purified rhSOD3 disappeared almost completely after 13 days in PBS buffer (Fig. 3C), but 0.1 % BSA prevented this disappearance. This result suggested that rhSOD3 loses its activity due to fragmentation or degradation.
Fig. 3. Stabilization of rhSOD3. (A) Purified rhSOD3 lost its catalytic activity over time. The catalytic activity of rhSOD3 stored in PBS at different temperatures was monitored for 13 days. (B) The catalytic activity of rhSOD3 stored in PBS, 0.1% BSA, or 10% FBS at 37℃ is shown. (C) After 13 days of incubation, the amount of rhSOD3 was analyzed by Western blotting. (D) Freshly purified rhSOD3 were dialyzed under three different conditions; dialysis into PBS buffer, dialysis into PBS buffer after mixing 0.1% BSA with purified rhSOD3, and dialysis into PBS buffer containing 0.1% PEG. (E) Purified rhSOD3 was stored in PBS, 0.1% BSA, 1% BSA, 10% glycerol, or a protein stabilizing cocktail, and their catalytic activities were measured after repeated freeze/thaw cycles.
Second, we examined whether rhSOD3 loses its activity during the purification procedure, which is performed at low temperature over 2 days. Right after elution from the affinity column, rhSOD3 was either mixed with 0.1% BSA, followed by dialysis into PBS, or it was dialyzed directly into PBS containing 0.1% PEG, another known protein stabilizer. Interestingly, rhSOD3 prepared with 0.1% BSA or 0.1% PEG showed much higher activity than with PBS only (Fig. 3D). Protein amount was not affected by the different dialysis methods (Fig. 3D enclosed figure), suggesting that BSA (or PEG) can also prevent the release of metal ions from purified rhSOD3.
Finally, the effects of freeze/thaw cycles on rhSOD3 were investigated. In general, repeated freezing/thawing is known to destabilize proteins. Freshly purified rhSOD3 were frozen in liquid nitrogen and stored at —80℃ within different buffer conditions. After thawing, the catalytic activity of rhSOD3 was immediately measured. A single freeze/thaw cycle reduced rhSOD3 activity by approximately 50% in PBS. However, BSA (1% and 0.1%) completely prevented rhSOD3 activity loss from the freeze/thaw cycle (Fig. 3E). 10% glycerol also partially stabilized rhSOD3 activity. However, a commercially available protein stabilizing cocktail (Thermo Fisher) failed to stabilize active rhSOD3.
DISCUSSION
In this study, we found that exogenous copper and zinc ions can maximize rhSOD3 activity post-translation and that BSA or PEG can stabilize active rhSOD3 either by protecting against fragmentation or the release of metal ions. However, rhSOD3 purified from bacteria did not show a significant increase in catalytic activity in the presence of exogenous Cu/Zn ions (data not shown), suggesting that the native-like conformations must be important for the chaperone free post-translational insertion of Cu/Zn ions into rhSOD3.
The heparin-binding domain of SOD3 is cleaved naturally, resulting in 209E (20, 29). Therefore, we investigated whether the heparin-binding domain affected rhSOD3 stability. However, recombinant 209E lost its activity similarly to rhSOD3 and was also protected by BSA or PEG during long term storage and a freeze/thaw cycle (data not shown). These results indicate that the heparin-binding domain contributes neither to the stability nor the activity loss of rhSOD3. Instead, it may regulate the plasma levels of rhSOD3 by interacting with the cell surface or the extracellular matrix (20, 29).
In this study, we optimized the preparative method for active rhSOD3. After purification from mammalian cell culture media, rhSOD3 was combined with 0.1% BSA (or 0.1% PEG) and 50 μM Cu/Zn ions to maximize and stabilize its catalytic activity, and then free Cu/Zn ions were removed by dialysis for future applications. The enhanced catalytic activity of rhSOD3 prepared using the above procedure was well maintained even after removing free Cu/Zn ions (data not shown). 209E and two cysteine mutants, C195S and C219S, prepared through the above procedure showed similar or better activity than wild-type rhSOD3 (Table 1). Although C195S is thought to be a hyperactive mutant due to a deficiency of the inhibitory disulfide bond (C107-C195) (30), its activity was only 1.33-fold higher than wild-type rhSOD3. Compared to rhSOD3 purified from bacteria or yeast, rhSOD3 prepared using our procedure displayed 100- to 1,000-fold higher activity (25, 31-34) (Table 1). The optimized procedure recommended in this study can help to overcome practical obstacles in the development of rhSOD3 as a novel biomedicine.
MATERIALS AND METHODS
Cloning and mammalian cell culture
The full length human SOD3 and 209E variant, from Met1 to Glu227, containing a C-terminal His6 tag or an enhanced green fluorescence protein (EGFP) tag was inserted into pcDNA3.1 (Invitrogen) using HindIII and EcoRI or HindIII and XbaI, respectively. Plasmids encoding hSOD3 and 209E variants were transfected into 293T-EBNA cells with Attractene (Qiagen) based on the manufacturer’s instructions. One day after transfection, the media were replaced with serum-free Dulbecco’s Modified Eagle Medium (DMEM). 293T-EBNA cells stably expressing rhSOD3-EGFP were selected using G418 (Invitrogen) and enriched by fluorescence-activated cell sorting (FACS). 293T-EBNA and Raw264.7 cells were maintained with DMEM containing 10% FBS.
Protein expression and purification
Five days after transfection, culture media containing rhSOD3 were collected, filtrated, and loaded onto HiTrap Chelating HP column (GE Healthcare). After loading, the column was washed with more than 50 column volumes of washing buffer, 50 mM NaPO4, 500 mM NaCl, and 30 mM imidazole. Then, rhSOD3 and 209E were eluted by increasing the elution buffer containing 500 mM imidazole (Fig. 1A), followed by dialysis in PBS or the indicated buffer conditions. The concentration of purified rhSOD3 was determined based on a BSA standard curve with a protein assay dye (Bio-Rad).
Activity assay for SOD
To measure the enzymatic activity of rhSOD3, the rate of superoxide radical formation was quantified spectrophotometrically. A 20 μl sample was mixed with 200 μl of 200 μM xanthine (Sigma) and 50 μM WST-1 (Dojindo) in PBS. After adding 0.0005 unit XOD (Sigma), the increase in the formazan dye signal was immediately recorded using a colorimetric method at A450. The generation of a formazan dye signal was determined kinetically, and absolute SOD activity was determined from the dilution factor exhibiting 50% inhibition (IC50) on the inhibition curve.
Anti-inflammatory effects of rhSOD3
At 70% confluence, Raw 264.7 cells were starved with serum-free DMEM for 6 h prior to treatment with 1 μg/ml lipopolysaccharide (LPS). rhSOD3 was added to cells with different ratios of holo-enzyme to apo-enzyme, but a constant amount corresponding to 100 units/ml concentration of 100% holo-enzyme. Cells were harvested after 24 h incubation by directly adding SDS sample buffer containing protease inhibitors. iNOS, GAPDH, and hSOD3 were analyzed by Western blot analysis with anti-NOS2 (Santa Cruz Biotechnology), anti-GAPDH (Santa Cruz Biotechnology), and anti-hSOD3 (AbCam) antibodies.
Monitoring activity loss of rhSOD3
Purified rhSOD3 was incubated in different conditions such as 0.1% BSA, or 10% fetal bovine serum (FBS) at 4℃, room temperature (RT), or 37℃. The activity of 10 μl purified rhSOD3 corresponding to 4 units of the initial activity was monitored for 13 days. To assess the effects of freeze/thaw cycles on activity loss, purified rhSOD3 in PBS supplemented with 1% BSA, 0.1% BSA, 10% glycerol, or protein stabilizing cocktail (Thermo Scientific) was quickly frozen with liquid nitrogen. After thawing, the activity of 2 μl rhSOD3 corresponding to 1 units initial activity was determined.
Supplementation of copper and zinc ions and refolding
In order to check the effects of Cu/Zn on expression of SOD3, 0, 10, and 100 μM CuSO4/ZnCl2 mixtures were added to the culture media used for 293T cells stably expressing rhSOD3. One day later, the amount of expressed and secreted rhSOD3 (-EGFP) in the culture media was determined by Western blotting with anti-SOD3 antibody or GFP fluorescence. To assess the effects of exogenous Cu/Zn on purified rhSOD3, 50 μM CuSO4/ZnCl2 was either directly combined with purified rhSOD3 or purified rhSOD3 was dialyzed into PBS containing 10 μM CuSO4/ZnCl2, followed by the removal of free Cu/Zn. For refolding, rhSOD3 was denatured using 6 M Guanidine HCl and refolded by dialysis into PBS with either 10 μM CuSO4/ZnCl2 or 10 mM EDTA.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2013R1A1A2008027) and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013M3A9A3050567). We give many thanks to the Integrative Research Support Center of the Catholic University of Korea for FACS.
References
- 1.Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. (2005);6:971–976. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- 2.Rosanna DP, Salvatore C. Reactive oxygen species, inflammation, and lung diseases. Curr Pharm Des. (2012);18:3889–3900. doi: 10.2174/138161212802083716. [DOI] [PubMed] [Google Scholar]
- 3.Niwa Y. Lipid peroxides and superoxide dismutase (SOD) induction in skin inflammatory diseases, and treatment with SOD preparations. Dermatologica. (1989);179(Suppl 1):101–106. doi: 10.1159/000248458. [DOI] [PubMed] [Google Scholar]
- 4.Kim BH, Na KM, Oh I, et al. Kurarinone regulates immune responses through regulation of the JAK/STAT and TCR-mediated signaling pathways. Biochem Pharmacol. (2013);85:1134–1144. doi: 10.1016/j.bcp.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Sunitha K, Hemshekhar M, Thushara RM, et al. N-Acetylcysteine amide: a derivative to fulfill the promises of N-Acetylcysteine. Free Radic Res. (2013);47:357–367. doi: 10.3109/10715762.2013.781595. [DOI] [PubMed] [Google Scholar]
- 6.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. (2011);51:1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamakura F, Kawasaki H. Post-translational modifications of superoxide dismutase. Biochim Biophys Acta. (2010);1804:318–325. doi: 10.1016/j.bbapap.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. (1995);64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 9.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. (2002);33:337–349. doi: 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- 10.Lee YS, Cheon IS, Kim BH, Kwon MJ, Lee HW, Kim TY. Loss of Extracellular Superoxide Dismutase Induces Severe IL-23-Mediated Skin Inflammation in Mice. J Invest Dermatol. (2013);133:732–741. doi: 10.1038/jid.2012.406. [DOI] [PubMed] [Google Scholar]
- 11.Asikainen TM, Huang TT, Taskinen E, et al. Increased sensitivity of homozygous Sod2 mutant mice to oxygen toxicity. Free Radic Biol Med. (2002);32:175–186. doi: 10.1016/S0891-5849(01)00776-6. [DOI] [PubMed] [Google Scholar]
- 12.Kwon MJ, Jeon YJ, Lee KY, Kim TY. Superoxide dismutase 3 controls adaptive immune responses and contributes to the inhibition of ovalbumin-induced allergic airway inflammation in mice. Antioxid Redox Signal. (2012);17:1376–1392. doi: 10.1089/ars.2012.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon MJ, Han J, Kim BH, Lee YS, Kim TY. Superoxide dismutase 3 suppresses hyaluronic acid fragments mediated skin inflammation by inhibition of toll-like receptor 4 signaling pathway: superoxide dismutase 3 inhibits reactive oxygen species-induced trafficking of toll-like receptor 4 to lipid rafts. Antioxid Redox Signal. (2012);16:297–313. doi: 10.1089/ars.2011.4066. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga T, Hachiya M, Shibata T, Sakamoto Y, Taki K, Akashi M. Exogenously-added copper/zinc superoxide dismutase rescues damage of endothelial cells from lethal irradiation. J Clin Biochem Nutr. (2012);50:78–83. doi: 10.3164/jcbn.11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DW, Hwang HS, Kim DS, et al. Effect of silk fibroin peptide derived from silkworm Bombyx mori on the anti-inflammatory effect of Tat-SOD in a mice edema model. BMB Rep. (2011);44:787–792. doi: 10.5483/BMBRep.2011.44.12.787. [DOI] [PubMed] [Google Scholar]
- 16.Na K, Kim KE, Park ST, Kim T. Y. EC-SOD suppresses contact hypersensitivity in mouse skin by impairing Langerhans cell migration. J Invest Dermatol. (2007);127:1930–1937. doi: 10.1038/sj.jid.5700802. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y, Kim BH, Lee H, et al. Regulation of skin inflammation and angiogenesis by EC-SOD via HIF-1alpha and NF-kappaB pathways. Free Radic Biol Med. (2011);51:1985–1995. doi: 10.1016/j.freeradbiomed.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Jaarsma D, Haasdijk ED, Grashorn JA, et al. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis. (2000);7:623–643. doi: 10.1006/nbdi.2000.0299. [DOI] [PubMed] [Google Scholar]
- 19.Uematsu T, Nagashima S, Umemura K, Kanamaru M, Nakashima M. Pharmacokinetics and safety of intravenous recombinant human superoxide dismutase (NK341) in healthy subjects. Int J Clin Pharmacol Ther. (1994);32:638–641. [PubMed] [Google Scholar]
- 20.Karlsson K, Sandstrom J, Edlund A, Edlund T, Marklund SL. Pharmacokinetics of extracellular-superoxide dismutase in the vascular system. Free Radic Biol Med. (1993);14:185–190. doi: 10.1016/0891-5849(93)90009-J. [DOI] [PubMed] [Google Scholar]
- 21.Bowler RP, Nicks M, Olsen DA, et al. Furin proteolytically processes the heparin-binding region of extracellular superoxide dismutase. J Biol Chem. (2002);277:16505–16511. doi: 10.1074/jbc.M105409200. [DOI] [PubMed] [Google Scholar]
- 22.Enghild JJ, Thogersen IB, Oury TD, Valnickova Z, Hojrup P, Crapo JD. The heparin-binding domain of extracellular superoxide dismutase is proteolytically processed intracellularly during biosynthesis. J Biol Chem. (1999);274:14818–14822. doi: 10.1074/jbc.274.21.14818. [DOI] [PubMed] [Google Scholar]
- 23.Petersen SV, Thogersen IB, Valnickova Z, et al. The concentration of extracellular superoxide dismutase in plasma is maintained by LRP-mediated endocytosis. Free Radic Biol Med. (2010);49:894–899. doi: 10.1016/j.freeradbiomed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Jeney V, Itoh S, Wendt M, et al. Role of antioxidant-1 in extracellular superoxide dismutase function and expression. Circ Res. (2005);96:723–729. doi: 10.1161/01.RES.0000162001.57896.66. [DOI] [PubMed] [Google Scholar]
- 25.Ahl IM, Lindberg MJ, Tibell LA. Coexpression of yeast copper chaperone (yCCS) and CuZn-superoxide dismutases in Escherichia coli yields protein with high copper contents. Protein Expr Purif. (2004);37:311–319. doi: 10.1016/j.pep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Kim JI, Jang HS, Park KM. Endotoxin-induced renal tolerance against ischemia and reperfusion injury is removed by iNOS, but not eNOS, gene-deletion. BMB Rep. (2010);43:629–634. doi: 10.5483/BMBRep.2010.43.9.629. [DOI] [PubMed] [Google Scholar]
- 27.Adachi T, Ohta H, Hayashi K, Hirano K, Marklund SL. The site of nonenzymic glycation of human extracellular-superoxide dismutase in vitro. Free Radic Biol Med. (1992);13:205–210. doi: 10.1016/0891-5849(92)90016-A. [DOI] [PubMed] [Google Scholar]
- 28.Arai K, Maguchi S, Fujii S, Ishibashi H, Oikawa K, Taniguchi N. Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vitro glycated sites. J Biol Chem. (1987);262:16969–16972. [PubMed] [Google Scholar]
- 29.Petersen SV, Olsen DA, Kenney JM, et al. The high concentration of Arg213-->Gly extracellular superoxide dismutase (EC-SOD) in plasma is caused by a reduction of both heparin and collagen affinities. Biochem J. (2005);385:427–432. doi: 10.1042/BJ20041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen SV, Oury TD, Valnickova Z, et al. The dual nature of human extracellular superoxide dismutase: one sequence and two structures. Proc Natl Acad Sci U S A. (2003);100:13875–13880. doi: 10.1073/pnas.2436143100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tibell L, Hjalmarsson K, Edlund T, Skogman G, Engstrom A, Marklund SL. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. (1987);84:6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Son YJ, Bae JY, Chong SH, et al. Expression, high cell density culture and purification of recombinant EC-SOD in Escherichia coli. Appl Biochem Biotechnol. (2010);162:1585–1598. doi: 10.1007/s12010-010-8940-1. [DOI] [PubMed] [Google Scholar]
- 33.Chen HL, Yen CC, Tsai TC, et al. Production and characterization of human extracellular superoxide dismutase in the methylotrophic yeast Pichia pastoris. J Agric Food Chem. (2006);54:8041–8047. doi: 10.1021/jf061379x. [DOI] [PubMed] [Google Scholar]
- 34.Bae JY, Koo BK, Ryu HB, et al. Cu/Zn incorporation during purification of soluble human EC-SOD from E. coli stabilizes proper disulfide bond formation. Appl Biochem Biotechnol. (2013);169:1633–1647. doi: 10.1007/s12010-012-0025-x. [DOI] [PubMed] [Google Scholar]