Introduction
Obesity, insulin resistance, and metabolic syndrome are escalating problems in developed and developing countries. High plasma triglyceride (TG) levels are commonly encountered in clinical practice; 30% of the adult US population has a TG level above 150 mg/dL.1 Hypertriglyceridemia is perceived to be associated with increased risk of coronary heart disease (CHD) in many epidemiological studies,2 however the independency of TG level as a causal risk factor in promoting cardiovascular (CV) disease has remained a matter of debate for more than 3 decades.1,3
Several mechanisms may link triglycerides to atherogenesis; (1) High very low density lipoprotein (VLDL) triglyceride output activates cholesterol ester transfer protein (CETP) which leads to triglyceride enrichment of low density lipoprotein (LDL) and the formation of small dense LDL particles which are more susceptible to oxidative modification.4 (2) Triglyceride enrichment of high density lipoprotein (HDL) particles makes them dysfunctional.5 (3) Postprandial triglycerides has been reported to induce apoptosis, and increase the expression of pro-inflammatory genes.6 (4) Remnant lipoprotein particles (by-products of triglyceride rich lipoproteins “TRL” hydrolysis) may interfere with the function of endothelial progenitor cells, and lead to foam cell formation in a manner analogous to modified LDL particles.1 (5) Liberation of free fatty acids, monoacylglycerols during TRL lipolysis could cause local injury and inflammation of vascular endothelium.
APOC3 – a 79-amino acid glycoprotein that is a major component of circulating TRL – (Figure 1) has received recently increasing attention. APOC3 inhibits TRL hydrolysis and promotes pro-atherogenic responses in macrophages and endothelial cells through activation of adhesion and pro-inflammatory molecules expression, and impairment of endothelial nitric oxide production and insulin signaling pathways.7,8 Carriers of a null mutation (R19X) in APOC3 gene in the genome-wide association study (GWAS) had significantly lower triglyceride levels, higher HDL-C level, and reduced coronary artery calcification compared to non-carriers, suggesting a potential cardioprotective effect for lifelong deficiency of APOC3.8
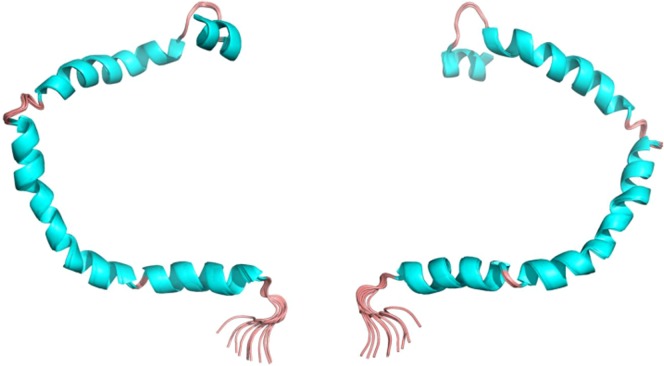
Figure 1.
3D structure of human apolipoprotein C3 (APOC3). Image shows the NMR structures of the front (left) and back (right). This representation shows the secondary structures [helices are cyan and turns, pink].
Data from the TG and HDL Working Group of the Exome Sequencing Project provide insights into the association between APOC3 genotype, plasma lipid profile, and risk of clinical CHD.
The study
This study was conducted as a part of the Exome Sequencing Project of the National Heart, Lung, and Blood Institute (NHLBI), and published in the New England Journal of Medicine in June 2014.9 Seven population-based cohorts (Atherosclerosis Risk in Communities, Coronary Artery Risk Development in Young Adults, the Cardiovascular Health Study, the Framingham Heart Study, the Jackson Heart Study, the Multiethnic Study of Atherosclerosis, and the Women's Health Initiative) and participants in the Myocardial Infarction Genetics Consortium were included.
Among the 6823 subjects for whom sequencing was performed through the project, 3734 participants were eligible for this study. The protein-coding regions (exons) of 18,666 genes – total of 256,143 exons, collectively named the exome - in each participant were sequenced. The gene encoding for APOC3 was identified to be strongly associated with plasma TG levels. Specifically, four rare mutations in the APOC3 gene (a nonsense mutation (R19X), two splice-site mutations (IVS2+1G → A and IVS3+1G → T), and a missense mutation (A43T)) were associated with lower plasma TG levels. Carriers of any of these mutations had 39% lower plasma TG levels (84.5 vs. 137.5 mg/dL respectively, p = 6 × 10− 9), 22% higher HDL-C levels (61.9 vs. 50.7 mg/dL respectively, p = 3 × 10− 6), and 16% lower LDL-C levels (122.8 vs. 146.2 mg/dL respectively, p = 0.05) than non-carriers.
Approximately 1 in 150 participants was a heterozygous carrier of at least one of these mutations. Carriers of APOC3 loss-of-function mutation had 40% lower risk of CHD than that in non-carriers (odds ratio 0.60; 95% confidence interval (CI): 0.47 to 0.75; p = 4 × 10− 6). Participants with values in the lowest third of the distribution of plasma APOC3 levels had a reduced risk of incident CV events, compared to those with values in the highest third. This association was abrogated after adjustment for other CV risk factors in the Framingham Heart Study, however it was maintained in the Verona Heart study (a study included in the Myocardial Infarction Genetics Consortium).
Discussion
Hypertriglyceridemia is typically heritable, and results from the cumulative burden of variants in more than 30 genes interacting together with several lifestyle factors, most importantly overweight and obesity.10 Growing evidence from genetic studies has renewed interest in the potential association between elevated triglycerides concentrations and increased risk of CV diseases, specifically after the grave doubts about the power of HDL-C as a causal CV risk factor. Recently published data from Copenhagen City Heart Study (CCHS) and Copenhagen General Population Study (CGPS) have confirmed a significantly lower incidence of CHD in participants with non-fasting triglyceride levels of less than 1.00 mmol/L (90 mg/dL), compared to those with levels of 4.00 mmol/L(350 mg/dL) or more (hazard ratio = 0.40; 95% CI = 0.31 to 0.52, p < 0.01).11 Moreover, an increase of 1 mmol/L in remnant lipoprotein cholesterol was associated with a 2.8 times increased risk of CHD in a recent Mendelian randomization study.12
Loss of APOC3 function seems to confer a cardioprotection against CHD. In accordance with a prior study by Pollin et al.8, data from the NHLBI Exome Sequencing Project, CCHS, and CGPS provide more evidence regarding the favorable impact of reduced APOC3 expression on plasma lipid profile and CV outcome.9,11 Interestingly, lipid lowering agents such as fibrates acts partially through indirect lowering of APOC3 expression.13 In addition, the use of statins, thiazolidinediones, ezetimibe, niacin, fish oil and weight loss, has been associated with decreases in plasma APOC3 levels.14
In the same context, Kathiresan et al.15 has recently confirmed a highly significant association between the effects of single nucleotide polymorphism (SNPs) on triglyceride levels and CHD risk, even after accounting for their confounding effect on HDL-C or LDL-C levels. Kathiresan et al. have previously applied the same approach to LDL and HDL in studies that confirmed a causal role for LDL-C and raised doubt on the independent role for HDL-C in the development of CHD.
Regulating APOC3 metabolism may be an important novel therapeutic approach in the near future to manage dyslipidemia and reduce CVD risk - in a manner similar to the development of monoclonal antibodies directed against proprotein convertase subtilisin/kexin type 9 (PCSK9) based on the original data derived from PCSK9 gene studies. In fact, an antisense oligonucleotide therapeutic agent that decreases the production of APOC3 has recently been investigated and shown to be associated with reduced plasma TG levels in mice, nonhuman primates, and healthy human volunteers.16 With all of these studies and the resurgence of new therapeutic targets comes the hope for more efficient management of dyslipidemia and subsequent elimination of residual CV risk.
What have we learned?
Genetic studies strongly support a causal association between plasma triglyceride concentrations and CV disease. Low HDL-C might merely be a marker of raised triglycerides and remnant cholesterol. These data may renew the potential benefit of therapeutic lowering of triglyceride levels to reduce the risk of CAD. Large scale randomized trials are urgently needed to assess whether triglyceride-lowering reduces CV disease in patients with raised triglycerides. Moreover, future studies are required to fully understand APOC3 complex physiology and pathophysiology and to establish the practical value of its routine assay in the laboratory, and most importantly the clinical efficacy and safety of novel drug therapy targeting APOC3.
References
- 1.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S, American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease Triglycerides and cardiovascular disease: A scientific statement from the american heart association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 2.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Heiss G, Cohn R, Cowan LD, Suchindran CM, Bangdiwala S, Kritchevsky S, Jacobs DR, Jr, O'Grady HK, Davis CE. Plasma triglyceride level and mortality from coronary heart disease. N Engl J Med. 1993;328(17):1220–1225. doi: 10.1056/NEJM199304293281702. [DOI] [PubMed] [Google Scholar]
- 4.Chait A, Brazg RL, Tribble DL, Krauss RM. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med. 1993;94(4):350–356. doi: 10.1016/0002-9343(93)90144-e. [DOI] [PubMed] [Google Scholar]
- 5.Greene DJ, Skeggs JW, Morton RE. Elevated triglyceride content diminishes the capacity of high density lipoprotein to deliver cholesteryl esters via the scavenger receptor class B type I (SR-BI) J Biol Chem. 2001;276(7):4804–4811. doi: 10.1074/jbc.M008725200. [DOI] [PubMed] [Google Scholar]
- 6.Norata GD, Grigore L, Raselli S, Redaelli L, Hamsten A, Maggi F, Eriksson P, Catapano AL. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: Molecular mechanisms and gene expression studies. Atherosclerosis. 2007;193(2):321–327. doi: 10.1016/j.atherosclerosis.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113(5):691–700. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
- 8.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322(5908):1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute, Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, DePristo M, Gupta N, Escher SA, Jansson JH, Van Zuydam N, Palmer CN, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O'Donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho-Melander M, Ardissino D, Loos RJ, McPherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin DY, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E, Kathiresan S. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, Borén J, Bruckert E, Catapano AL, Descamps OS, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AF, Stroes E, Taskinen MR, Tybjærg-Hansen A, Watts GF, Wiklund O, European Atherosclerosis Society Consensus Panel The polygenic nature of hypertriglyceridaemia: Implications for definition, diagnosis, and management. lancet. Diabetes Endocrinol. 2014;2(8):655–666. doi: 10.1016/S2213-8587(13)70191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 12.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61(4):427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 13.Auwerx J, Schoonjans K, Fruchart JC, Staels B. Transcriptional control of triglyceride metabolism: Fibrates and fatty acids change the expression of the LPL and apo C-III genes by activating the nuclear receptor PPAR. Atherosclerosis. 1996;124:S29–S37. doi: 10.1016/0021-9150(96)05854-6. [DOI] [PubMed] [Google Scholar]
- 14.Ooi EMM, Barrett PHR, Chan DC, Watts GF. Apolipoprotein C-III: Understanding an emerging cardiovascular risk factor. Clin Sci (Lond) 2008;114(10):611–624. doi: 10.1042/CS20070308. [DOI] [PubMed] [Google Scholar]
- 15.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Döring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Altshuler D, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Mohlke KL, Ingelsson E, Abecasis GR, Daly MJ, Neale BM, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45(11):1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham MJ, Lee RG, Bell TA, 3rd, Fu W, Mullick AE, Alexander VJ, Singleton W, Viney N, Geary R, Su J, Baker BF, Burkey J, Crooke ST, Crooke RM. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112(11):1479–1490. doi: 10.1161/CIRCRESAHA.111.300367. [DOI] [PubMed] [Google Scholar]