Abstract
Introduction:
Craniopharyngiomas treatment has been challenging because of their anatomical location. The endoscopic endonasal (EE) trans-sphenoidal approach is indicated in sellar, supra sellar, selected intraventricular lesions in adults and children. We are reporting our initial experience of 44 patients managed by EE approach.
Materials and Methods:
This is a retrospective study of 44 craniopharyngiomas. The goal of surgery was gross-total resection in all cases. All patients underwent pre- and post-operative comprehensive ophthalmological and endocrinological evaluation. Lumbar drain at the start of the operation was used in all cases with tumor larger than 3 cm maximum diameter. Binostril technique vascularized nasoseptal flap and multilayer closure of the dural defect were used. Wide sphenoidotomy, posterior ethmoidectomy, tuberculum selle, and planum removal were performed in all cases. Perioperative antibiotic prophylaxis was used for 72 h.
Results:
There were 44 patients of age ranging from 8 to 65 (mean: 42) years. Diameter of the tumor varied from 3.1 cm to 6.6 cm (average: 4.3 cm). Visual and pituitary dysfunctions were observed in 44 and 33, respectively, before surgery. Vision improvement, gross-total removal, cerebrospinal fluid (CSF) leak and recurrence were observed in 34, 26, four and six patients, respectively. Average follow-up was 19 months.
Conclusion:
Endoscopic endonasal trans-sphenoidal approach for craniopharyngioma is safe and effective alternative to transcranial approach in selected patients. Although this technique is associated with effective tumor removal and improved visual outcome, CSF leak, and endocrine dysfunctions remain a major challenge.
Keywords: Brain neoplasm, craniopharyngioma, endoscopy, intracranial neoplasm, neoplasm, surgical endoscopy
Introduction
Endoscopic surgeries are increasingly being used in various cranial and spine conditions.[1,2,3,4] Craniopharyngiomas most frequently arise in the pituitary stalk and project into the hypothalamus. They can extend anteriorly, posteriorly, superiorly or laterally. The endoscopic endonasal (EE) approach allows improved visualization, avoids brain retraction, and there are no external scars.[5] Extended endoscopic approaches should be performed after specialized training.[6] Collaboration between otolaryngologists and neurosurgeons has helped in better management of sellar and suprasellar craniopharyngiomas.[7] Development of newer and better repair technique for cerebrospinal fluid (CSF) leak, angled suction tips, single shaft tools, slim and longer forceps etc., has allowed tumor removal under direct visualization.[8] Although there are many advantages of endoscopic technique, surgeons should understand its limitations and indication.[9] Larger lesions with more lateral extension may be more suitable for an open transcranial approach.[10] This article is aimed to evaluate our results of endoscopic trans nasal management of 44 patients of craniopharyngiomas.
Materials and Methods
This is a retrospective evaluation of 44 craniopharyngiomas that were treated via a fully endoscopic, endonasal, extended trans-sphenoid approach at our institute between January 2010 and December 2013. The senior author performed all surgeries. Goal of surgery was gross-total resection (GTR) in all cases. All patients underwent pre- and post-operative comprehensive ophthalmological and endocrinological evaluation. Ophthalmological assessment consisted of visual acuity and visual field testing. Endocrinological assessment consisted of pre- and post-operative endocrinological evaluation including fasting morning cortisol, adrenocorticotropic hormone, thyroid function testing, follicle-stimulating hormone, luteinizing hormone, growth hormone, prolactin, serum sodium, and urine specific gravity. Endocrinological assessment was performed during the immediate postoperative period and then repeated at 4–6 weeks postoperatively. Preoperative computed tomography (CT) scans were performed in all patients [Figure 1], and magnetic resonance imaging (MRI) scans were done in 34 patients due to financial constrains [Figure 2]. Postoperative MRI scan with contrast were done to detect the extent of resection after 6 weeks of surgery [Figure 3].
Figure 1.
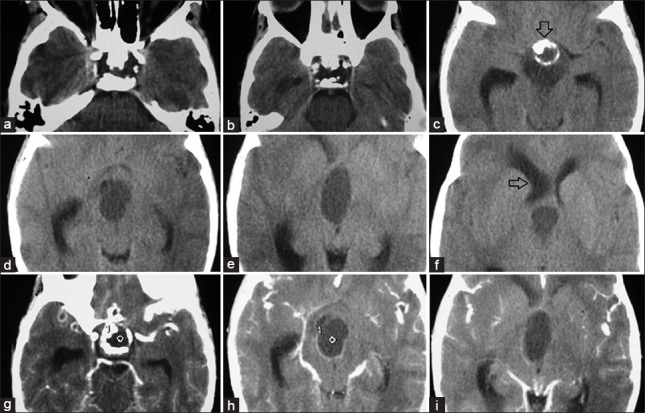
Preoperative plain (a-f) and contrast (g-i)-computed tomography scan showing craniopharyngioma with calcification (arrow down) extending up to right side foramen of Monro causing asymmetrical dilatation of lateral ventricle (arrow to left)
Figure 2.
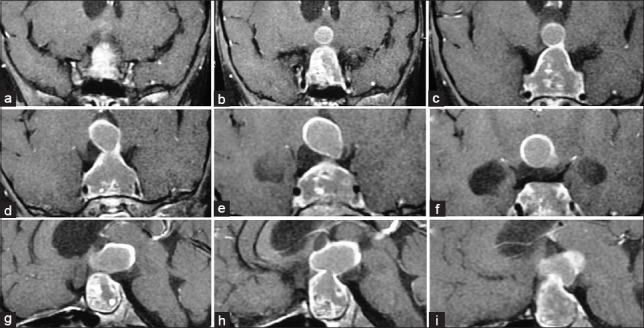
Preoperative contrast coronal (a-f) and sagittal (g-i) magnetic resonance imaging scan showing sellar, suprasellar, and intraventricular craniopharyngioma
Figure 3.
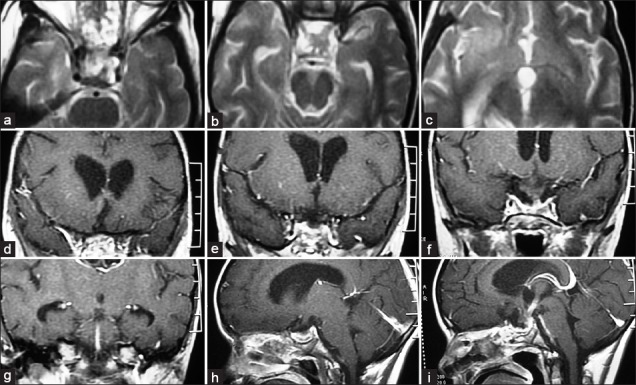
Postoperative axial (a-c), coronal (d-g) and sagittal (h and i) magnetic resonance imaging scan of the same patient shown in Figures 1 and 2 with gross-total excision of craniopharyngioma
Postoperative follow-up was done at 2 weeks, 3, 6, and 12 months after surgery. Patients were also seen by an otolaryngologist for scheduled nasal debridement at 10 days, 3 weeks, 6 weeks, 3 months, and 6 months after surgery. Surgical excision of the tumor was considered as GTR when there was per operative impression of total excision along with no tumor detection on postoperative MRI or CT scan. Subtotal resection was defined when >95% tumor excision was performed.
Surgical procedure
Details of the EE procedure and the technique of CSF leak closure are described in the literature.[11] We placed a lumbar drain at the start of the operation in all cases larger than 3 cm maximum tumor diameter. Binostril technique was used which provided more space and was useful in manipulations of more instruments. Vascularized nasoseptal flap was harvested in all patients for reconstruction. One side middle turbinate was excised while other side was lateralized. A wide sphenoidotomy, posterior ethmoidectomy, tuberculum selle, and planum removal were performed in all cases. Posterior clinoid process along with clivus excision was required in the retrochiasmatic and posterior fossa extension. The dura above and below the superior intercavernous sinus was opened, and the sinus was cauterized and cut.
Surgical corridor was between optic chiasm and stalk in majority of cases. The tumor was removed after decompression and careful dissection from adjacent critical neurovascular structures [Figures 4 and 5]. Stalk and superior hypophyseal arteries could be identified and preserved in all cases. Space created after tumor removal around the stalk provided adequate exposure to retroinfundibular region.[12] Bony removal after drilling of posterior clinoid process along with part of clivus helped in soft tissue retraction in tumor removal. Angled scope was used to detect residual lesion and for inspection of larger residual cavities. Multilayered closure using onlay vascularized pedicled septal flap, onlay fascia lata graft, onlay fat, and intradural fascia lata graft was used in larger bony and dural defects. We used large fascia lata graft, which was kept partly intradural and partly on bony defect margin. 4–0 silk sutures were tied to all 4 margins of the fascia lata graft, which were fixed using tissue glue to prevent graft migration. Piece of fat was kept intracranial on fascia lata to eliminate dead space. Perioperative antibiotic prophylaxis was used for 72 h.
Figure 4.
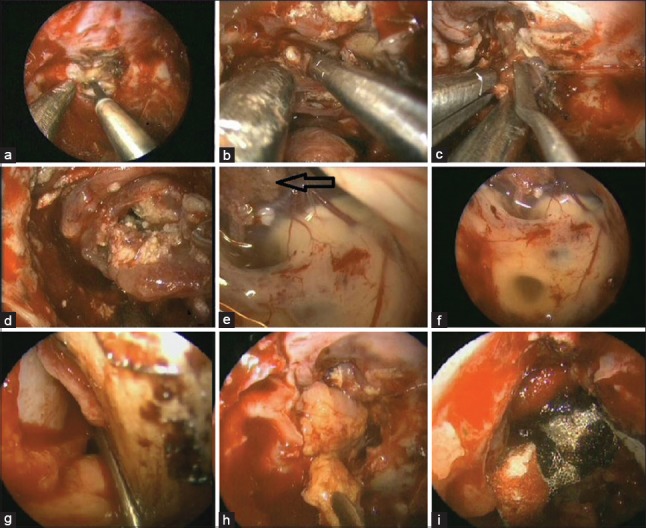
Endoscopic images showing (a) coagulation of sellar and part of anterior cranial fossa duramatter, (b-d) dissection of craniopharyngioma, (e and f) visualized third ventricle with choroid plexus (arrow), (g and h) placement of vascularized flap in dural defect, (i) surgicel and tissue glue being used
Figure 5.

Endoscopic images showing (a) removal of floor of sella, (b) duramatter incision over the sellar floor, (c) removal of tumor and (d) view after gross-total excision, (e) surgicel being laid in the cavity after tumor excision, and (f) fat used for obliteration of cavity
Results
There were 44 patients out of which 26 were male patients. Age ranged from 8 to 65 years (mean: 42 years). A lumbar drain was inserted in all patients just before nasal part of surgery. Diameter of the tumor varied from 3.1 cm to 6.6 cm (average: 4.3 cm). Only 13 patients had tumor extension in sella while in rest lesion was suprasellar with (n = 31) or without third ventricle extension (n = 13). Duration of symptoms ranged from 6 months to 2 years.
Although all patients had visual dysfunction either as decreased vision (n = 37) or defective visual field (n = 7), decreased vision was presenting feature in 37 patients. There was no vision in six patients. Thirty-three patients had pituitary dysfunction out of which 6 had posterior, and 23 had anterior pituitary dysfunction while 4 had pan hypopituitarism. There was no improvement in pituitary functions in any of the patients. 29 patients had postoperative diabetes insipidus (DI) out of them 6 continue to have permanent DI.
Vision improvement was observed in 34 patients while 10 patients did not show any improvement. Of 10 patients who did not show improvement, 6 were blind preoperative, and 4 had only perception of light with optic atrophy before surgery. Visual field defect improvement was seen in all 34 patients while the acuity improved in 20 patients only. Although total excision was aimed in all cases, GTR, subtotal and partial excisions were done in 26, 11 and 7 patients, respectively. All 7 patients in partial excision and 3 elderly patients in subtotal group had postoperative radiotherapy. Out of 8 patients (3 children and 5 adults) in subtotal excision, 2 were re-operated (GTR), and 6 were observed. There was 1, 2, and 3 recurrences in GTR, subtotal and partial excision group at an average of 19 months (range: 6–37 months) follow-up. CSF leak and meningitis developed in 4 and 1 patients, respectively. All 4 CSF leak patients were successfully re-operated using endonasal technique. One patient died within 1-month of surgery due to progression of the disease.
Discussion
There was no improvement in pituitary functions in any of the patients in our series. Similar observations were made in the previous reports.[13,14,15,16] Although the pituitary stalk can be preserved in the majority of the patients, DI along with other endocrine deficiencies are common complications.[13] Preoperative pituitary dysfunction usually does not show any improvement, on the contrary, new postoperative endocrine deficiency may occur.[14,15,16] Posterior pituitary dysfunctions are more common than anterior pituitary malfunction.[14,17] DI was reported in 8%, 8%, 14%, 30%, 42%, 43%, 44%, 47%, 48%, and 67% patients in Gardner et al. 2008,[15] Campbell et al. 2010,[17] current series 2014, Leng et al. 2012,[18] de Divitiis et al. 2007,[16] Jane et al. 2010,[19] Koutourousiou et al. 2013,[20] Cavallo et al., 2014[21] and Cavallo et al., 2013,[12] series, respectively [Table 1]. Hypopituitarism developed in 9%, 17%, 18%, 25%, 38%, 58% and 67% patients in current series 2014, de Divitiis et al. 2007,[16] Gardner et al. 2008.[15] Cavallo et al., 2013,[12] Leng et al. 2012,[18] Koutourousiou et al. 2013[20] and Jane et al. 2010,[19] series respectively [Table 1]. Panhypopituitarism without preexisting hypopituitarism developed in 18% and 67% patients in Gardner et al.[15] and Jane et al.[19] series respectively. Endocrine functions are better preserved after less tissue removal.[22,23]
Table 1.
Comparison of various series in terms of number of patients, amount of tumor resection, postoperative improvement in vision, CSF leak, diabetes insipidus, and hypopituitarism
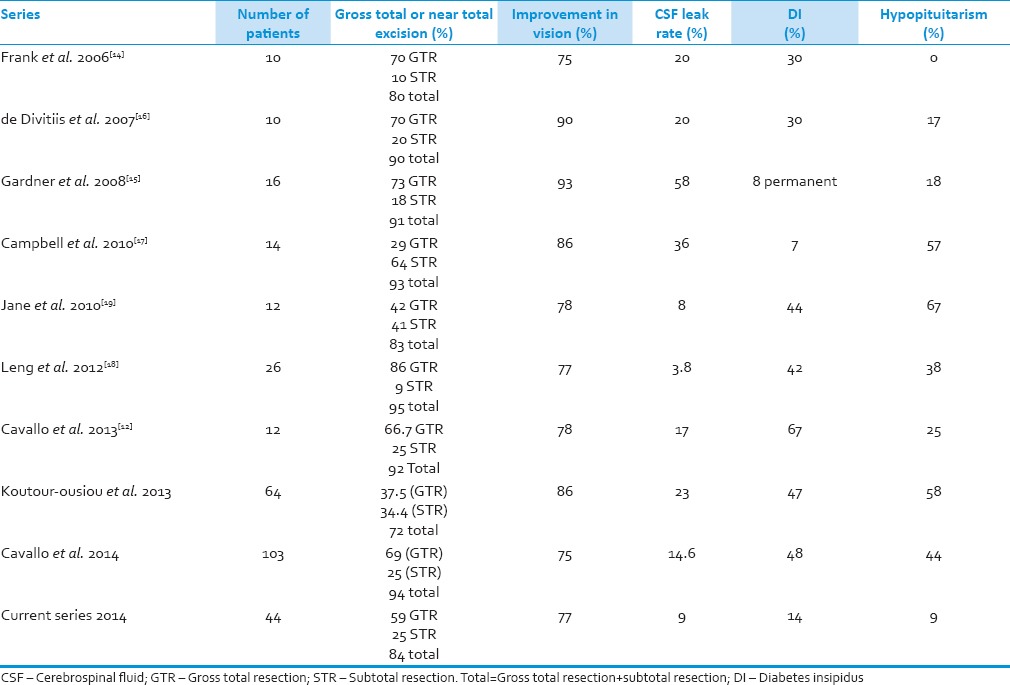
Visual field defect improvement was seen in 34 patients while the acuity improved in 20 patients only in our series. Similar results were observed in other series.[13,17] Improvement in vision was reported in 71%, 75%, 75%, 77%, 77%, 78%, 78%, 86%, 86%, and 93% de Divitiis et al. 2007,[16] Frank et al. 2006,[14] Cavallo et al. 2014,[21] current series 2014, Leng et al. 2012,[18] Jane et al. 2010,[19] Cavallo et al., 2013,[12] Campbell et al. 2010,[17] Koutourousiou et al. 2013[20] and Gardner et al. 2008[15] series respectively [Table 1]. The endoscopic group usually has a significantly greater rate of improvement compared to open group.[10] Although visual improvement occurs in the majority of patients, worsening though rare are also observed.[16,17]
Gross-total resection was done in 26 (59%) patients while GTR plus subtotal excision was performed in 84% in our series. GTR was achieved in 29%, 38%, 42%, 59%, 67%, 69%, 70%, 70%, 73%, and 86% patients in Campbell et al. 2010,[17] Koutourousiou et al. 2013,[20] Jane et al. 2010,[19] current series 2014, Cavallo et al., 2013,[12] Cavallo et al. 2014,[21] Frank et al. 2006,[14] de Divitiis et al. 2007,[16] Gardner et al. 2008,[15] and Leng et al. 2012,[18] respectively. Gross-total plus near total removal was achieved in about 72%, 80%, 83%, 84%, 90%, 91%, 92%, 93% and 95% in Koutourousiou et al. 2013,[20] Frank et al. 2006,[14] Jane et al. 2010,[19] current series 2014, de Divitiis et al. 2007,[16] Gardner et al. 2008,[15] Cavallo et al., 2013,[12] Campbell et al. 2010,[17] and Leng et al. 2012,[18] respectively [Table 1]. Endoscopic cyst drainage, 4–6 weeks prior to definitive surgery, is helpful in improving subsequent surgical excision in large cystic tumors.[24] The endoscopic group usually has a significantly greater resection compared to the microscopic group.[10,25]
There were 4%, 18%, and 43% recurrences in GTR, subtotal and partial excision removal group at an average of 19 months follow-up in our series. Less recurrence rate in our series compared to about 13%, 19% and 51% reported recurrence at 5 years follow-up in GTR, subtotal and partial excision group, respectively, could be due to short follow-up in our series. The rate of recurrence depends on the amount of tumor excision. Addition of postoperative radiotherapy reduces the recurrence rate in subtotal or partial excision group. The recurrence rate after cyst fenestration combined with GKS was higher than that of subtotal resection and GKS group.[22] Presence of residual tumor on the first postoperative MRI, male sex, and patients without postoperative radiation therapy are usually associated with a higher tumor recurrence.[26] Proper management of residual or recurring disease by radiotherapy, repeat surgery, or a combination of both is usually successful in controlling further tumor growth.[26]
Cerebrospinal fluid leak rate was 9% in our series. It was 0%, 4%, 9%, 15%, 17%, 20%, 20%, 23%, 36%, and 58% in Jane et al. 2010,[19] Leng et al. 2012,[18] current series 2014, Cavallo et al. 2014,[21] Cavallo et al., 2013,[12] Frank et al. 2006,[14] de Divitiis et al. 2007,[16] Koutourousiou et al. 2013,[20] Campbell et al. 2010,[17] and Gardner et al. 2008,[15] series, respectively [Table 1]. It varies from 0% in Jane et al. 2010,[19] to 58% in Gardner et al. series.[15] Endoscopic skull base reconstruction with a vascularized nasoseptal flap has dramatically reduced the incidence of CSF leak.[27,28,29] We also used nasoseptal flap in all our patients which could be responsible for comparative low CSF leak. Postoperative CSF leak is higher in larger tumors, longer duration surgery, and longer hospitalization.[30] The CSF leakage has been reported to be more in endoscopic trans-sphenoid (18.4%) and microscopic trans-sphenoid techniques (9.0%) as compared to transcranial approach (2.6%).[10]
All the types of sellar,[19,31,32] supra sellar craniopharyngiomas[13,19,33,34] can be better approached by endonasal surgery as compared to any single open transcranial technique. It is difficult to compare transcranial and trans-sphenoid approaches because later approach is increasingly used for smaller and intrasellar tumors, while transcranial surgery is performed in large tumors with significant lateral extension, vascular encasement, and those with significant peripheral calcification.[27,35] Severe adverse events are more frequently reported after transcranial approach (37%) as compared to trans-sphenoidal technique (5.6%).[26] EE surgery allows good infrachiasmatic exposure without manipulation of surrounding neurovascular structures. It has advantages especially during tumor dissection from the inferior aspect of the chiasm, infundibulum, and third ventricle, retrosellar areas. These benefits can be best appreciated in prior transcranial surgery cases.[36] High definition wide-angle visualization and good bimanual techniques facilitate a better endocrine function preservation and a higher rate of visual improvement.[28,29,37] Reduced postoperative hospital stay, low cost of management,[38] surgeon and the patient comfort[39] are some of the advantages of endoscopic technique. Adding endoscopy to the microsurgical technique improved the total resection rate by nearly 10%.[25]
Although more and more neurosurgical operations are being performed due to the advantages of endoscopic approaches,[40,41,42,43,44] EE procedures may be associated with problems such as control of the hemorrhage, closure of the dural and bony defects, postoperative CSF leak, tension pneumocephalus, and meningitis etc.[45] The lack of stereoscopic visualization, the constant need for manual control of the endoscope, and steep learning curve are other limitations.[37] A narrow surgical corridor, restricted lateral suprasellar access, and a more demanding cranial base repair are some disadvantages.[46] Although none heat generating, oscillating, cutting, and tissue-removing instrument can be used for fibrous tissue resection, large vascular, and dense fibrous tumors are difficult to remove.[47] Bleedings in craniopharyngioma surgery can occur from the venous sinuses, small arteries, the tumor bed and internal carotid artery. Avoiding pulling of tumor tissue from the brain or neurovascular structure should prevent such bleeding. Although management of intradural bleeding is challenging, it can be controlled by application of thrombin gelatin hemostatic matrix and head end elevation. Thrombin gelatin hemostatic matrix can be useful in oozing, focal hemorrhage and even in high flow bleeding.[48] Short follow-up and a small number of patients in our series are another limitation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Yadav YR, Parihar V, Pande S, Namdev H. Endoscopic management of colloid cysts. J Neurol Surg A Cent Eur Neurosurg. 2014;75:376–80. doi: 10.1055/s-0033-1343984. [DOI] [PubMed] [Google Scholar]
- 2.Charalampaki P, Igressa A, Mahvash M, Pechlivanis I, Schick B. Optimal invasive key-hole neurosurgery with a miniaturized 3D chip on the tip: Microendoscopic device. Asian J Neurosurg. 2013;8:125–31. doi: 10.4103/1793-5482.121681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadav YR, Parihar V, Namdev H, Agarwal M, Bhatele PR. Endoscopic interlaminar management of lumbar disc disease. J Neurol Surg A Cent Eur Neurosurg. 2013;74:77–81. doi: 10.1055/s-0032-1333127. [DOI] [PubMed] [Google Scholar]
- 4.Zohdi AZ, El Damaty AM, Aly KB, El Refaee EA. Success rate of endoscopic third ventriculostomy in infants below six months of age with congenital obstructive hydrocephalus (a preliminary study of eight cases) Asian J Neurosurg. 2013;8:147–52. doi: 10.4103/1793-5482.121686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oostra A, van Furth W, Georgalas C. Extended endoscopic endonasal skull base surgery: From the sella to the anterior and posterior cranial fossa. ANZ J Surg. 2012;82:122–30. doi: 10.1111/j.1445-2197.2011.05971.x. [DOI] [PubMed] [Google Scholar]
- 6.Cappabianca P, Cavallo LM, Esposito F, De Divitiis O, Messina A, De Divitiis E. Extended endoscopic endonasal approach to the midline skull base: The evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008;33:151–99. doi: 10.1007/978-3-211-72283-1_4. [DOI] [PubMed] [Google Scholar]
- 7.Gardner PA, Prevedello DM, Kassam AB, Snyderman CH, Carrau RL, Mintz AH. The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg. 2008;108:1043–7. doi: 10.3171/JNS/2008/108/5/1043. [DOI] [PubMed] [Google Scholar]
- 8.Saeki N, Murai H, Hasegawa Y, Horiguchi K, Hanazawa T, Fukuda K. Endoscopic endonasal surgery for extrasellar tumors: Case presentation and its future perspective. No Shinkei Geka. 2009;37:229–46. [PubMed] [Google Scholar]
- 9.Sun J, Ye F, Wang M, Jin Z, Liu R. Trannasal-transsphenoidal endoscopic surgery of the sphenoid sinus and the sella turcica. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2000;35:369–70. [PubMed] [Google Scholar]
- 10.Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg. 2012;77:329–41. doi: 10.1016/j.wneu.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Yadav Y, Sachdev S, Parihar V, Namdev H, Bhatele P. Endoscopic endonasal trans-sphenoid surgery of pituitary adenoma. J Neurosci Rural Pract. 2012;3:328–37. doi: 10.4103/0976-3147.102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavallo LM, Solari D, Esposito F, Cappabianca P. The endoscopic endonasal approach for the management of craniopharyngiomas involving the third ventricle. Neurosurg Rev. 2013;36:27–37. doi: 10.1007/s10143-012-0403-4. [DOI] [PubMed] [Google Scholar]
- 13.Laufer I, Anand VK, Schwartz TH. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106:400–6. doi: 10.3171/jns.2007.106.3.400. [DOI] [PubMed] [Google Scholar]
- 14.Frank G, Pasquini E, Doglietto F, Mazzatenta D, Sciarretta V, Farneti G, et al. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery. 2006;59:ONS75–83. doi: 10.1227/01.NEU.0000219897.98238.A3. [DOI] [PubMed] [Google Scholar]
- 15.Gardner PA, Kassam AB, Snyderman CH, Carrau RL, Mintz AH, Grahovac S, et al. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: A case series. J Neurosurg. 2008;109:6–16. doi: 10.3171/JNS/2008/109/7/0006. [DOI] [PubMed] [Google Scholar]
- 16.de Divitiis E, Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A. Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery. 2007;61:219–27. doi: 10.1227/01.neu.0000303220.55393.73. [DOI] [PubMed] [Google Scholar]
- 17.Campbell PG, McGettigan B, Luginbuhl A, Yadla S, Rosen M, Evans JJ. Endocrinological and ophthalmological consequences of an initial endonasal endoscopic approach for resection of craniopharyngiomas. Neurosurg Focus. 2010;28:E8. doi: 10.3171/2010.1.FOCUS09292. [DOI] [PubMed] [Google Scholar]
- 18.Leng LZ, Greenfield JP, Souweidane MM, Anand VK, Schwartz TH. Endoscopic, endonasal resection of craniopharyngiomas: Analysis of outcome including extent of resection, cerebrospinal fluid leak, return to preoperative productivity, and body mass index. Neurosurgery. 2012;70:110–23. doi: 10.1227/NEU.0b013e31822e8ffc. [DOI] [PubMed] [Google Scholar]
- 19.Jane JA, Jr, Kiehna E, Payne SC, Early SV, Laws ER., Jr Early outcomes of endoscopic transsphenoidal surgery for adult craniopharyngiomas. Neurosurg Focus. 2010;28:E9. doi: 10.3171/2010.1.FOCUS09319. [DOI] [PubMed] [Google Scholar]
- 20.Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Tyler-Kabara EC, Wang EW, Snyderman CH. Endoscopic endonasal surgery for craniopharyngiomas: Surgical outcome in 64 patients. J Neurosurg. 2013;119:1194–207. doi: 10.3171/2013.6.JNS122259. [DOI] [PubMed] [Google Scholar]
- 21.Cavallo LM, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, et al. The endoscopic endonasal approach for the management of craniopharyngiomas: A series of 103 patients. J Neurosurg. 2014;121:100–13. doi: 10.3171/2014.3.JNS131521. [DOI] [PubMed] [Google Scholar]
- 22.Park YS, Chang JH, Park YG, Kim DS. Recurrence rates after neuroendoscopic fenestration and Gamma Knife surgery in comparison with subtotal resection and Gamma Knife surgery for the treatment of cystic craniopharyngiomas. J Neurosurg. 2011;114:1360–8. doi: 10.3171/2009.9.JNS09301. [DOI] [PubMed] [Google Scholar]
- 23.Elowe-Gruau E, Beltrand J, Brauner R, Pinto G, Samara-Boustani D, Thalassinos C, et al. Childhood craniopharyngioma: Hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab. 2013;98:2376–82. doi: 10.1210/jc.2012-3928. [DOI] [PubMed] [Google Scholar]
- 24.Mallucci C, Pizer B, Blair J, Didi M, Doss A, Upadrasta S, et al. Management of craniopharyngioma: The Liverpool experience following the introduction of the CCLG guidelines. Introducing a new risk assessment grading system. Childs Nerv Syst. 2012;28:1181–92. doi: 10.1007/s00381-012-1787-8. [DOI] [PubMed] [Google Scholar]
- 25.Kadri H, Mawla AA. Endoscopy-assisted microsurgical total resection of craniopharyngioma in childhood. Minim Invasive Neurosurg. 2006;49:369–72. doi: 10.1055/s-2006-961820. [DOI] [PubMed] [Google Scholar]
- 26.Mortini P, Losa M, Pozzobon G, Barzaghi R, Riva M, Acerno S, et al. Neurosurgical treatment of craniopharyngioma in adults and children: Early and long-term results in a large case series. J Neurosurg. 2011;114:1350–9. doi: 10.3171/2010.11.JNS10670. [DOI] [PubMed] [Google Scholar]
- 27.Elliott RE, Jane JA, Jr, Wisoff JH. Surgical management of craniopharyngiomas in children: Meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery. 2011;69:630–43. doi: 10.1227/NEU.0b013e31821a872d. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Strojan P, Suárez C, et al. Craniopharyngioma: A pathologic, clinical, and surgical review. Head Neck. 2012;34:1036–44. doi: 10.1002/hed.21771. [DOI] [PubMed] [Google Scholar]
- 29.Stamm AC, Vellutini E, Balsalobre L. Craniopharyngioma. Otolaryngol Clin North Am. 2011;44:937–52. doi: 10.1016/j.otc.2011.06.015. viii. [DOI] [PubMed] [Google Scholar]
- 30.Tabaee A, Anand VK, Brown SM, Lin JW, Schwartz TH. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope. 2007;117:1133–7. doi: 10.1097/MLG.0b013e31805c08c5. [DOI] [PubMed] [Google Scholar]
- 31.Cavallo LM, Prevedello D, Esposito F, Laws ER, Jr, Dusick JR, Messina A, et al. The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev. 2008;31:55–64. doi: 10.1007/s10143-007-0098-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Ni Z, Sun H, Zhang J. Endoscopic intrasellar tumor surgery. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2002;37:363–5. [PubMed] [Google Scholar]
- 33.de Divitiis E, Cavallo LM, Cappabianca P, Esposito F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: Part 2. Neurosurgery. 2007;60:46–58. doi: 10.1227/01.NEU.0000249211.89096.25. [DOI] [PubMed] [Google Scholar]
- 34.Gaab MR, Schroeder HW. Neuroendoscopic approach to intraventricular lesions. Neurosurg Focus. 1999;6:e5. [PubMed] [Google Scholar]
- 35.Zimmer LA, Theodosopoulos PV. Anterior skull base surgery: Open versus endoscopic. Curr Opin Otolaryngol Head Neck Surg. 2009;17:75–8. doi: 10.1097/moo.0b013e328325a525. [DOI] [PubMed] [Google Scholar]
- 36.Cavallo LM, Prevedello DM, Solari D, Gardner PA, Esposito F, Snyderman CH, et al. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg. 2009;111:578–89. doi: 10.3171/2009.2.JNS081026. [DOI] [PubMed] [Google Scholar]
- 37.Gondim J, Schops M, Tella OI., Jr Transnasal endoscopic surgery of the sellar region: Study of the first 100 cases. Arq Neuropsiquiatr. 2003;61(3B):836–41. doi: 10.1590/s0004-282x2003000500024. [DOI] [PubMed] [Google Scholar]
- 38.Cappabianca P, Cavallo LM, Colao A, Del Basso De Caro M, Esposito F, Cirillo S, et al. Endoscopic endonasal transsphenoidal approach: Outcome analysis of 100 consecutive procedures. Minim Invasive Neurosurg. 2002;45:193–200. doi: 10.1055/s-2002-36197. [DOI] [PubMed] [Google Scholar]
- 39.Rudnik A, Zawadzki T, Wojtacha M, Bazowski P, Gamrot J, Galuszka-Ignasiak B, et al. Endoscopic transnasal transsphenoidal treatment of pathology of the sellar region. Minim Invasive Neurosurg. 2005;48:101–7. doi: 10.1055/s-2004-830185. [DOI] [PubMed] [Google Scholar]
- 40.Yadav YR, Madhariya SN, Parihar VS, Namdev H, Bhatele PR. Endoscopic transoral excision of odontoid process in irreducible atlantoaxial dislocation: Our experience of 34 patients. J Neurol Surg A Cent Eur Neurosurg. 2013;74:162–7. doi: 10.1055/s-0032-1327441. [DOI] [PubMed] [Google Scholar]
- 41.Yadav YR, Mukerji G, Shenoy R, Basoor A, Jain G, Nelson A. Endoscopic management of hypertensive intraventricular haemorrhage with obstructive hydrocephalus. BMC Neurol. 2007;7:1. doi: 10.1186/1471-2377-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav YR, Mukerji G, Parihar V, Sinha M, Pandey S. Complex hydrocephalus (combination of communicating and obstructive type): An important cause of failed endoscopic third ventriculostomy. BMC Res Notes. 2009;2:137. doi: 10.1186/1756-0500-2-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadav YR, Parihar V, Sinha M, Jain N. Endoscopic treatment of the suprasellar arachnoid cyst. Neurol India. 2010;58:280–3. doi: 10.4103/0028-3886.63772. [DOI] [PubMed] [Google Scholar]
- 44.Yadav YR, Shenoy R, Mukerji G, Parihar V. Water jet dissection technique for endoscopic third ventriculostomy minimises the risk of bleeding and neurological complications in obstructive hydrocephalus with a thick and opaque third ventricle floor. Minim Invasive Neurosurg. 2010;53:155–8. doi: 10.1055/s-0030-1263107. [DOI] [PubMed] [Google Scholar]
- 45.Ceylan S, Koc K, Anik I. Extended endoscopic approaches for midline skull-base lesions. Neurosurg Rev. 2009;32:309–19. doi: 10.1007/s10143-009-0201-9. [DOI] [PubMed] [Google Scholar]
- 46.Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery. 2009;64:269–84. doi: 10.1227/01.NEU.0000327857.22221.53. [DOI] [PubMed] [Google Scholar]
- 47.Dlouhy BJ, Dahdaleh NS, Greenlee JD. Emerging technology in intracranial neuroendoscopy: Application of the NICO Myriad. Neurosurg Focus. 2011;30:E6. doi: 10.3171/2011.2.FOCUS10312. [DOI] [PubMed] [Google Scholar]
- 48.Cappabianca P, Esposito F, Esposito I, Cavallo LM, Leone CA. Use of a thrombin-gelatin haemostatic matrix in endoscopic endonasal extended approaches: Technical note. Acta Neurochir (Wien) 2009;151:69–77. doi: 10.1007/s00701-008-0172-6. [DOI] [PubMed] [Google Scholar]


