Abstract
Neurons in the developing brain form the cortical plate (CP) in an inside-out manner, in which the late-born neurons are located more superficially than the early-born neurons. Fyn, a member of the Src family kinases, plays an important role in neuronal migration by binding to many substrates. However, the role of the Src-homology 2 (SH2) domain in function of Fyn in neuronal migration remains poorly understood. Here, we demonstrate that the SH2 domain is essential for the action of Fyn in neuronal migration and cortical lamination. A point mutation in the Fyn SH2 domain (FynR176A) impaired neuronal migration and their final location in the cerebral cortex, by inducing neuronal aggregation and branching. Thus, we provide the first evidence of the Fyn SH2 domain contributing to neuronal migration and neuronal morphogenesis. [BMB Reports 2015; 48(2): 97-102]
Keywords: Fyn, In utero electroporation, Mice, Neuronal migration, SH2 domain
INTRODUCTION
The mammalian neocortex is composed of a birthdate-dependent inside-out alignment of six layers of neurons. During corticogenesis, new-born neurons derive from the ventricular zone (VZ)/subventricular zone (SVZ) pass through a multipolar stage to become bipolar and then undergo radial glia-dependent migration to their final destination. The migration of bipolar neurons requires elaborate coordination of leading process extension and somal translocation (1, 2). Defects in neuronal migration and cell morphogenesis in the cerebral cortex cause specific neurological syndromes, such as epilepsy, schizophrenia, and Alzheimer’s disease (3-5).
Fyn is a non-receptor protein tyrosine kinase that belongs to Src family kinases (SFK) and plays a key role in regulating cell proliferation, differentiation, and cell migration (6, 7). The double knockout mouse of Fyn and Src shows a reeler-like phenotype (8). Fyn is involved in the Reelin-dependent tyrosine phosphorylation of Dab1, which controls the positioning of radially migrating neurons in the cerebral cortex (9, 10). Fyn has also been identified as a signal factor in the organization of the cytoskeleton (11). Neuronal migration requires the coordination of dynamics of F-actin, microtubule, and nucleokinesis. However, the underlying molecular mechanism(s) of Fyn controlling neuronal migration remain(s) poorly understood.
Fyn is composed of multiple domains. The short N-terminal region has a unique function and is most divergent among different SFK members. Following the N-terminal is the Src homology 3 (SH3) domain (85-142 aa), which binds to target proteins through sequences containing proline and hydrophobic amino acids (the classic ‘PXXP’ consensus). The Src homology 2 (SH2) domain (147-237 aa) recognizes the pYEEI consensus and the kinase domain (271-520 aa) is responsible for the enzymatic activity (12-15). The SH2 domain can bind to phosphotyrosine motifs that play an essential role in Fyn signal transduction.
An intriguing question is whether the SH2 domain of Fyn is essential for regulating neuronal migration during brain development. Here, we show that the SH2 domain of Fyn is required for neuronal migration in vivo. SH2-phosphotyrosine recognition generally involves a highly conserved SH2 arginine, forming an electrostatic interaction with the phosphate moiety of the ligand (16). Thus, we constructed a point mutation (R176A) in the SH2 domain. Using in utero electroporation, we first identified that the FynR176A mutant showed impaired neuronal migration, in a dose-dependent manner. Furthermore, we observed numerous transfected neurons aggregated in the cortical plate (CP) or intermediate zone (IZ). Finally, we found that the transfected neurons in the CP gave rise to many branches. Thus, our findings indicated that the SH2 domain of Fyn controlled many aspects of neuronal migration and neuronal morphogenesis.
RESULTS
FynR176A impaired neuronal migration
Several studies have reported that Fyn is required for neuronal migration (8-10, 17). We hypothesized that the SH2 domain of Fyn might play an important role in cortical neuronal migration. To examine this, we transfected the FynR176A plasmid into the newly generated neurons in the neocortex at E15.5 and analyzed 5 days later (at P1), while the pCAG-MCS-GFP plasmid was used as a control. As expected, many transfected cells were mislocated beneath the primitive cortical zone (PCZ), while most of them were located in the PCZ in the control group (Fig. 1). In the FynR176A -transfected group, many neurons were in the CP and IZ, where very few GFP-positive neurons were found in the control group. Statistical analysis showed that the ratio of neurons in PCZ to CP was significantly different between the two groups. These findings suggested that point mutation of the Fyn SH2 domain impaired migration and the final location of the cortical neurons.
Fig. 1. FynR176A mutant impairs neuronal migration. (A) Coronal sections of P1 brains that had been transfected with GFP plasmid and FynR176A mutant plasmid at E15.5. The GFP-positive cells showed the transfected cells; many neurons were arrested in the low-CP and IZ in the FynR176A group. (B) the ratio of neurons in PCZ to CP of the two groups (FynR176A group compared with GFP control group using the t-test. *P < 0.05). (C) Average fluorescent intensity in each layer was analyzed. Neurons in IZ showed significantly stronger fluorescent intensity (***P < 0.001). Bars indicate mean ± SEM. (A) Scale bar = 100 μm.
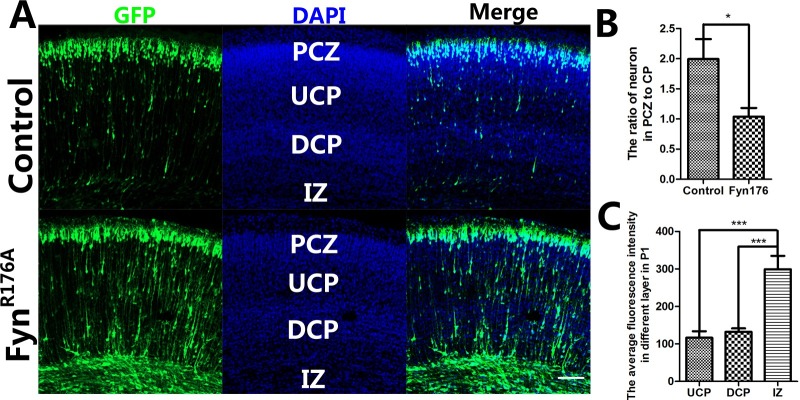
FynR176A impaired neuronal migration in a dose-dependent manner
In the FynR176A -transfected group, some neurons also reached the PCZ at P1. Why were only some arrested? We further analyzed the expression of Fyn in different layers, such as the upper CP (UCP), deeper CP (DCP), and IZ. We used GFP as a reporter to indicate the expression of Fyn. To determine average fluorescence intensity of the different layers of the cortex at P1, the fluorescence intensities of GFP-positive cells were measured with ZEN 2011 across a 300 μm-wide column and normalized with the number of cells. The statistical analysis showed the average fluorescence intensity of the IZ (299.1 ± 35.69) was significantly higher than the UCP (116.5 ± 17.39) and DCP (132.2 ± 9.238). These data indicated that FynR176A impaired neuronal migration in a dose-dependent manner. The neurons expressing this mutant at a high level were arrested in the lower layer, while those expressing it at a lower level migrated correctly to the PCZ (Fig. 1). Thus, we demonstrated that Fyn is necessary for neuronal migration, because the SH2 domain mutant resulted in a change in Fyn activity.
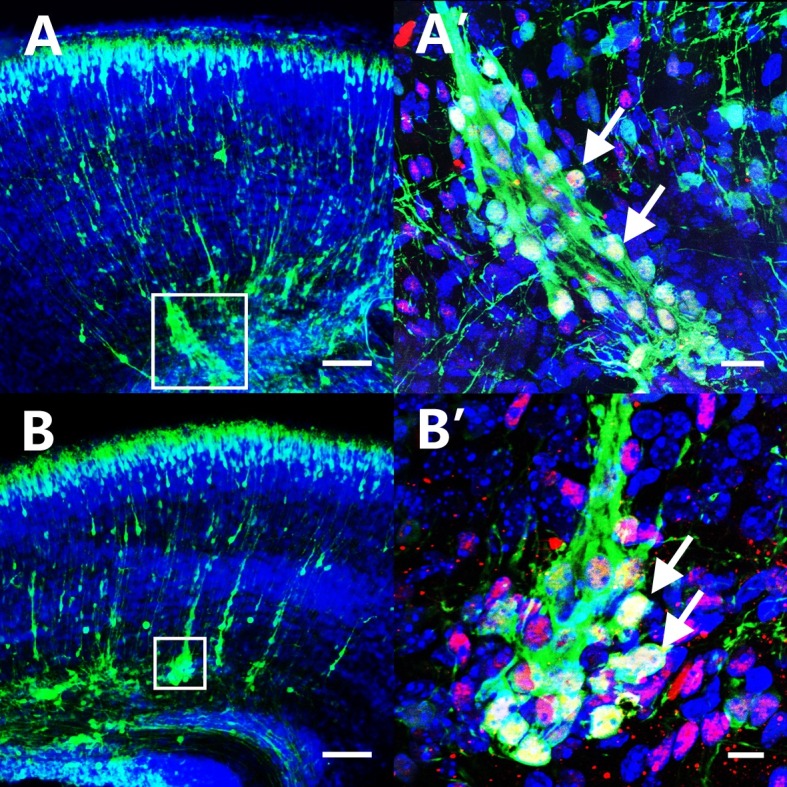
FynR176A induced neuronal aggregation in the CP and IZ
Our study showed that overexpression of the SH2 domain mutant form of Fyn also induced neuronal aggregation in the CP and IZ of mouse cerebral cortex (Fig. 2). The upper layer neuronal marker ‘Brn2’ staining suggested that the aggregated neurons in the CP/IZ belonged to layer II/III. The aggregation zone was packed densely with cells and clearly demarcated from the surroundings. There was an average of six aggregations in every section. The average cell number in the aggregation zone was 18.6 ± 4.32. The average fluorescence intensity of aggregated neurons, normalized to the number of aggregated cells, was 231.1 ± 14.49. These results indicated that Fyn could promote neuronal aggregation and adhesion, even when its SH2 domain was inactive.
Fig. 2. Over-expression of FynR176A induced neuronal aggregation in the DCP and IZ. (A, B) Sections of P1 brain transfected with FynR176A at E15.5 showed many aggregations formed in the DCP and IZ. (A’, B’) Higher magnification of FynR176A positive neurons formed aggregations in the DCP and IZ. The arrow points to the GFP+/DAPI+/Brn2+ (red). (A, B) Scale bar = 100 μm, (A’) Scale bar = 20 μm, (B’) Scale bar = 10 μm.
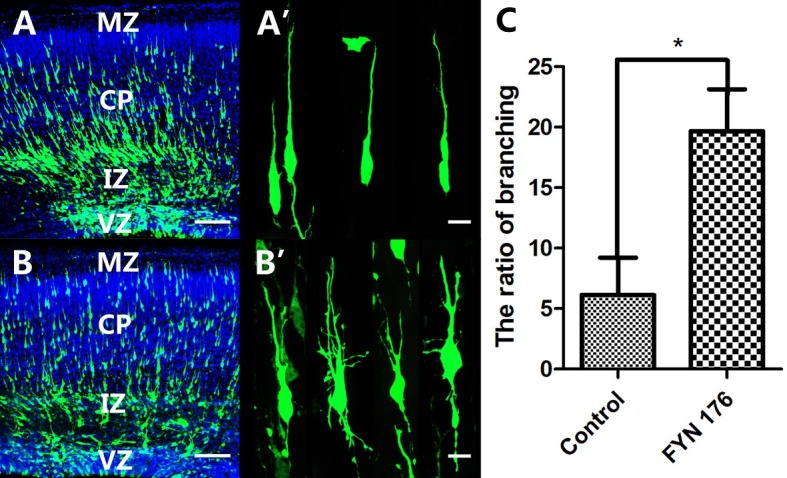
FynR176A promoted the branching of migrating neurons
As a member of the non-receptor tyrosine kinases, Fyn plays an essential role in actin cytoskeletal organization and cell migration (18-20). The results above show that overexpression of FynR176A arrested neuronal migration. We hypothesized that Fyn impaired neuronal migration by controlling actin cytoskeleton dynamics and neuronal morphogenesis. To assess this, we examined the morphology of the migrating neurons in the CP. Compared with the GFP control group, striking morphological changes occurred in the neurons transfected with FynR176A. As shown in Fig. 3 the transfected migrating neurons extended many branches and some of them lost the ‘classical’ neuronal morphology. The ratio of neurons having branches in the FynR176A expression group reached 19.67 ± 3.47%, while the GFP control group showed 6.13 ± 3.08%. Thus, our findings demonstrated that FynR176A impaired neuronal migration by controlling neuronal morphogenesis.
Fig. 3. Expression of FynR176A mutant induced the branching of cortical neurons. The brains were transfected with GFP or FynR176A at E15.5 and analyzed at E18.5. (A, A’) Normal morphology of migratory neurons in the GFP control group. (B, B’) Branching of cortical neurons in the FynR176A group. (C) Ratio of neurons with branches in the FynR176A mutant group compared with the GFP control group. Bars represent means ± standard deviation (*P < 0.05). (A, B) Scale bar = 100 μm, (A’ and B’) Scale bar = 10 μm.
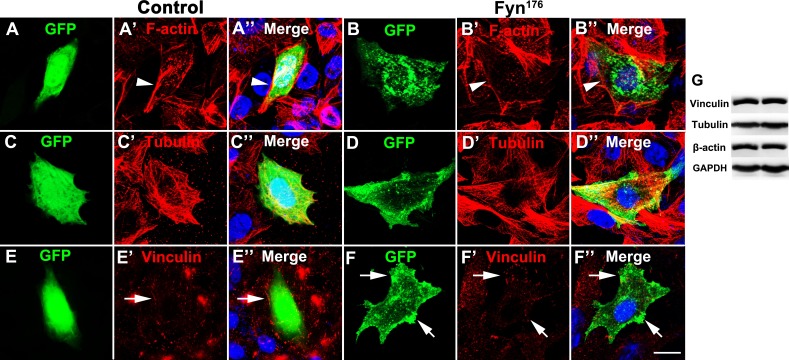
FynR176A causes reorganization of F-actin and focal accumulation of vinculin
We expressed FynR176A in CHO cells and stained cytoskeletal components, microtubules and F-actin, as well as the focal adhesion molecule, vinculin. We also transfected 293T cells for Western blot analysis. As shown in Fig. 4G, the expression of FynR176A did not affect the total amount of tubulin, actin, or vinculin. Immunochemistry results showed that FynR176A induced the depolymerization of F-actin. Well-organized F-actin was detected in the control group, while very little typical F-actin was found in the FynR176A group (Fig. 4). Expression of FynR176A also induced focal accumulation of the adhesion molecule, vinculin. As shown in Fig. 4, FynR176A partly colocalized with bundled vinculin, while no ‘typical’ bundled vinculin was observed in the control group (Fig. 4E). The expression and organization of another cytoskeletal component, microtubules, was not affected by the expression of FynR176A . Thus, we further demonstrated the mechanism by which FynR176A controls neuronal aggregation, adhesion, and branching.
Fig. 4. FynR176A induced reorganization of F-actin and focal accumulation of vinculin CHO cells were cultured in F12K medium containing 10% FBS and transfected with GFP or FynR176A . (A, B) CHO cells were stained with phalloidin and DAPI, FynR176A induced the depolymerization of F-actin. (C, D) CHO cells were stained with tubulin; no difference was observed between the GFP group and FynR176A group. (E, F) CHO cells were stained with vinculin, and FynR176A partially colocalized with vinculin and induced focal accumulation of vinculin. (G) Western blotting analysis of vinculin, tubulin, and actin in the GFP group and FynR176A group; no difference was observed between these two groups. Scale bar = 20 μm.
DISCUSSION
The conserved arginine in the phosphotyrosyl-binding pocket of the SH2 domain forms essential electrostatic interactions with the bound phosphorylated tyrosine (16). It is typically the case that mutation of this arginine to other residues will greatly weaken the interaction with the ligand but otherwise be tolerated structurally (21). This indicates that a ‘negative’ mutant may or may not influence the activation of Fyn.
Neuronal migration requires the elaborate coordination of many molecules in multiple steps (1, 22, 23). Previous studies have shown that Fyn acts as an intracellular signal molecule of Reelin, an extracellular matrix protein known to be essential for brain development (10). By binding to its two receptors, VLDLR and ApoER2, Reelin induces the tyrosine phosphorylation of the cytoplasmic protein Dab1 via SFKs (9). Fyn, a member of the SFKs, binds to the phosphorylated Dab1 with its SH2 domain to promote Dab1 phosphorylation. Phosphorylated Dab1 transduces the signal to downstream molecules (9). Our results showed that expression of FynR176A disturbed neuronal migration, and many neurons were arrested in the CP or IZ. As FynR176A is the SH2 domain mutant form, it may disturb the interaction between Fyn and downstream molecules, such as Dab1, Lis1, and FAK. These perhaps contribute to the defects in cortical neuronal migration.
Many FynR176A overexpressing neurons arrested in the CP or IZ aggregated to form a big column. Brn2 staining demonstrated that they were later-born neurons, belonging to the upper layers. Previous studies have shown that Fyn interact with many adhesion molecules including integrin β1, neural cell adhesion molecule (NCAM), and FAK (20, 24, 25). Fyn is a molecule downstream of NCAM, and is enriched in lipid rafts for the interactions of NCAM with both signaling and cytoskeletal components (26-28). Detailed analyses on the expression level of FynR176A showed that the aggregated neurons strongly expressed Fyn protein; these neurons were the major group of the migration-defective neurons. These results indicated that Fyn probably promotes neuronal adhesion to control neuronal migration in a dose- and activity-dependent manner. Also, overexpression of the FynR176A mutant induced the branching of migrating neurons. It is crucial for migrating neurons to keep the dynamics of F-actin organization in the growth cone of the leading process, and Fyn-regulated reorganization of the actin cytoskeleton is probably involved in the process. Fyn regulates F-actin dynamics through Rac1, Cdc42, RhoA, Arp2/3, N-WASP, and Cofilin, which induces the formation of filopodia and lamellipodia (29-31). These indicate the importance of the non-receptor tyrosine kinase Fyn in Reelin-induced neuronal branching in the MZ.
The SH2 domain has been found to participate in protein-protein interactions involving phosphotyrosine motifs. However, the aggregation and branching suggest that the interaction of Fyn with other proteins was not abolished. The SH2 domain functions mainly in signal transduction, even though the SH2-negative form of Fyn still can induce neuronal aggregation and branching. An in vitro analysis also showed that FynR176A can introduce the reorganization of F-actin and the adhesion molecule, vinculin. These results confirmed that inactivation of the SH2 domain did not completely prevent the activation of Fyn.
In summary, we have shown that the SH2 domain of Fyn plays an essential role in Reelin signaling transduction, and an SH2 domain mutant disturbs neuronal migration in a dose-dependent manner. We found that SH2 domain mutant perturbed neuronal final localizations and induced neuronal aggregation. A detailed analysis revealed that Fyn impaired neuronal morphology. Thus, Fyn regulates neuronal migration by controlling neuronal morphogenesis in a SH2 domain-dependent manner.
MATERIALS AND METHODS
Animals
C57BL/6J mice were housed in our lab. All animals were fed in clean environment with food and water available freely. All animal experiments were performed according to the institutional guidelines for animal experiments.
Plasmid construction
Fyn coding sequences were cloned from mouse brain cDNA using PCR. The specific primers used were Fyn-wt-forward (5’-CGGAATTCATGGGCTGTGTGCAA-3’) and Fyn-wt-reverse (5’-ACGCGTCGACTCACAGGTTTTCACCG-3’); EcoR I and Sal I sites were added to the primers, respectively. We used the pCAG-MCS-Fyn-wt vector as a template to enable preparation of the Fyn mutant using the MutanBEST Kit system (TaKaRa, Japan). The specific primers for FynR176A were FynR176A -forward (5’-TACATTCTCAGCAATTACG-3’), and FynR176A -reverse (5’-ACCAGTTTCCCCGGTTGTC-3’).
In utero electroporation
In utero electroporation was performed as previous described (32). Briefly, pregnant mice were anesthetized with sodium pentobarbitone (50 mg/g), and the uterine horns were exposed. Plasmid DNA at a concentration of 5 μg/μl, with Fast Green solution (0.1%) to monitor the injection, was injected into the embryos’ lateral ventricle with a glass micropipette. For electroporation, five pulses separated by 950 ms were applied at 35 V. The uterine horns were placed back into the abdominal cavity to allow the embryos to continue normal development.
Slice preparation and staining
At postnatal day 1 (P1) or embryo day 18.5 (E18.5), the brains were fixed with 4% paraformaldehyde (PFA) for 48 h at 4℃. Brains were cut in 50 μm sections with a Leica VT 1000S vibratome (Leica Microsystems). The tissue sections were incubated with blocking solution (containing 0.1 M PBS, 5% serum, 1% BSA and 0.2% Triton X-100) at RT for 2 h. The sections were incubated with the rabbit-anti-GFP (1:1,000, Millipore) and goat-anti-Brn2 (1:1,000, Santa Cruz Biotechnology Inc.) overnight at 4℃. After rinsing in 0.1 M PBS, the sections were incubated with donkey-anti-rabbit 488 (1:300, Millipore) and donkey-anti-goat 555 (1:300, Millipore) for 3 h at RT. Then, the sections were counterstained with DAPI for 20 min and rinsed in 0.1 M PBS for 1h. The sections were mounted in Dako Fluorescent Mounting Medium on glass slides and placed overnight at 4℃.
Cell culture and immunochemistry
After transfecting with the plasmid pCAG-MCS-GFP and the FynR176A plasmid, CHO cells were plated on glass coverslips in a 24-well dish. When confluency reached 50-60%, the cells were fixed with 4% paraformaldehyde and immunostained with mouse anti-tubulin (1:1,000, Millipore), mouse anti-vinculin (1:1,000, Millipore), or TRITC-phalloidin (1:1,000, Millipore) at 4℃ overnight. The cells were then rinsed and stained with donkey anti-mouse 568 (1:300, Invitrogen) diluted in 2% BSA for 2 h in the dark, then counterstained with DAPI (1:500, Invitrogen). Following three additional rinses, coverslips were mounted with Dako fluorescent mounting medium and photographed by a structured-illumination microscope (Zeiss observer Z1).
Immunoblotting
After transfecting with the plasmid pCAG-MCS-GFP and the FynR176A plasmid, soluble 293T cell lysates (equivalent to 5 × 106 cells/condition) were subjected to immunoblotting with the following antibodies: mouse anti-vinculin (1:1,000, Millipore), mouse anti-β-actin (1:2,000, Sigma), mouse antitubulin (1:5,000, Sigma), and mouse anti-GAPDH (1:5,000, Sigma). Antibody complexes were detected with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (1:2,000, Cell signaling Technology) and enhanced chemiluminescence (ECL) reagents.
Statistical analyses
At least six slices from three brains in separate experiments were analyzed for each group. Image analysis/cell counting was done with the ZEN 2011 program. Statistical differences were analyzed with unpaired t-tests, using GraphPad Prism 5. In all cases, confidence intervals were set at 95% and P values less than 0.05 were considered significant.
Acknowledgments
This work was supported by Foundation for Special Talent of NWSUAF (No. Z111021101) and The National Natural Science Foundation of China (No. 31071873).
References
- 1.Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. (2007);128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. (2001);4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 3.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. (2003);13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- 4.Valiente M, Marin O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. (2010);20:68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. (2003);26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 6.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. (1996);1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 7.Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. (1999);18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo G, Arnaud L, Kronstad-O'Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. (2005);25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. (2003);13:9–17. doi: 10.1016/S0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- 10.Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. (2003);13:18–26. doi: 10.1016/S0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- 11.Xu D, Kishi H, Kawamichi H, Kajiya K, Takada Y, Kobayashi S. Involvement of Fyn tyrosine kinase in actin stress fiber formation in fibroblasts. FEBS Lett. (2007);581:5227–5233. doi: 10.1016/j.febslet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Engen JR, Wales TE, Hochrein JM, et al. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. (2008);65:3058–3073. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. (2004);23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 14.Brickell PM. The p60c-src family of protein-tyrosine kinases: structure, regulation, and function. Crit Rev Oncog. (1992);3:401–446. [PubMed] [Google Scholar]
- 15.Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. (2008);1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Waksman G, Shoelson SE, Pant N, Cowburn D, Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. (1993);72:779–790. doi: 10.1016/0092-8674(93)90405-F. [DOI] [PubMed] [Google Scholar]
- 17.Forster E, Jossin Y, Zhao S, Chai X, Frotscher M, Goffinet AM. Recent progress in understanding the role of Reelin in radial neuronal migration, with specific emphasis on the dentate gyrus. Eur J Neurosci. (2006);23:901–909. doi: 10.1111/j.1460-9568.2006.04612.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Cofreces NB, Sancho D, Fernandez E, et al. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J Immunol. (2006);176:4201–4207. doi: 10.4049/jimmunol.176.7.4201. [DOI] [PubMed] [Google Scholar]
- 19.Klein C, Kramer EM, Cardine AM, Schraven B, Brandt R, Trotter J. Process outgrowth of oligodendrocytes is promoted by interaction of Fyn kinase with the cytoskeletal protein Tau. J Neurosci. (2002);22:698–707. doi: 10.1523/JNEUROSCI.22-03-00698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. (1996);109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Shen K, Lu W, Cole PA. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J Biol Chem. (2003);278:4668–4674. doi: 10.1074/jbc.M210028200. [DOI] [PubMed] [Google Scholar]
- 22.Sekine K, Kawauchi T, Kubo K, et al. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin alpha5beta1. Neuron. (2012);76:353–369. doi: 10.1016/j.neuron.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. (2011);69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodrikov V, Leshchyns'ka I, Sytnyk V, Overvoorde J, den Hertog J, Schachner M. RPTP alpha is essential for NCAM-mediated p59(fyn) activation and neurite elongation. J Cell Biol. (2005);168:127–139. doi: 10.1083/jcb.200405073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy KB, Smith DM, Plow EF. Analysis of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling. J Cell Sci. (2008);121:1641–1648. doi: 10.1242/jcs.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santuccione A, Sytnyk V, Leshchyns'ka I, Schachner M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J Cell Biol. (2005);169:341–354. doi: 10.1083/jcb.200409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ditlevsen DK, Povlsen GK, Berezin V, Bock E. NCAM-induced intracellular signaling revisited. J Neurosci Res. (2008);86:727–743. doi: 10.1002/jnr.21551. [DOI] [PubMed] [Google Scholar]
- 28.Samayawardhena LA, Kapur R, Craig AW. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood. (2007);109:3679–3686. doi: 10.1182/blood-2006-11-057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. (2004);24:7140–7149. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suetsugu S, Tezuka T, Morimura T, et al. Regulation of actin cytoskeleton by mDab1 through N-WASP and ubiquitination of mDab1. Biochem J. (2004);384:1–8. doi: 10.1042/BJ20041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin acts as a stop signal for radially migrating neurons by inducing phosphorylation of n-cofilin at the leading edge. Commun. Integr Biol. (2009);2:375–377. doi: 10.4161/cib.2.4.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura YV, Shinoda T, Inaguma Y, Ito H, Nagata K. Application of in utero electroporation and live imaging in the analyses of neuronal migration during mouse brain development. Med Mol Morphol. (2012);45:1–6. doi: 10.1007/s00795-011-0557-0. [DOI] [PubMed] [Google Scholar]