Abstract
Objective and Background:
Unruptured aneurysm surgery is a challenge to all vascular neurosurgeons as the patient is asymptomatic and no even slight neurological deficits should be expected postoperatively. To this aim, multi-modality checking of the vessels during the surgery is highly recommended to assure of the patency of the parent and perforator arteries next to an aneurysm. In this paper, we present our experience in the last 1.5 years with emphasis on the role of endoscope assisted microsurgery.
Methods:
One hundred and seventy-five patients with unruptured intracranial aneurysms were operated in our institute in the last 1½ years. All patients underwent endoscope assisted microsurgery with pre- and post-clipping indocyanine green angiography. In selected cases, motor evoked potential monitoring was implemented.
Results:
No mortality was observed in this period, and only 6 patients (3.4%) suffered new permanent neurological deficits postoperatively. Our illustrative cases show how endoscopy may help the surgeon to visualize hidden vessels behind and medial to an aneurysm.
Conclusions:
Our results indicated that multi-modality monitoring during unruptured aneurysm surgeries is associated with excellent outcome. Endoscope is able to show blind corners during aneurysm surgery which cannot be routinely observed with microscope and its application in aneurysm surgery assists the surgeon to make certain of complete neck clipping and preservation of perforating arteries around the aneurysm.
Keywords: Brain, clipping, endoscopy, monitoring, unruptured aneurysm
Introduction
Published surveys show that unruptured aneurysms can be a real threat if left untreated[1] and recent reports indicate a dramatic increase in the number of unruptured aneurysms being treated in the last years with favorable surgical or interventional outcome.[2,3] When treating an incidental lesion with probable detrimental impact in the long-term, the outcome of our therapeutic strategies must outweigh the natural course of the disease. Hence, we should take every possible measure to reduce the morbidity caused by our therapy. In treating unruptured aneurysms, preservation of adjacent perforating arteries and maintaining blood flow in the parent vessel are of paramount importance. Various monitoring strategies are applied during an aneurysm surgery to perform a safe clipping such as recording motor evoked potential (MEP), intra-operative angiography, Doppler ultrasonography, and endoscopy.
Motor evoked potential monitoring during aneurysm surgery has been advocated and showed to predict postoperative neurological function.[4] Changes in MEPs should be dealt with appropriately during temporary clipping of the parent artery or after permanent clipping of the aneurysm. Indocyanine green (ICG) video angiography (VA) is an alternative to the conventional intra-operative angiographies to evaluate the patency of the vessels and complete clipping. It has been previously reported how observing ICG under the microscope may change surgical planning to adjust an aneurysm clip.[5] Sometimes, when the ICG-VA is repeated in a short time, the ICG has not washed out through the aneurysm and the clipped aneurysm also appears bright under the infra-red camera. We usually perform blood flow analysis with FLOW 800 software (Carl Zeiss, Oberkochen, Germany) in the clipped aneurysm to make sure there is no flow inside the sac after observing an almost constant intensity curve. Hence, FLOW 800 software is considered as a valuable addition to the monitoring armamentarium during aneurysm surgery.[5] Endoscopic re-checking of the aneurysm neck and the clip is valuable in this scenario as well.
Unfortunately, ICG-VA visualization is limited to the vessels directly observed under microscope. To have a better view of the vessels hidden beyond the aneurysm, some authors have utilized Yasargil mirror in combination with ICG-VA.[6] Another alternative to evaluate the hidden vessels is endoscopy. So far, endoscope-assisted microsurgery of aneurysms has become a routine practice in many centers, but only small case series have been published addressing its value in neurovascular operations.[7,8,9] In one of these reports, 64 patients were included while 30 of them were ruptured and the rest unruptured aneurysms.[10] To our best knowledge, the present study is the largest case series published so far which pertains to endoscope-assisted microsurgery of unruptured aneurysms.
Methods
A retrospective analysis of all patients with unruptured cerebral aneurysm operated in our hospital in the last 1½ years was performed. Patients’ demographic data, site of the aneurysm, operation notes, and postoperative morbidities or mortalities were reviewed and recorded. According to our department protocol, all patients diagnosed with unruptured cerebral aneurysms who are candidate for treatment, will be discussed with neuro-intervention team of our department. If not considered a good candidate for intervention, the patient will be offered surgery. We use reconstructed computed tomography angiography (CTA) routinely for surgical planning and in case of any ambiguity request a digital subtraction angiography.[11] In our hospital, all neurovascular surgeries are performed with OPMI Pentero Microscope (Carl Zeiss, Oberkochen, Germany) with infra-red 800 camera equipped with FLOW 800 software (Carl Zeiss, Oberkochen, Germany). The camera allows us real-time intra-operative ICG-VA while the latter software provides hemodynamic information about blood flow. Upon exposure of aneurysm, we perform ICG-VA to clarify the aneurysm and its relation to all the surrounding vessels. Furthermore, to evaluate any perforating artery or other structures hidden behind the aneurysm we introduce a rigid endoscope (Machida, Japan) under microscopic guidance. With this technique, we check for the estimated final location of the aneurysm clip tips to be away from any critical structure. Technical nuances of aneurysm surgery have been discussed elsewhere in great detail.[12] After applying the aneurysm clip (s), the vessels and the aneurysm are re-checked with ICG-VA. Whenever the distal blood flow is in question, blood flow measurements with Doppler ultrasound (DVM 4300, Hadeco, Japan) and FLOW 800 software are made. Once again, the endoscope is used to check for the final position of the blades and their relations to other structure and also to make certain that the aneurysm neck is completely obliterated. Based on the location of the aneurysm and probable risks, other monitoring systems such as MEP may be implemented. We do not use MEP monitoring for all patients although it has been advocated by some authors.[4] Postoperatively, patients are kept for at least one night in neurosurgical intensive care unit under cardiac and neurological monitoring where any new neurological deficit is evaluated by CTA and/or magnetic resonance imaging (MRI) according to which necessary interventions are performed. Furthermore, CTA and CT-perfusion studies will be performed on 7th day postoperatively as a routine to evaluate any subclinical vasospasm and make sure of the proper placement of the clip.
Results
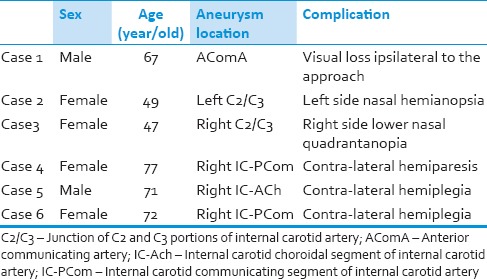
From January 2012 to June 2013, 175 patients with single unruptured aneurysm were operated by the senior author in our hospital. The mean age at diagnosis was 62.0 years (range of 24–79 years). There were 115 female and 60 male patients (female: Male ratio 1.92:1). Distribution of the aneurysms based on their site is shown in Table 1. The most common location of aneurysms was middle cerebral artery (MCA) followed by internal cerebral artery (ICA) and anterior cerebral artery (ACA). We observed no postoperative mortalities, but 6 patients suffered permanent and another ten transient morbidities during this period [Table 2]. Data for the patients who suffered permanent deficits are depicted in Table 3. All transient morbidities in 2013 were postoperative seizures fully controlled with anti-epileptic drugs. Postoperative CTAs did not reveal any neck remnant (unless deliberately) and no patients required further interventional or surgical treatment.
Table 1.
Distribution of the aneurysms according to their site
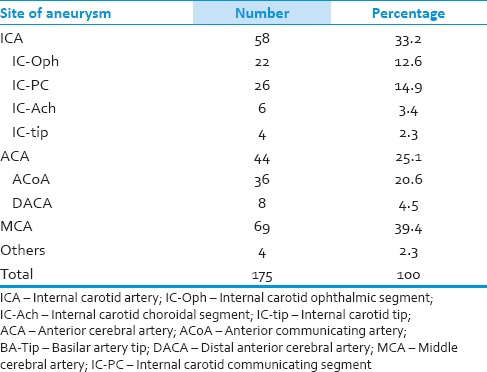
Table 2.
Morbidity and mortality of the patients with unruptured aneurysm operated in our hospital in the last 1.5 years
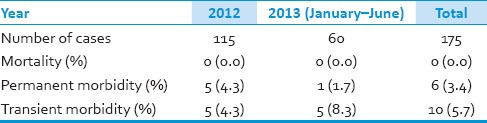
Table 3.
Characteristics of six patients with permanent complications in our case series
Discussion
Surgical outcome
Our results are in concordance with the recently published case series reporting 0.0–1.6% mortality and 6.2–9.4% morbidity for unruptured aneurysm clipping.[13,14,15] In their recent review, Bacigaluppi et al. reported that a comprehensive intraoperative monitoring during aneurysm surgery reduces ischemia-related morbidities.[16] We also believe that multi-modality intra-operative monitoring of the vascular patency before and after clipping is crucial for a safe aneurysm surgery. This is especially true of unruptured aneurysms where only a narrow margin of error is acceptable. Though endoscope is used commonly in many centers to check for a safe aneurysm clipping, our case series as the largest one addressing this issue, manifested its benefits in unruptured aneurysm surgeries with good outcome.
Advantages of endoscope assisted microsurgery
The main goals of a vascular surgeon while performing aneurysm surgery are to achieve a complete obliteration of the aneurysm while preserving normal neural and vascular elements. Endoscopy assists the surgeon to achieve both these goals [Figure 1] and its function turns vital in certain aneurysm locations when the aneurysm neck is usually surrounded by a bunch of perforators. These locations according to our experience and the literature include but not limited to the ICA-choroidal segment, ICA-communicating segment, MCA-M1, and MCA bifurcation. In one recent publication, the percentage of the different aneurysms with perforators originating from the neck was as follows: Basilar 7%, ICA bifurcation 17%, MCA main stem 12%, and anterior communicating artery (AComA) 11%.[17] Although the author concluded that perforators originate infrequently from the aneurysm neck, these proportions are still so high that any negligence during the operation may increase morbidity of the patients dramatically.
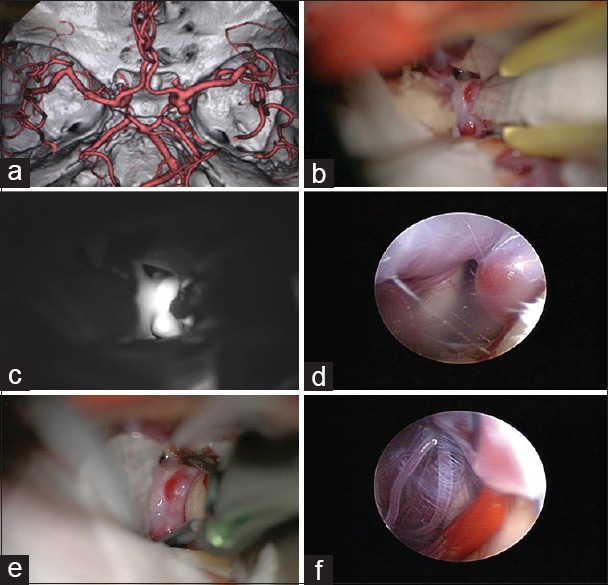
Figure 1.
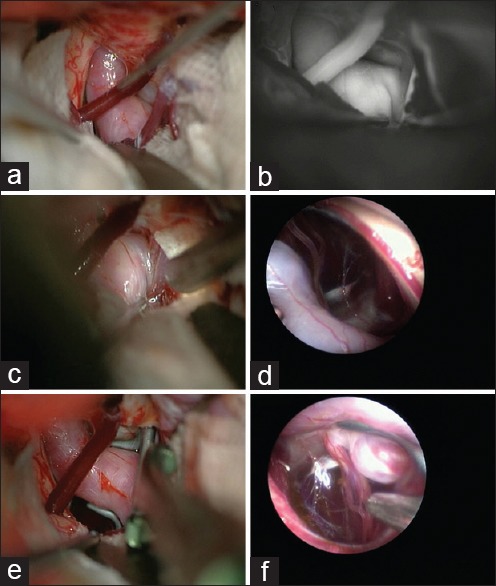
An internal carotid artery (ICA) postcommunicating segment aneurysm with the neck medially located. (a) Despite gentle retraction of the brain, the aneurysm neck and the surrounding vessels cannot be visualized well. (b) Some slight manipulation of the ICA with a suction tip to open a corridor is practiced but still indocyanine green angiography is not able to show the perforating arteries. (c) An endoscope is introduced to the field under microscopic vigilance. (d) Neck of the aneurysm as well as the medially located perforators come into the endoscopic view. (e) An angled fenestrated clip is inserted in parallel to the ICA to obstruct the medially located neck. (f) Endosopic view after insertion of the aneurysm clip to confirm complete obliteration of the neck without engulfment of the perforators
M1 aneurysms have specific anatomical proximities which increase the chance of injury during surgical or interventional treatments.[18] Aneurysms projecting superiorly are associated with lateral lenticulostriate artery (LSA) and frontal cortical branches while those projecting inferiorly accompany temporal cortical branches. Yeon et al. also showed that the closer an MCA-M1 aneurysm to the ICA bifurcation, the higher the chance of perforators injury.[19] In another report by Iwama et al., the authors underlined the importance of the LSAs while operating a superior wall type aneurysm at the MCA-M1.[20] Actually, we recommend and perform intraoperative MEP monitoring for all cases with this aneurysm location. Aneurysms in proximal MCA require special attention during localization and dissection of the aneurysm as some tiny perforators located at the posterior MCA wall and obscured by the temporal lobe may be damaged. In this scenario, endoscopy may come to help the surgeon with checking the final results after clipping.
Another location for the hidden perforators is the ventromedial wall of ICA when addressing posterior communicating artery (PComA) aneurysms particularly when the neck of the aneurysm extends medially [Figure 1a]. Wilson et al. reported their experience with the use of a Yarargil mirror during ICG-VA to elucidate medially located vessels in two cases with PComA aneurysms.[6] PComA and anterior choroidal artery (AChA) are sometimes elusive but must be clearly visualized before and after clipping of these aneurysms as their injury may lead to the grave situation.[21,22] Besides these two main branches by which a PComA aneurysm is flanked, there are important medially located perforators from PComA and ICA, which cannot be seen under microscope without significant retraction of the ICA and the aneurysm.[23,24] By introducing an endoscope into the optico-carotid or carotid-oculomotor windows, one may visualize all these perforators and their relations to the aneurysm neck before and after aneurysm clipping [Figure 1d and e].
Surgery of AChA and ICA bifurcation aneurysms is also associated with an increased chance of morbidity[25] which may be explained by their proximities to the early ACA-A1 and MCA-M1 perforating arteries. These locations are among the hot spots where endoscopy may assist the surgeon to identify perforators attached to the medial wall of the aneurysms and prevent morbidity.
Although the most frequent site of the aneurysm in our case series was MCA, none of these patients developed postoperative deficits. In fact, supraclinoid ICA aneurysms composed the majority of neurological deficits. This finding was in accordance with the previous literature reviewed above.
Current literature
The first report of endoscope guided clipping in aneurysm surgery was published by Fischer and Mustafa.[26] They used this method in 24 patients with 30 aneurysms and emphasized the benefits of endoscopy in improving the visualization of the pertinent anatomy. Taniguchi et al. reported benefits of endoscopy to clarify the anatomy in 81.5% of 44 cases operated for cerebral aneurysms.[7] Interestingly, in 16.7% of their cases, the anatomy could be recognized only after endoscopy leading to clip adjustment in 9.3% of the surgeries. Wang et al. performed endoscope-assisted microsurgery to treat 24 cases of ruptured aneurysms and found it very practical for providing the surgeon with good definition of surrounding structures such as cranial nerves, parent arteries, and perforators.[9] In another report by Profeta et al. 52 patients with 58 aneurysms were operated with applying endoscope-assisted microsurgery over a period of 3 years.[8] In four cases, endoscopy disclosed inappropriate clip placement requiring re-positioning or addition of an extra clip. The authors recommended endoscopy for certain aneurysm surgeries especially for those developed from the AComA. In another clinical study of endoscope assisted microsurgery of 50 cerebral aneurysms, the endoscopy showed incomplete clipping in two cases leading to additional clipping.[27]
Above-mentioned results should be interpreted cautiously: Usually studies evaluating a safety measure by performing an operation with and without a new tool (e.g., intra-operative MRI) in one session are biased in favor of the newly introduced device because the surgeon attempts to keep on the safe side by not exploring or removing the lesion without the safety device as he would do previously. This may improve the overall functional outcome but decreases intra-operatively measured goals before using the device (e.g., extent of tumor resection before performing intra-operative MRI). The ideal study for impact analysis is a prospective randomized controlled study, but as the technique we use here (i.e., endoscopy) is widely available, cost-effective, and with minimum harm to the patient it may be more ethical to rely on the results of such before-after studies.
Limitations and complications
Like any instrument, endoscopy has its limitations. Just confirming the correct placement of the blades is not always a proof of complete obliteration of the aneurysm. Conversely, sometimes even a partial clipping excludes the aneurysm from the circulation. Recent utilization of endoscopic ICG-VA may compensate for these shortcomings to some extent[28,29,30] because it shows not only the external anatomy of the vessels but also the blood flow inside them.
When doing endoscope assisted microsurgery, usually the chief surgeon has to perform the endoscopy under microscopic guidance to avoid any unwanted damage to the surrounding structures, and the assistant surgeon will look at the endoscopy monitor. To come up with a solution, some companies offered a picture-in-picture mode for their microscopes.[10,31] Furthermore, Hiramatsu et al. introduced a new endoscopic image display attached to the microscope eyepieces that allows the chief surgeon to watch both endoscopic and microscopic views almost simultaneously.[29] We would like to videotape the endoscopic images rather than use either of the mentioned methods. The endoscopy is monitored by the assistant, and on its completion, the chief surgeon reviews the video if needed.
In spite of normal monitoring after clipping, sometimes a postoperative neurological deficit happens. Distal emboli during the vascular manipulation, irreversible brain ischemia due to prolonged proximal closure or permanent damage of perforators due to a misplaced clip can be the underlying causes. However, we still do not know whether discovering these events (i.e., emboli or irreversible ischemia) intra-operatively improves the outcome. To overcome these obstacles, we use intraoperative MEP especially when there is a high risk of distal emboli (e.g., severe atherosclerosis of the parent artery) or the aneurysm is placed in proximity to numerous perforators (e.g., proximal MCA aneurysms). This is in concordance with the studies reporting more difficult clipping and worse outcome for atherosclerotic calcified aneurysms.[32,33] Vasospasm of a major or perforating artery can be another source of postoperative deficits despite a safe and uneventful clipping of the aneurysm [case 5 in Table 3] [Figure 2]. Although rare, but isolated symptomatic perforators vasospasm has been reported and it further signifies the importance of these vessels.[34] Current technology does not offer surgeons an opportunity to monitor and prevent arterial spastic events during the operation, and in fact, the vasospasm related infarction develops in the postoperative period in most cases. In spite of aggressive medical or interventional management, the functional outcome is usually poor in this situation.
Figure 2.
(a) Internal carotid artery choroidal segment aneurysm in 71-year-old man [case 5 in Table 3]. (b) Intra-operative view of the aneurysm and (c) the same view after fluorescence angiography. (d) Endoscopic view showing the perforators behind the aneurysm. (e) Clipping of the aneurysm and (f) postaneurysm clipping demonstrating perforators. The patient recovered fully from the anesthesia when 6 h later he developed contra-lateral incomplete hemiparesis which despite aggressive medical therapy turned into a complete hemiplegia after 36 h
Another pitfall with both ICG-VA and endoscopy is that observing a patent vessel does not necessarily equal adequate blood perfusion. When suspected, we prefer to use FLOW 800 or Doppler ultrasound to measure distal blood flow. Though some efforts have been made to measure tissue perfusion with FLOW 800,[35] its accuracy and reliability have not been proved so far.
Endoscope-assisted microsurgery may result in some complications as well, such as cerebral contusion or optic nerve damage.[7] The problem with endoscopy is that the surgeon may not view the structures superficial to the tip of the endoscope which may lead to inadvertent damage to the structures already passed by. This fact emphasizes the importance of a watchful monitoring of the endoscope lens under microscopic view.
Purely endoscopic approach to aneurysms
Although some authors have applied endoscopy for transcranial aneurysm dissection in addition to checking the safety of clipping,[36,37] we highly recommend microscope for dissecting the aneurysm: It gives us a three-dimensional view and high magnification which are indispensable advantages in aneurysm surgery. Furthermore, due to the probable complications discussed above, applying a purely endoscopic approach without microscopic monitoring might be perilous.
On the other hand, there are some reports of endoscopic endonasal approach to aneurysms.[38] We do not consider such approaches to be safe for several reasons. Intra-operative premature rupturing is always a concern even at the hands of experienced neurovascular surgeons.[8,15] Such a mishap may lead to a catastrophe in endonasal corridor as the intense bleeding obscures the view and securing a proximal control is very difficult if not impossible. Furthermore, current endoscopes do not provide surgeons with enough magnification to do microdissection around the aneurysm whilst it is an absolute necessity to dissect the perforators off the neck. Finally, though some progresses have been made in devising long instruments to perform trans-nasal surgeries, they are still less than perfect for microsurgical dissections.
We applied different monitoring tools to make a safe and secure aneurysm clipping as much as possible. Endoscopy demonstrates blind corners of the surgical field when different lenses are applied. Compared with ICG-VA, endoscopy can demonstrate the aspects that are concealed from our view normally. It is useful to show the position of clip and length of its blades, location of perforators before and after clipping, and the absence of the aneurysm neck remnant after clipping. As discussed above, each monitoring device has its advantages and shortcomings during aneurysm surgery and only a multi-modality approach help the surgeon reduce the chance of possible morbidities and have a favorable surgical outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Lin N, Cahill KS, Frerichs KU, Friedlander RM, Claus EB. Treatment of ruptured and unruptured cerebral aneurysms in the USA: A paradigm shift. J Neurointerv Surg. 2012;4:182–9. doi: 10.1136/jnis.2011.004978. [DOI] [PubMed] [Google Scholar]
- 2.Aydin Y, Cavusoglu H, Kahyaoglu O, Müslüman AM, Yilmaz A, Türkmenoglu ON, et al. Clip ligation of unruptured intracranial aneurysms: A prospective midterm outcome study. Acta Neurochir (Wien) 2012;154:1135–44. doi: 10.1007/s00701-012-1397-y. [DOI] [PubMed] [Google Scholar]
- 3.Choi SW, Ahn JS, Park JC, Kwon do H, Kwun BD, Kim CJ. Surgical treatment of unruptured intracranial middle cerebral artery aneurysms: Angiographic and clinical outcomes in 143 aneurysms. J Cerebrovasc Endovasc Neurosurg. 2012;14:289–94. doi: 10.7461/jcen.2012.14.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motoyama Y, Kawaguchi M, Yamada S, Nakagawa I, Nishimura F, Hironaka Y, et al. Evaluation of combined use of transcranial and direct cortical motor evoked potential monitoring during unruptured aneurysm surgery. Neurol Med Chir (Tokyo) 2011;51:15–22. doi: 10.2176/nmc.51.15. [DOI] [PubMed] [Google Scholar]
- 5.Oda J, Kato Y, Chen SF, Sodhiya P, Watabe T, Imizu S, et al. Intraoperative near-infrared indocyanine green-videoangiography (ICG-VA) and graphic analysis of fluorescence intensity in cerebral aneurysm surgery. J Clin Neurosci. 2011;18:1097–100. doi: 10.1016/j.jocn.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 6.Wilson J, Screven R, Volk J, Payner T. Use of a Yasargil mirror as an adjunct to indocyanine green angiography to evaluate the patency of elusive posterior communicating arteries during aneurysm clipping: Case report. Neurosurgery. 2012;71:195–7. doi: 10.1227/NEU.0b013e318257d0c6. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi M, Takimoto H, Yoshimine T, Shimada N, Miyao Y, Hirata M, et al. Application of a rigid endoscope to the microsurgical management of 54 cerebral aneurysms: Results in 48 patients. J Neurosurg. 1999;91:231–7. doi: 10.3171/jns.1999.91.2.0231. [DOI] [PubMed] [Google Scholar]
- 8.Profeta G, De Falco R, Ambrosio G, Profeta L. Endoscope-assisted microneurosurgery for anterior circulation aneurysms using the angle-type rigid endoscope over a 3-year period. Childs Nerv Syst. 2004;20:811–5. doi: 10.1007/s00381-004-0935-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang E, Yong NP, Ng I. Endoscopic assisted microneurosurgery for cerebral aneurysms. J Clin Neurosci. 2003;10:174–6. doi: 10.1016/s0967-5868(02)00320-x. [DOI] [PubMed] [Google Scholar]
- 10.Kato Y, Sano H, Nagahisa S, Iwata S, Yoshida K, Yamamoto K, et al. Endoscope-assisted microsurgery for cerebral aneurysms. Minim Invasive Neurosurg. 2000;43:91–7. doi: 10.1055/s-2000-12260. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Kato Y, Motoharu H, Sifang C, Junpei O, Takeya W, et al. An update on three-dimensional ct angiography in aneurysms: A useful modality for a neurosurgeon. Turk Neurosurg. 2013;23:304–11. doi: 10.5137/1019-5149.JTN.5958-12.0. [DOI] [PubMed] [Google Scholar]
- 12.Kato Y, Kumar A, Chen S. Surgical nuances of clipping after coiling: Looking beyond the international subarachnoid aneurysm trial. J Clin Neurosci. 2012;19:638–42. doi: 10.1016/j.jocn.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, et al. Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke. 2010;41:1471–6. doi: 10.1161/STROKEAHA.110.580647. [DOI] [PubMed] [Google Scholar]
- 14.Brinjikji W, Rabinstein AA, Lanzino G, Kallmes DF, Cloft HJ. Effect of age on outcomes of treatment of unruptured cerebral aneurysms: A study of the National Inpatient Sample 2001-2008. Stroke. 2011;42:1320–4. doi: 10.1161/STROKEAHA.110.607986. [DOI] [PubMed] [Google Scholar]
- 15.Sharma M, Brown B, Madhugiri V, Cuellar-Saenz H, Sonig A, Ambekar S, et al. Unruptured intracranial aneurysms: Comparison of perioperative complications, discharge disposition, outcome, and effect of calcification, between clipping and coiling: A single institution experience. Neurol India. 2013;61:270–6. doi: 10.4103/0028-3886.115067. [DOI] [PubMed] [Google Scholar]
- 16.Bacigaluppi S, Fontanella M, Manninen P, Ducati A, Tredici G, Gentili F. Monitoring techniques for prevention of procedure-related ischemic damage in aneurysm surgery. World Neurosurg. 2012;78:276–88. doi: 10.1016/j.wneu.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Pritz MB. Perforator and Secondary Branch Origin in Relation to the Neck of Saccular, Cerebral Bifurcation Aneurysms. World Neurosurg. 2014;82:726–32. doi: 10.1016/j.wneu.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 18.Cho YD, Lee WJ, Kim KM, Kang HS, Kim JE, Han MH. Endovascular coil embolization of middle cerebral artery aneurysms of the proximal (M1) segment. Neuroradiology. 2013;55:1097–102. doi: 10.1007/s00234-013-1190-5. [DOI] [PubMed] [Google Scholar]
- 19.Yeon JY, Kim JS, Hong SC. Angiographic characteristics of unruptured middle cerebral artery aneurysms predicting perforator injuries. Br J Neurosurg. 2011;25:497–502. doi: 10.3109/02688697.2010.535924. [DOI] [PubMed] [Google Scholar]
- 20.Iwama T, Yoshimura S, Kaku Y, Sakai N. Considerations in the surgical treatment of superior-wall type aneurysm at the proximal (M1) segment of the middle cerebral artery. Acta Neurochir (Wien) 2004;146:967–72. doi: 10.1007/s00701-004-0325-1. [DOI] [PubMed] [Google Scholar]
- 21.Abbie AA. The clinical significance of the anterior choroidal artery. Brain. 1933;56:233–46. [Google Scholar]
- 22.Rand RW, Brown WJ, Stern WE. Surgical occlusion of anterior choroidal arteries in parkinsonism; clinical and neuropathologic findings. Neurology. 1956;6:390–401. doi: 10.1212/wnl.6.6.390. [DOI] [PubMed] [Google Scholar]
- 23.Saeki N, Rhoton AL., Jr Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg. 1977;46:563–78. doi: 10.3171/jns.1977.46.5.0563. [DOI] [PubMed] [Google Scholar]
- 24.Gibo H, Lenkey C, Rhoton AL., Jr Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55:560–74. doi: 10.3171/jns.1981.55.4.0560. [DOI] [PubMed] [Google Scholar]
- 25.Houkin K, Ito M, Miyamoto M, Hokari M, Kazumata K, Nakayama N, et al. Systematic review of complications for proper informed consent. Surgery for unruptured internal carotid artery aneurysm. No Shinkei Geka. 2012;40:365–75. [PubMed] [Google Scholar]
- 26.Fischer J, Mustafa H. Endoscopic-guided clipping of cerebral aneurysms. Br J Neurosurg. 1994;8:559–65. doi: 10.3109/02688699409002948. [DOI] [PubMed] [Google Scholar]
- 27.Takaishi Y, Yamashita H, Tamaki N. Cadaveric and clinical study of endoscope-assisted microneurosurgery for cerebral aneurysms using angle-type rigid endoscope. Kobe J Med Sci. 2002;48:1–11. [PubMed] [Google Scholar]
- 28.Nishiyama Y, Kinouchi H, Senbokuya N, Kato T, Kanemaru K, Yoshioka H, et al. Endoscopic indocyanine green video angiography in aneurysm surgery: An innovative method for intraoperative assessment of blood flow in vasculature hidden from microscopic view. J Neurosurg. 2012;117:302–8. doi: 10.3171/2012.5.JNS112300. [DOI] [PubMed] [Google Scholar]
- 29.Hiramatsu K, Inui T, Okada M, Takeshima T, Mishima H, Sakaki T, et al. New device for endoscopic image display during microsurgical clipping of cerebral aneurysms – Technical note. Neurol Med Chir (Tokyo) 2005;45:487–90. doi: 10.2176/nmc.45.487. [DOI] [PubMed] [Google Scholar]
- 30.Bruneau M, Appelboom G, Rynkowski M, Van Cutsem N, Mine B, De Witte O. Endoscope-integrated ICG technology: First application during intracranial aneurysm surgery. Neurosurg Rev. 2013;36:77–84. doi: 10.1007/s10143-012-0419-9. [DOI] [PubMed] [Google Scholar]
- 31.Kalavakonda C, Sekhar LN, Ramachandran P, Hechl P. Endoscope- assisted microsurgery for intracranial aneurysms. Neurosurgery. 2002;51:1119–26. doi: 10.1097/00006123-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Szelényi A, Beck J, Strametz R, Blasel S, Oszvald A, Raabe A, et al. Is the surgical repair of unruptured atherosclerotic aneurysms at a higher risk of intraoperative ischemia? Clin Neurol Neurosurg. 2011;113:129–35. doi: 10.1016/j.clineuro.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia S, Sekula RF, Quigley MR, Williams R, Ku A. Role of calcification in the outcomes of treated, unruptured, intracerebral aneurysms. Acta Neurochir (Wien) 2011;153:905–11. doi: 10.1007/s00701-010-0846-8. [DOI] [PubMed] [Google Scholar]
- 34.Salunke P, Gupta SK. Symptomatic bilateral isolated perforator infarction following aneurysmal subarachnoid hemorrhage. J Neurosci Rural Pract. 2013;4:45–7. doi: 10.4103/0976-3147.105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamp MA, Slotty P, Turowski B, Etminan N, Steiger HJ, Hänggi D, et al. Microscope-integrated quantitative analysis of intraoperative indocyanine green fluorescence angiography for blood flow assessment: First experience in 30 patients. Neurosurgery. 2012;70:65–73. doi: 10.1227/NEU.0b013e31822f7d7c. [DOI] [PubMed] [Google Scholar]
- 36.Perneczky A, Boecher-Schwarz HG. Endoscope-assisted microsurgery for cerebral aneurysms. Neurol Med Chir (Tokyo) 1998;38(Suppl):33–4. doi: 10.2176/nmc.38.suppl_33. [DOI] [PubMed] [Google Scholar]
- 37.Xia X, Ramanathan M, Orr BA, Salmasi V, Salvatori R, Reh DD, et al. Expanded endonasal endoscopic approach for resection of a growth hormone-secreting pituitary macroadenoma coexistent with a cavernous carotid artery aneurysm. J Clin Neurosci. 2012;19:1437–41. doi: 10.1016/j.jocn.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 38.Germanwala AV, Zanation AM. Endoscopic endonasal approach for clipping of ruptured and unruptured paraclinoid cerebral aneurysms: Case report. Neurosurgery. 2011;68:234–9. doi: 10.1227/NEU.0b013e318207b684. [DOI] [PubMed] [Google Scholar]


