Abstract
Thiamine pyrophosphate (TPP), the biologically active form of thiamine (also known as vitamin B1), is an essential cofactor for several important enzymes involved in carbohydrate metabolism, and therefore, it is required for all living organisms. We recently found that a thiamine-binding protein (TDE_0143) is essential for the survival of Treponema denticola, an important bacterial pathogen that is associated with human periodontitis. In this report, we provide experimental evidence showing that TP_0144, a homolog of TDE_0143 from the syphilis spirochete Treponema pallidum, is a thiamine-binding protein that has biochemical features and functions that are similar to those of TDE_0143. First, structural modeling analysis reveal that both TDE_0143 and TP_0144 contain a conserved TPP-binding site and share similar structures to the thiamine-binding protein of Escherichia coli. Second, biochemical analysis shows that these two proteins bind to TPP with similar dissociation constant (Kd) values (TDE_0143, Kd of 36.50 nM; TP_0144, Kd of 32.62 nM). Finally, heterologous expression of TP_0144 in a ΔTDE_0143 strain, a previously constructed TDE_0143 mutant of T. denticola, fully restores its growth and TPP uptake when exogenous thiamine is limited. Collectively, these results indicate that TP_0144 is a thiamine-binding protein that is indispensable for T. pallidum to acquire exogenous thiamine, a key nutrient for bacterial survival. In addition, the studies shown in this report further underscore the feasibility of using T. denticola as a platform to study the biology and pathogenicity of T. pallidum and probably other uncultivable treponemal species as well.
INTRODUCTION
Spirochetes comprise a monophyletic phylum that exhibits distinct long and coiled cell shapes and a characteristic corkscrew-like motility (1). The phylum comprises nine genera. One of these genera is Treponema, which contains several important human pathogens such as Treponema pallidum, the causative agent of syphilis (2, 3). The genus of Treponema consists of more than 60 different species, and many of these species cannot be cultivated in vitro (e.g., T. pallidum can be grown experimentally only in rabbit testes) (4–7). The lack of reliable in vitro cultivation systems has substantially hindered our understanding of the biology of T. pallidum and syphilis pathogenesis (3). Identifying virulence determinants of T. pallidum still depends on expressing potential virulence genes in heterologous systems such as Escherichia coli (8–11). However, the physiological differences between the spirochetes and E. coli have limited the use of E. coli systems for functional investigations.
Among the treponemal species, Treponema denticola, an oral spirochete associated with periodontitis, can be easily cultivated and is genetically manipulable (12–14). Due to the high physiological similarities between T. denticola and T. pallidum, the oral spirochete has been explored to study the physiology, biosynthetic pathways, and virulence determinants of T. pallidum (e.g., heterologous expression of T. pallidum genes in T. denticola) (15–17). ATCC 33520 and ATCC 35405 are two laboratory strains of T. denticola. The first one can be genetically manipulated more easily, and several vectors for heterologous gene expression have been developed (15, 17, 18). However, the genome of this strain has not been decoded, which has limited its application. The genome of ATCC 35405 has been sequenced (4). Comparative genomic studies revealed that more than two-thirds (728/1,041) of the predicted open reading frames (ORFs) in T. pallidum can be found in the genome of ATCC 35405. On average, these homologues share 53% amino acid sequence identities and 71% similarities (4), which makes T. denticola a plausible model for T. pallidum research. Furthermore, we recently developed several new genetic tools for T. denticola, including a genetically modified strain that is more amenable to genetic manipulations (19), a modified gentamicin resistance marker (aacCm) for targeted mutagenesis (20), and a thiamine-riboswitch regulatory element (21). These tools make it possible to express T. pallidum homologues and then carry out functional studies in T. denticola.
Thiamine pyrophosphate (TPP) is essential for all living systems due to its cofactor roles for several important enzymes involved in carbohydrate and branched-chain amino acid metabolism (22–25). TPP biosynthesis is often linked to other central metabolic pathways (e.g., purine biosynthesis). Thus, studying TPP biosynthesis in a microorganism can help us to dissect its metabolic integrations (26). Bacteria either synthesize TPP via de novo biosynthesis pathways or import exogenous TPP from environments via specific transporters (22, 27). Both T. denticola and T. pallidum lack a de novo thiamine biosynthesis pathway (2, 4, 21). Thus, they must take up exogenous thiamine, a key nutrient for their survival. Bacteria typically import exogenous thiamine and its derivatives via primary ABC transporters, which consist of a thiamine-binding protein (Tbp), a transmembrane permease, and a cytosolic ATPase (28). The role of Tbp is to secure the ligand and deliver it to the permease, which uses energy from ATP hydrolysis to transport the ligand against a concentration gradient into the cytoplasm (25). TbpA, the thiamine-binding protein of E. coli, is essential for growth when its de novo thiamine biosynthesis pathway is disrupted (29). We recently identified a thiamine-binding protein (TDE_0143) in T. denticola. Mutagenesis studies revealed that deletion of TDE_0143 dramatically impaired the ability of T. denticola to import exogenous TPP, and the mutant failed to grow in a medium with limited amounts of TPP (21). Similar to T. denticola, the genome of T. pallidum also encodes a thiamine-ABC transporter, which consists of TP_0144 (thiamine-binding protein), TP_0143 (transmembrane permease), and TP_0142 (ATPase), suggesting that T. pallidum also needs to import exogenous thiamine for its metabolism (2, 21). The experiments in this report were specifically designed to address the following two questions. (i) Does TP_0144 function as a thiamine-binding protein? (ii) By studying the function of TP_0144, can we advance the genetics toolbox of T. denticola to allow us to establish a universal platform for expressing T. pallidum homologues and then conduct functional studies in T. denticola?
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Treponema denticola ATCC 35405 (wild type) and its derivative mutant strains were grown in an oral bacterial growth medium (OBGM) supplemented with 10% heat-inactivated rabbit serum at 37°C in an AS-580 anaerobic chamber (Anaerobe Systems, Morgan Hill, CA) in the presence of 85% nitrogen, 5% carbon dioxide, and 10% hydrogen, as previously described (30). For the semisolid growth medium, 0.7% low-melting-point SeaPlaque agarose (Lonza, Rockland, ME) was incorporated into the medium, which was then plated after bacterial suspensions were inoculated into the medium at 37°C (31). Treponema pallidum subsp. pallidum Nichols was extracted from rabbit testes after intratesticular infection and further purified by Percoll density gradient centrifugation as described before (5). The Escherichia coli TOP10 strain (Invitrogen, Carlsbad, CA) was used for routine plasmid construction and preparation. The E. coli dam dcm mutant strain (New England BioLabs, Ipswich, MA) was used to amplify and prepare unmethylated plasmids. The E. coli B21 Star(DE3) strain (Invitrogen) was used for recombinant protein expression and preparations. The E. coli strains were grown in lysogeny broth (LB) supplemented with appropriate concentrations of antibiotics.
Preparations of TDE_0143 and TP_0144 recombinant proteins and antibodies.
To prepare the recombinant TDE_0143 (rTDE_0143) and TP_0144 (rTP_0144) proteins, the entire genes were PCR amplified using Platinum Pfx DNA polymerase (Invitrogen). The resulting amplicons were cloned into a pET100/D-TOPO expression vector (Invitrogen) with an N-terminal six-His (His6) fusion tag. To prepare mutated recombinant TDE_0143 and TP_0144 proteins, the codons for tyrosine 227 (TAC) in these two genes were mutated to alanines (GCC) (yielding rTP_0143227Y/A and rTP_0144227Y/A, respectively) using a QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). The obtained plasmids were transformed into E. coli B21 Star(DE3) cells and then induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The recombinant proteins were purified using Ni-nitrilotriacetic acid (NTA)–agarose (Qiagen, Valencia, CA) under native conditions as previously described (21). The final purified proteins were dialyzed in 10 mM Tris buffer (pH 8.0) at 4°C overnight. Protein concentrations were measured using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). To generate antibodies against TDE_0143 and TP_0144, mice were immunized with 1 mg of the recombinant proteins in three inoculations during a 1-month period (the antisera were produced by General Bioscience Corporation, Brisbane, CA). The obtained antisera were tested by immunoblotting as previously described (21).
TPP-binding assay.
Protein-TPP binding affinity was measured using an equilibrium dialysis method as described before (32). The assay was carried out using DispoBiodialyzer cassettes (The Nest Group, Southborough, MA), which have two chambers separated by a 5-kDa-cutoff membrane. Briefly, 60 μl of TPP (0 to 25 μM) was injected into one chamber, and then 60 μl (10 μM) of either the wild-type recombinant proteins or the mutated ones was injected into the other chamber. The loaded cassettes were maintained for 2 h with gentle rocking at room temperature to reach equilibrium. Then, 50 μl of samples from both sides of the cassettes was taken, pretreated, and subjected to reverse-phase high-performance liquid chromatography (HPLC) for measuring TPP concentrations as previously documented (21). The binding constants were calculated with GraphPad Prism, version 5, software (GraphPad Software, San Diego, CA).
Complementation of a TDE_0143 deletion mutant using TDE_0143 and TP_0144.
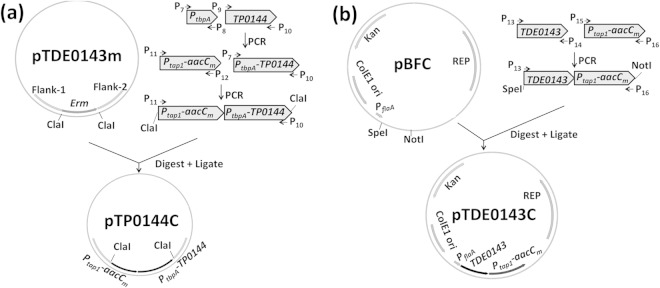
In our previous report, the TDE_0143 gene was deleted in frame (21), and the obtained mutant was designated ΔTDE_0143. We first attempted to cis complement ΔTDE_0143 using TP_0144. The complementation vector pTP_0144C was constructed by multiple-step PCR as illustrated in Fig. 1a. Briefly, the previously identified TDE_0143 promoter (PtbpA) (21), the TP_0144 gene, and the Ptap1-aacCm cassette (20, 33) were PCR amplified with primers P7/P8, P9/P10, and P11/P12, respectively (Table 1 lists the primer sequences). PtbpA and TP_0144 were fused together by PCR with primers P7/P10. The resulting PtbpA-TP_0144 fragment was fused to Ptap1-aacCm by PCR with primers P11/P10. The resulting fragments were cloned into a pGEM-T vector and then treated with ClaI to release Ptap1-aacCm-PtbpA-TP_0144. The released fragment was finally inserted into pTDE_0143m, a previously constructed vector for inactivation of TDE_0143 (21), at an engineered ClaI cut site, yielding pTP_0144C (Fig. 1a).
FIG 1.
Schematic illustration of vector construction. Construction of vectors pTP_0144C (a) and pTDE_0143C (b) is shown. pTP_0144C was constructed to cis complement ΔTDE_0143 using TP_0144, and pTDE_0143C was constructed to trans complement the mutant using TDE_0143. The vector pTDE_0143m was previously constructed to delete TDE_0143 in frame (21). pBFC is a shuttle vector of T. denticola (17). Arrows indicate the relative positions of PCR primers for constructing these vectors. The sequences of these primers are listed in Table 1. Ptap1, tap1 promoter; PtbpA, tbpA promoter; aacCm, modified gentamicin resistance marker; REP, replication origin of T. denticola.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequencea | Orientationb | Description and/or purpose |
|---|---|---|---|
| P1 | CACCGCTCACCGTATGCGTGTT | F | TP_0144 recombinant protein |
| P2 | TCATGGGGTTGATGCGTG | R | TP_0144 recombinant protein |
| P3 | GCCGCACTGGTACTTAGTGCCACCACAAGTGC | F | Site mutagenesis of TP_0144 |
| P4 | GCACTTGTGGTGGCACTAAGTACCAGTGCGGC | R | Site mutagenesis of TP_0144 |
| P5 | GCTCCCATAGCCATTTCTGCCACAAGCAGCC | F | Site mutagenesis of TDE_0143 |
| P6 | GGCTGCTTGTGGCAGAAATGGCTATGGGAGC | R | Site mutagenesis of TDE_0143 |
| P7 | CGCCACCTAAGGACAACTTGTTATTATGCAC | F | Complementation, PtbpA |
| P8 | CATACGGTGAGCCATATGCTCCCTCCGCCG | R | Complementation, PtbpA |
| P9 | GGGAGCATATGGCTCACCGTATGCGTGTTGTCTTC | F | Complementation, TP_0144 |
| P10 | ACTAGTTCATGGGGTTGATGCGTGTAATGC | R | Complementation, TP_0144 |
| P11 | ACTAGTCATCGTTCATATTATACTTCTCC | F | Complementation, Ptap1-aacCm |
| P12 | CAAGTTGTCCTTAGGTGGCGGTACTTGGGTC | R | Complementation, Ptap1-aacCm |
| P13 | ACTAGTATGAAAAATCATTTACGCTC | F | Complementation, TDE_0143 |
| P14 | GAACGATGTTATTTTTGTAAAACATTGATTACGGC | R | Complementation, TDE_0143 |
| P15 | CAATGTTTTACAAAAATAACATCGTTCATATTATATTCTCC | F | Complementation, Ptap1-aacCm |
| P16 | GCGGCCGCTTAGGTGGCGGTACTTGGGTC | R | Complementation, Ptap1-aacCm |
| P17 | CGGTTGTGCCGCTCTTTTTGCAG | F | TDE_0143 DNA probe |
| P18 | GCAAAATAGCCGTAGTCAAAGGG | R | TDE_0143 DNA probe |
| P19 | CTCACCGTATGCGTGTTGTC | F | TP_0144 DNA probe |
| P20 | CGGATGGCACGATGAATAGC | R | TP_0144 DNA probe |
The engineered restriction enzyme sites are underlined.
F, forward; R, reverse.
We also attempted to complement the mutant with TDE_0143 using pBFC, a shuttle vector of T. denticola (17), as illustrated in Fig. 1b. The TDE_0143 gene and the tap1 promoter-driven gentamicin resistance marker (Ptap1-aacCm) were PCR amplified with primers P13/P14 and P15/P16, respectively. The resulting fragments were fused together by PCR using primers P13/P16. The fused TDE_0143-Ptap1-aacCm fragment was cloned into a pGEM-T Easy vector and then released using SpeI and NotI. The released DNA fragment was finally cloned into pBFC downstream of the flaA promoter (Pfla) (17, 33), yielding the pTDE_0143C vector (Fig. 1b). The obtained plasmids were amplified and purified from the E. coli dam dcm mutant strain (19). For complementation of the ΔTDE_0143 mutant, the mutant was separately transformed with 10-μg amounts of unmethylated pTP_0144C and pTDE_0143C and then plated on OBGM semisolid agar plates containing 40 μg ml−1 erythromycin and/or 20 μg ml−1 gentamicin for screening of positive colonies as previously documented (32, 34).
Southern blotting.
Total genomic DNA of T. denticola was isolated using an Illustra Bacteria Genomic Prep kit (GE Healthcare, Little Chalfont, United Kingdom). Southern blot analysis was carried out as previously described (19). Briefly, the purified genomic DNA was first digested with HindIII, separated on a 1.0% agarose gel, and transferred to Hybond-N+ membranes (GE Healthcare). To prepare DNA probes, aacCm, TP_0144, and TDE_0143 were amplified by PCR, and the obtained amplicons were labeled with digoxigenin (DIG). The DNA labeling, hybridization, and detection were carried out using DIG High Prime DNA Labeling and Detection Starter Kit I (Roche Diagnosis GmbH, Mannheim, Germany), according to the manufacturer's instructions.
SDS-PAGE and Western blotting.
Equal amounts of T. denticola and T. pallidum whole-cell lysates (ranging from 10 to 20 μg) were separated on SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories). The blots were probed using antibodies against TDE_0143 (anti-TDE_0143), TP_0144 (anti-TP_0144), or FlaA (anti-FlaA) and then developed using horseradish peroxidase-conjugated secondary antibody with an enhanced chemiluminescence (ECL) luminol assay as previously described (21). Signals were quantified using a Molecular Imager ChemiDoc XRS system with Image Lab software (Bio-Rad Laboratories).
Measuring T. denticola growth rates.
The previously developed TPP assay medium (TA medium) (21) was used to measure growth of T. denticola. Briefly, 5 μl of late-logarithmic-phase T. denticola cultures (∼5 × 108 cells ml−1) was inoculated into 5 ml of TA medium supplemented with different amounts of thiamine or TPP and grown at 37°C in an anaerobic chamber. The cultures were enumerated every 24 h using a Petroff-Hausser counting chamber (Hausser Scientific, Horsham, PA) for up to 7 days. Counts were repeated in triplicate with at least three independent experiments. The results were expressed as mean cell numbers ± standard deviations (SD).
Detection of intracellular thiamine and its derivatives by HPLC.
Concentrations of thiamine and its derivatives (i.e., thiamine monophosphate [TMP] and TPP) in the T. denticola cells were measured by HPLC as previously described (21, 35). Briefly, T. denticola strains were cultivated in the TA medium with different amounts of TPP to stationary phase. Bacterial cells from 50 ml of the cultures were harvested by centrifugation at 6,000 × g for 10 min. The collected cell pellets were washed twice using phosphate-buffered saline (PBS, pH 7.4) and then resuspended in PBS. The final cell densities were adjusted to 1010 cells ml−1. The obtained samples were lysed with sonication and fractionated by centrifugation at 10,000 × g for 10 min. The supernatants were collected and pretreated for HPLC analysis as previously reported (21). Quantification of thiamine and its derivatives was conducted on an Agilent 1260 Infinity series HPLC system (Agilent) equipped with a Zobex Eclipse Plus C18 Rapid Resolution HT analytical column (3.0 by 50 mm; 1.8-μm particle size) under the same detection conditions as previously documented (35). The calibration linear range of thiamine, TMP, and TPP was 10 to 500 nmol on the column. Three independent experiments were conducted, and the intracellular thiamine and its derivative concentrations were expressed in molecules per cell.
Construction of a homology model of TP_0144 and TDE_0143.
Chain A from the crystal structure of E. coli TbpA (Protein Data Bank identification code [PDB ID] 2QRY) (36) together with TPP was used as the template for constructing both homology models in Modeler, version 9v7 (37). Sequence alignment among TP_0144, TDE_0143, and the template was conducted using Clustal X. The automodel module with the conjugate gradient optimization method was adopted to produce the final model with the best objective function value among the five resultant candidates. Thiamine-binding residues were selected at the relative positions comparable to those in 2QRY. All the diagrams were produced in VMD, version 1.8.7.
RESULTS AND DISCUSSION
TP_0144 is a thiamine-binding protein.
TP_0144 is annotated as a thiamine-binding periplasmic protein in the genome of T. pallidum (Nichols) (2). It consists of 335 amino acids (aa) with a predicted molecular mass of 36.8 kDa. The amino acid sequence of TP_0144 was used as a query to search the Pfam database (38) and the Conserved Domain Database (CDD) (39). The results showed that TP_0144 is a member of PRK11205, a family of thiamine transporter substrate-binding subunits. Within this family, TP_0144 shares a high amino acid sequence similarity to TDE_0143 (38% identity and 59% similarity). Structure homology modeling further revealed that TP_0144 could possibly bear a structure similar to that of TDE_0143 and TbpA of E. coli (Fig. 2a) and that TPP could conformably sit in the active site with well-conserved binding residues (Fig. 2b) (36), suggesting that TP_0144 is a thiamine-binding protein.
FIG 2.
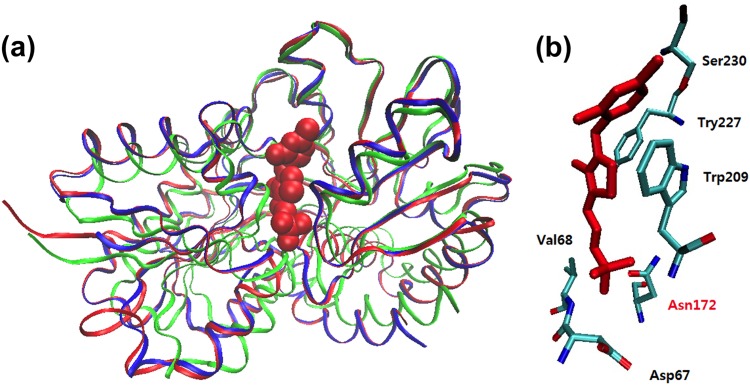
Structural modeling of TP_0144. (a) Comparison of overall structures of TDE_0143 (blue), TP_0144 (red), and the TbpA protein of E. coli (green). The TbpA protein of E. coli (PDB ID 2QRY) (36) was used as the template in structural modeling. The TPP ligand is shown in a Van der Waals representation. (b) A putative thiamine-binding site is displayed with predicted binding residues and the bound TPP (red).
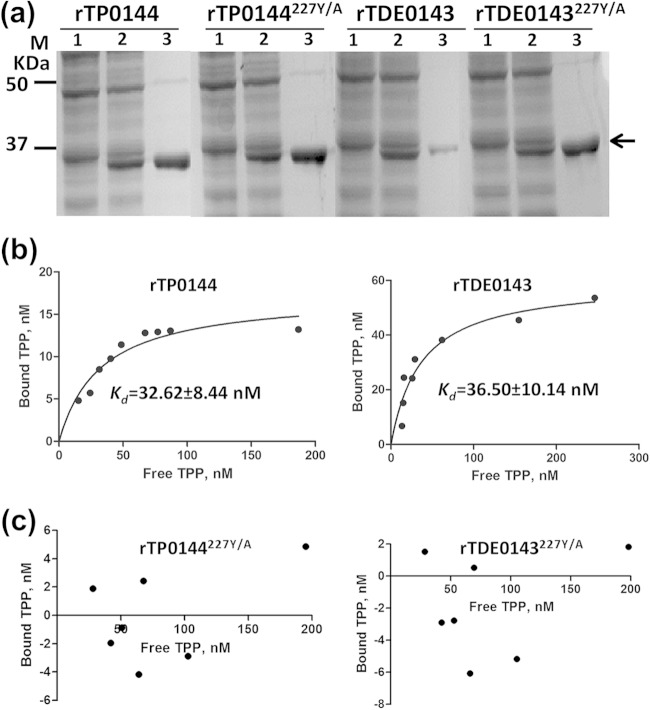
To further ascertain that TDE_0143 and TP_0144 are thiamine-binding proteins, the binding affinities of purified rTDE_0143 and rTP_0144 to TPP were measured. As shown in Fig. 3, these two recombinant proteins bound to TPP with similar affinities: the dissociation constant (Kd) value of rTP_0144 is 32.62 ± 8.44 nM, and the Kd value of rTDE_0143 is 36.50 ± 10.14 nM. Previous mutational and structural studies indicate that tyrosine 215 (Y215) in the TPP-binding site of TbpA is essential for TPP binding activity (36). To confirm that the observed binding activity is specific, the corresponding tyrosine 227 (Y227) residues in the predicted TPP-binding sites of rTDE_0143 and rTP_0144 (Fig. 2b) were replaced with alanines, yielding two mutated recombinant proteins (rTDE_0143227Y/A and rTP_0144227Y/A). We found that the mutation completely abolished their ability to bind TPP (Fig. 3c). Collectively, these results indicate that TDE_0143 and TP_0144 are thiamine-binding proteins with similar binding affinities to TPP.
FIG 3.
Measuring the TPP-binding affinity of TP_0144 and TDE_0143. (a) SDS-PAGE analysis of purified recombinant TP_0144 (rTP_0144) and TDE_0143 (rTDE_0143) proteins and their mutated versions (rTP_0144227Y/A and rTDE_0143227Y/A). For each protein, lanes 1 and 2 represent uninduced and induced E. coli lysates, respectively; lane 3 for each protein represents the purified recombinant protein. (b and c) Plots of equilibrium binding of the recombinant proteins and TPP. Equilibrium dialysis was performed as previously described (21). The binding constants were determined with GraphPad Prism, version 5 (one site, specific binding).
Heterologous expression of TP_0144 in T. denticola.
In our previous studies, we constructed a TDE_0143 mutant (designated ΔTDE_0143) in which the entire gene was replaced in frame by an erythromycin resistance marker (erm). This mutant is defective in TPP transport and is unable to grow when exogenous TPP in the growth medium is limited (21). T. pallidum cannot be continuously cultivated in vitro and is not genetically manipulable (5). To study the biological role of TP_0144 in vivo, we decided to complement the ΔTDE_0143 mutant using TP_0144. If it plays a role similar to that of TDE_0143, we anticipated that heterologous expression of TP_0144 in the mutant would restore its wild-type phenotype. The strategy illustrated in Fig. 1a was developed to cis complement ΔTDE_0143 with TP_0144. The suicide plasmid pTP_0144C was specifically designed to replace the erm cassette in the mutant chromosome with Ptap1-aacCm-PtbpA-TP_0144. To complement the ΔTDE_0143 strain, purified unmethylated pTP_0144C was transformed into the mutant by electroporation. Seven days after the transformation, more than 20 gentamicin-resistant colonies appeared on the plates. Six colonies were randomly selected and subjected to PCR using a pair of primers specific to the TP_0144 gene. All six colonies were positive (data not shown), indicating that TP_0144 was integrated into the chromosomes of these colonies. One colony (TP_0144com, where com indicates the complemented strain) was selected for further confirmation by Southern blotting. The chromosomal DNA of the TP_0144com strain was extracted, digested with HindIII, and then hybridized with two DNA probes: one is a TP_0144-specific probe (P144), and the other is an aacCm-specific probe (PCm). If the erm cassette in TP_0144com is replaced by Ptap1-aacCm-PtbpA-TP_0144 as expected, a 2.4 kb-HindIII-digested DNA fragment should be detected by P144, and a 2.7-kb DNA fragment should be detected by PCm (Fig. 4a). As shown in Fig. 4b, these two DNA fragments were detected in the TP_0144com strain (lane 3) but not in its parental mutant strain (lane 1), indicating that the Ptap1-aacCm-PtbpA-TP_0144 fragment had integrated to the original locus of TDE_0143 in the chromosome of T. denticola as designed.
FIG 4.
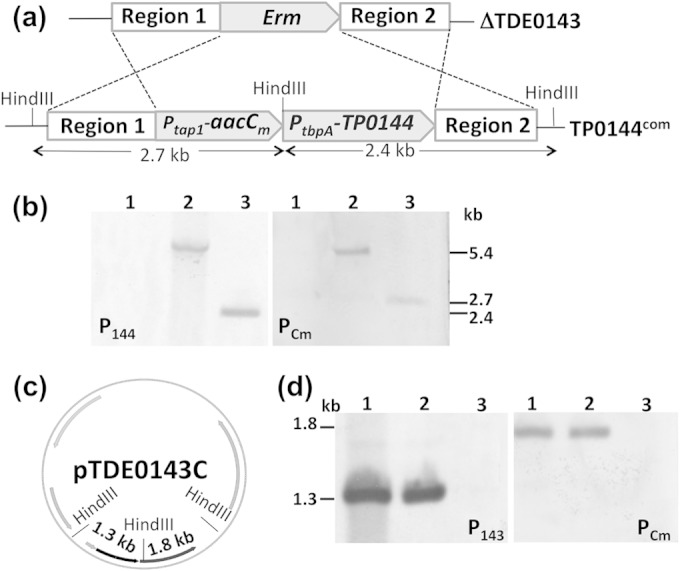
Southern blot analysis of TP_0144com and TDE_0143com. (a) The diagram shows how the erm cassette in the ΔTDE_0143 strain is replaced by Ptap1-aacCm-PtbpA-TP_0144 via allelic exchange recombination, generating the TP_0144com strain. Predicted HindIII cut sites and the sizes of DNA fragments generated by HindIII near the locus of TDE_0143 are labeled. (b) Southern blot analysis. Purified chromosomal DNA and plasmids were treated with HindIII and then hybridized against two DNA probes: one is specific to TP_0144 (P144), and the other is specific to aacCm (PCm). Lane 1, ΔTDE_0143 chromosomal DNA (negative control); lane 2, pTP_0144C (positive control); lane 3, TP_0144com chromosomal DNA. The numbers on the right represent the sizes of detected DNA fragments. (c) Physical map of pTDE_0143C. Predicted HindIII cut sites and the sizes of HindIII-digested DNA fragments are shown on the map. Additional details are depicted in Fig. 1b. (d) Southern blot analysis. The isolated plasmid DNAs were digested with HindIII and then hybridized against PCm and a TDE_0143-specific probe (P143). Lanes 1, pTDE_0143C isolated from E. coli (positive control); lanes 2, the plasmids isolated from TDE_0143com; lanes 3, pBFC (negative control). The numbers on the left represent the sizes of detected DNA fragments.
As a control, we also complemented the ΔTDE_0143 mutant with TDE_0143 using the vector pTDE_0143C shown in Fig. 1b. After the transformation, only two gentamicin-resistant colonies appeared on the plates. Restriction digestion analysis of the plasmids isolated from these two colonies revealed that they both harbored pTDE_0143C. The plasmids isolated from one of these colonies (TDE_0143com) were further digested with HindIII and then hybridized with a TDE_0143-specific probe (P143) and PCm. As shown in Fig. 4d, both of these probes hybridized to the plasmids (a 1.3-kb DNA fragment was detected by P143, and a 1.8-kb fragment was detected by PCm) isolated from the TDE_0143com (lane 2) strain and to the pTDE_0143C vectors isolated from E. coli (lane 1, positive control) but not to the shuttle vector pBFC (lane 3, negative control). These results indicate that pTDE_0143C was successfully transformed into ΔTDE_0143.
TP_0144 is expressed in the TP_0144com strain.
Western blot analysis was carried out to determine if TP_0144 is expressed in the TP_0144com strain. We first raised an antiserum against TP_0144 (anti-TP_0144) in mice and then carried out the Western blot analysis. As shown in Fig. 5, a positive band near 37 kDa was detected in the whole-cell lysates of the TP_0144com strain (lane 1) and T. pallidum (lane 3), indicating that TP_0144 was successfully expressed in the complemented strain. Western blotting also detected a positive band (approximate 36 kDa) in the whole-cell lysates of the TDE_0143com (lane 2) and T. denticola wild-type (lane 4) strains but not in the lysate of the ΔTDE_0143 mutant (lane 5), indicating that TDE_0143 was cross-reactive with anti-TP_0144 and that the expression of TDE_0143 was restored in the TDE_0143com strain. We repeated this experiment using an antibody against TDE_0143 (anti-TDE_0143), and a similar pattern was observed (data not shown), suggesting that there is an antigenic cross-reaction between TP_0144 and TDE_0143. This result is consistent with the high sequence similarity between these two proteins.
FIG 5.
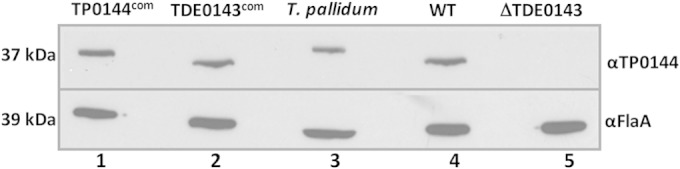
Detection of TP_0144 and TDE_0143 by Western blotting. The blots were probed with an antibody against T. pallidum TP_0144 (αTP_0144) or an antibody against T. denticola flagellar filament sheath protein FlaA (αFlaA). FlaA was used as a loading control. WT, wild type.
Heterologous expression of TP_0144 in ΔTDE_0143 restores its growth and TPP uptake.
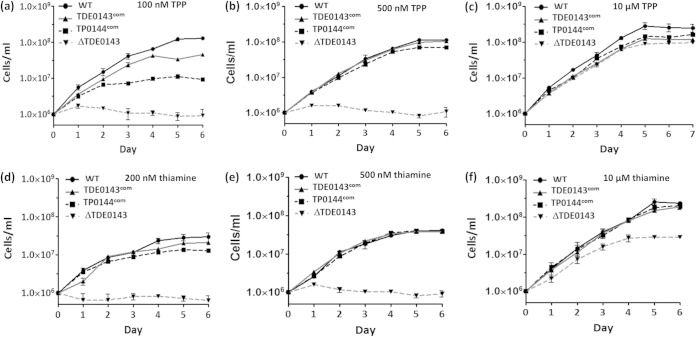
Our previous studies demonstrated that inactivation of TDE_0143 impaired TPP uptake, and the mutant failed to grow when exogenous TPP was limited in the growth medium (21). These results indicate that T. denticola primarily depends on its thiamine-ABC transporter to acquire TPP for its survival and growth. To determine if TP_0144 plays a role similar to that of TDE_0143 in vivo, we cultivated the TP_0144com and TDE_0143com strains in TA medium containing defined amounts of TPP (100 nM, 500 nM, and 10 μM) or thiamine (200 nM, 500 nM, and 10 μM). Similar to our previous report, the ΔTDE_0143 strain could grow only when a high concentration of TPP or thiamine (i.e., 10 μM) was supplemented in the medium (Fig. 6c and f). Under the low concentrations of TPP (100 nM and 500 nM) or thiamine (200 nM and 500 nM), the mutant completely stopped growing (Fig. 6a, b, d, and e). In contrast, the TP_0144com and TDE_0143com strains partially restored growth at a concentration of 100 nM TPP or 200 nM thiamine (Fig. 6a and d); they restored growth almost to the same rate as that of the wild type at a concentration of 500 nM TPP or thiamine (Fig. 6b and e). These results indicate that heterologous expression of TP_0144 in the mutant could compensate for the absence of TDE_0143, demonstrating that TP_0144 plays a role similar to that of TDE_0143 in vivo.
FIG 6.
Measuring T. denticola growth in TA medium with a defined amount of TPP and thiamine. Four T. denticola strains were cultivated in TA medium supplemented with three different amounts of TPP (a, b, and c) or thiamine (d, e, and f). Cell counting was repeated in triplicate from at least three independent experiments, and the results shown here are representatives of one experiment. The results are expressed as mean cell numbers ± standard deviations. WT, wild type.
The T. denticola wild-type and complemented strains but not the ΔTDE_0143 mutant could grow in TA medium supplemented with a limited amount of thiamine (Fig. 6d and e), suggesting that TDE_0143 and TP_0144 are also involved in thiamine uptake. As mentioned earlier, TPP is the active form of thiamine. Besides the de novo biosynthesis pathways, some bacterial species can take up exogenous thiamine and then convert it to TPP through different salvage pathways (27). In E. coli, thiamine is converted to TPP by a two-step reaction that is catalyzed by ThiK (thiamine kinase) and ThiL (thiamine phosphate kinase) (40). In Bacillus subtilis, YloS (also known as ThiN), a thiamine pyrophosphokinase, catalyzes the direct conversion of thiamine to TPP (41). Interestingly, genome mining revealed that while T. denticola contains a homolog of ThiK (TDE_1260), T. pallidum encodes a homolog of YloS (TP_0518), suggesting that they might convert thiamine to TPP by different mechanisms.
To further investigate whether the heterologous expression of TP_0144 restores TPP uptake, the intracellular level of thiamine, TMP, and TPP in the TP_0144com strain was measured by HPLC and compared to that of the wild-type and TDE_0143com strains. Parallel to the measurement of growth curves (Fig. 6), the bacterial cells were harvested and subjected to HPLC analysis. As shown in Table 2, with the supplement of 10 μM TPP, the intracellular level of TPP in the TP_0144com strain (18.89 × 103 ± 0.18 × 103 molecules/cell) was almost identical to that of the wild-type (19.91 × 103 ± 0.51 × 103 molecules/cell) and TDE_0143com (20.44 × 103 ± 0.66 × 103 molecules/cell) strains. With supplements of 100 nM and 500 nM, the intracellular level of TPP in TP_0144com (3.72 × 103 and 10.57 × 103 molecules/cell, respectively) was slightly lower than that in the wild type (4.79 × 103 and 14.08 × 103 molecules/cell, respectively), but the difference was not statistically significant. Of note, with the supplement of 100 nM, the intracellular level of TPP in the TDE_0143com strain (8.11 × 103 ± 0.45 × 103 molecules/cell) was higher than that of the wild type (4.79 × 103 ± 0.56 × 103 molecules/cell). This is probably due to the trans complementation, which might elevate the expression level of TDE_0143. This, in turn, could increase TPP uptake. The ΔTDE_0143 strain was able to grow when 10 μM TPP was supplemented (Fig. 6c). However, its intracellular TPP level (14.23 × 103 ± 0.16 × 103 molecules/cell) was lower than the levels of the wild type and the complemented strains (Table 2), suggesting that the lack of TDE_0143 compromised the function of the TPP-ABC transporter in TPP uptake. Taken together, these results indicate that heterologous expression of TP_0144 in the ΔTDE_0143 strain restores its growth and TPP uptake when exogenous TPP is limited, which is indicative of the important role of TP_0144 in TPP acquisition of T. pallidum.
TABLE 2.
Detection of intracellular TPP and its analogs in T. denticola using HPLC
| Treatment and strainb | Intracellular concn (103 molecules/cell)a |
||
|---|---|---|---|
| TPP | TMP | Thiamine | |
| 100 nM TPP | |||
| WT | 4.79 ± 0.56 | 0.69 ± 0.09 | 2.76 ± 0.41 |
| TP0_144com | 3.72 ± 0.82 | 0.14 ± 0.04 | 0.56 ± 0.12 |
| TDE_0143com | 8.11 ± 0.45 | 0.32 ± 0.01 | 0.56 ± 0.04 |
| ΔTDE_0143 | ND | ND | ND |
| 500 nM TPP | |||
| WT | 14.08 ± 0.13 | 0.50 ± 0.02 | 4.10 ± 0.23 |
| TP0_144com | 10.57 ± 0.26 | 0.55 ± 0.58 | 1.30 ± 0.03 |
| TDE_0143com | 15.17 ± 0.14 | 1.22 ± 0.03 | 0.86 ± 0.02 |
| ΔTDE_0143 | ND | ND | ND |
| 10 μM TPP | |||
| WT | 19.11 ± 0.51 | 1.35 ± 0.13 | 1.05 ± 0.11 |
| TP0_144com | 18.89 ± 0.18 | 4.32 ± 0.14 | 1.30 ± 0.04 |
| TDE_0143com | 20.44 ± 0.66 | 5.39 ± 0.50 | 1.42 ± 0.17 |
| ΔTDE_0143 | 14.23 ± 0.16 | 4.73 ± 0.17 | 1.31 ± 0.01 |
Parallel to the growth curve measurements shown in Fig. 6, T. denticola cells were harvested at the stationary growth phase, lysed by ultrasonication, pretreated, and subjected to HPLC analysis as previously described (21). Three independent assays were conducted. ND, not detected due to the mutant incapable of growth.
Strains were grown in TA medium supplemented with the indicated concentration of TPP.
Conclusion.
Although tremendous efforts have been undertaken during the last several decades, T. pallidum still cannot be continuously cultivated in vitro (5, 42, 43). This is primarily due to a lack of understanding of its nutrient requirements and metabolism. Thiamine is a key nutrient that is required by all living organisms (25). However, little is known about its metabolism in T. pallidum. To our knowledge, this report is the first attempt to investigate thiamine metabolism of T. pallidum. Based on the genomic information of T. pallidum (2) and the evidence provided here, we conclude (i) that it lacks a de novo thiamine biosynthesis pathway, which means that T. pallidum must acquire this key nutrient from the host, and (ii) that it most likely depends on its thiamine-ABC transporter (TP_0144-TP_0143-TP_0142) to import exogenous thiamine. The studies from this report also tell us that thiamine, as an essential nutrient, should be supplemented in the growth medium in future attempts to develop in vitro cultivation methods for T. pallidum.
It has long been of high interest to use cultivable Treponema species as a vehicle to express heterologous genes of T. pallidum and then study their biological and/or pathogenic functions (1, 15, 44). To date, T. denticola ATCC 33520 and Treponema phagedenis biotype Kazan strains have been used (15, 44). However, the genome information of these two strains remains unknown, which makes it more difficult to conduct genetic operations and interpret the functions of heterologous genes. Moreover, in the process of infection, a given virulence factor often acts in concert with other virulence determinants (45). T. phagedenis is nonpathogenic, which limits its usefulness to study the virulence factors of T. pallidum. In contrast to ATCC 33520 and T. phagedenis, the genome of T. denticola ATCC 35405 has been sequenced (4). This strain is pathogenic, and its pathogenicity can be tested in animal models (46–48). Most importantly, more than two-thirds of T. pallidum genes can be found in the genome of ATCC 35405 (4), including the majority of known T. pallidum virulence factors (10, 16, 44, 49–53). These advantages make this strain an ideal model for T. pallidum research. However, this strain is notoriously challenging for genetic manipulation (20, 54). For example, it contains multiple DNA modification systems and does not accept the shuttle vectors developed for ATCC 33520 (19). Fortunately, the genetic tools that we recently developed allow us to genetically manipulate this strain (19, 20, 30, 54). In this report, by investigating the function of TP_0144, we demonstrate that ATCC 35405 can be a useful platform to study the biology and pathogenicity of T. pallidum and probably other uncultivable treponemal species as well.
ACKNOWLEDGMENTS
We thank Ronald J. Limberger for providing the pBFC plasmid and Justin Radolf and Steven J. Norris for providing Treponema pallidum cell pellets.
This research was supported by Public Health Service grants DE018829 and DE019667 to C.L.
REFERENCES
- 1.Lafond RE, Lukehart SA. 2006. Biological basis for syphilis. Clin Microbiol Rev 19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, Gwinn M, Hickey EK, Clayton R, Ketchum KA, Sodergren E, Hardham JM, McLeod MP, Salzberg S, Peterson J, Khalak H, Richardson D, Howell JK, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton MD, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith HO, Venter JC. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 3.Ho EL, Lukehart SA. 2011. Syphilis: using modern approaches to understand an old disease. J Clin Invest 121:4584–4592. doi: 10.1172/JCI57173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, Dodson RJ, Davidsen TM, DeBoy RT, Fouts DE, Haft DH, Selengut J, Ren Q, Brinkac LM, Madupu R, Kolonay J, Durkin SA, Daugherty SC, Shetty J, Shvartsbeyn A, Gebregeorgis E, Geer K, Tsegaye G, Malek J, Ayodeji B, Shatsman S, McLeod MP, Smajs D, Howell JK, Pal S, Amin A, Vashisth P, McNeill TZ, Xiang Q, Sodergren E, Baca E, Weinstock GM, Norris SJ, Fraser CM, Paulsen IT. 2004. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci U S A 101:5646–5651. doi: 10.1073/pnas.0307639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris SJ. 1982. In vitro cultivation of Treponema pallidum: independent confirmation. Infect Immun 36:437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paster BJ, Dewhirst FE. 2000. Phylogenetic foundation of spirochetes. J Mol Microbiol Biotechnol 2:341–344. [PubMed] [Google Scholar]
- 7.Chan EC, McLaughlin R. 2000. Taxonomy and virulence of oral spirochetes. Oral Microbiol Immunol 15:1–9. doi: 10.1034/j.1399-302x.2000.150101.x. [DOI] [PubMed] [Google Scholar]
- 8.Giacani L, Denisenko O, Tompa M, Centurion-Lara A. 2013. Identification of the Treponema pallidum subsp. pallidum TP0092 (RpoE) regulon and its implications for pathogen persistence in the host and syphilis pathogenesis. J Bacteriol 195:896–907. doi: 10.1128/JB.01973-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand A, Luthra A, Dunham-Ems S, Caimano MJ, Karanian C, LeDoyt M, Cruz AR, Salazar JC, Radolf JD. 2012. TprC/D (Tp0117/131), a trimeric, pore-forming rare outer membrane protein of Treponema pallidum, has a bipartite domain structure. J Bacteriol 194:2321–2333. doi: 10.1128/JB.00101-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houston S, Hof R, Francescutti T, Hawkes A, Boulanger MJ, Cameron CE. 2011. Bifunctional role of the Treponema pallidum extracellular matrix binding adhesin Tp0751. Infect Immun 79:1386–1398. doi: 10.1128/IAI.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desrosiers DC, Anand A, Luthra A, Dunham-Ems SM, LeDoyt M, Cummings MA, Eshghi A, Cameron CE, Cruz AR, Salazar JC, Caimano MJ, Radolf JD. 2011. TP0326, a Treponema pallidum beta-barrel assembly machinery A (BamA) orthologue and rare outer membrane protein. Mol Microbiol 80:1496–1515. doi: 10.1111/j.1365-2958.2011.07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Kuramitsu HK. 1996. Development of a gene transfer system in Treponema denticola by electroporation. Oral Microbiol Immunol 11:161–165. doi: 10.1111/j.1399-302X.1996.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 13.Ellen RP, Galimanas VB. 2005. Spirochetes at the forefront of periodontal infections. Periodontology 2000 38:13–32. doi: 10.1111/j.1600-0757.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuramitsu HK. 2003. Molecular genetic analysis of the virulence of oral bacterial pathogens: an historical perspective. Crit Rev Oral Biol Med 14:331–344. doi: 10.1177/154411130301400504. [DOI] [PubMed] [Google Scholar]
- 15.Chi B, Chauhan S, Kuramitsu H. 1999. Development of a system for expressing heterologous genes in the oral spirochete Treponema denticola and its use in expression of the Treponema pallidum flaA gene. Infect Immun 67:3653–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand A, Luthra A, Edmond ME, Ledoyt M, Caimano MJ, Radolf JD. 2013. The major outer sheath protein (Msp) of Treponema denticola has a bipartite domain architecture and exists as periplasmic and outer membrane-spanning conformers. J Bacteriol 195:2060–2071. doi: 10.1128/JB.00078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slivienski-Gebhardt LL, Izard J, Samsonoff WA, Limberger RJ. 2004. Development of a novel chloramphenicol resistance expression plasmid used for genetic complementation of a fliG deletion mutant in Treponema denticola. Infect Immun 72:5493–5497. doi: 10.1128/IAI.72.9.5493-5497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi B, Limberger RJ, Kuramitsu HK. 2002. Complementation of a Treponema denticola flgE mutant with a novel coumermycin A1-resistant T. denticola shuttle vector system. Infect Immun 70:2233–2237. doi: 10.1128/IAI.70.4.2233-2237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian J, Li C. 2011. Disruption of a type II endonuclease (TDE0911) enables Treponema denticola ATCC 35405 to accept an unmethylated shuttle vector. Appl Environ Microbiol 77:4573–4578. doi: 10.1128/AEM.00417-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bian J, Fenno JC, Li C. 2012. Development of a modified gentamicin resistance cassette for genetic manipulation of the oral spirochete Treponema denticola. Appl Environ Microbiol 78:2059–2062. doi: 10.1128/AEM.07461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bian J, Shen H, Tu Y, Yu A, Li C. 2011. The riboswitch regulates a thiamine pyrophosphate ABC transporter of the oral spirochete Treponema denticola. J Bacteriol 193:3912–3922. doi: 10.1128/JB.00386-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begley TP, Downs DM, Ealick SE, McLafferty FW, Van Loon AP, Taylor S, Campobasso N, Chiu HJ, Kinsland C, Reddick JJ, Xi J. 1999. Thiamin biosynthesis in prokaryotes. Arch Microbiol 171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
- 23.Downs DM. 2006. Understanding microbial metabolism. Annu Rev Microbiol 60:533–559. doi: 10.1146/annurev.micro.60.080805.142308. [DOI] [PubMed] [Google Scholar]
- 24.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2002. Comparative genomics of thiamin biosynthesis in procaryotes New genes and regulatory mechanisms. J Biol Chem 277:48949–48959. doi: 10.1074/jbc.M208965200. [DOI] [PubMed] [Google Scholar]
- 25.Manzetti S, Zhang J, van der Spoel D. 2014. Thiamin function, metabolism, uptake, and transport. Biochemistry 53:821–835. doi: 10.1021/bi401618y. [DOI] [PubMed] [Google Scholar]
- 26.Koenigsknecht MJ, Downs DM. 2010. Thiamine biosynthesis can be used to dissect metabolic integration. Trends Microbiol 18:240–247. doi: 10.1016/j.tim.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurgenson CT, Begley TP, Ealick SE. 2009. The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem 78:569–603. doi: 10.1146/annurev.biochem.78.072407.102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb E, Claas K, Downs D. 1998. thiBPQ encodes an ABC transporter required for transport of thiamine and thiamine pyrophosphate in Salmonella typhimurium. J Biol Chem 273:8946–8950. doi: 10.1074/jbc.273.15.8946. [DOI] [PubMed] [Google Scholar]
- 29.Iwashima A, Matsuura A, Nose Y. 1971. Thiamine-binding protein of Escherichia coli. J Bacteriol 108:1419–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Stewart PE, Shi X, Li C. 2008. Development of a transposon mutagenesis system in the oral spirochete Treponema denticola. Appl Environ Microbiol 74:6461–6464. doi: 10.1128/AEM.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Ruby J, Charon N, Kuramitsu H. 1996. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol 178:3664–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bian J, Liu X, Cheng YQ, Li C. 2013. Inactivation of cyclic Di-GMP binding protein TDE0214 affects the motility, biofilm formation, and virulence of Treponema denticola. J Bacteriol 195:3897–3905. doi: 10.1128/JB.00610-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limberger RJ, Slivienski LL, Izard J, Samsonoff WA. 1999. Insertional inactivation of Treponema denticola tap1 results in a nonmotile mutant with elongated flagellar hooks. J Bacteriol 181:3743–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuramitsu HK, Chi B, Ikegami A. 2005. Genetic manipulation of Treponema denticola. Curr Protoc Microbiol Chapter 12:Unit 12B.2. doi: 10.1002/9780471729259.mc12b02s00. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Frank EL. 2008. Rapid HPLC measurement of thiamine and its phosphate esters in whole blood. Clin Chem 54:901–906. doi: 10.1373/clinchem.2007.099077. [DOI] [PubMed] [Google Scholar]
- 36.Soriano EV, Rajashankar KR, Hanes JW, Bale S, Begley TP, Ealick SE. 2008. Structural similarities between thiamin-binding protein and thiaminase-I suggest a common ancestor. Biochemistry 47:1346–1357. doi: 10.1021/bi7018282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sali A, Blundell TL. 1993. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 38.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, Sonnhammer EL, Tate J, Punta M. 2014. Pfam: the protein families database. Nucleic Acids Res 42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Lu S, Marchler GH, Song JS, Thanki N, Yamashita RA, Zhang D, Bryant SH. 2013. CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41:D348–D352. doi: 10.1093/nar/gks1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb E, Downs D. 1997. Characterization of thiL, encoding thiamin-monophosphate kinase, in Salmonella typhimurium. J Biol Chem 272:15702–15707. doi: 10.1074/jbc.272.25.15702. [DOI] [PubMed] [Google Scholar]
- 41.Melnick J, Lis E, Park JH, Kinsland C, Mori H, Baba T, Perkins J, Schyns G, Vassieva O, Osterman A, Begley TP. 2004. Identification of the two missing bacterial genes involved in thiamine salvage: thiamine pyrophosphokinase and thiamine kinase. J Bacteriol 186:3660–3662. doi: 10.1128/JB.186.11.3660-3662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley BS, Cox DL. 1988. Cultivation of cottontail rabbit epidermal (Sf1Ep) cells on microcarrier beads and their use for suspension cultivation of Treponema pallidum subsp. pallidum. Appl Environ Microbiol 54:2862–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norris SJ, Edmondson DG. 1988. In vitro culture system to determine MICs and MBCs of antimicrobial agents against Treponema pallidum subsp. pallidum (Nichols strain). Antimicrob Agents Chemother 32:68–74. doi: 10.1128/AAC.32.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron CE, Kuroiwa JM, Yamada M, Francescutti T, Chi B, Kuramitsu HK. 2008. Heterologous expression of the Treponema pallidum laminin-binding adhesin Tp0751 in the culturable spirochete Treponema phagedenis. J Bacteriol 190:2565–2571. doi: 10.1128/JB.01537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson JW. 1996. Chapter 7. Bacterial pathogenesis. In Baron S. (ed), Medical microbiology, 4th ed University of Texas Medical Branch, Galveston, TX. [PubMed] [Google Scholar]
- 46.Kesavalu L, Walker SG, Holt SC, Crawley RR, Ebersole JL. 1997. Virulence characteristics of oral treponemes in a murine model. Infect Immun 65:5096–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimizuka R, Kato T, Ishihara K, Okuda K. 2003. Mixed infections with Porphyromonas gingivalis and Treponema denticola cause excessive inflammatory responses in a mouse pneumonia model compared with monoinfections. Microbes Infect 5:1357–1362. doi: 10.1016/j.micinf.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Lee SF, Andrian E, Rowland E, Marquez IC. 2009. Immune response and alveolar bone resorption in a mouse model of Treponema denticola infection. Infect Immun 77:694–698. doi: 10.1128/IAI.01004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houston S, Russell S, Hof R, Roberts AK, Cullen P, Irvine K, Smith DS, Borchers CH, Tonkin ML, Boulanger MJ, Cameron CE. 2014. The multifunctional role of the pallilysin-associated Treponema pallidum protein, Tp0750, in promoting fibrinolysis and extracellular matrix component degradation. Mol Microbiol 91:618–634. doi: 10.1111/mmi.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deka RK, Goldberg MS, Hagman KE, Norgard MV. 2004. The Tp38 (TpMglB-2) lipoprotein binds glucose in a manner consistent with receptor function in Treponema pallidum. J Bacteriol 186:2303–2308. doi: 10.1128/JB.186.8.2303-2308.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox DL, Luthra A, Dunham-Ems S, Desrosiers DC, Salazar JC, Caimano MJ, Radolf JD. 2010. Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect Immun 78:5178–5194. doi: 10.1128/IAI.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaFond RE, Centurion-Lara A, Godornes C, Van Voorhis WC, Lukehart SA. 2006. TprK sequence diversity accumulates during infection of rabbits with Treponema pallidum subsp. pallidum Nichols strain. Infect Immun 74:1896–1906. doi: 10.1128/IAI.74.3.1896-1906.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. 2000. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis 181:1401–1413. doi: 10.1086/315399. [DOI] [PubMed] [Google Scholar]
- 54.Goetting-Minesky MP, Fenno JC. 2010. A simplified erythromycin resistance cassette for Treponema denticola mutagenesis. J Microbiol Methods 83:66–68. doi: 10.1016/j.mimet.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]