Introduction
A low level of high-density lipoprotein cholesterol (HDL-C) is an established predictor of the risk of coronary heart disease (CHD).1 HDL promotes cellular cholesterol efflux and reverse cholesterol transport from lipid-laden macrophages (Figure 1), and prevents lipoprotein oxidation. A linear inverse relation has been reported in many observational studies between plasma HDL-C level and incident CHD events, with a plateau effect at HDL-C values >90 mg/dL in men and 75 mg/dL in women.2 However, it remains uncertain whether raising HDL-C levels therapeutically (by either niacin or cholesterol ester transfer protein “CETP” inhibitors) would reduce subsequent cardiovascular (CV) events beyond that achieved with intensive statin therapy.
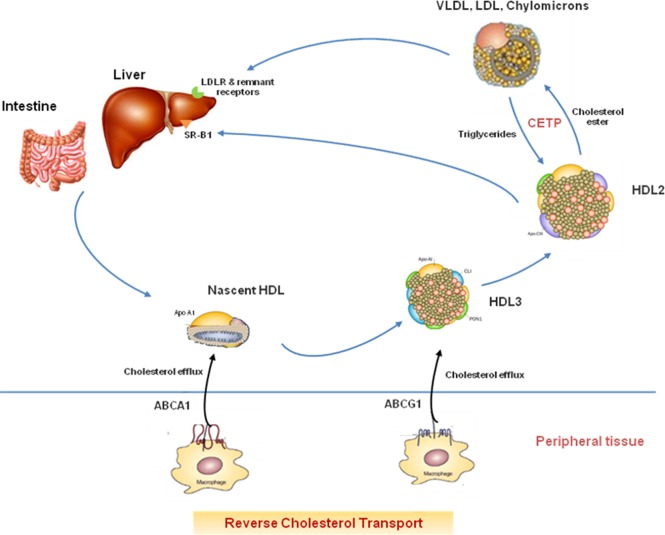
Figure 1.
Reverse Cholesterol Transport. ABCA1, ATP binding cassette A1 transporter; ABCG1, ATP binding cassette G1 transporter; CETP, cholesterol ester transfer protein; SR-B1: Scavenger receptor-B1; LDLR, low density lipoprotein receptor.
Niacin has multiple beneficial effects on serum lipoproteins;3 (1) it raises HDL-C level primarily by promoting the production and retarding the catabolism of Apo A1 in the liver, and also by stimulating ATP-binding cassette transporter A1 which augments reverse cholesterol transport. (2) It decreases the secretion of very low density lipoprotein (VLDL) particles from the liver with subsequent reduction of both intermediate density lipoprotein (IDL) and low density lipoprotein (LDL) particles; an effect mediated by inhibition of hormone-sensitive lipase in the adipose tissue, and hepatocyte diacylglycerol acyltransferase-2 (DGAT-2). (3) It is unique in lowering lipoprotein (a) levels by up to 30%. (4) It inhibits oxidative stress, key inflammatory genes, cytokines involved in atherosclerosis.
Inhibition of CETP by torcetrapib, dalcetrapib, anacetrapib, and evacetrapib is another attractive strategy to raise plasma HDL-C level.4,5 CETP promotes the transfer of cholesterol esters from HDL to triglyceride-rich lipoprotein particles, in exchange for triglyceride, thereby reducing the circulating HDL-C concentration.
Over the past years, numerous randomized clinical studies have been conducted to assess the clinical efficacy and safety of these drugs.
Hps2-thrive Study
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study was a randomized, multicenter, double-blind, prospective, controlled clinical trial that recruited patients at 245 sites in the United Kingdom, Scandinavia, and China and was published in the New England Journal of Medicine in July 2014.6
This study was designed to evaluate the clinical efficacy and safety of adding a combination of extended-release niacin and laropiprant - a selective antagonist of prostaglandin D2 at the receptor level to reduce flushing and improve adherence to therapy - to effective LDL-C lowering therapy.
A total of 25,673 high-risk patients, 50 to 80 years of age, with prior CV disease (myocardial infarction “MI”, cerebrovascular disease, peripheral arterial disease, or diabetes mellitus with evidence of symptomatic CHD) were enrolled. No entry criteria regarding lipid levels were adopted in this study. After LDL-C lowering therapy had been standardized in the run-in phase using simvastatin 40 mg with or without ezetemibe 10 mg, patients were randomly assigned to receive two niacin-laropiprant combination tablets (a total of 2 g of niacin and 40 mg of laropiprant) daily or matching placebo.
The primary end-point was the first major vascular event (either major coronary event “non-fatal MI or death from coronary causes”, stroke, or coronary or non-coronary arterial revascularization. Secondary end-points included the components of the primary end-point, different types of stroke, and mortality (overall and in specific categories), the primary end-point after the exclusion of hemorrhagic stroke, or the primary end-point after the exclusion of both hemorrhagic stroke, and any arterial revascularization procedure.
Niacin-laropiprant was discontinued after randomization in 25.4% compared to 16.6% in the placebo group (p < 0.001). Over a median follow up period of 3.9 years, HDL-C levels increased by 6 mg/dL, triglycerides levels decreased by 33 mg/dL, and LDL-C levels decreased by 10 mg/dL in the niacin-laropiprant group. No significant difference in the incidence of major vascular events “primary end-point” was demonstrated between patients assigned to niacin-laropiprant and those assigned to placebo (13.2% vs. 13.6% respectively, p = 0.29). Similarly, no statistically significant differences were detected between both groups in terms of incidence of major coronary events (5.2% vs. 5.4% respectively; p = 0.5), stroke (3.9% vs. 3.9%; p = 0.56), major vascular events excluding hemorrhagic stroke (12.4% vs. 13.1% respectively, respectively; p = 0.12) or excluding both hemorrhagic stroke and revascularization procedures (7.9% vs. 8.4%, respectively; p = 0.20). Lack of efficacy was uniform in all subgroups defined according to different types of vascular disease or diabetes. Compared to placebo, niacin–laropiprant was associated with a statistically nonsignificant increase in all-cause mortality (6.2% vs. 5.7%, respectively; p = 0.08).
Patients assigned to niacin-laropiprant were more likely to suffer from disturbances in diabetes control (11.1% vs. 7.5%; p < 0.001), and had a new diagnosis of diabetes more frequently (5.7% vs. 4.3%, p < 0.001) compared to those assigned to placebo. Furthermore, in the niacin-laropiprant group, there was significant excess in serious adverse events associated with the gastrointestinal system (mostly bleeding and ulcerations) (4.8% vs. 3.8%, p < 0.001), myopathy (3.7% vs. 3.0%, p < 0.001), and skin rash/ulceration (0.7% vs. 0.4%, p = 0.003), as well as excess bleeding events -mostly gastrointestinal and intracranial (2.5% vs. 1.9%, p < 0.001), and infections (8.0% vs. 6.6%, p < 0.001).
Aim-high Study
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study was a randomized, multicenter, prospective, controlled clinical trial that was conducted at 92 centers in the United States and Canada, and was published in the New England Journal of Medicine in December 2011.7
This study was designed to test whether extended-release niacin added to intensive statin therapy, as compared to statin therapy alone, would reduce the risk of CV events in patients with established atherosclerotic CV disease (defined as stable coronary, cerebrovascular or peripheral arterial disease) and atherogenic dyslipidemia (low baseline levels of HDL-C < 40 mg/dL for men; < 50 mg/dL for women, and elevated triglyceride levels 150 to 400 mg/dL).
A total of 3414 patients, 45 years of age or older, were randomly assigned to receive high dose (1500–2000 mg) extended release niacin or placebo. Both groups received simvastatin adjusted to maintain LDL-C level below 80 mg/dL. The primary end-point was the composite of death from CHD, non-fatal MI, ischemic stroke, hospitalization (>23 h) for an acute coronary syndrome (ACS), or symptom-driven coronary or cerebral revascularization. Secondary end-points included the composite of death from CHD, non-fatal MI, ischemic stroke, or hospitalization for a “high-risk” ACS (characterized by accelerating ischemic symptoms or prolonged chest pain with electrocardiographic evidence of ischemia or biomarker values greater than, but less than 2 times, the upper limit of the normal range); death from CHD; non-fatal MI; ischemic stroke; or death from CV causes. The study was terminated after a mean follow up period of 3 years due to lack of clinically meaningful efficacy. Over 3 years, HDL-C levels increased by 9.6 mg/dl (35%) in the niacin group compared to 4.2 mg/dl (9.8%) in the placebo group (p < 0.001), whereas triglycerides levels decreased by 28.6% in the niacin group compared to 8.1% in the placebo group. The use of aspirin, beta-blockers, and inhibitors of renin angiotensin system was similar in both groups. Niacin was discontinued after randomization in 25.4% compared to 20.1% in the placebo group (p < 0.001). Flushing and itching was the primary reason for discontinuation in the niacin group (6.1%).
The primary end-point occurred in 16.4% in the niacin group and in 16.2% in the placebo group (hazard ratio with niacin = 1.02; 95% CI = 0.87–1.21, p = 0.8). There was no statistically significant difference in the composite secondary end point between patients assigned to niacin and those assigned to placebo (hazard ratio = 1.08; 95% CI = 0.87 to 1.34, p = 0.49). Among the components of primary end-point, unexpected increase in the rate of ischemic stroke was noticed in the niacin group (1.6%) compared to placebo group (0.9%).
Because of the large number of serious adverse events especially infection and bleeding which have been observed in the HPS2-THRIVE study, the pattern of serious side effects associated with niacin in the AIM-HIGH study was reported in a recent issue in the New England Journal of Medicine in July 2014.8 A significant increase in the rate of gastrointestinal side effects (7.4% vs. 5.5%, p = 0.02), and infections (8.1% vs. 5.8%, p = 0.008) were reported in the niacin group. There was however no significant difference in the rates of bleeding events compared to placebo (3.4% vs. 2.9%, p = 0.36).
Dal-outcomes Study
The Effects of the Cholesterol Ester Transfer Protein Inhibitor Dalcetrapib in Patients with Recent Acute Coronary Syndrome (dal-OUTCOMES) study was a randomized, multi-center, randomized, double-blind, controlled clinical study that was published in the New England Journal of Medicine in November 2012.5 This study was designed to determine whether the CETP inhibitor dalcetrapib reduces CV mortality or morbidity in stable CHD patients who had experienced recent ACS.
Dalcetrapib has minimal effects on levels of LDL-C and triglycerides; thereby the dal-OUTCOMES trial provided an important opportunity to test the clinical benefit of raising HDL-C level. A total of 15,600 patients were randomly assigned to receive dalcetrapib, at a dose of 600 mg daily, or placebo. Both groups received a statin adjusted to achieve a target LDL-C of 100 mg/dL or less and preferably < 70 mg/dL.
The primary efficacy end-point was a composite of death from CHD, non-fatal MI, ischemic stroke, unstable angina, or cardiac arrest with resuscitation. Secondary efficacy end-points included each component of the primary composite end-point, unanticipated coronary revascularization, death from any cause, and changes in levels of circulating lipoproteins and inflammatory markers.
The study was terminated for futility. Over a follow up period of 31 months, HDL-C levels substantially increased by 31 to 40% in the dalcetrapib group and by 4 to 11% in the placebo group with minimal effect on LDL-C and fasting blood glucose levels. Dalcetrapib had no significant effect on the primary end-point, as compared to placebo (8.3% vs 8.0% respectively, hazard ratio with dalcetrapib, 1.04; 95% CI, 0.93–1.16; p = 0.52). Dalcetrapib also had no significant effect on the rate of any component of the primary end-point, on the rate of unanticipated coronary revascularization, or on the rate of death from any cause. C reactive protein (CRP) level was significantly higher in the dalcetrapib group than in the placebo group (1.6 vs. 1.4 mg/L respectively, p < 0.001).
Regarding the safety profile of dalcetrapib, the mean systolic blood pressure (BP) was approximately 0.6 mmHg higher in the dalcetrapib group than in the placebo group (p < 0.001). There were no significant differences in diastolic blood pressure (BP), pulse rate, plasma aldosterone level, or the number of prescribed antihypertensive medications between both groups.
Discussion
Despite advances in optimal medical treatment – especially intensive statin therapy – randomized trials have demonstrated a residual 20–25% incidence of major CV events in the 2 years following an ACS.9 In the Treating to New Targets (TNT) study, Low HDL-C has been reported to predict residual CV risk after optimal statin therapy,10 thereby modification of HDL-C may be an important therapeutic target. Unfortunately, the string of failures for HDL therapies continues unabated. It is disappointing that all existing HDL-C boosting drugs consistently failed to have an impact on clinical outcomes in several large randomized clinical trials. In accordance with the results of the AIM-HIGH study, data by the HPS2-THRIVE collaborative group revealed no incremental clinical benefit from niacin-laropiprant combination to standard LDL-C lowering therapy.
Moreover excess serious adverse events were unexpectedly demonstrated in HPS2-THRIVE study, which were at least partially confirmed, by a recent report from the AIM-HIGH investigators. Of further concern, a trend towards 9% increase in the risk of death (number needed to harm 200, p = 0.08) was reported in patients assigned to niacin–laropiprant in HPS2-THRIVE study which strikingly tackle the safety profile of this drug.
The initial enthusiasm for CETP inhibitors has been also tempered by the surprising failure of torcetrapib in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) study,4 and then failure of dalcetrapib to show any reduction in CV risk despite significant increase in plasma HDL-C levels in the dal-OUTCOMES study. Torcetrapib has been also associated with significantly increased risk of death, hyperaldosteronism, and hypertension which most likely attributed to its off-target toxicity rather than the inhibition of CETP.4 Despite these negative results, the clinical efficacy and safety of other two CETP inhibitors, evacetrapib and anacetrapib, are currently being evaluated in two large phase III studies; ACCELERATE (Assessment of Clinical Effects of Cholesterol Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High-Risk for Vascular Outcomes) and REVEAL (Randomized EValuation of the Effects of Anacetrapib through Lipid-modification) studies. Importantly and in contrast to dalcetrapib, both evacetrapib and anacetrapib significantly decrease LDL-C and lipoprotein (a) in addition to marked increase in HDL-C. Thus, it will be very difficult to discern what causes the benefit if they have positive impact on CV risk.
In light of these studies, certain points should be raised to conclude whether the recent data had cast a pall over the HDL hypothesis or it is still valid:
-
(1)
It seems that raising HDL-C level when LDL-C is very low is not going to be beneficial. It is worth mentioning that patients in all studies were pre-treated with high intensity statin therapy that made baseline lipid profile was relatively normal before randomization. Of note, in the original Coronary Drug Project,11 before the statin era, high dose immediate release niacin was associated with a significant reduction (14%) in the rate of death from CHD or MI and a reduction (26%) in the rate of strokes or transient ischemic attacks.
-
(2)
The studies are too far from reality. It is very unlikely that niacin (or any other HDL-C raising medications) would be prescribed to patients with lipoprotein profile similar to that in these studies before randomization. In the HPS2-THRIVE study, average baseline LDL-C was 63 mg/dL, HDL-C was 44 mg/dL, and triglycerides was125 mg/dL before study drug treatment. Of further concern, major vascular event reduction in the niacin-laropiprant group was strongly predicted by baseline LDL-C (p = 0.02), with apparent clinical benefit if baseline LDL-C level was above 58 mg/dL.12 Indeed, it might not be expected to get clinical benefit if patients were on the flat part of the event curve. However it is still uncertain whether niacin or CETP inhibitor would benefit patients without very low LDL-C level.
-
(3)
HPS2-THRIVE and dal-OUTCOMES studies are all-comers study with no specific entry criteria regarding lipid levels, thus these studies didn't really target specific group of patients in whom benefit is anticipated from the studied drug.
-
(4)
Not all HDL is created equal. HDL particles are very complex and heterogeneous in composition and function. Moreover, the composition and function of HDLs might have been altered in patients with established CV disease. It has been reported recently that Low HDL3 cholesterol, but not HDL2 cholesterol, is associated with increased risk of death and MI, highlighting the potential value of subclassifying HDL-C in patients with established CHD.13 Indeed, changes in HDL-C levels may not reflect changes in the physiologic functions of HDLs.14
-
(5)
Patients in the placebo group in AIM-HIGH study had received 50 mg immediate release niacin in each placebo tablet in order to mask the identity of treatment to patients or study personnel. Per protocol, the placebo group in all studies received also high dose of statin with or without ezetimibe.
-
(6)
The early termination of the AIM-HIGH and dal-OUTCOMES studies might not allow detection of positive results. In this context, the initial results of the Veterans Affairs Cooperative Studies Program High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) was also negative at 3 years, however gemfibrozil therapy resulted in a significant reduction in the risk of major CV events at 5 years in patients with CAD whose HDL-C less than or equal 40 mg/dL.15
-
(7)
Half the patients in the HPS2-THRIVE study were recruited from the centre of China and it is well known that Asians have higher rate of side effects with niacin.
-
(8)
The favorable effect of CETP inhibition by dalcetrapib may be offset by its unfavorable effects on BP, CRP. The more potent anacetrapib and evacetrapib that lead to near complete inhibition of CETP activity may have a better effect on clinical outcome than dalectrapib that leads to only 50% inhibition of CETP activity.
What have we learned?
Current evidence reveals doubts about the safety profile of niacin and accordingly it should not be used routinely to reduce CV risk. Until further data is available and In agreement with the recent American College of Cardiology guidelines,16 no additional benefit has been established from the addition of non-statin therapy to further treat non-HDL-C, once LDL-C goal is achieved. However, niacin may be reserved cautiously to patients with contraindications for statin therapy, statin intolerance, or as a valuable adjunct to lifestyle modification, fibrates and omega 3 fatty acids in patients with severe hypertriglyceridemia to prevent pancreatitis. Treatment should be individualized and the potential benefit must be weighed against its off-target hazards.
It is uncertain whether HDL-C is a causal risk factor or a risk marker. We are looking forward the results of ACCELERATE and REVEAL studies in order to have a final conclusion. Ultimately, new therapeutic targets should be investigated such as lowering apoC3 which has been shown to significantly reduce plasma levels of triglycerides and increase HDL-C level. Interestingly, loss of function mutation of apoC3 has been recently reported to be associated with reduced risk of CHD.17
References
- 1.Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl):S11–S20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins JT, Ning H, Stone NJ, Criqui MH, Zhao L, Lloyd-jones DM. Coronary heart disease risks associated with high levels of HDL cholesterol. J Am Hear Assoc. 2014;3:e000519. doi: 10.1161/JAHA.113.000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101(8A):20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, ILLUMINATE Investigators Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz G, Chaitman BBR, Holme IM, Kallend DD, Leiter LA, Leitersdorf E, Mcmurray JJV. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 6.HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 7.AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2012;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.Anderson TJ, Boden WE, Desvigne-Nickens P, Fleg JL, Kashyap ML, McBride R, Probstfield JL, AIM-HIGH Investigators Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371(3):288–290. doi: 10.1056/NEJMc1311039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM, Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 10.Waters DD, Guyton JR, Herrington DM, McGowan MP, Wenger NK, Shear C. Treating to New Targets (TNT) Study: does lowering low-density lipoprotein cholesterol levels below currently recommended guidelines yield incremental clinical benefit? Am J Cardiol. 2004;93(2):154–158. doi: 10.1016/j.amjcard.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- 12.HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 13.Martin SS, Khokhar AA, May HT, Kulkarni KR, Blaha MJ, Joshi PH, Toth PP, Muhlestein JB, Anderson JL, Knight S, Li Y, Spertus JA, Jones SR, on behalf of the Lipoprotein Investigators Collaborative (LIC) HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the lipoprotein investigators collaborative. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu264. pii: ehu264. http://dx.doi.org/10.1093/eurheartj/ ehu264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenson RS, Bryan Brewer H, Jr, Sean D, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang X-C, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Contemporary reviews in cardiovascular medicine: Cholesterol efflux and atheroprotection. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high density lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 16.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 17.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute, Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, DePristo M, Gupta N, Escher SA, Jansson JH, Van Zuydam N, Palmer CN, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O'Donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho-Melander M, Ardissino D, Loos RJ, McPherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin DY, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E, Kathiresan S. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;(371):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]