Abstract
An 11-year-old boy presented with holocranial headache associated with vomiting and heaviness in right paranasal region. On examination, he had left sided upper motor neuron type facial palsy along with a subtle ipsilateral hemiparesis (grade 4/5 Medical Research Council). Magnetic resonance imaging brain revealed a contrast enhancing large well-defined extra-axial mass lesion in right middle cranial fossa with extension into the infratemporal fossa. The mass was excised in two stages. Histopathology was suggestive of clear cell meningioma (CCM). Supratentorial pediatric CCM is a rare entity. The case is discussed along with review of literature on this rare clinico-pathological entity.
Keywords: Brain tumor, clear cell meningioma, meningioma
Introduction
CCM is an uncommon histological variant of meningioma and have been classified by WHO as Grade II neoplasm. These tumors behave more aggressively than Grade I meningiomas, irrespective of their location and degree of excision. Most CCMs are seen involving the spine and posterior fossa and the supratentorial location is relatively uncommon.[1,2,3] CCM involving the supra tentorial area in children is extremely uncommon and to the best of our knowledge, only 5 such cases have been reported till date.[1,2,4,5,6] In this report, we present a case of CCM in the temporal base with extracranial extension in a child. Apart from the case, a brief review of the literature is also presented.
Case Report
An 11-year-old boy presented with holocranial headache associated with vomiting for the last 1-month. He also complained of heaviness in right paranasal region for the last 10 days. On examination, he was conscious, oriented with intact higher mental functions. There was left upper motor neuron type of facial palsy along with ipsilateral subtle hemiparesis (grade 4/5 Medical Research Council). Magnetic resonance imaging (MRI) of the brain revealed a large well-defined extra-axial mass lesion in the right middle cranial fossa with extension into the infratemporal fossa. The lesion was isointense on T1 and T2-weighted images and was heterogeneously enhancing in postgadolinium scan. There was perilesional edema causing mass effect in the form of effacement of ipsilateral lateral ventricle and midline shift [Figure 1a–c]. He was planned for surgical excision of the tumor in two stages. In the first stage, intracranial tumor was excised with right fronto-temporal craniotomy with zygomatic osteotomy approach. Intraoperatively the tumor was extra-axial, greyish pink and highly vascular. The tumor was involving the dura of middle fossa base through which it was extending into the infratemporal fossa. The intracranial part of the tumor was excised completely at the first setting. The infratemporal part was removed at a later stage by trans facial trans maxillary approach. Postoperative MRI brain revealed no residual tumor [Figure 2a and b]. Histopathological examination showed tumor dispersed in diffuse sheets and in nests separated by thick fibrovascular septa. The tumor cells were round to polygonal in shape, had round nucleus, dispersed nuclear chromatin, inconspicuous nucleoli and moderate amount of clear cytoplasm, entrapped bony trabeculae were also seen in between the tumor cells with occasional mitotic activity. Tumor cells were positive for vimentin, S-100 and focally positive for epithelial membrane antigen. On imunohistochemistry, Ki-67 index was 7% suggestive of CCM (aggressive variant) [Figure 3a and b]. Following surgery weakness on the left side improved. In view of complete excision, no radiotherapy was advised, and patient was kept in close follow-up. At follow-up of 9 months patient, was doing well.
Figure 1.
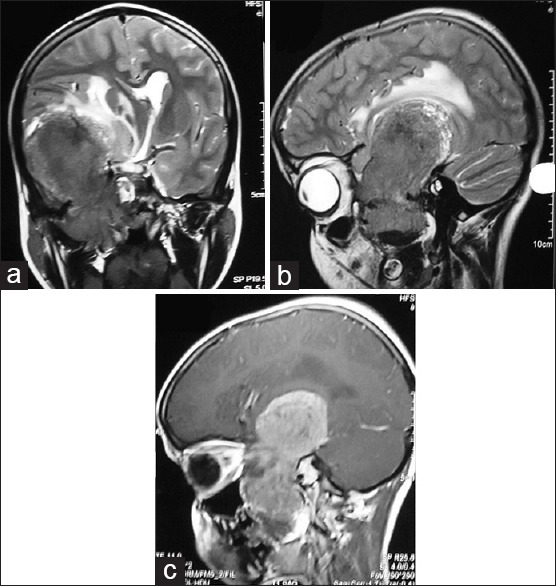
Magnetic resonance imaging brain: T2-weighted coronal section (a), sagittal section (b) and T1-weighted contrast sagittal section (c), showing T2-weighted hyperintense and heterogeneously contrast enhancing, well defined extra-axial mass lesion in right middle cranial fossa with extension into the infratemporal fossa
Figure 2.
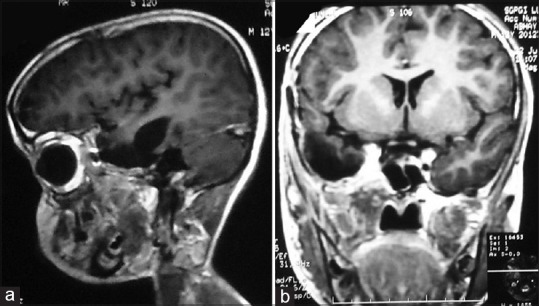
Postoperative contrast magnetic resonance imaging brain sagittal (a) and coronal image (b) showing complete excision of tumor
Figure 3.
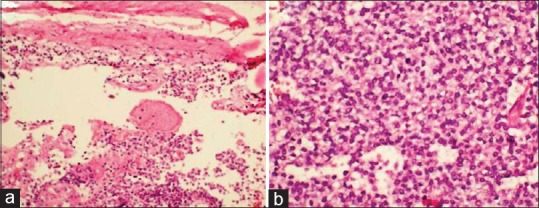
H and E stained sections showing tumor arising from meninges (×100) (a). Cells displaying round to polygonal cells, central round nucleus, dispersed nuclear chromatin, inconspicuous nucleoli and moderate amount of clear cytoplasm (×400) (b)
Discussion
Clear cell meningioma is recognized as WHO grade II meningioma. Grade II meningiomas are intermediate grades as far as aggressive clinical behavior is concerned. These tumors may recur, spread locally, and even metastasize despite their rather innocuous histological appearance. CCM is reported to occur more frequently in the pediatric age group.[2,7,8] However, usual sites of occurrence are the spinal canal (intradural, 48%), the cerebellopontine angle, tentorium, skull base and foramen magnum.[7,9,10] Supratentorial location of pediatric CCM is rare. Only 5 cases of supratentorial pediatric CCM have been reported till date.[1,2,4,5,6] In a review of 35 cases of intracranial CCM, Ma et al.[5] reported only two pediatric patients with supratentorial intraparenchymal CCM. They treated these patients with intracranial and spinal irradiation after surgical removal. Zorludemir et al.[1] first described this neoplasm in 1995 and since then, <50 cases have been reported. It was subsequently listed as a distinct entity in the current WHO classification of brain tumors in 2000. Most cases were single case reports, with >50% reported by Zorludemir et al., Pimentel et al., and Jain et al.[1,2,11] Most patients of CCM are young, usually in the first three decades of life, although occasional cases in older patients have also been reported. A slight female predominance has been noted. Our patient was an 11-year-old boy and the tumor was located in middle cranial fossa with infratemporal extension.
Prior to the acceptance of CCM as a distinct variant of meningioma, tumors with clear cell morphology were often reported as metastatic renal cell carcinoma, oligodendroglioma, hemangioblastoma, and clear cell ependymoma, especially so when diagnoses were based solely on histomorphology.[1,2,8] The absence of IHC facility during the initial biopsy, compounded with the lack of sufficient data pertaining to CCM possibly attributed to misinterpretation of initial tumor as an oligodendroglioma.[3]
The biological behavior of CCM may be inordinately aggressive, despite its benign histological appearance, and may display inconsistent correlation with MIB-1 proliferation. Zorludemir noted high MIB-1 LI (range: 3.3–25.7%; mean 13.3%) with 61% recurrence, while, Jain series noted 22% recurrence despite low MIB-1 LI, which was similar to the present case. In meningioma, factors with prognostic significance generally include histological subtype, tumor grade, MIB-1 proliferation, mitotic index, brain invasion, and the extent of surgical resection.[12] Zorludemir et al.[1] in their series found a recurrence rate of 61%, but failed to note any definite correlation between tumor recurrence and factors as mitotic activity, proliferating cell nuclear antigen proliferation indices, percent S-phase determination, or DNA ploidy status. However, Pimentel et al.[11] noted that CCMs, which recurred were in the intracranial location, and ones with subtotal resection, while Lee et al.[7] documented a case of CCM, in which initial MRI showed leptomeningeal enhancement that had progressed into entire subarachnoid space after surgical resection of the primary tumor.
Currently, molecular genetics and karyotypic studies have also found consistent correlation between meningioma recurrence and loss of heterozygosity 22q, 1p, and 14q.[12] In order to gain insight into the pathophysiology behind the aggressive behavior of CCM, it is imperative to have a detailed cytogenetic evaluation, especially in a large series, prior to attributing aggressiveness in CCM to any definite factor.
Treatment of choice in CCM is maximal surgical resection, with radiosurgery and radiotherapy being reserved for residual/recurrent cases.[9,10] Staged excision can be an excision in children considering the extensive nature of the procedure in large tumors like in our patient. Since there are concerns of radiotherapy in children, including neuro-cognitive complications and late malignancies, our personal take is to excise residual or recurrent tumors whenever possible. Radiotherapy remains the only alternative when excision is not feasible. Follow-up is essential in these patients considering their propensity for loco-regional and distant relapse.
Conclusion
Supratentorial CCM in children is indeed rare. Meticulous and detailed histopathological examination, including proliferation index estimation, is of paramount importance for making the right diagnosis in such cases. Complete excision remains the treatment of choice and radiotherapy may be administered in irresectable recurrences. A long-term follow-up is mandatory to truly decipher the natural course in these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Zorludemir S, Scheithauer BW, Hirose T, Van Houten C, Miller G, Meyer FB. Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol. 1995;19:493–505. [PubMed] [Google Scholar]
- 2.Jain D, Sharma MC, Sarkar C, Suri V, Garg A, Singh M, et al. Clear cell meningioma, an uncommon variant of meningioma: A clinicopathologic study of nine cases. J Neurooncol. 2007;81:315–21. doi: 10.1007/s11060-006-9237-7. [DOI] [PubMed] [Google Scholar]
- 3.Deb P, Datta SG. An unusual case of clear cell meningioma. J Cancer Res Ther. 2009;5:324–7. doi: 10.4103/0973-1482.59902. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Zhang Y, Wang E, Wang Z, Li W, Huang S, et al. Intracranial clear cell meningioma in two children with blood relations: Two case reports and literature review. Childs Nerv Syst. 2012;28:2143–51. doi: 10.1007/s00381-012-1840-7. [DOI] [PubMed] [Google Scholar]
- 5.Ma L, Liu WK, Wang K, Shrestha B, Zhang YK. Intracranial clear-cell meningioma. Acta Neurochir (Wien) 2009;151:373–8. doi: 10.1007/s00701-009-0236-2. [DOI] [PubMed] [Google Scholar]
- 6.King J, Cusimano M, Hawkins C, Dirks P. Extradural middle fossa approach to a clear cell meningioma in a child. Can J Neurol Sci. 2009;36:257–61. doi: 10.1017/s0317167100120311. [DOI] [PubMed] [Google Scholar]
- 7.Lee W, Chang KH, Choe G, Chi JG, Chung CK, Kim IH, et al. MR imaging features of clear-cell meningioma with diffuse leptomeningeal seeding. AJNR Am J Neuroradiol. 2000;21:130–2. [PMC free article] [PubMed] [Google Scholar]
- 8.Oviedo A, Pang D, Zovickian J, Smith M. Clear cell meningioma: Case report and review of the literature. Pediatr Dev Pathol. 2005;8:386–90. doi: 10.1007/s10024-005-0119-3. [DOI] [PubMed] [Google Scholar]
- 9.Colen CB, Rayes M, McClendon J, Jr, Rabah R, Ham SD. Pediatric spinal clear cell meningioma. Case report. J Neurosurg Pediatr. 2009;3:57–60. doi: 10.3171/2008.10.17668. [DOI] [PubMed] [Google Scholar]
- 10.Jallo GI, Kothbauer KF, Silvera VM, Epstein FJ. Intraspinal clear cell meningioma: Diagnosis and management: Report of two cases. Neurosurgery. 2001;48:218–21. doi: 10.1097/00006123-200101000-00042. [DOI] [PubMed] [Google Scholar]
- 11.Pimentel J, Fernandes A, Pinto AE, Fonseca I, Moura Nunes JF, Lobo Antunes J. Clear cell meningioma variant and clinical aggressiveness. Clin Neuropathol. 1998;17:141–6. [PubMed] [Google Scholar]
- 12.Louis DN, Scheithauer BW, Budka H. World Health Organization Classification of Tumors. In: Kleihues P, Cavenee WK, editors. Pathology and Genetics of Tumors of the Nervous System. Lyon: International Agency for Research on Cancer (IARC) Press; 2000. pp. 176–84. [Google Scholar]


