Abstract
A 68-year-old man presented to our hospital with a 1-month history of slowly progressing altered mental status and gait disturbance. Magnetic resonance imaging and abdominal computed tomography revealed advanced pancreatic cancer (PC) with brain and para-aortic lymph node metastases. Gross total resection of the brain metastatic tumor was performed. Although symptoms improved, the patient died 3 months postoperatively. In general, the prognosis for PC patients with brain metastasis is very poor. Surgical resection of brain metastasis may play a very limited role in allowing long-term survival of patients for whom the primary PC is controlled or with particular oncocytic-type tumors.
Keywords: Brain metastasis, pancreatic cancer, surgical resection
Introduction
Pancreatic cancer (PC) is a disease with a 5-year survival rate below 5%, and most patients die within 2 years of diagnosis.[1,2] Even with curative resection, the median survival time for patients with resected PC is only 8–12 months.[3,4,5] Given the absence of early signs or symptoms of PC, 65–70% of patients show nodal or distant metastases to the liver, peritoneum, lungs or bone by the time of diagnosis.[6] Metastasis to the brain is quite rare and usually predicts very poor prognosis.[6,7,8] Although the treatment strategy tends to be palliative for advanced PC with brain metastasis, several reports have described aggressive surgery for brain metastasis resulting in long-term survival.[7,9,10,11]
We describe a case of advanced PC in which brain metastasis was the first manifestation and was treated surgically. Although gross total resection of the tumor was performed, the patient died 3 months postoperatively. We review the literature and discuss the indications for surgical resection.
Case Report
A 68-year-old man presented to our hospital with a 1-month history of slowly progressing altered mental status and gait disturbance. On neurological examination, the patient displayed disorientation with mild left hemiparesis and facial palsy. Magnetic resonance imaging revealed a mass lesion comprising solid parts and multiple cysts in the right temporoparietal region. The mass demonstrated irregular ring-enhancement on contrast administration and was accompanied by extensive perifocal edema [Figure 1a–c]. We suspected metastatic brain tumor and performed whole-body computed tomography (CT). Although no obvious lung cancer was identified, abdominal contrast-enhanced CT revealed multiple hypodense lesions at the head of the pancreas with enlargement of the para-aortic lymph nodes [Figure 2a and b]. Laboratory testing showed: Glutamic oxaloacetic transaminase, 30 U/L; glutamic pyruvic transaminase, 66 U/L; alkaline phosphatase, 606 U/L; lactate dehydrogenase, 347 U/L; and g-glutamyl transpeptidase, 362 U/L. These findings were consistent with biliary obstruction and liver damage. The carbohydrate antigen 19–9) level was 87 U/mL (normal, <37 U/mL) and the carcinoembryonic antigen level was 193 ng/mL (normal, <5 ng/mL). Advanced PC was diagnosed (International Union against Cancer tumor-node-metastasis classification, T3N3M1 stage 4) and very poor prognosis was predicted due to the presence of brain metastasis.
Figure 1.
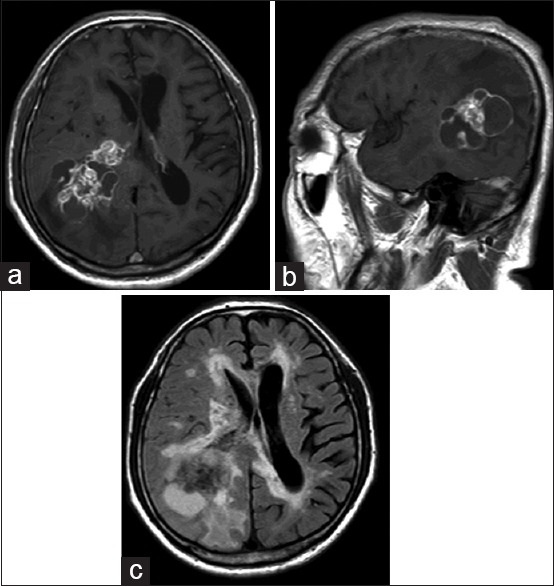
(a and b) T1-weighted imaging after gadolinium administration revealed a tumor with irregular ring-enhancement in the right temporoparietal region (a: axial, b: sagittal). (c) Axial fluid-attenuated inversion recovery imaging revealed the tumor accompanied extensive perifocal edema
Figure 2.
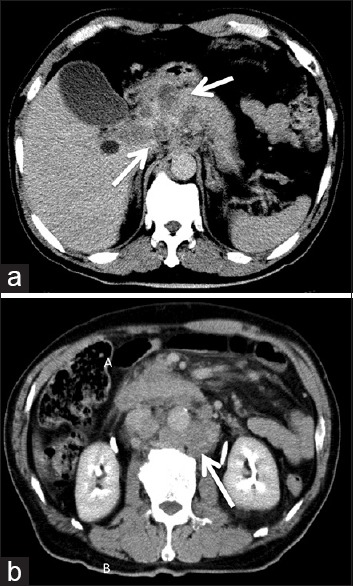
Contrast-enhanced computed tomography revealed multiple hypodense lesions at the head of the pancreas (a), and para-aortic lymph nodes enlargement (b), as indicated the arrows
The patient underwent craniotomy with gross total resection of the tumor. Histopathological analysis revealed moderately differentiated tubular adenocarcinoma and immunohistochemical studies showed positive results for cytokeratin 7 (CK7) and negative results for CK20 and thyroid transcription factor 1, consistent with pancreatic adenocarcinoma [Figure 3a and b]. Although symptoms improved postoperatively, the patient declined aggressive chemotherapy and radiotherapy because of the poor prognosis. The clinical condition of the patient deteriorated and he died under hospice care 3 months postoperatively.
Figure 3.
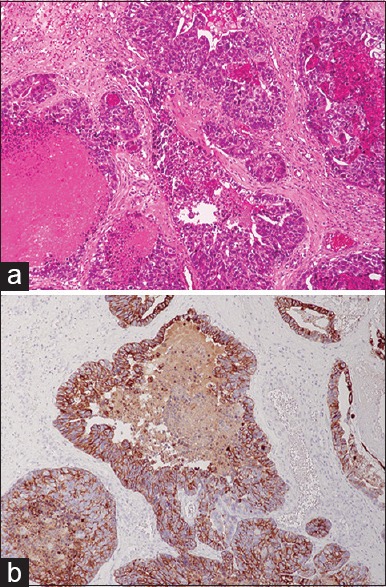
(a) Microscope analysis revealed the tumor to be a moderately differenciated tubular adenocarcinoma surrounded by an extracellular matrix (H and E, ×200). (b) Immunohistochemical stains showed that the tumor cells were positive for cytokeratin-7 (×200)
Discussion
Pancreatic cancer metastases to the brain are quite rare, occurring in 0.33% of PC cases.[12] Similarly, metastatic brain tumor from PC comprises only 0.1–0.4% of all metastatic brain tumors.[13,14] Although several cases of PC with brain metastases have been reported, the majority of such cases have been identified postmortem.[6,7,8,15,16] To the best of our knowledge, only 20 cases in which brain metastases from PC were identified ante-mortem have been reported until date, including the present case.[8,9,10,11,12,14,17,18,19,20,21,22,23,24] Table 1 summarizes the clinical features. Patients have been predominantly female (17 female, 3 male), and 17 (85%) have shown other remote metastases such as the liver, lungs, lymph nodes or bone. In six patients (30%), brain metastases represented the first manifestation of PC, while all other patients developed brain metastases later in the clinical course. Prognosis was very poor, and most patients died within 3 months after the diagnosis of brain metastases. Only two patients had survived >2 years at time their cases were reported.
Table 1.
Summary of reported cases of BM in PC
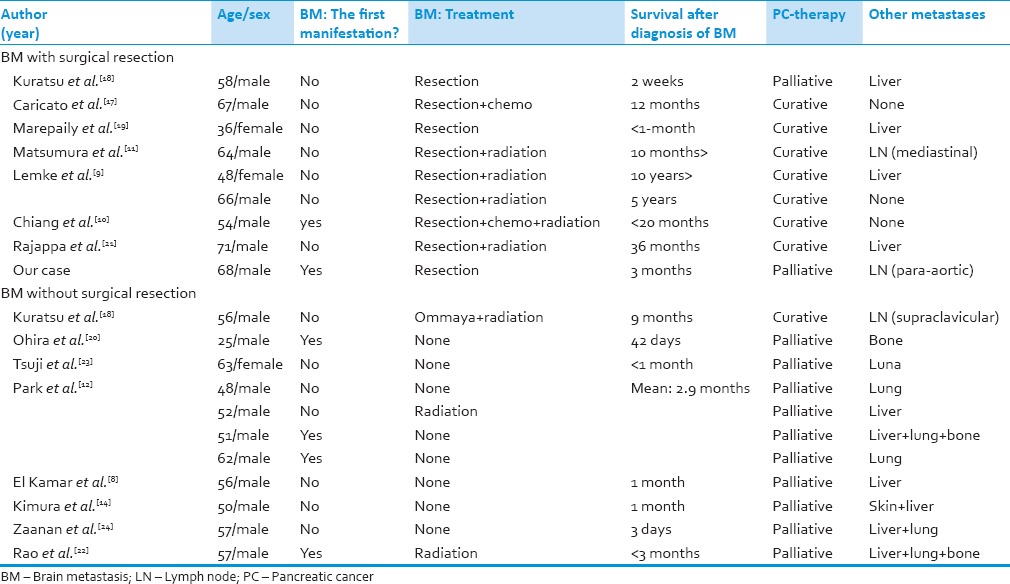
In general, the treatment strategy tends to be palliative for brain metastases from PC, because of the extremely poor prognosis. Treatments of metastatic brain tumor from PC have included surgical resection, whole-brain radiotherapy, stereotactic radiosurgery and placement of an ommaya reservoir for cystic tumor.[9,10,11,17,18,19,21] Given the radioresistant nature of most PCs, radiotherapy appears to offer little benefit.[6,12] On the other hand, aggressive surgical resection may prove effective. Nine reports have described surgical resection for metastatic brain tumor from PC, including our case [Table 1]. Survival periods appear significantly longer in cases with surgical resection (mean, 883.7 days) than in cases without (mean, 75 days; P = 0.03, Student's t-test). Moreover, the 5-year survival rate after surgical resection for brain metastasis (22%) was better than that of general advanced PC (<5%). In our case, the patient died 3 months postoperatively due to due to worsening of the primary lesion. The prognosis was the same as for cases without surgical resection. However, the patient showed improved neurological symptoms and was able to spend the remainder of his life meaningfully and without neurological deficits. In this regard, surgical resection might be of benefit to some patients.
Contrasting with our case, Lemke et al.[7,9] reported two cases with long-term survival of >5-year after surgical resection. To identify factors contributing to the prognosis, we analyzed the cases of 9 patients who underwent surgical resection, and divided patients into groups according to whether they lived >8 months, as the median survival time for resected PC patients. The most significant factor was whether curative therapy for PC was performed (P = 0.02, Chi-square test). The presence of other remote metastases was not significant (P = 0.1, Chi-square test). Park et al.[12] reported that cause of death was not usually related to nervous system metastasis. Hence, in patients for whom the primary tumor is uncontrolled, surgery for brain metastasis may not improve the prognosis. In our case, the patient had untreated PC with para-aortic lymph node metastases. Para-aortic lymph node metastasis is another factor associated with poor prognosis.[3,25,26] This patient thus had very poor prognosis despite gross total resection of a brain metastasis.
On the other hand, oncocytic-type intraductal papillary mucinous neoplasm of the pancreas (IPMN)-derived PC may be a factor associated with good prognosis. IPMN has been deemed a precursor of PC with a very slow rate of progression.[10] Chiang et al.[10] reported a case of IPMN-derived PC with brain metastasis, in which brain metastasis represented the first manifestation and aggressive treatments, including surgical resection of the brain metastasis, radiation, and chemotherapy for PC, achieved long-term survival. They therefore recommended an aggressive therapeutic strategy for IPMN-derived PC.
Conclusion
In general, the prognosis for PC patients with brain metastasis is very poor. Surgical resection of brain metastasis may play a very limited role and may allow long-term survival in patients for whom the primary PC is controlled or with particular oncocytic-type tumors such as IPMN-derived PC.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Masui T, Kubota T, Aoki K, Nakanishi Y, Miyamoto T, Nagata J, et al. Long-term survival after resection of pancreatic ductal adenocarcinoma with para-aortic lymph node metastasis: Case report. World J Surg Oncol. 2013;11:195. doi: 10.1186/1477-7819-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: A population-based study (United States) Cancer Causes Control. 2006;17:403–9. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 5.Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer. 1995;76:1671–7. doi: 10.1002/1097-0142(19951101)76:9<1671::aid-cncr2820760926>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: A rare and ominous sign. Cancer. 2011;117:3630–40. doi: 10.1002/cncr.25940. [DOI] [PubMed] [Google Scholar]
- 7.Lemke J, Scheele J, Kapapa T, Wirtz CR, Henne-Bruns D, Kornmann M. Brain metastasis in pancreatic cancer. Int J Mol Sci. 2013;14:4163–73. doi: 10.3390/ijms14024163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Kamar FG, Jindal K, Grossbard ML, Mizrachi HH, Kozuch PS. Pancreatic carcinoma with brain metastases: Case report and literature review. Dig Liver Dis. 2004;36:355–60. doi: 10.1016/j.dld.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Lemke J, Barth TF, Juchems M, Kapapa T, Henne-Bruns D, Kornmann M. Long-term survival following resection of brain metastases from pancreatic cancer. Anticancer Res. 2011;31:4599–603. [PubMed] [Google Scholar]
- 10.Chiang KC, Yu CC, Chen JR, Huang YT, Huang CC, Yeh CN, et al. Oncocytic-type intraductal papillary mucinous neoplasm (IPMN)-derived invasive oncocytic pancreatic carcinoma with brain metastasis – A case report. World J Surg Oncol. 2012;10:138. doi: 10.1186/1477-7819-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumura T, Ohzato H, Yamamoto T, Ota K, Mabuchi E, Miwa H, et al. A case of postoperative brain metastasis originated from pancreatic cancer which was successfully treated by resection and postoperative irradiation. Gan To Kagaku Ryoho. 2009;36:2433–5. [PubMed] [Google Scholar]
- 12.Park KS, Kim M, Park SH, Lee KW. Nervous system involvement by pancreatic cancer. J Neurooncol. 2003;63:313–6. doi: 10.1023/a:1024337020884. [DOI] [PubMed] [Google Scholar]
- 13.The Committee of Brain Tumor Registry of Japan. Report of brain tumor registry of Japan (2001-2004) Neurol Med Chir (Tokyo) (13th editon) 2014;54(Suppl):1–102. [Google Scholar]
- 14.Kimura H, Furukawa Y, Kuwada Y, Hananoki M, Matsumoto N, Yamamoto M, et al. A patient with pancreatic cancer associated with brain and skin metastases. Suizou. 2008;23:74–82. [Google Scholar]
- 15.Yamada K, Miura M, Miyayama H, Sakashita N, Kochi M, Ushio Y. Brain metastases from asymptomatic adenocarcinoma of the pancreas: An autopsy case report. Surg Neurol. 2002;58:332–6. doi: 10.1016/s0090-3019(02)00805-4. [DOI] [PubMed] [Google Scholar]
- 16.Lee YT, Tatter D. Carcinoma of the pancreas and periampullary structures. Pattern of metastasis at autopsy. Arch Pathol Lab Med. 1984;108:584–7. [PubMed] [Google Scholar]
- 17.Caricato M, Borzomati D, Ausania F, Garberini A, Rabitti C, Tonini G, et al. Cerebellar metastasis from pancreatic adenocarcinoma. A case report. Pancreatology. 2006;6:306–8. doi: 10.1159/000092693. [DOI] [PubMed] [Google Scholar]
- 18.Kuratsu J, Murakami M, Uemura S, Ushio Y. Brain and skull metastases of hepatic or pancreatic cancer – Report of six cases. Neurol Med Chir (Tokyo) 1990;30:476–82. doi: 10.2176/nmc.30.476. [DOI] [PubMed] [Google Scholar]
- 19.Marepaily R, Micheals D, Sloan A, Hatfield J, Adsay V, Joyrich R, et al. Octreotide uptake in intracranial metastasis of pancreatic ductal adenocarcinoma origin in a patient with a prolonged clinical course. Dig Dis Sci. 2009;54:188–90. doi: 10.1007/s10620-008-0531-4. [DOI] [PubMed] [Google Scholar]
- 20.Ohira Y, Kaga K, Kodama A. A case of bilateral sudden hearing loss and vertigo caused by bilateral temporal bone metastasis from pancreatic carcinoma – Comparison of clinical findings and temporal bone pathological findings. Nihon Jibiinkoka Gakkai Kaiho. 1991;94:9–15. doi: 10.3950/jibiinkoka.94.9. [DOI] [PubMed] [Google Scholar]
- 21.Rajappa P, Margetis K, Wernicke G, Ginter P, Cope W, Sherr DL, et al. Stereotactic radiosurgery plays a critical role in enhancing long-term survival in a patient with pancreatic cancer metastatic to the brain. Anticancer Res. 2013;33:3899–903. [PubMed] [Google Scholar]
- 22.Rao R, Sadashiv SK, Goday S, Monga D. An extremely rare case of pancreatic cancer presenting with leptomeningeal carcinomatosis and synchronous intraparenchymal brain metastasis. Gastrointest Cancer Res. 2013;6:90–2. [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji Y, Ohigashi H, Ishikawa O, Yasuda T, Nakano H, Nakamori S, et al. A case of non-resectable pancreatic cancer surviving more than 4 years by intra-arterial infusion chemotherapy with angiotensin-II. Gan To Kagaku Ryoho. 1996;23:1617–20. [PubMed] [Google Scholar]
- 24.Zaanan A, Lequoy M, Landi B, Lievre A, Franco D, Taïeb J. Brain metastases from pancreatic adenocarcinoma. BMJ Case Rep 2009. 2009 doi: 10.1136/bcr.08.2008.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Yuasa Y, Sueda T. Prognostic impact of para-aortic lymph node metastasis in pancreatic ductal adenocarcinoma. World J Surg. 2010;34:1900–7. doi: 10.1007/s00268-010-0577-2. [DOI] [PubMed] [Google Scholar]
- 26.Sakai M, Nakao A, Kaneko T, Takeda S, Inoue S, Kodera Y, et al. Para-aortic lymph node metastasis in carcinoma of the head of the pancreas. Surgery. 2005;137:606–11. doi: 10.1016/j.surg.2005.02.009. [DOI] [PubMed] [Google Scholar]


