Abstract
Leptomeningeal spinal metastases from supratentorial high-grade glioma are relatively rare. Authors report an unusual case of metachronous, symptomatic, dual spinal drop metastases in a 20-year-old male patient who was operated for right insular anaplastic astrocytoma 20 months earlier. Surgical decompression of the symptomatic D11-L2 drop metastasis was carried out. Histo-pathological examination revealed features suggestive of glioblastoma multiforme. Patient was advised postoperative radiotherapy. The pertinent literature is reviewed regarding this uncommon entity.
Keywords: Anaplastic astrocytoma, glioblastoma multiforme, leptomeningeal metastases, spine
Introduction
Glioblastoma multiforme (GBM) is the most common primary malignant tumor of the central nervous system. Leptomeningeal metastases from intracranial high-grade gliomas including GBM are relatively rare. Autopsy series suggest that approximately 25% of patients with intracranial glioblastoma have evidence of spinal subarachnoid seeding, although the exact incidence is not known as postmortem examination of the spine is not routinely performed.[1,2] Symptomatic spinal metastases from supratentorial anaplastic astrocytoma are unusual. We here describe an extremely rare presentation of supratentorial anaplastic astrocytoma degenerating into GBM post-radiotherapy and presenting with dual spinal drop metastases. The index case was a 20-year-old male operated for right insular anaplastic astrocytoma 20 months earlier and received adjuvant chemo-radiotherapy.
Case Report
A 20-year-old male patient presented to our outpatient services with 1-month history of low backache with radiation to both lower limbs and progressive paraparesis. Patient had developed urinary and fecal incontinence from last 15 days. History revealed that he was operated for right insular glioma in March, 2010. Gross total excision of the tumor was carried out at that time via a right fronto-temporal craniotomy and trans-sylvian, trans-insular approach. Histo-pathological examination was suggestive of anaplastic astrocytoma World Health Organizations (WHO) Grade III. Patient received postoperative radiotherapy and chemotherapy including Temozolomide. Patient was functionally preserved since then.
Neurological examination revealed a paraparesis with a power of 2/5 (Medical Research Council grading). Patient had sensory impairment to all modalities of sensation below L2 level. Gadolinium – enhanced magnetic resonance imaging (MRI) of the spine revealed dual, well defined, intra-dural extramedullary drop metastases at D11-L2 and S1-S2 levels. They were hypointense on T1weighted, hyperintense on T2-weighted images with intense, heterogenous postcontrast enhancement [Figure 1]. MRI brain with contrast did not reveal any evidence of residual/recurrent tumor.
Figure 1.
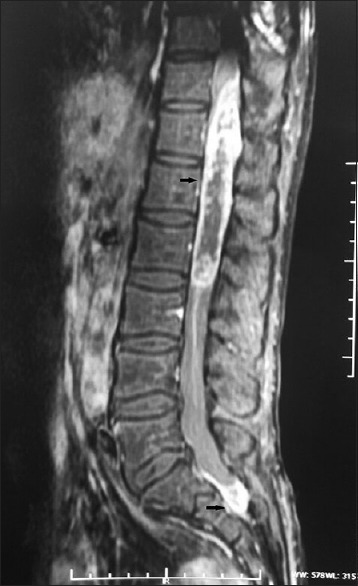
Sagittal T1-postgadolinium contrast enhancement magnetic resonance imaging suggestive of two heterogeneously contrast enhancing intra-dural, extramedullary lesions at D11-L2 and S1-S2 spinal levels (arrows)
In view of the diffuse nature of the disease, it was decided to surgically decompress the symptomatic lesion at D11-L2 level. D10-L2 laminoplasty and tumor decompression was carried out. Intra-operative findings revealed that the tumor was intra-dural, extramedullary with infiltration of the cord tissue. It was heterogeneous, moderately vascular and suckable. However, there was no definite plane between the tumor and the cord tissue. Maximum safe decompression was carried out. Patient had an uneventful postoperative course. Power in both lower limbs improved to 3/5 at the time of discharge.
Histo-pathological examination (H and E, ×100) [Figure 2] showed a highly cellular astrocytic tumor with frequent mitosis, necrosis and hyperchromatic pleomorphic tumor cells. Endothelial cell proliferation and necrosis were also noted. Immunohistochemical staining showed positivity for P53 and positivity for glial fibrillary acidic protein. Tumor cells were negative for epidermal growth factor receptor, and MIB 1 labeling index was 55% in highest mitotic areas. Based on these findings, diagnosis of GBM (WHO Grade IV) was rendered. Patient was referred to the radiotherapy department for further management.
Figure 2.
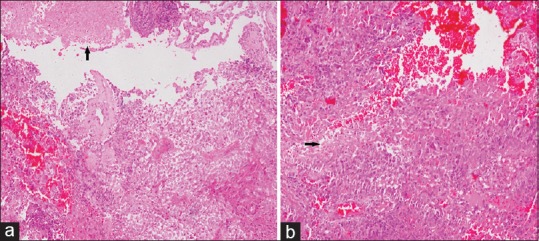
(a) Microphotograph of H and E section showing highly cellular tumor with focal endothelial proliferation along with small focus of necrosis (arrow) (H and E, ×100). (b) Microphotograph of H and E section showing highly cellular tumor with pleomorphic cells and small foci of necrosis (arrow) (H and E, ×100)
Discussion
The ability of supratentorial high-grade glioma to metastasize along cerebrospinal fluid (CSF) pathways to the spinal cord was first described in 1931.[3] CSF dissemination occurs in 15–25% of cases of supratentorial GBM.[1,4] However, a higher incidence of spinal metastases (up to 60%) has been noted for infratentorial GBM.[5] The rate of leptomeningeal metastasis in cases of GBM has been reported variably. In a study involving 600 patients with intracranial GBM, only 2% had symptomatic CSF tumor dissemination.[6] A review of the literature by Erlich and Davis in 1978 revealed only 14 well-documented cases of spinal subarachnoid seeding.[1] Intramedullary spinal metastases are still rarer.[7] These results suggest that the symptomatic involvement occurs relatively late in the course of GBM. In our case, the patient presented with symptomatic drop metastases 20 months after surgery for intracranial glioma. Probably, the tumor in our case has decompensated to a higher grade (from WHO Grade III to WHO Grade IV) and although there is no residual/recurrent tumor on contrast MRI brain, it may happen that few microscopic foci of the tumor remains, followed by metachronous presentation as a spinal leptomeningeal drop metastases.
The most common sites for spinal GBM metastases are the lower thoracic, upper lumbar, and lumbosacral regions.[8,9] The most common clinical features of leptomeningeal metastases are radiculopathy and backache. This is frequently followed by paraparesis or, infrequently, quadriparesis.[9] This symptomatology have been exemplified in our case.
Most of the large series describe patients with meningeal and spinal spread discovered at autopsy.[1,2] Metastases, which are associated with pain and/or neurological deficit, are usually verified by imaging studies. Until recently, computed tomography (CT) myelography was the diagnostic test of choice for leptomeningeal metastases,[2] but CT myelography has now been superseded by MRI. Spinal MRI with gadolinium enhancement is currently the investigation of choice for leptomeningeal metastases.
Ventricular entry at operation, multiple resections and male sex were all associated with a statistically significant increased incidence of CSF dissemination in a study of supratentorial gliomas in children by Grabb et al.[10] Ependymal invasion, fissuring of the ependyma due to hydrocephalus, and fragmentation of the tumor in contact with CSF are also risk factors for CSF dissemination.[3] Repeated tumor resection is associated with an even greater risk of CSF dissemination, because of repeated tumor manipulation, more aggressive tumor cell types, and depressed immune function after radiotherapy and chemotherapy.[10]
Treatment options for leptomeningeal metastasis are limited. Surgery may be attempted if there is a symptomatic, large metastatic deposit causing cord compression as in our case, but usually leptomeningeal metastases are not amenable to surgery due to the diffuse nature of the disease.[11] The treatment modality for these patients is mainly palliative. RT is the most commonly used treatment modality, with a total dose of 25–40 Gy.[12]
Leptomeningeal metastasis with CSF tumor dissemination almost always results in a fatal outcome. The median time from diagnosis of the primary intracranial GBM to diagnosis of CSF tumor dissemination ranges from 8 to 14 months, median survival ranges from 11 to 17 months and the average time interval between diagnosis of leptomeningeal metastasis and death is 2 to 3 months.[10]
Conclusion
Leptomeningeal symptomatic spinal drop metastases from supratentorial high-grade gliomas are relatively rare. In cases of high-grade gliomas with recent onset extremity and back pain and/or associated spinal root nerve signs should alert the clinicians to consider leptomeningeal and/or spinal dissemination of GBM. The treatment of spinal drop metastases remains palliative and usually prognosis dismal.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Erlich SS, Davis RL. Spinal subarachnoid metastasis from primary intracranial glioblastoma multiforme. Cancer. 1978;42:2854–64. doi: 10.1002/1097-0142(197812)42:6<2854::aid-cncr2820420647>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Lam CH, Cosgrove GR, Drislane FW, Sotrel A. Spinal leptomeningeal metastasis from cerebral glioblastoma. Appearance on magnetic resonance imaging. Surg Neurol. 1991;35:377–80. doi: 10.1016/0090-3019(91)90049-f. [DOI] [PubMed] [Google Scholar]
- 3.Cairns H, Russell DS. Intracranial and spinal metastases in gliomas of the brain. Brain. 1931;54:377–420. [Google Scholar]
- 4.Arita N, Taneda M, Hayakawa T. Leptomeningeal dissemination of malignant gliomas. Incidence, diagnosis and outcome. Acta Neurochir (Wien) 1994;126:84–92. doi: 10.1007/BF01476415. [DOI] [PubMed] [Google Scholar]
- 5.Salazar OM, Rubin P. The spread of glioblastoma multiforme as a determining factor in the radiation treated volume. Int J Radiat Oncol Biol Phys. 1976;1:627–37. doi: 10.1016/0360-3016(76)90144-9. [DOI] [PubMed] [Google Scholar]
- 6.Vertosick FT, Jr, Selker RG. Brain stem and spinal metastases of supratentorial glioblastoma multiforme: A clinical series. Neurosurgery. 1990;27:516–21. doi: 10.1097/00006123-199010000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Scoccianti S, Detti B, Meattini I, Iannalfi A, Sardaro A, Leonulli BG, et al. Symptomatic leptomeningeal and intramedullary metastases from intracranial glioblastoma multiforme: A case report. Tumori. 2008;94:877–81. doi: 10.1177/030089160809400620. [DOI] [PubMed] [Google Scholar]
- 8.Onda K, Tanaka R, Takahashi H, Takeda N, Ikuta F. Cerebral glioblastoma with cerebrospinal fluid dissemination: A clinicopathological study of 14 cases examined by complete autopsy. Neurosurgery. 1989;25:533–40. [PubMed] [Google Scholar]
- 9.Stanley P, Senac MO, Jr, Segall HD. Intraspinal seeding from intracranial tumors in children. AJR Am J Roentgenol. 1985;144:157–61. doi: 10.2214/ajr.144.1.157. [DOI] [PubMed] [Google Scholar]
- 10.Grabb PA, Albright AL, Pang D. Dissemination of supratentorial malignant gliomas via the cerebrospinal fluid in children. Neurosurgery. 1992;30:64–71. doi: 10.1227/00006123-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Shah A, Redhu R, Nadkarni T, Goel A. Supratentorial glioblastoma multiforme with spinal metastases. J Craniovertebr Junction Spine. 2010;1:126–9. doi: 10.4103/0974-8237.77678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaca M, Andrieu MN, Hicsonmez A, Guney Y, Kurtman C. Cases of glioblastoma multiforme metastasizing to spinal cord. Neurol India. 2006;54:428–30. doi: 10.4103/0028-3886.28122. [DOI] [PubMed] [Google Scholar]


