Abstract
Background:
Medulloblastoma is a common malignancy in the pediatric population, accounting for 25% of all childhood brain tumors and relatively uncommon in adults. This review was to investigate treatment outcome and prognostic factors after treatment of medulloblastoma.
Materials and Methods:
A total of 53 patients with histological confirmed medulloblastoma cases treated at our institute between 2006 and 2012 were included in the study. Demographic variables, clinical variables, radiological findings and treatment details with respect to age, sex, signs and symptoms, location of tumor, extent of surgical resection, histopathology type, radiotherapy dose, follow-up period and outcomes were recorded. Survival was analyzed by using these parameters.
Results:
Thirty-one (58.5%) patients were pediatric (<14 years), and 22 (41.5%) patients were adults (≥14 years). Duration of symptoms were <3 months in 19 (36%) and more than 3 months in 34 (64%) patients. Tumor resection was performed in all patients with gross total resection in 8 (15%) patients, near total resection in 34 (64%) patients and subtotal resection in 11 (21%) patients. All patients underwent postoperative craniospinal irradiation (CSI) delivering a median craniospinal dose of 36 Gy with additional boosts to the posterior fossa up to 54 Gy. Median overall survival was 50 months for the total group whereas 36 months for pediatric age group and 70 months for adult group. Desmoplastic histology showed an improved outcome compared with other histologies with a median survival of 71 months compared with that of classical medulloblastoma histology being 36 months and other histologies shown a median survival of 34 only.
Conclusions:
Treatment of medulloblastoma with surgery and CSI yields long survival rates in both children and adults. Adult age group and desmoplastic histology were associated with a favorable outcome.
Keywords: Desmoplastic medulloblastoma, medulloblastoma, pediatric brain tumor
Introduction
Medulloblastoma is a common malignancy in the pediatric population, accounting for 25% of all childhood brain tumors.[1] Originally classified a glioma, medulloblastoma is referred to now as a primitive neuro-ectodermal tumor. It is a highly invasive tumor arising from the cerebellum with tendency to disseminate throughout the central nervous system (CNS) early in its course. The median age of diagnosis is 5 years, with 80% of cases being diagnosed in the first 15 years. A retrospective analysis of our operated series of medulloblastoma was carried out to study the various clinicoradiological features and overall surgical outcome. An attempt was made to identify predictors of poor outcome and to assess patterns of relapse.
Materials and Methods
Fifty-three patients with histological confirmed medulloblastoma cases were treated at our institute of between 2006 and 2012 were included in the study. Demographic variables, clinical variables, radiological findings and treatment details with respect to age, sex, signs and symptoms, location of tumor, extent of surgical resection, histopathology type, radiotherapy (RT) dose, follow-up period and outcomes were recorded.
Statistical analysis
Descriptive and inferential statistical analysis has been carried out in this study. Results on continuous measurements are presented on mean ± standard deviation (min-max) and results on categorical measurements are presented in number (%). Significance is assessed at 5% level of significance. The following assumptions on data is made, assumptions: (1) Dependent variables should be normally distributed, (2) Samples drawn from the population should be random, cases of the samples should be independent. Student's t-test (two-tailed, independent) has been used to find the significance of study parameters on a continuous scale between two groups (intergroup analysis) on metric parameters. Chi-square/Fisher exact test has been used to find the significance of study parameters on the categorical scale between two or more groups. Kaplan–Meier function was used to find the significance of survival in months according to age. Significant figures + suggestive significance (0.05< P < 0.10), *moderately significant (0.01< P ≤ 0.05), **strongly significant (P ≤ 0.01). Statistical software: The statistical software namely SAS 9.2, SPSS version 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0 and R environment ver.2.11.1 were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs, tables etc.[2,3,4,5]
Results
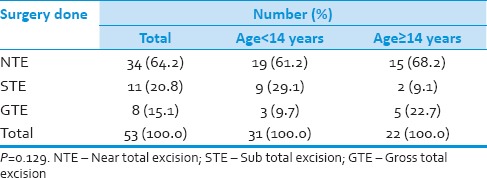
Thirty-one (58.5%) patients were pediatric (<14 years), and 22 (41.5%) patients were adults (≥14 years). Duration of symptoms were <3 months in 19 (36%) and more than 3 months in 34 (64%) patients. Vomiting was present in 47 (90%) cases, 23 (42%) had visual blurring and 23 (40%) presented with diplopia. Cerebellar symptoms in the form of gait unsteadiness were present in 36 (69%). History of seizures or loss of consciousness was present in 12 (23%) patients. Cranial nerve palsies were seen in 23 (42%) patients and 21 patients (40%) had nystagmus. While 6 patients presented with hemiparesis, cerebellar signs were observed in 48 patients. All patients underwent preoperative magnetic resonance imaging (MRI) scan of head. The postoperative analysis however was only on the basis of computed tomography scan. MRI was done in few patients postoperatively, but all the films could not be retrieved and hence was not included in the study. The tumor was located in midline in 37 patients and periphery in 17 patients. Calcification was observed in 10 cases (18.8%). MRI showed hydrocephalus in 42 cases (79%). MRI evidence of brainstem infiltration was present in five cases (9.4%). All patients underwent surgical excision of lesion. Tumor resection was performed in all patients and gross total resection in 8 (15%) patients, near total resection in 34 (64%) patients and subtotal resection in 11 (21%) patients. Thirty-two patients (60%) required external ventricular drain in the perioperative period. Histopathological examination showed classical medulloblastoma in 33 cases (62.3%), while desmoplastic variant was observed in 16 (30%) cases. Other histopathological variants include anaplastic large cell variant in two cases (3.8%) and nodular variant in two cases (3.8%). Operative complications included gait unsteadiness in 15 cases (19%).
All patients underwent postoperative craniospinal irradiation (CSI) delivering a median craniospinal dose of 36 Gy with additional boosts to the posterior fossa up to 54 Gy and adjuvant chemotherapy. On follow-up one patient was developed spinal metastasis. After detection of spinal metastasis this patient subsequently was subjected to chemotherapy. A comparison between the adult and pediatric population is given in [Tables 1–6]. The following variables were assessed by bivariate analysis for adult and pediatric study groups: Age, sex, duration of symptoms, location of the lesion, brainstem invasion, extent of surgical excision of the tumor, histological subtype.
Table 1.
Age distribution of patients studied
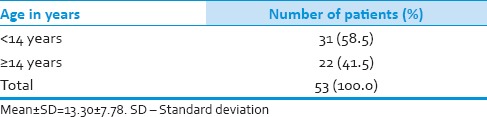
Table 6.
Postoperative HPE
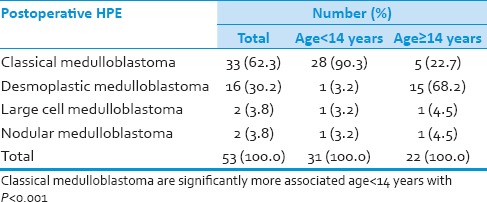
Table 2.
Gender distribution of patients studied
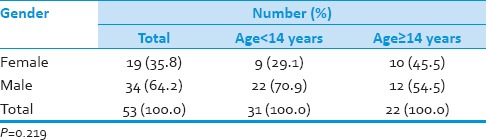
Table 3.
Duration of symptoms
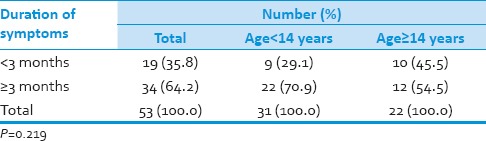
Table 4.
Lesion site involved of patients studied
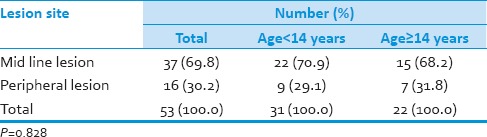
Table 5.
Surgery done
Median overall survival was 50 months for the total group whereas 36 months for pediatric age group and 70 months for adult group [Figure 1, Table 7 and 8]. Desmoplastic histology showed an improved outcome compared to other histologies with median survival 71 months compared to classical medulloblastoma histology median survival 36 and other histologies median survival 34 months [Figure 2]. Other factors include durations of symptoms, sex, location of the tumor and extent of surgical resection did not have any statistically significant effect on the outcome in our series.[6,7,8,9] The effects of RT in outcome were difficult to assess as all the patients received the same dose.
Figure 1.
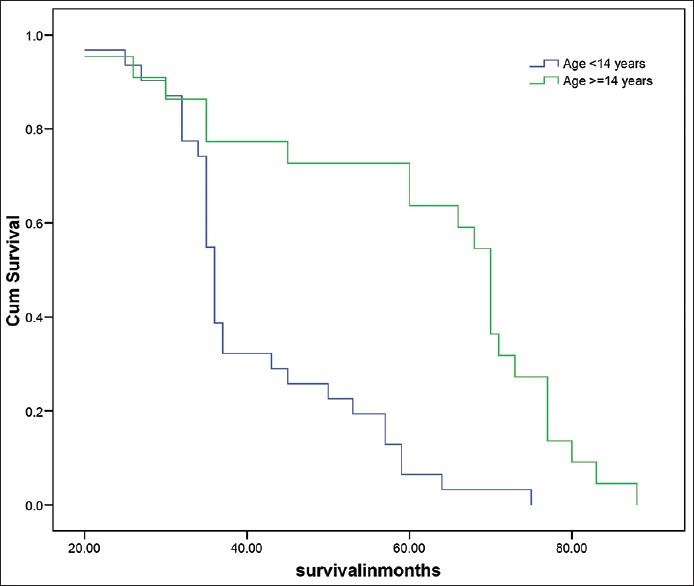
Survival analysis according to age groups
Table 7.
Survival in months**
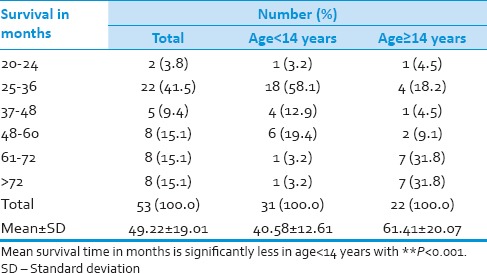
Table 8.
Survival analysis
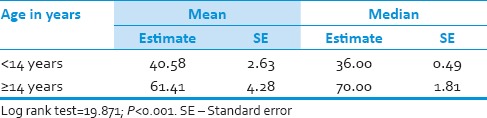
Figure 2.
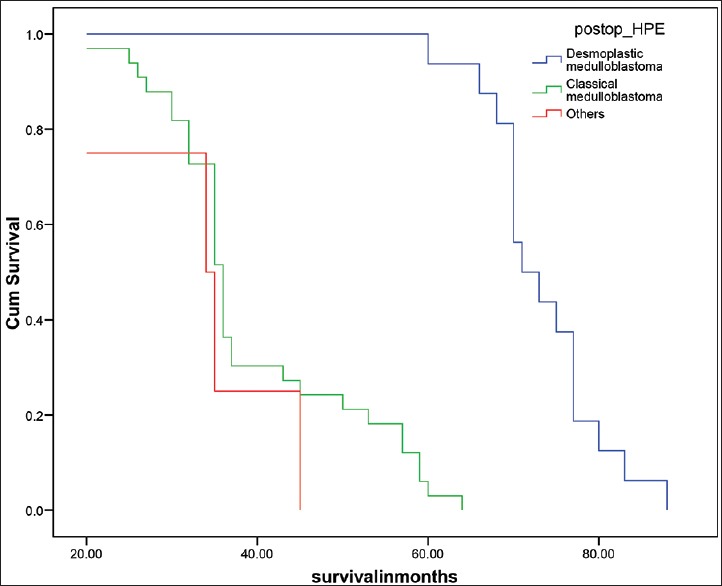
Survival analysis according to Histology
Discussion
Medulloblastoma is the most common malignant brain tumor of childhood, accounting for 20–25% of pediatric CNS neoplasms. Medulloblastomas are undifferentiated embryonal neuroepithelial tumors of the cerebellum[10] arising predominantly from the cerebellar vermis and primarily affecting children in the first decade of life. The cell of origin and the exact histological classification of this highly malignant tumor are still controversial.[11]
Although the majority cases occur as sporadic cases, hereditary conditions have been associated with medulloblastoma, including (1) gorlin syndrome (nevoid basal cell carcinoma syndrome) (2) blue rubber-bleb nevus syndrome, (3) Turcot syndrome (e.g. glioma polyposis syndrome) and (4) Rubenstein-Taybi syndrome. The most frequent cytogenetic abnormality in sporadic medulloblastoma is an isochromosome 17q[12] of tumors analyzed, 40–50% have a deletion of the short arm of chromosome 17, implicating the presence of a tumour suppressor gene that maps to 17p, which is distinct from the p53 gene. These p53 mutations may be important in the pathogenesis of human medulloblastoma.[13] Gender is a debatable prognostic factor in pediatric series. Weil et al.[14] and Prados et al.[15] found female gender to be a significant favorable prognostic factor in medulloblastoma. Sex did not reveal any bearing on the outcome in our series. Complete resection should be performed if possible as several studies have correlated outcome with extent of resection and amount of residual tumor.[16] In our pediatric population, near total to near total excision of the lesion could be performed in 74.6%. We noted a lesser incidence of the brain stem infiltration in our series, unlike Packer RJ et al.[17] who found 36% of patients with radiological evidence of brainstem infiltration. Brain stem infiltration could be one of the major reasons of a relatively high incidence of subtotal excision in our series. However, infiltration of the brain stem glioma not effected survival in our series. Histopathological examination of tumor in the present study showed clear predominance of classical medulloblastoma in children. Available literature suggests desmoplastic histological variant to be of favorable prognostic significance.[18,19] Our series too could establish a statistically significant correlation with a better outcome in children having desmoplastic medulloblastoma. Transcriptional profiling studies of medulloblastoma cohorts from several research groups around the globe have suggested the existence of multiple distinct molecular subgroups that differ in their demographics, transcriptomes, somatic genetic events, and clinical outcomes. Variations in the number, composition, and nature of the subgroups between studies brought about a consensus conference in Boston in the fall of 2010. Discussants at the conference came to a consensus that the evidence supported the existence of four main subgroups of medulloblastoma (Wnt, Shh, Group 3, and Group 4). Participants outlined the demographic, transcriptional, genetic, and clinical differences between the four subgroups. While it is anticipated that the molecular classification of medulloblastoma will continue to evolve and diversify in the future as larger cohorts are studied at greater depth.[20]
Standard therapy consists of total surgical removal of the tumor followed by radiation to the entire craniospinal axis with boost to both the primary tumor size and focal CNS.
In contrast to a report by Yock et al.[21] we did not find an influence of the interval between surgery and start of RT on survival. Certain studies have shown a correlation between improved posterior fossa control and shorter periods for the completion of RT.[22] As we followed a uniform protocol of RT, we failed to observe any difference in the outcome related either to the interval between surgery and RT or to the total duration of RT. Recently, adjuvant chemotherapy also has been shown to be beneficial in children with medulloblastoma. The efficacy of chemotherapy in the treatment of medulloblastoma has been assessed previously in two large randomized trials conducted by the International Society of Pediatric Oncology.[21] The addition of chemotherapy for high-risk patients seems to improve their survival and has contributed better outcome even in average-risk patients.
Conclusions
Treatment of medulloblastoma with surgery and CSI yields long survival rates in both children and adults. In our series of medulloblastomas, among the classical predictors of the outcome age and histological subtype were found to have any significant correlation on outcome. We observed a favorable outcome in adults and desmoplastic medulloblastoma variant. Classical outcome determinants like extent of excision and residual tumor did not show any correlation with outcome in our series.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rorke LB. The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumors. J Neuropathol Exp Neurol. 1983;42:1–15. [PubMed] [Google Scholar]
- 2.Rosner B. 5th ed. Duxbury; 2000. Fundamentals of Biostatistics; pp. 80–240. [Google Scholar]
- 3.Riffenburg RH. 2nd ed. Academic Press; 2005. Statistics in Medicine; pp. 85–125. [Google Scholar]
- 4.Sunder Rao PS, Richard J. 4th ed. New Delhi: Prentice Hall of India; 2006. An Introduction to Biostatistics. A Manual for Students in Health Sciences; pp. 86–160. [Google Scholar]
- 5.Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5:7–13. doi: 10.4103/0974-1208.97779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Rosner B. 5th ed. Pacific Grove, CA: Duxbury; 2000. Fundamentals of Biostatistics; pp. 80–240. [Google Scholar]
- 7.Riffenburg RH. 2nd ed. Waltham, Massachussetts, United States of America: Academic Press; 2005. Statistics in Medicine; pp. 85–125. [Google Scholar]
- 8.Sunder Rao PSS, Richard J. 4th ed. New Delhi: Prentice hall of India; 2006. An Introduction to Biostatistics, A manual for students in health sciences; pp. 86–160. [Google Scholar]
- 9.Suresh KP, Chandrasekhar S. Sample Size estimation and Power analysis for Clinical research studies. Journal Human Reproduction Science. 2012;5:7–13. doi: 10.4103/0974-1208.97779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Jakacki RI. Treatment strategies for high-risk medulloblastoma and supratentorial primitive neuroectodermal tumors. Review of the literature. J Neurosurg. 2005;102:44–52. doi: 10.3171/ped.2005.102.1.0044. [DOI] [PubMed] [Google Scholar]
- 11.Tomita T. Youmans JR, editor. Medulloblastomas. Neurological Surgery. 1996;4:2570–92. [Google Scholar]
- 12.James TR, Harold H. A critical review of medulloblastoma: From a difficult past to a promising future. Neurosurg Q. 1991;1:54. [Google Scholar]
- 13.Chan AW, Tarbell NJ, Black PM, Louis DN, Frosch MP, Ancukiewicz M, et al. Adult medulloblastoma: Prognostic factors and patterns of relapse. Neurosurgery. 2000;47:623–31. doi: 10.1097/00006123-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Weil MD, Lamborn K, Edwards MS, Wara WM. Influence of a child's sex on medulloblastoma outcome. JAMA. 1998;279:1474–6. doi: 10.1001/jama.279.18.1474. [DOI] [PubMed] [Google Scholar]
- 15.Prados MD, Warnick RE, Wara WM, Larson DA, Lamborn K, Wilson CB. Medulloblastoma in adults. Int J Radiat Oncol Biol Phys. 1995;32:1145–52. doi: 10.1016/0360-3016(94)00476-2. [DOI] [PubMed] [Google Scholar]
- 16.Chatty EM, Earle KM. Medulloblastoma. A report of 201 cases with emphasis on the relationship of histologic variants to survival. Cancer. 1971;28:977–83. doi: 10.1002/1097-0142(1971)28:4<977::aid-cncr2820280422>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Packer RJ, Sutton LN, Rorke LB, Littman PA, Sposto R, Rosenstock JG, et al. Prognostic importance of cellular differentiation in medulloblastoma of childhood. J Neurosurg. 1984;61:296–301. doi: 10.3171/jns.1984.61.2.0296. [DOI] [PubMed] [Google Scholar]
- 18.Gajjar A, Sanford RA, Bhargava R, Heideman R, Walter A, Li Y, et al. Medulloblastoma with brain stem involvement: The impact of gross total resection on outcome. Pediatr Neurosurg. 1996;25:182–7. doi: 10.1159/000121121. [DOI] [PubMed] [Google Scholar]
- 19.Skolyszewski J, Glinski B. Results of postoperative irradiation of group (POG 9031) Int J Radiat Oncol Biol Phys. 2001;51:120–1. doi: 10.1016/0360-3016(89)90346-5. [DOI] [PubMed] [Google Scholar]
- 20.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yock TI, Friedman H, Kun L, Kepner J, Barnes P, Tarbell NJ. Response to pre-radiation chemotherapy is predictive of improved survival in high risk medulloblastoma: Results from the paediatric oncology group (POG 9031) Int J Radiat Oncol Biol Phys. 2001;51:120–1. [Google Scholar]
- 22.Prados MD, Wara W, Edwards MS, Ater J, Rabbit J, Lamborn K, et al. Treatment of high-risk medulloblastoma and other primitive neuroectodermal tumors with reduced dose craniospinal radiation therapy and multi-agent nitrosourea-based chemotherapy. Pediatr Neurosurg. 1996;25:174–81. doi: 10.1159/000121120. [DOI] [PubMed] [Google Scholar]


