Abstract
Phosphodiesterase inhibitors (PDE) can be used as therapeutic agents for various diseases such as dementia, depression, schizophrenia and erectile dysfunction in men, as well as congestive heart failure, chronic obstructive pulmonary disease, rheumatoid arthritis, other inflammatory diseases, diabetes and various other conditions. In this review we will concentrate on one type of PDE, mainly PDE5 and its role in pulmonary vascular diseases.
Keywords: pulmonary vascular disease, pulmonary phosphodiesterase inhibitors, pulmonary hypertension
Introduction
Phosphodiesterase inhibitors (PDE) can be used as therapeutic agents for various diseases such as dementia, depression, schizophrenia and erectile dysfunction in men,1 as well as congestive heart failure,2 chronic obstructive pulmonary disease, rheumatoid arthritis, other inflammatory diseases,3,4, diabetes5 and various other conditions.6 In this review we will concentrate on one type of PDE, mainly PDE5 and its role in pulmonary vascular diseases (PVDs).7 The PDE5 has been targeted by specific inhibitors (mostly cGMP analogs), such as sildenafil, vardenfil, and tadalafil (See Figure 1).8
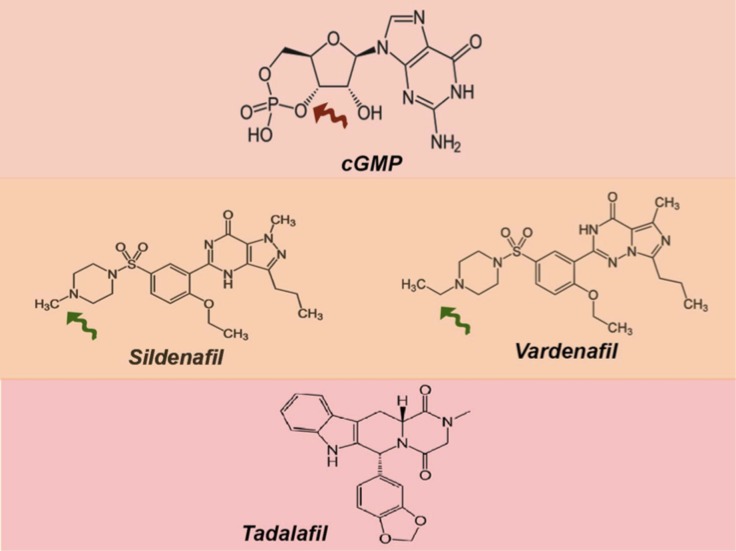
Figure 1.
The chemical structure of the cGMP (brown arrow indicates phosphodiester bond). The lower panel shows the chemical structure of sildenafil and vardenfil. Notice the similarity in structure, except the nitrogen atom's position and the change of sildenafil's piperazinering from a methyl group to an ethyl group (green arrows). The bottom structure is for tadalafil which is more different the other PDE5 inhibitors, which explains the binding affinity to the PDE5 enzyme and thus its longer half-life.
NO-cGMP signaling pathway and role cGMP as a regulator of pulmonary vascular homeostasis
Unlike the systemic circulation, the pulmonary circulation is a low pressure system, and therefore regulation may differ from the systemic circulation. The regulation and adaptation of the pulmonary circulation can be regulated by various remodelling mechanisms, including the process of angiogenesis (a physiological process through which new blood vessels are formed from pre-existing vessels) or vasculogenesis (the formation of new blood vessels when there are no pre-existing ones). The disturbance of these mechanisms can lead to the abnormal increase in blood pressure and to the development of pulmonary hypertension.
A major regulatory mechanism involved the discovery of the endothelium-derived relaxing factor (EDRF) by Furchgott and Zawadzki in 1980.9 EDRF contributes to the relaxation of arterial smooth muscle by acetylcholine, and was identified to be nitric oxide in 1987.10 Nitric oxide (NO) is a highly reactive molecule, which is synthesized from l-arginine by a nitric oxide synthase. There are usually a few forms of nitric oxide synthase, namely endothelial nitric oxide synthase (eNOS) in the vascular smooth muscle endothelial cells, and inducible nitric oxide synthase (iNOS) in the neuronal ends. When the nitric oxide is synthesized in the endothelial cells, it diffuses into the vascular smooth muscles, where it will stimulate another protein called soluble guanylate cyclase. In turn, this protein helps produce cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP). The other mechanism for stimulating production of cyclic GMP is through natriuretic peptide (the atrial natriuretic peptide (ANP)) and brain natriuretic peptide (BNP). These ligands are linked to specific cell membrane receptors called natriuretic peptide receptors.11 In essence, these receptors are trans-membrane guanylyl cyclase which converts GTP to cGMP. cGMP will then stimulate cGMP-dependent protein kinase (PKG), which has many effects including smooth muscle relaxation. cGMP which plays a very important role in the physiology of the vascular system (see Figure 2 for more details. For full review see references12,13).
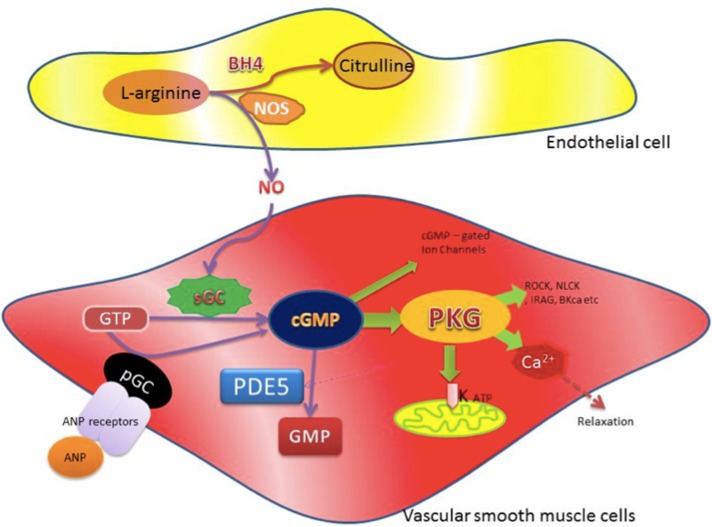
Figure 2.
Schematic diagram shows the synthesis of cGMP and its physiological effects. NO is synthesized from L-arginine by NO synthases (NOS) with the cofactor tetrahydrobiopterin (BH4). In vascular smooth muscle cells, NO interacts with soluble guanylyl cyclase (sGC) to convert guanosine triphosphate (GTP) to cyclic GMP. Alternatively, cGMP can also be produced from GTP via particulate guanylyl cyclase (pGC), which is part of the Atrial Natriuretic Peptide (ANP) receptors on the cell members that can be active via ANP. cGMP has a wide variety of physiological activities, in particular its activation of protein Kinase G (PKG). Activation of PKG results in the regulation, contraction, and proliferation as well as survival of vascular smooth muscles by various signaling mechanism, including Rho kinase (ROCK), inositol 1,4,5-triphosphate receptor-associated PKG substrate (IRAG), myosin light chain kinase (MLCK), calcium-sensitive potassium channels (BKCa), etc. PKG also plays a part in regulating subsequent potassium channel openings and mitochondrial ion channels homeostasis as well as hemostasis of Ca2+, which regulates vascular smooth muscle contraction and thus mediates relaxation of vascular smooth muscle. It also activates phosphodiesterases (PDE5), which regulates the cGMP by enhancing its breakdown.
The endothelial cells line the blood vessels, and play a crucial role in the physiology of blood vessels and the regulation of blood flow and pressure. They are similarly important in the remodeling process, which can be the result of pathological changes that take place during the pathogenesis of pulmonary hypertension.14,15
Therefore cGMP plays a crucial part in the regulation and development of the pulmonary circulation,12,16–18 and in the regulation of pulmonary vascular tone, structure and survival. Thus its intracellular regulation is a very important step in the signaling mechanism. One of the major regulator of the cGMP is phosphodiesterase, and in particular PDE5.
Chemistry and enzymology of PDE5
In chemistry, the hydroxyl groups in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds called phosphodiester bond. This reaction is catalyzed by ligases. This type of bond is widely seen in biology, for example in the backbone of DNA (DNA ligase during DNA replication) and RNA. It is also observed in some second messengers, such as cyclic nucleotide, and in particular in the case of cyclic nucleotide like cAMP (synthesized from ATP by adenylyl cyclase) or cGMP by guanylate cyclase catalyzes, which support the reaction of guanosine triphosphate (GTP) to 3′,5′-cyclic guanosine monophosphate (cGMP) and pyrophosphate, which act as secondary messenger signaling pathways (Figure 3).
Figure 3.
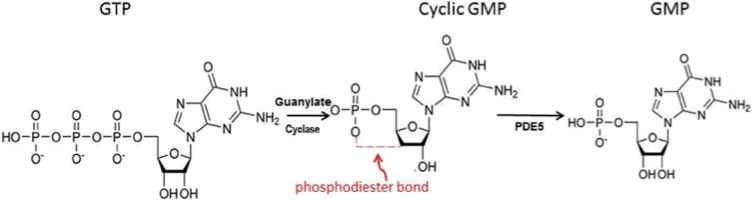
The formation of phosphodiester bond and formation of the cGMP from GTP, and the role of PDE5 in breaking this bond.
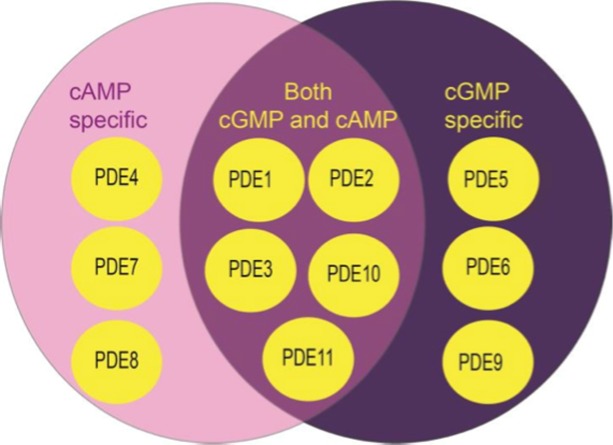
A phosphodiesterase (PDE) is any enzyme that breaks a phosphodiester bond. There are many families of phosphodiesterase enzymes, mainly grouped on their target, the cyclic nucleotide. This family of enzymes degrades the phosphodiester bond in the second messenger molecules. Thus PDE plays a vital role in the regulation of cell cyclic nucleotide signaling within subcellular domains.19–23 Different PDEs of the same family are functionally related despite the fact that their amino acid sequences can show considerable divergence. Eleven PDEs have been identified in this family, each withdifferent substrate specificities (hydrolysis of cAMP or cGMP) (Figure 4).
Figure 4.
The PDEs family and the classification according to their target.
Each family is encoded by 1–4 genes, thus a total of 21 PDE Isoforms are recognized, and they are classified and named by a common convention. The most widely accepted nomenclature signifies the PDE family with an Arabic numeral,1–5,7–12 then a capital letter denotes the gene in that family, and a second and final Arabic numeral indicates the splice variant derived from a single gene (e.g., PDE1C3: family 1, gene C, splicing variant 3).24–26
The molecular structure of the PDE5
PDE5 enzyme in the cell is a homodimer, which means it is comprised of two identical units approximately equal in size. Each monomer contains a carboxyl-terminal catalytic domain, with a highly conserved zinc-binding motif, and regulatory domain with two allosteric binding pockets for cGMP, a phosphorylation site (Figure 5).6,25,27–29
Figure 5.
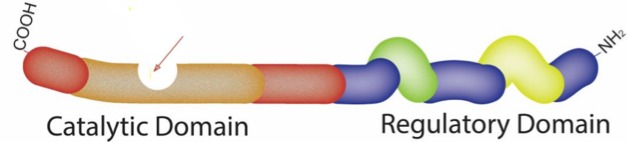
The schematic diagram of the PDE5 monomer, the PDE5 enzyme in the cell is a homodimer, which means it is comprised of two identical units approximately equal in size (see Figure 7). Each monomer contains a carboxyl-terminal catalytic domain (left) with a special pocket to occupy cGMP (or PDE5 inhibitor) for the catalytic role of enzyme (arrow) the detail of the pocket is shown in Figure 6.The other half (to the right is regulatory domain with two allosteric binding pockets for cGMP the regulatory domain GAF-A (yellow) and GAF –B (green), and, a phosphorylation site.
Catalytic domains. All PDEs contain a conserved catalytic domain of approximately 270 amino acids at the carboxyl terminus. This domain contains the active pocket that accommodates ligands, such as cyclic nucleotides and PDE inhibitors, where catalysis hydrolysis of the phosphodiesterase bond takes place The crystal structures of the catalytic domains suggest that this domain is composed of three subdomains. In PDE5 these domains are an N-terminal cyclin-fold domain (residues 537–678 about 141 amino acids), a linker helical domain (residues 679–725 about 46 amino acids), and a C-terminal helical bundle domain (residues 726–860 about 140 amino acids).6,19,21,23,25
Functionally, the catalytic core can be also be divided into sub-sites, these include metal-binding site (M site), which has some metal atoms (mainly zinc and magnesium), which bind to residues that are completely conserved in all PDE family members. Metal ions bound can stabilize binding of the ligands into the catalytic pocket and support conformational changes (see below).30 The other functional sites are the core pocket (Q pocket), hydrophobic pocket (H pocket) and Lid region (L region).1,14
In this catalytic pocket, ligands like cyclic nucleotide or PDE5 inhibitors have special bind with various amino acids that contribute to the affinity of the ligand to this site. Cyclic GMP will bind by hydrogen bond of various amino acids (Figure 6A). One of the most important amino acids is Gln817 (Glutamine at the site of 817 in the sequence of amino acid of the protein). This amino acid also contributes to the PDE5 selectivity toward cyclic nucleotide (cGMP vs cAMP). This is done by special orientation of the side chain of Gly817 that binds the purine moiety in cAMP or cGMP. The orientation can be done with the help of another amino (Gly775) in the Q core. This special mechanism has been dubbed “glutamine switch”.31,32 Furthermore, cGMP in particular is sandwiched between Gly817 and Phe820, described as “hydrophobic clamp”. Other amino acid are involved with other inhibitors which are highly conserved in all PDEs. The other import binding location within the catalytic pocket for cGMP is the H pocket. Although this pocket is of great importance to the cGMP binding, it is of less use to PDE5 inhibitors such as sildenafil.
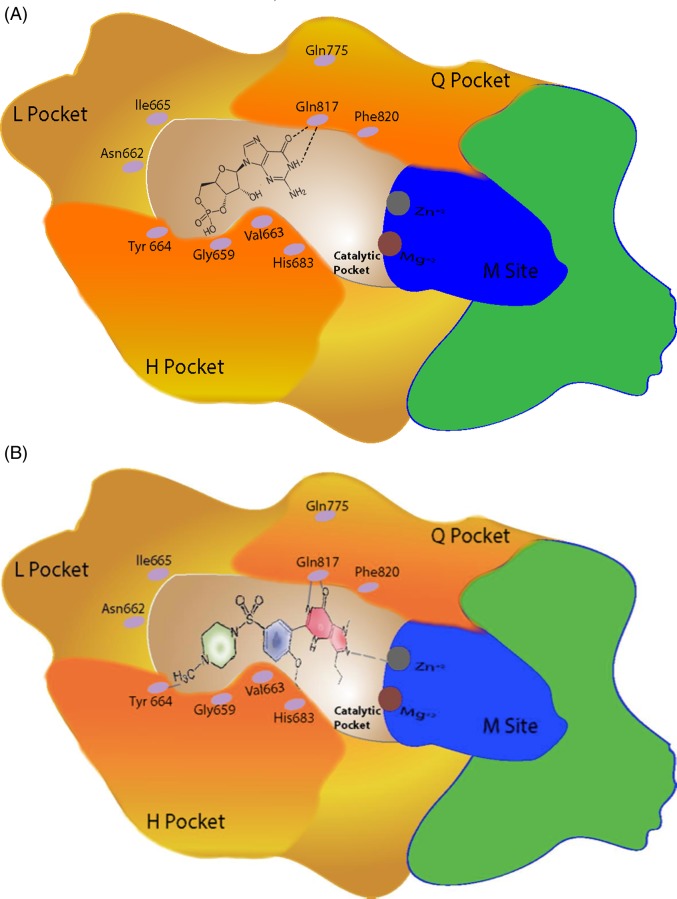
Figure 6.
schematic diagram of the surrounding of the Catalytic Pocket (arrow in Figure 5) See details in the text. (A) shows cGMP occupies the Catalytic Pocket and in (B) when PDE5 inhibitor (sildenafil) occupies the pocket. Note that the pyrazolopyrimidinone group (in red) is connected to the sildenafil. With the amino acid Gln 817 similar to that of cGMP (Glutamine switch with Gln775) with the support of, ZN2+ in the M site. The ethoxyphenyl group (blue) of sildenafil fits into the hydrophobic H pocket. The methylpiperazine group (green) of sildenafil is surrounded by other amino in particularly Tyr 664.
The crystal structure of PDE5A in complex with sildenafil showed that the Q pocket accommodates the heterocyclic ring of the pyrazolopyrimidinone group of sildenafil. The amide moiety of the pyrazolopyrimidinone group forms a bidentate hydrogen bond with Gln 817 similar to that of cGMP. In this region, ZN2+ is also involved and can contribute to the site and stability of the binding, and thereby contribute to the PDE catalytic function These is also some movement and conformational changes in this loop during activation of the enzyme, which plays an important part in increasing the affinity to the ligands.32,33 The ethoxyphenyl group of sildenafil fits into the hydrophobic H pocket,; but to sildenafil, this pocket is of less importance when compared to CGMP, as detailed above. The methylpiperazine group of sildenafil is surrounded by various other amino acid from the L and H pockets, particularly Tyr 664 (Figure 6B).31
Regulatory domains: The N-terminal regulatory domain varies widely among the PDE classes and includes regions that auto-inhibit the catalytic domains, and may play part in controlling the subcellular localizations of this enzyme. In certain PDEs, phosphorylation of the enzyme can take place in this domain. The reader can consult various recent reviews on this topic.19,21,25,34 However, in this review we will concentrate on the Phosphodiesterase 5. The PDE5 regulatory domain has two domain tandems, GAF-A and GAF -B. The GAF acronym is derived from the names of the first three classes of proteins recognized to be contained in this domain: mammalian cGMP-binding PDEs, Anabaenaadenylyl cyclases, and Escherichia coli FhlA. These are a type of protein domain that is found in a wide range of proteins from all species.35,36 cGMP binds to the GAF-A, but GAF-B is still a questionable site for the binding of cGMP. In addition, it contains a single phosphorylation site (serine-102 in the human enzyme) that can be phosphorylated by Protein kinase G (PKG).37
PDE5 isoforms: At present, only one gene for PDE5 has been discovered. Furthermore, the chromosomal location of the PDE5A gene was defined as chromosome 4q26.38 However, 3 variants (PDE5A1, 5A2, and 5A3) differ at their N-terminal regions. It is assumed, though it has not yet been clearly shown, that the different promoters for the PDE5 isoforms allow physiologically relevant differential control of PDE5 gene expression, thereby providing an additional mechanism for longer-term feedback regulation.39,40 In vitro tests have shown little differences among the three isoforms in cGMP catalytic activities and in sensitivities to PDE5-specific inhibitors, but may have a tissue distribution pattern.41,42
Localization of the PDE5 enzyme
Early identifications of PDE5 were reported in the 1970s and the early 1980s by various centers, and in particular by investigators from the Department of Physiology at Vanderbilt University in Nashville, Tennessee. Most of these are identified in many species and in various tissues with different concentration activity. There were high concentrations in the extracts of the lung, cerebellum, and Purkinje neurons, small intestine and platelets, and in certain tissue of the kidneys, particularly the proximal renal tubules and collecting duct. However, the concentration was low in extracts of the liver, adipose tissue, and skeletal muscle.43–50
By 1990, most of the various forms of phosphodiesterases known today were recognized.51 However, there is also a differential quantity difference among the three isoforms. PDE5A1 and PDE5A2 are ubiquitous in many tissues, but PDE5A3 is specific to smooth muscle52 to maintain the contracted state of contractile organs such as the uterus and penis (penile corpus cavernosum). PDE5 is abundant in the lung,48,53 mainly in the pulmonary vessel smooth muscles as well as in pulmonary artery endothelial cells. However, the expression of PDE5 is greater in lung tissues from patients with pulmonary hypertension compared with controls, especially the expression of PDE5A1. In particular, the cells of intimal lesions and neomuscularised distal vessels see greater PDE5 expression, and this is true also in smooth muscle cells in the medial layer of the diseased pulmonary vasculature.54 In fact, PDE5 expression is 15 times higher in the lung than in the heart.
The subject of PDE5 extracts in the heart has long been controversial, as it may be present at very low levels in normal hearts, but PDE5 is normally expressed in the coronary vasculature and not in myocytes. Yet induction of PDE5 expression happens in the right and left ventricular hypertrophy. Similarly, heart failure of patients with pulmonary hypertension or other causes of left ventricle failure were reported,55–57 which suggests that right ventricle PDE5 expression could contribute to the pathogenesis of tight ventricular failure, probably via an increase in the myocardial oxidative stress which causes a rise of PDE5 expression in the failing heart.58 These findings suggest that right ventricle PDE5 expression could contribute to the pathogenesis of RV failure, and that PDE5 inhibitors increase RV inotropy and decrease RV afterload without significantly affecting systemic hemodynamics.
Cellular distribution and subcellular localization: PDE5A is generally considered to be a cytosolic protein in the smooth muscle of all vascular beds. There is evidence that PDE5A may be compartmentalized, and that at least a portion of PDE5 may be concentrated around various intracellular organelles. PDE5A has been found at the level of caveolin-rich lipid rafts, where it allows for a feedback loop between endothelial PDE5A and nitric oxide synthase (NOS3) via cGMP primary location of PDE5A at or near caveolae in vascular endothelial cells.59 Furthermore, PDE5A does not always maintain its sarcomeric localization but can take on a more diffuse distribution in the cytosol. In the hypertrophied LV myocytes, immmunohistology has shown PDE5A normally localizes to the sarcomere z-disk.60,61
The dynamicity of the PDE5 enzyme
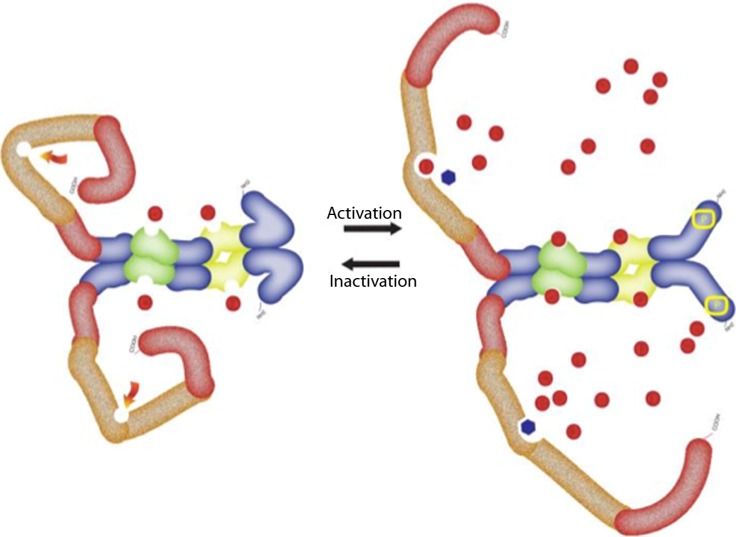
In the last decade, much has been discovered about the dynamic of the PDE5 enzyme. The PDE5 holoenzyme can be present in inactive and active forms associated with conformational changes which are important in the PDE5 function.34 These changes undergo both positive and negative regulatory feedback (Figure 7).
Figure 7.
The dynamic conformational changes of the PDE5 showing active and inactive form. For full details see text. The structure of the PDE5 as explained in Figure 5. The red dots represent cGMP and black dots represent PDE5 inhibitor.
In general when cGMP is elevated, there will be a need to increases the activity of the PDE5 to lower the cGMP level, which then leads to a decrease of the activity of PDE5. Thus PDE5 obeys the Michaelis-Menten kinetics so that mass action applies only when the cGMP level is near or below the Km of the enzyme for cGMP. cGMP occupy the allosteric binding sites in the regulatory domain, mainly in GAF-A whenever there is an increase in the intracellular cGMP. This allosterical activation is brought about by either a change in the dynamics of a protein or alteration in its mean structure, which is the beginning of the conformational changes of the PDE5 enzyme. It also exposes the enzyme for phosphorylation by protein kinase G (PKG). Phosphorylation itself will enhance the affinity of the allosteric binding in the GAF for more binding with cGMP.37 The conformational changes and phosphorylation of the PDE5 will increase the exposure of the catalytic site, and enhance the affinity of the catalytic site to cGMP or PDE5 inhibitors such as sildenafil.62–64
Recent findings, however, showed that the conformational change is in fact multiform, and dynamically differs if the cGMP or PDE5 inhibitor occupy the catalytic site. For example, in the case of sildenafil occupation, the catalytic site will delay the reversal of these changes.65 However, it was also observed that PDE5 inhibitors would stimulate their own affinities for PDE5, partly by enhancing phosphorylation of PDE5, and further by enhancing conformational changes of the PDE5 holoenzyme.66 This action would also block the cGMP-lowering negative-feedback process initiated by cGMP elevation since they would occupy the catalytic site to prevent cGMP breakdown. This is important in consideration of the pharmacodynamics of these drugs, as it may be partly responsible for the prolongation of the PDE5 inhibitors action even beyond the pharmacological half-life of the inhibitors. These unique features of the PDE5 catalytic domain and the sildenafil configuration are key considerations for understanding the action of sildenafil and for development of PDE5 inhibitors.67 Further details can be found in Francis et al.68 A reverse mechanism of deactivation of the enzyme can happen by dephosphorylating the enzyme with the help of the protein phosphatase type I (PP1 phosphatase) enzyme.69
Selective PDE5 inhibitors: Pharmacology and pharmacokinetics
The number of drugs that have been shown to inhibit the PDE5 selectively has been demonstrated since the early days of the discovery of this enzyme.70 The first drugs, including IBMX, dipyridamole and coffee, are all known to be potent but non-selective inhibitors. However, during the 1990s, a more specific drug, like Zaprinast, was described71–73 but could not find its way into clinical practice. Since then more specific enzymes have been developed,74 in particular sildenafil (Viagra, UK-92,480),73,75–77 tadalafil,78 vardenafil,79 Udenafil,80 Avanafil,81 and Mirodenafil.82 These drugs will inhibit the PDE5, and therefore increase cyclic GMP and the blood flow to the penile corpus cavernosum.83–85 Thus the last six drugs have been approved in the erectile dysfunction indication. It is very obvious that although they have very high specificity to PDE5, they still have effects on some other PDEs (Table 1), in particular PDE6 and PDE1, which has consequences for the side effects of these drugs- particularly in the retina, wherein PDE6 is the predominant enzyme. There are also differential effects on the other PDEs, like PDE1C and PDE11, but these do not have clinical significance as far as we currently know. Most of the licensed drugs differ significantly in their chemical structure, but they all inhibit median inhibitory concentration (IC50) values in the low nM range (Table 1).86–88 Some of the basic pharmacology can also be seen in Table 1.
Table 1.
Median inhibitory concentration values in nM (fold selectivity versus PDE5).
| Compound | PDE1 | PDE5 | PDE6 (rod) | PDE6 (cone) | Onset (min) | Bio-avilability | Tmax | plasma half life | Route of Clearance | available dose range |
| Sildenafil | 281 (80) | 3.5 | 37 (11) | 34 (10) | 30–60 | ∼40% | ∼1 h | ∼4 h | hepatic | “20–100” |
| Vardenafil | 70 (500) | 0.14 | 3.5 (25) | 0.6 (4) | “8–12” | 14.50% | ∼40 min | ∼4 h | hepatic | “5–20” |
| Tadalafil | >30000 (>4450) | 6.74 | 1260 (187) | 1300 (193) | “15–45” | ∼36% | ∼2 h | 15.5 h | hepatic | “5–20” |
The drugs are mainly metabolized in the liver by cytochrome P450 (CYP) 3A4, and to a lesser extent by CYP2C9. Thus significant drug–drug interaction through CYP3A4 has been reported in many studies. This results in significant increases in the peak plasma concentration and area under curve of most PDE5 inhibitors with no effect on plasma half-life,89 in particular with co-administration of potent CYP3A4 inhibitors, such as erythromycin, ketoconazole and saquinavir. One important interaction pertinent to the topic of this review is the collaboration with another pulmonary hypertension drug, namely bosentan. Bosentan decreased the maximum plasma concentration of sildenafil by 55.4%, whilst sildenafil increased bosentan's maximum plasma concentration by 42.0%.90 These combinations were very well tolerated in the clinical setting described below. The same can be applied to tadalafil where co-administration with bosentan decreased tadalafil exposure by 41.5% but incurred no significant changes in the bosentan level.91 However, these interactions are much smaller with ambrisentan, another once-a-day endothelial antagonist. HIV-infected patients taking protease inhibitors (saquinavir, indinavir and particularly ritonavir) significantly modify the pharmacokinetics of PDE5 inhibitors (sildenafil, tadalafil or vardenfil). This results in increased plasma concentration of both drug and metabolite,92–96 although a few recent papers do not report any significant adverse reactions to sildenafil during the follow-up period.97 Therefore, it is advisable to consider therapeutic drug monitoring during treatment in order to avoid possible over-dosage. On the basis of these interactions, phosphodiesterase inhibitors, when used for pulmonary arterial hypertension in HIV patients, should be used with caution and with considerable consideration, as it should be avoided in combination with many HIV drugs such as boceprevir and telaprevir.
PDE5 inhibitors in the experimental models in pulmonary vascular diseases
During the 1990s, there was some interest in the role of PDE5 inhibitors regulating or influencing the pulmonary pressures. In one of the early studies in awake lambs with U-46619-induced pulmonary hypertension, intravenous and aerosolized zaprinast or dipyridamole potentiated the vasodilating effects or prolonged the duration of action of intermittent NO inhalation.98–102
It is well known that hypoxia-induced vasoconstriction and pulmonary hypertension occurs in response to low vascular oxygen tension.103,104 This represents the main physiological mechanism to match ventilation and profusion in the lung. The hypoxic pulmonary vasoconstriction response diverts regional pulmonary blood flow away from poor ventilation to a better ventilated lung region, and thus preserves the ventilation perfusion mechanism and systemic oxygenation.105–107 Therefore, hypoxia-induced vasoconstriction and hypoxia-induced pulmonary hypertension is a very interesting model to study the effects of the selected PDE5 inhibitors. This model can be used as an acute model or chronic model.103,108–110 In the acute model, it has been shown that sildenafil significantly inhibits the increase in the pulmonary pressure produced by hypoxia, without affecting resting pulmonary pressure.111–113 One interpretation of this is that the hypoxia pulmonary vasoreaction normally involves retroactive inhibition of nitric oxide, therefore resulting in the subsequent decline of cGMP which normally exerts a calcium desensitization action.114 Exposure to chronic hypoxia induces a sustained pulmonary hypertension associated with structural and functional changes in the pulmonary arterial bed.
Zhao et al.103 showed in the isolated perfused lung of wild-type and endothelial nitric oxide synthase deficient mice that sildenafil markedly blunted acute hypoxic pulmonary vasoconstriction. Wild-type mice that dosed sildenafil orally throughout 3 weeks of exposure to hypoxia of 10% O2 exhibited a significant reduction in right ventricular systolic pressure, coupled with a small reduction in right ventricular hypertrophy and inhibition of pulmonary vascular remodeling. In endothelial nitric oxide synthase mutant mice, sildenafil attenuated the increase in right ventricular systolic pressure but without a significant effect on right ventricular hypertrophy or vascular remodeling, suggesting that sildenafil attenuates hypoxia-induced pulmonary hypertension in humans. This happens most likely via the endothelial NOS-NO-cGMP pathway which contributes to the response to sildenafil, but other biochemical sources of cGMP also play a role. Sildenafil has beneficial pulmonary hemodynamic effects even when endothelial nitric oxide synthase activity is impaired. Later the same group of investigators noticed the identical phenomenon and documented that PDE5 is also found in newly masculinized distal pulmonary arteries exposed to hypoxia. PDE5 inhibition attenuates the rise in PAP and vascular remodeling when given before chronic exposure to hypoxia, and when administered as a treatment during ongoing hypoxia-induced pulmonary hypertension.108 NO-cGMP signaling is compensatory up-regulated in the hypoxic mouse model of PH, and sildenafil further augments this pathway to functionally alleviate pulmonary vasoconstriction.115
There are many other signaling mechanisms that are involved with PDE5 inhibitors actions, indicating a major role of the NO/cyclic GMP pathway. Chronic hypoxia-induced alteration in RhoA signaling in the pulmonary artery leads to the abolition of RhoA/Rho kinase–mediated Ca2+ sensitization of contraction, and is responsible for the decreased responses to contracting agonists in the pulmonary artery of rats. However, these alterations are prevented.116,117 Consequently, pharmacological inhibition of sildenafil-sensitive PDE5 down-regulates the Ca2+ signaling pathway involved in this model of pulmonary hypertension.118
Rats with monocrotaline-induced pulmonary hypertension were used extensively to understand the pathophysiological changes exerted by a PDE5 inhibitor. When sildenafil is chronically administered, the rats significantly increased plasma cGMP and inhibited the development of pulmonary hypertension and right heart hypertrophy, with preservation of gas exchange and systemic arterial pressure. It was also noticed that the death rate significantly decreased in those animals treated with sildenafil and prevented and reversed the development of pulmonary hypertension in monocrotaline-treated rats by improving the function of the endothelin system in pulmonary arteries.119–122 These models also demonstrated the number of lung arterioles was lowest, whereas the number of arterioles with muscularization of the medial layer was highest in the monocrotaline model that was not treated with sildenafil. Similarly, pulmonary mRNA expressions of tumor necrosis factor-α, caspase-3, plasminogen activator inhibitor-1, and transforming growth factor were higher, whereas bone morphogenetic protein type II (BMPR2) receptor, Bcl-2, and endothelial nitric oxide synthase were lower in the non-treated group, which also measured higher oxidative stress in the right ventricle.123,124 In another study using this model, vardenafil significantly increased endothelial NO synthase expression and superoxide dismutase activity, and down-regulated nicotinamide adenine dinucleotide phosphate oxidase expression in rat lung tissue.122
The early clinical observation with PDE5 inhibitors
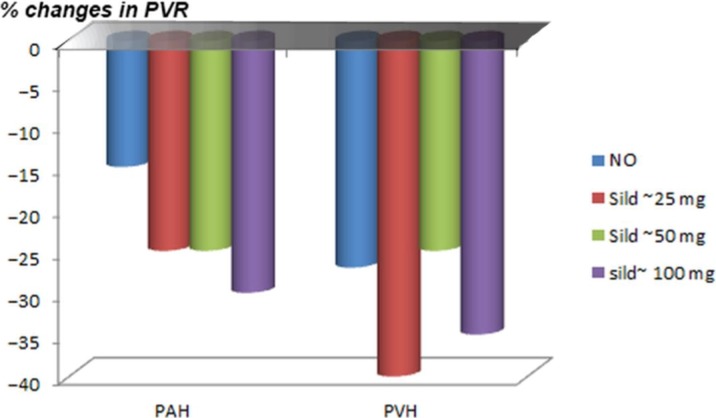
Early studies supervised by the author and other workers to evaluate the effect of intravenous administration of sildenafil at various doses were conducted between 1998 and 2000. In particular Pfizer study 1024 was the first intravenous placebo control study. Various doses of intravenous sildenafil in the placebo-controlled study in the cardiac catheterization lab showed that sildenafil selectively reduced pulmonary pressure and pulmonary vascular resistance in more than 80 patients with pulmonary arterial hypertension. Additionally, pulmonary venous hypertension and pulmonary hypoxic hypertension placebos have shown (in unpublished data) that the intravenous administration of sildenafil can cause a reduction in the pulmonary pressure, which suggests more selectivity to the reduction of systemic blood pressure. It has been observed that there was no dose relationship noticed in early studies, although a decrease in the pulmonary pressure in congestive heart failure patients and patients with idiopathic pulmonary hypertension was noticed. Another observation stated that the effect reached a plateau at a plasma concentration of 100 ng per ml of sildenafil (Butrous et al. unpublished data) (Figure 8).
Figure 8.
The initial results of first unpublished data of the role of intravenous sildenafil on pulmonary vascular resistance (PVR) in 12 patients with either pulmonary arterial hypertension (PAH)(left) or patients with venous pulmonary hypertension (heart failure patients (PVH) (right); using three doses of sildenafil to achieve a plasma level equivalent to taking oral tablets of 25, 50 or 100 mg of sildenafil.
During this period, interest in the role of sildenafil in pulmonary hypertension gained significant momentum.125 These results have encouraged many early investigators to use sildenafil, and the first case report focused126 on a 21-year-old man who presented with a three year history of worsening dyspnea. He was diagnosed with primary pulmonary hypertension with severe heart failure and a four-month history of being unable to walk more than 90 meters. After declining treatment with continuous infusion of epoprostenol and lung transplantation, the patient instead complied with a maintenance dose of 100 mg five times per day. At follow-up at three months, the patient's condition had improved dramatically, and he was able to perform one hour of regular aerobic exercise. As a result of these findings, many clinicians began to prescribe sildenafil on a compassionate basis, and it showed success, which by word of mouth encouraged more people to consider this modality. One of the early studies on the use of sildenafil in the clinical setting was done by Wilkins et al.127 and Ghofrani et al.128 In both studies, sildenafil was used in combination with a short acting iloprost (prostanoids) to enhance its activity. Long-term treatment of patients with pulmonary arterial hypertension was investigated in a number of single-center studies. Kothari and Duggal129 were amongst the first, and treated 14 patients with severe pulmonary hypertension. They showed that oral sildenafil was well tolerated and led to an improved clinical condition and exercise performance. Their experiment was followed by several small studies that started to give credence to theidea that this sort of treatment could be used for the management of pulmonary hypertension.130–137
Phase III clinical trials on the role of PDE5 inhibitors in pulmonary arterial hypertension
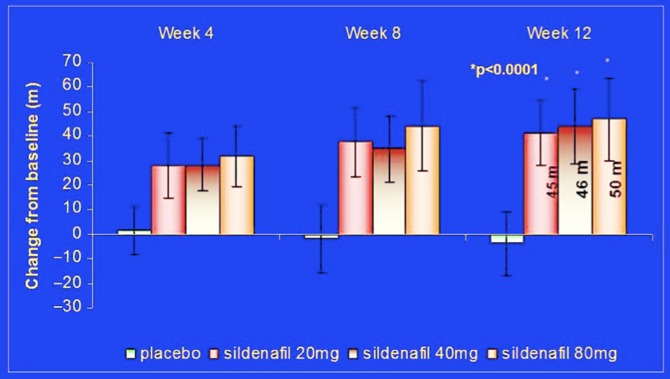
The growing body of evidence from various small studies, the increase of compassionate use of sildenafil, and the proof of the mechanistic activities in the experimental setting between 1998 and2003 led to the decision to start the first Phase 3 study trial involving PDE5 inhibitor SUPER-1 (Sildenafil Use in Pulmonary HypERtension).138 The trial was set to prove that sildenafil is effective and safe for the management of pulmonary arterial hypertension. It was a randomized, placebo-controlled, multinational trial. It included 278 patients with symptomatic pulmonary arterial hypertension (idiopathic or associated with connective tissue disease or with repaired congenital systemic-to-pulmonary shunts). Patients were randomized to either placebo or sildenafil (20, 40 or 80 mg), to be taken orally three times daily for 12 weeks. Patients completing the 12-week randomized study could enter a long-term extension study.138 The study showed an increase in the distance walked in six minutes in patients receiving all doses of sildenafil with no significant difference between the three doses, compared to placebo. The mean placebo-corrected treatment effects were 45 to 50 meters (p < 0.001). (Figure 9 results of SUPER 1).
Figure 9.
The initial result of 6-minutes' walking distance changes from placebo, in SUPER-1 study modified from the results published in Galiè et al.138
These changes were observed as early as week 4, and were maintained at weeks 8 and 12. Sildenafil was also demonstrated to reduce mean pulmonary artery pressure and World Health Organization (WHO) functional class. Most adverse side-effects, such as flushing, dyspepsia, and diarrhea, were mild to moderate in intensity for all treatment groups.138 Based on these results, sildenafil was approved by the FDA and the EMEA in 2005 for the treatment of patients suffering from pulmonary arterial hypertension and was marketed as Revatio® with 20 mg tablets based on a flat (non-significant) dose–effect relationship between 20–80 mg TID. It is worth noting that even the unpublished data from the Pfizer 1024 study, mentioned previously, showed a flat effect of mean pulmonary pressure for all doses between 25–100 mg equivalents in plasma level (Figure 1 iv data sildenafil). It is similarly worth mentioning that the three times per day dosing may not reflect the pharmacokinetics of sildenafil. The therapeutic window of sildenafil could be framed at approximately 30 minutes (median onset of action) and 4 hours (plasma half-life [t1/2]) post-dose. However, some data suggested that the duration of sildenafil activity might not be accurately reflected by plasma levels and the applied dosage, reflecting the discussion above that the conformational changes in the PDE5 enzyme can prolong the intracellular action of sildenafil despite the shorter half-life in the plasma. Moncada et al.139 noticed that in the majority of patients with erectile dysfunction sildenafil remains clinically active 12 hours after administration. Improvement of the erectile response persists beyond the time required for plasma clearance of the drugs (t1/2 values of 4 and 18 h, respectively) to sub-therapeutic levels. These clinical observations may be partly explained by an unexpected persistence of the pharmacological effects of PDE5 inhibitors, which are likely caused by the conformational changes of the PDE5 which may remain as shown above. Additionally, sildenafil binding (and mostly likely other PDE5 inhibitors) to the catalytic site of PDE5 could occur at higher affinity intracellular than estimated previously, which might retard clearance of the inhibitor from the cells.125
The 259 patients (out of 277) who finished the 12 weeks study of SUPER-1 were entered into an open-label uncontrolled extension study (SUPER-2) 140 that continued until the last patient completed 3 years of sildenafil treatment. Patients titrated to sildenafil 80 mg TID. The reason for the higher dosage was the belief of the investigators that 80 mg would provide full PDE5 occupancy as shown by in-vitro enzymology of sildenafil. At 3 years from SUPER-1 baseline, 187 patients were alive. The overall Kaplan-Meier survival estimate was 79%. The most conservative figure for 3-year survival (i.e., assuming that all censored patients had died) is 68%. Patients with idiopathic pulmonary arterial hypertension had higher Kaplan-Meier 3-year survival rates than patients with pulmonary arterial hypertension associated with connective tissue disease (81% vs 72%), and patients walking ≥ 325 m at SUPER-1 baseline had better 3-year survival rates compared with those walking < 325 m at SUPER-1 baseline (84% and 70%, respectively).140 However, this open label extension study showed poor walkers ( < 325 m) at SUPER-1 enrollment whose 6-minute walking distance failed to improve after the first 12 weeks of sildenafil treatment. Their reduced survival at 3 years may aid in the early identification of patients who have particularly poor prognoses, and therefore identify appropriate candidates for additional early therapy. Overall, most adverse events were of mild or moderate severity. At 3 years, 53 patients had died (censored, n = 37).140
The second major phase 3 clinical trials was carried out with tadalafil based on the encouraging outcome of the SUPER-1 results and published case reports. Affuso et al. reported on a middle-aged woman affected by idiopathic pulmonary arterial hypertension whose quality of life and exercise tolerance improved remarkably after a six-month course of treatment with the long-acting phosphodiesterase-5 inhibitor tadalafil.141 PHIRST study (Pulmonary Arterial Hypertension and Response to Tadalafil))142 was a 16-week, double-blind, placebo-controlled study, involving 405 patients with pulmonary arterial hypertension either treatment-naive or on background therapy with the endothelin receptor antagonist bosentan. Patients were randomized to placebo or tadalafil 2.5, 10, 20, or 40 mg orally once daily. Tadalafil increased the distance walked in 6 minutes in a dose-dependent manner; only the 40 mg dose met the pre-specified level of statistical significance (p < 0.01). In the bosentan-naive group, the treatment effect was 44 m (95% confidence interval, 20 to 69 m), compared with 23 m (95% confidence interval, − 2 to 48 m) in patients on background bosentan therapy. Tadalafil 40 mg improved the time to clinical worsening (p = 0.041), incidence of clinical worsening (68% relative risk reduction; p = 0.038), and health-related quality of life. The changes in World Health Organization functional class were not statistically significant. The most common treatment-related adverse events reported with tadalafil were headache, myalgia, and flushing (Figure 10).142
Figure 10.
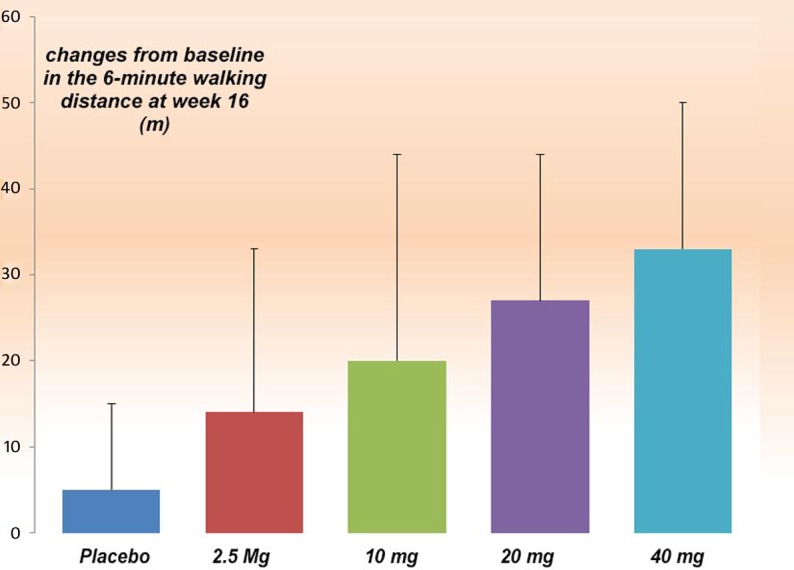
The result of 6-minute walking distance changes from placebo, in PHIRST study modified from the results published in Galiè et al.142.
357 eligible patients entered into the PHIRST-1 received once-daily tadalafil at 20 mg or 40 mg in the double-blind, 52-week, uncontrolled extension study (PHIRST-2).143 293 patients completed PHIRST-2 and showed significantly improved exercise capacity, clinical worsening, and quality of life. PHIRST-2 demonstrated that this dose was generally well tolerated up to 68 weeks. The addition of pulmonary arterial hypertension-specific therapy other than bosentan was not permitted in PHIRST-2. Despite this, the 161 patients on 20 mg and 40 mg in PHIRST-2 had an estimated survival of 95% and 97% respectively, and were associated with fewer clinical worsening events. The safety profile of tadalafil in PHIRST-2 was similar to that in PHIRST-1.143 Conversion from Sildenafil to Tadalafil in Pulmonary Arterial Hypertension (SITAR) Study144 has demonstrated that it is usually well tolerated, and may enhance clinical outcome.
In both SUPER -2 and PHIRST-2, the patients who started on placebo or a lower ineffective dose of tadalafil and were later treated with active medications during the extension study, showed less long term effect than patients taking randomized active doses, consistent with the hypothesis that a delay in the initiation of an effective drug or dose may result in less long-term efficacy.
Major clinical trials on pulmonary arterial hypertension of PDE5 inhibitors in combination therapy
The combination therapy has been advocated recently to add additive or synergistic effects, as there is currently no cure for the disease. Treatment with single agents remains far from satisfactory though it may maximize the therapeutic benefits such as symptom control and increased rate of survival.145–147 Most current clinical practice guidelines recommend combination therapy in functional classes III and IV if there is no improvement or if there is deterioration.148–151 The REVEAL registry demonstrated that nearly 50% of patients with pulmonary arterial hypertension received combination treatment.152 The majority of combination therapy involves PDE5 inhibitors. A few early clinical observations suggested that adding sildenafil to endothelin receptor antagonists bosentan, ambrisentan or prostacyclin caused additional clinical improvement in combination with sildenafil or tadalafil.153–161
The first Phase 3 clinical trial to address the combination therapy by adding sildenafil to the prostacyclin derivatives162,163 was the PACE-1 study (Pulmonary Arterial Hypertension Combination Study of Epoprostenol and Sildenafil).164 Prostacyclin derivatives were always considered the main therapy for pulmonary arterial hypertension. PACE-1 was designed to investigate the safety and efficacy of adding oral sildenafil to long-term intravenous epoprostenol treatment. It involved 267 patients with pulmonary arterial hypertension who were receiving long-term intravenous epoprostenol therapy. The patients were randomized to receive placebo or sildenafil 20 mg three times daily, titrated to 40 mg and 80 mg three times daily as tolerated, at 4-week intervals. 97% of the patients completed the study. The results showed that a placebo-adjusted increase of 28.8 meters (95% CI, 13.9 to 43.8 meters) in the 6-minute walking distance occurred in patients in the sildenafil group (Figure 11). These improvements were most prominent amongst patients with baseline distances of 325 meters or more. By the end of week 16, fewer patients in the sildenafil group compared to the placebo group had clinical worsening events. Furthermore, sildenafil resulted in a greater change in mean pulmonary arterial pressure by 3.8 mm Hg relative to epoprostenol monotherapy. Overall, the combination was well tolerated.164
Figure 11.
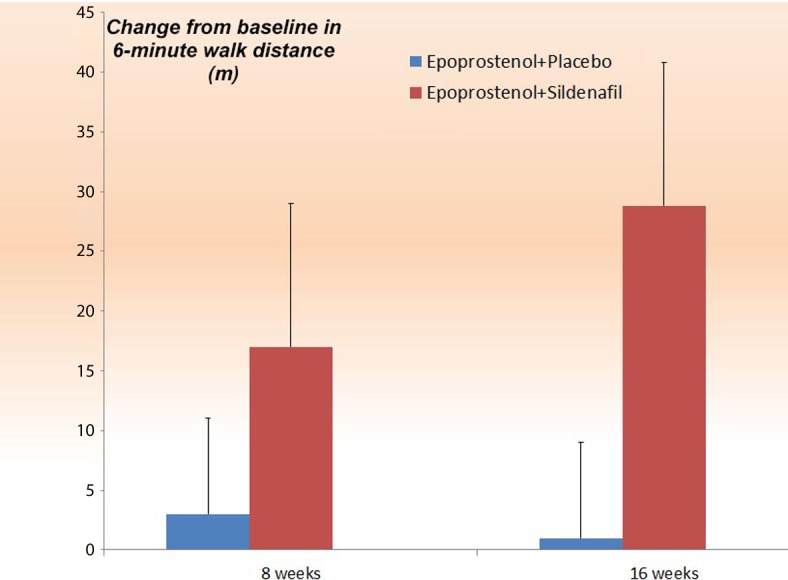
The remove of the additive effect of sildenafil on top of Epoprostenol (PACE-1).164
The study was followed by an open extension study (PACES-2) for a duration of more than 3 years.165 Patients on placebo were given sildenafil. The study showed that the 6-minute walking distance improvement in PACE-1 was maintained in 59%, 44%, and 33% of patients at 1, 2, and 3 years respectively; the same was noticed with functional class. However, patients with PACES-1 baseline 6-minutes walking distance < 325 meters without 6-minutes walking distance improvement during the first 20 weeks of sildenafil treatment subsequently had poorer survival. Thus the PACE-2 provided some clinical assurance that the addition of sildenafil to patients on epoprostenol may carry some additional clinical benefits, as it appears generally well tolerated in pulmonary arterial hypertension patients.165,166
Further works were done with patients treated with subcutaneous treprostinil (which is another prostacyclin derivative) by adding sildenafil as an add-on therapy. Patients were evaluated in a 12-week open-label study. The combination was well tolerated with improvement in the mean treadmill time from 465 ± 167 seconds to 656 ± 205 seconds after 12 weeks of treatment with statistical improvement in the dyspnea–fatigue score.167
The combination of oral bosentan and sildenafil proved to be one of the most common combinations used in clinical practice as shown by the REVEAL database.152 Clearly, randomized, double-blind, placebo-controlled studies are warranted to define the role and type of combination therapies in pulmonary arterial hypertension.168,169 Two clinical studies in particular are worth mentioning here, namely COMPASS-1 and COMPASS-3. The former was an open-label study which investigated the acute pharmacodynamic effects of a single oral dose of sildenafil (25 mg) in 45 patients with PAH on the background of bosentan during right heart catheterization to evaluate the acute hemodynamic effects. Further reduction was observed in the PVR following sildenafil, and the amount of reduction was comparable to that nitric oxide (NO). The totality of the findings suggests that the addition of sildenafil to bosentan treatment can have additive effects.157 Additionally, COMPASS-3 assessed the benefits of a bosentan-based stepped approach using a 6-minute walking distance of 380 meters as the functional threshold. By study's end, 100 patients were enrolled in COMPASS-3 and 31patients (31%) reached the 380 meter threshold, either at week 16 (monotherapy, n = 16) or week 28 (combination, n = 15).170 Combination of Bosentan and Sildenafil vs Sildenafil Monotherapy on Morbidity and Mortality in Symptomatic Patients with Pulmonary Arterial Hypertension (COMPASS-2) was a phase IV multicenter, double-blinded, randomized, placebo-controlled, event-driven study enrolling 180 patients on sildenafil by adding bosentan to assess morbidity and mortality. However, in March 2014 the sponsoring companies announced COMPASS-2 did not meet the primary endpoint of time to first morbidity or mortality event, despite the fact that adding bosentan on top of sildenafil showed an improvement of 21.8 meters in 6 minutes walking distance at week 16 (p = 0.01). Furthermore, a placebo-corrected incidence of 15.4% in liver enzyme elevations was reported, which remains greater than three times the upper limit of normal over a median exposure to double-blind treatment of 23 months.171
Due to the potential combination use, the pharmacokinetic interactions between these two drugs were assessed in a crossover study in 26 healthy adults. Single-dose pharmacokinetics of ambrisentan (10 mg) and its metabolite, 4-hydroxymethyl ambrisentan, were determined in the absence and presence of multiple doses of tadalafil (40 mg QD). Similarly, single-dose pharmacokinetics of tadalafil (40 mg) was evaluated in the absence and presence of multiple doses of ambrisentan (10 mg QD). In the presence of tadalafil, ambrisentan maximum plasma concentration (Cmax) was similar (105.0% [90% CI: 95.9–115.0%]) and systemic exposure (AUC0–∞) was slightly decreased (87.5% [84.0–91.2%]), compared with ambrisentan alone. The safety profile of the drugs combined was similar to that of either drug alone. No dose adjustments should be necessary when these drugs are co-administered. These results are in contrast to previous reports that the sulfonamide-based ERA bosentan can cause marked decreases in the exposure of tadalafil.172
The ATHENA-1 study is an open-label, multicenter study evaluating the effect of ambrisentan in patients with pulmonary arterial hypertension and a suboptimal response to monotherapy with a PDE-5 inhibitor. The primary end point will examine reductions in pulmonary vascular resistance at 24 weeks, with change in functional capacity as a secondary end point.173 ATHENA-1 has recently completed enrollment. Initial results suggest that 33 patients who received ambrisentan in addition to PDE5 inhibitors showed some numerical improvement as well as high survival rates over 48 weeks.174
One large, ongoing, randomized clinical trial, AMBITION (A Study of First-Line Ambrisentan and Tadalafil Combination Therapy in Subjects With Pulmonary Arterial Hypertension),175 is designed to address whether up-front combination therapy with ambrisentan and tadalafil is superior to monotherapy with either of these agents in terms of the primary end point of time to clinical worsening and secondary end points of 6 minutes walking distance and WHO functional class upfront combination strategy. The results of this study are expected in 2015 and should further help in evaluating the benefit of upfront combination strategy.
A retrospective analysis of medical journals from pulmonary arterial hypertension patients from 2000–2011 suggests that first-line combination therapy may more potently improve haemodynamics and may represent a new prognostic marker in pulmonary arterial hypertension.176
PDE5 inhibitors in sickle cell anemia patients
Sickle cell anemia has been attributed as a risk factor for the development of pulmonary hypertension.177,178 It has been estimated that pulmonary hypertension can be found 10% patients during right heart catheterizations,179,180 and about 32–40% patients when assessed by the elevation of tricuspid regurgitant jet velocity echocardiography.181–183 It is a global disease, but the burden of sickle cell disease is highest in sub-Saharan Africa.184
It was hypothesized that PDE5 inhibitors, by increased nitric oxide availability, may be beneficial in patients with sickle cell disease and/or other hemolytic disorders, characterized by a state of relative NO deficiency. This NO deficiency may be due to the decreased NO synthesis by haemoglobin released during the process of haemolysis.185–188 In the first open label uncontrolled pilot trial, sildenafil was evaluated in 12 patients with sickle cell disease and pulmonary hypertension and was found to decrease the estimated pulmonary artery systolic pressure and increase the 6-min walking distance by an average of 78 m, p = 0.0012.189 Sildenafil has also been tried in a number of patients with other haemoglobinopathies (for example, thalassemia) who were suffering from severe pulmonary hypertension. In these cases, investigators noticed a significant decrease in pulmonary pressure and improvement in exercise capacity and functional class, and reported no significant adverse events.190 These findings were confirmed by others in other countries, such as Mehari et al.191,192 from USA, and Fonseca et al.179 from Brazil. The latter studied more than 160 patients with sickle cell disease and clinical suspicion of PH and found that 10% of the total cohort had pulmonary hypertension. PH was the major independent risk factor for death and risk in both studies, and the risk of pulmonary hypertension was associated with the severity of hemolytic anemia, suggesting that patients with all forms of chronic hemolytic anemia should be evaluated for possible pulmonary hypertension.193
The scientific background and these early observations led to the design of a multi-center, placebo-controlled, double-blind, 16-week trial evaluating sildenafil therapy, sponsored by the National Institute of the United States of America. This study was called PHaSST (treatment of Pulmonary Hypertension and Sickle cell disease with Sildenafil Therapy) which originally planned to screen approximately 1000 patients with sickle cell disease, ages 12 and above, with an enrollment goal of 132 patients. The pulmonary hypertension in this trial was Doppler-defined (TRV ≥ 2.7 m/s), based on the premise that patients within this criteria would have a higher risk of mortality.194 However, the NHLBI study was prematurely stopped due to a significant increase in serious adverse events, specifically vaso-occlusive crises in the sildenafil arm (46% vs. 22% in placebo arm; p = 0.048) after 29 subjects had completed the 16 week assessments and 74 subjects had been randomized. All 33 subjects with TRV ≥ 3.0 m/s underwent right heart catheterization (RHC) and had acute testing with sildenafil 60 mg; although mean pulmonary arterial pressure decreased from baseline (p = 0.01), change in PVR was not significant. After 16 weeks of sildenafil, there was no difference in the TRV change and no difference was detected in 6 minutes walking distance for those experiencing a vaso-occlusive crisis versus those who were crisis-free.195,196
PDE5 inhibitors in scleroderma patients
Although scleroderma is part of WHO class I, the effect of PDE5 inhibitors is less compared to its effect in idiopathic pulmonary arterial hypertension, suggesting that scleroderma patients may respond differently to pulmonary arterial hypertension therapy than idiopathic pulmonary arterial hypertension patients. Differences in therapy response found in scleroderma-associated pulmonary hypertension patients may involve the unique pathophysiology and disease progression associated with scleroderma, as opposed to idiopathic pulmonary arterial hypertension. This could suggest that the cohort in this study represented a population less likely to respond to additional therapy because of a more rapid disease progression and clinical deterioration. Mathai et al.155 studied a small cohort of patients (both idiopathic and scleroderma pulmonary hypertension) who had failed bosentan monotherapy, and were subsequently treated with bosentan–sildenafil combination therapy. Patients with idiopathic pulmonary arterial hypertension who failed bosentan monotherapy experienced a significant increase in the mean 6 minutes walking distance after 3 months of receiving additional sildenafil therapy, whereas no significant difference in the mean 6minute walking distance was observed in the scleroderma associated pulmonary arterial hypertension subjects. The results of this study show that SSD-associated pulmonary arterial hypertension was not responsive to additional sildenafil therapy, although beneficial effects were found in the idiopathic pulmonary arterial hypertension group after failing bosentan monotherapy.
PDE5 inhibitors in HIV patients
HIV infection increases the likelihood of developing pulmonary hypertension in both adult and pediatric age groups.197–200 The French national study reports that two-thirds of their patients with HIV-associated pulmonary hypertension used pulmonary arterial hypertension-specific therapy,199,201 yet none of these drugs have been specifically studied in a randomized clinical trial that warrants high confidence. Moreover, concomitant medication with current HAART may cause some interaction. It is now recognized that most of these patients need specific treatment in the form of established current therapies of drugs that interfere with endothelial receptors, nitric oxide or prostacyclin pathways.150,202
PDE5 inhibitors, in particular sildenafil, have been amongst the first oral therapies implemented in the treatment of pulmonary hypertension secondary to HIV, but there are very few case reports and no proper clinical trials.203 Nonetheless, it was observed that HIV-infected patients taking protease inhibitors (saquinavir, indinavir and particularly ritonavir) significantly modify the pharmacokinetics of sildenafil, presumably through inhibition of CYP3A4. This results in increased plasma concentration of both drug and metabolite,92 although a few recent papers do not report any significant adverse reactions to sildenafil during the follow-up period.97 Therefore, it is advisable to consider therapeutic drug monitoring of sildenafil during treatment in order to avoid possible over-dosage.
PDE5 inhibitors in adult congenital heart diseases patients
Unfortunately, most of the landmark trials for pulmonary arterial hypertension included only a small number of congenital heart disease (CHD) patients. However, due to the similarities in pathophysiology between PAH and PAH-CHD, all of the targeted therapies are used and approved for the treatment of pulmonary arterial hypertension- congenital heart disease. Randomized trials with sildenafil (SUPER-1) and tadalafil (PHIRST) included 7% and 12% congenital heart diseases patients, respectively.138,142 They both found improvements in exercise capacity in the treatment arms.204 Eisenmenger syndrome is characterized by elevated pulmonary vascular resistance and right-to-left shunting of blood through a systemic to pulmonary circulation connection. Some favorable results have also been reported in patients with Eisenmenger syndrome treated with the sildenafil or tadalafil.205–210 Furthermore, sildenafil may help in improving exercise performance in children and young adults with single-ventricle physiology after the Fontan operation.211
Recent observations confirm that patients with Eisenmenger syndrome had the highest baseline pulmonary vascular resistance and the lowest exercise capacity. Upon treatment with pulmonary arterial hypertension-specific drugs, either monotherapy or in combination, the survival of the patients with idiopathic pulmonary arterial hypertension appeared to be worse when compared with the pulmonary arterial hypertension-congenital heart diseases subgroups.212
In addition, PDE5 inhibitors were also considered in combination in adult congenital heart disease, in particular in Eisenmenger syndrome. A report on the case of a 68-year-old woman with Eisenmenger syndrome related to congenital heart disease shows that she was treated with inhaled iloprost and oral sildenafil for 2 years.213 A crossover study in Eisenmenger syndrome patients included 21 patients on open-label bosentan. After 3 months of therapy, the addition of sildenafil did not add significant improvement in the 6 minutes walking distance, but did show some improvement in oxygenation by 5%.214
PDE5 inhibitors in chronic thromboembolic pulmonary hypertension patients
Chronic thromboembolic pulmonary hypertension (CTEPH) affects up to 3.8% of patients with acute major pulmonary embolism. If left untreated, CTEPH leads to progressive pulmonary hypertension with additional pulmonary vascular remodeling. Pulmonary endarterectomy is considered the first choice of treatment for selected CTEPH patients, yet only a small percentage of these patients are eligible for pulmonary thrombendarterectomy. Patients deemed non-operable have to be treated with targeted therapies.215–218 PDE5 inhibitors, in particular sildenafil, have been tested in several small trials. The first uncontrolled clinical trial had 12 non-operable chronic thromboembolic pulmonary hypertension patients who were treated by sildenafil. Ghofrani et al. found that after approximately 6 months of sildenafil treatment, pulmonary hemodynamics and exercise capacity improved significantly.219 This was followed by a larger open-label uncontrolled clinical trial. 104 patients from the same center with inoperable CTEPH were treated with 50 mg sildenafil three times a day, which resulted in significant long-term functional improvement. Yet the acute effect of sildenafil may not predict the long-term outcome of therapy.220 In a small, double-blind, placebo-controlled pilot study, 19 subjects with inoperable CTEPH were randomly assigned to sildenafil or placebo for 12 weeks. Overall, investigators found no significant differences between the two groups with respect to change in exercise capacity, but significant improvements were seen in WHO class and pulmonary vascular resistance (PVR). This suggests partial beneficial effect and may necessitate further larger-scale trials of sildenafil in inoperable CTEPH.221 None of the PDE5 inhibitors available now are licensed for CTEPH patients, although recently Riociguat, a soluble stimulator which shares the same NO-cGMP pathway by stimulating guanylate cyclase enzyme, has been approved for this indication with chronic thromboembolic pulmonary hypertension.222
PDE5 inhibitors in high altitude pulmonary vascular diseases
There is interest in the use of PDE5 inhibitors for the treatment of pulmonary hypertension associated with hypoxia. Several studies have been conducted in subjects during hypoxia and at high altitude. Acute mountain sickness and high altitude pulmonary hypertension are two syndromes of high altitude illness. Recent clinical studies showed the beneficial effects of phosphodiesterase type 5 (PDE-5) inhibitors on the treatment of pulmonary hypertension. Meta-analysis to evaluate the clinical efficacy of PDE-5 inhibitors on high altitude hypoxia and its complications suggested that exaggerated hypoxic pulmonary vasoconstriction is a key factor in the development of high altitude pulmonary edema (HAPE). Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia.223
Richalet et al. studied the effects of oral sildenafil in 12 normal subjects who were exposed for 6 days at 4,350 meter in a randomized, double-blind, placebo-controlled study, and found that sildenafil protects against the development of altitude-induced pulmonary hypertension and improves gas exchange, limiting the altitude-induced hypoxemia and decrease in exercise performance.224
Another study included 188 natives from the Naryn region in Kyrgyzstan, which is situated 2500 meters above sea level. 44 natives underwent cardiac catheterization whilst 29 (66%) had a resting mean pulmonary artery pressure (PAP) above 25 mm Hg. 22 patients with a raised mean PAP were randomized to receive sildenafil (25 or 100 mg) or matching placebo taken 8 hourly for 12 weeks. Patients on sildenafil lowered their mean PAP compared to those on placebo (n = 8) (95% CI − 12.4 to − 1.3). Both doses improved the 6-minute walking distance by about 40 meters overall.225 During a planned trip, 14 healthy volunteers (12 men and 2 women) were enrolled to evaluate the effect of sildenafil's exercise capacity at Mount Everest Base Camp at an altitude of 5400 meters. Half of the participants were experienced mountaineers the other half were well-trained, experienced trekkers who had repeatedly traveled to altitudes above 3500 meters. Oral sildenafil reduced pulmonary hypertension both at rest and during exercise while maintaining gas exchange and systemic arterial pressure.226
In further analysis, the 14 members of a Mount Everest expedition were monitored during an acute hypoxic challenge at sea level, and further environmental hypoxia exposure at altitudes of 3440 m and 5245 m and 2 weeks after return to sea level. They received either placebo or 50 mg sildenafil in a double-blind randomized cross-over design. Reportedly, sildenafil significantly decreased systolic pulmonary arterial pressure and improved right ventricular function.227
However, another study used 62 healthy lowland volunteers (36 male; median age 21 years, range 18 to 31) on the Apex 2 research expedition, who were flown to La Paz, Bolivia (3650 m), and after 4–5 days acclimatization ascended over 90 min to 5200 m. Chronic sildenafil administration showed no significant difference in PASP at 5200 m between sildenafil and placebo groups. Median AMS score on day 2 at 5200 m was significantly higher in the sildenafil group (placebo 4.0, sildenafil 6.5; p = 0.004) but there was no difference in prevalence of acute mountain sickness between groups on pulmonary artery systolic pressure and symptoms of acute mountain sickness.228
A further 16 healthy subjects were included in a randomized, double-blind, placebo-controlled, cross-over study on the effects of 50 mg sildenafil on echocardiographic indexes of the pulmonary circulation and on cardiopulmonary cycle exercise in normoxia, in acute normobaric hypoxia (fraction of 10% inspired O2), and then again after 2 weeks of acclimatization at 5000 m on Mount Chimborazo (Ecuador). In normoxia, sildenafil had no effect on maximum VO2 or O2 saturation but in acute hypoxia, sildenafil increased maximum VO2. In chronic hypoxia, sildenafil did not affect maximum VO2 or O2 saturation, but did decrease pulmonary vascular resistance by 30% to 50% in different conditions. It was concluded that sildenafil increases exercise capacity in acute normobaric hypoxia and that this is explained by improved arterial oxygenation, rather than by a decrease in afterload of the right ventricle.229
However, in a single randomized placebo-controlled study, 10 high-altitude pulmonary edema-susceptible subjects received tadalafil 10 mg twice daily during a rapid ascent to 4559 m.230 Only 1 of the remaining 8 subjects who received tadalafil developed high-altitude pulmonary edema, but 2 of the subjects receiving tadalafil withdrew from the study due to severe acute mountain sickness on arrival at 4559 m. In the placebo group, 7 of 9 subjects developed high-altitude pulmonary edema. The occurrence of acute mountain sickness with tadalafil may have discouraged further trials for the prevention of high-altitude pulmonary edema.
In a recently published paper on Meta-Analysis of Clinical Efficacy of Sildenafil, on High Altitude Hypoxia and Its Complications, Yu et al.231 reviewed five clinical trials.224,226–229 A total of 60 subjects received sildenafil, and 72 subjects were given placebo. In accordance with previous reports, short-term treatment with sildenafil (1–2 days) significantly reduced pulmonary artery systolic pressure at rest (MD − 4.53; 95% CI − 6.72, − 2.34; p < 0.0001). However, treatment with sildenafil (1–2 days) did not improve oxygen saturation after exposure to high altitude (MD 0.07; 95% CI − 1.26, 1.41; p = 0.91). Moreover, no significant difference was observed in heart rate between the sildenafil and placebo-treated groups (MD 6.95; 95% CI − 3.53, 17.43; p = 0.19). AMS score did not improve after treatment at different time points. In this case, the investigators concluded that short-term treatment with sildenafil can attenuate the altitude-induced high pulmonary systolic arterial pressure, but has no significant beneficial effects on arterial oxygen saturation, heart rate, and acute mountain sickness.
PDE5 inhibitors in pediatric population
Animal and clinical data suggested that PDE5 is involved in the regulation of pulmonary vascular reactivity during the perinatal period and may potentiate birth-related pulmonary vasodilator stimuli.16,232–234 In late-gestation fetal lambs with chronic pulmonary hypertension, the prophylactic use of sildenafil prevents the rise in pulmonary vascular tone and altered vasoreactivity caused by ductus arteriosus compression. These results support the hypothesis that elevated PDE5 activity is involved in the consequences of chronic pulmonary hypertension in the perinatal lung.235 Additionally, it was noticed that sildenafil induces a transient pulmonary vasodilation and mediates a sustained change in vascular reactivity, especially to birth-related stimuli in the ovine fetal lung.234 The first observation by Atz and his colleagues236 suggested that sildenafil may potentiate pulmonary vasodilation with NO or ameliorate the deleterious effects of abrupt discontinuation of NO (pulmonary hypertension crisis) by increasing intracellular and circulating cGMP, preventing rapid depletion of cGMP when the gas is withdrawn.236,237 This was confirmed during the treatment of pulmonary hypertension crises after withdrawal of NO in study with 103 eligible patients.238 In turn, this led to interest in using sildenafil in a pediatric age group for patients with primary pulmonary hypertension.239 These studies have generally suggested favorable effects and outcomes in infants and young children with pulmonary arterial hypertension, but these reports are mainly uncontrolled observations except for one single-center, placebo-controlled trial for PPHN.240
The report of sildenafil treatment in critically ill infants with persistent pulmonary hypertension in the neonate ward in five centers involved a total of 36 neonates. Intravenous sildenafil was well tolerated with short-term improvements in oxygenation. Acute and sustained improvements in oxygenation were noted in those neonates who received the higher infusion doses.241,242
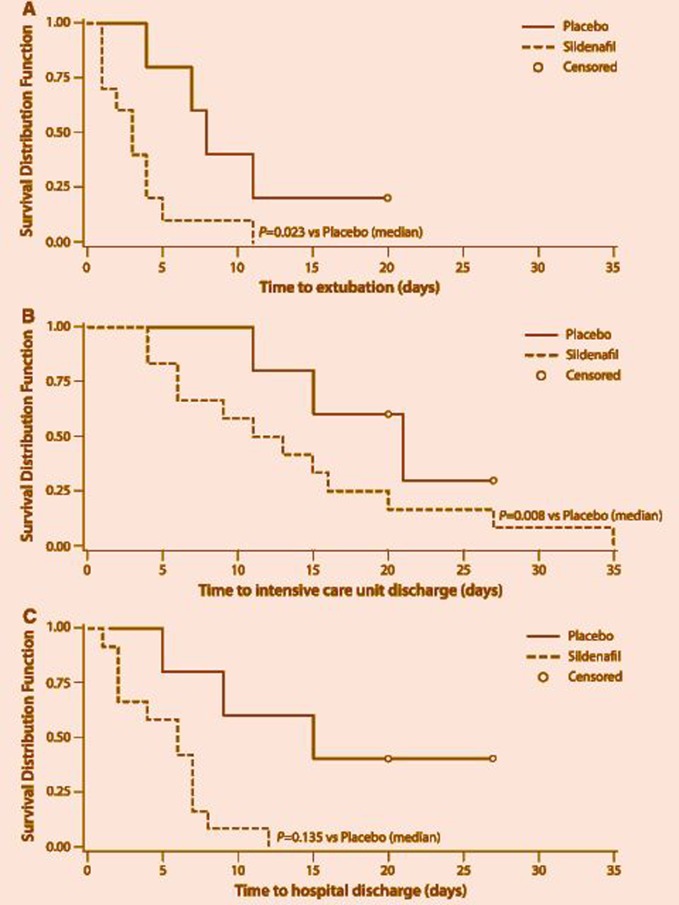
A separate study on patients with congenital heart disease compared the effects of inhaled NO before and after the specific inhibition of intravenous sildenafil. Investigators noticed that intravenous sildenafil is as effective as inhaled NO as a pulmonary vasodilator in children with congenital heart disease.243 In another study it was noticed that intravenous sildenafil reduced pulmonary artery pressure and shortened time to extubation and intensive care unit stay in children with postoperative PH with congenital heart disease (Figure 12).244 After Fontan surgery, sildenafil infusion acutely improves cardiopulmonary hemodynamics, increasing cardiac index. For the range of doses studied, exposure was within the acute safety range reported in adult subjects.245 Other smaller series suggested that sildenafil can be beneficial for children as an add-on to a failure or lesser response to bosentan monotherapy.246
Figure 12.
Kaplan–Meier analysis of a time to extubation, b time to intensive care unit discharge, and c length of hospital stay for patients in the placebo group versus those in the combined sildenafil group (from Fraisse A et al., Intensive Care Med. 2011.244
Many treating physicians have extrapolated from adult trials that the disease and its response to treatment can differ between children and adults.247–252 Sildenafil has been used extensively off-label for the treatment of neonates, infants, and children with pulmonary arterial hypertension associated with diverse heart and lung diseases. In a small open label study with sildenafil at 0.25 to 1 mg/kg 4 times daily to 14 children, group diagnoses were primary (n = 4) and secondary (n = 10).
Investigators found that sildenafil therapy was well tolerated and resulted in a significant increase of the distance walked in the 6-minute walking test.251 Therefore, a 16-week, placebo-controlled, dose-ranging study evaluated the effects in a trial of oral Sildenafil in Treatment-Naive Children, Aged 1 to 17 Years, With Pulmonary Arterial Hypertension (STARTS-1).252 Children (n = 235; weight ≥ 8 kg) were randomized to low-, medium-, or high-dose sildenafil or placebo orally 3 times daily for 16 weeks. The primary comparison was a percent change from baseline in peak oxygen consumption (PVO2) for the 3 sildenafil doses combined versus placebo. Exercise testing was performed in 115 children able to exercise reliably; the study was powered for this population. Secondary end points (assessed in all patients) included hemodynamics and functional class. The results showed that low-dose sildenafil was ineffective but PVO2, functional class, and hemodynamics improved with medium and high doses versus placebo, whilst most adverse events were mild to moderate in severity. STARTS-1 completers could enter the STARTS-2 extension study, wherein patients who received sildenafil in STARTS-1 continued the same dose, whilst placebo-treated patients were randomized to low-, medium-, or high-dose sildenafil. Because STARTS-2 did not include a placebo arm, the impact of sildenafil monotherapy on long-term survival is difficult to discern.
In STARTS-2, however, the statistical analysis suggested increased mortality with higher doses and appeared to occur after 2 years of treatment and in children with idiopathic pulmonary arterial hypertension/ with a baseline values above median values for PVRI (15.1 Wood units/m2), mean pulmonary arterial pressure of 62 mm Hg, and right atrial pressure of 7 mm Hg. With all patients having the potential to complete ≥ 3 years of treatment (including some receiving 7 years of treatment), 35 deaths have been reported. Deaths were reported on treatment (n = 26) or during follow-up (n = 9). The incidence of deaths is currently 9% (5 of 55), 14% (10 of 74), and 20% (20 of 100) for patients randomized in STARTS-1 or -2 to low-, medium-, and high-dose sildenafil, respectively.253,254 This led to some regulatory concern with the FDA warning that “children taking a high dose of Revatio had a higher risk of death than children taking a low dose”. For this reason, sildenafil has not been approved for the treatment of pulmonary arterial hypertension in children.255
Nevertheless, despite these concerns the European Medicines Agency (EMA) approved sildenafil therapy for use in children with pulmonary arterial hypertension, but added caveats regarding dosage. The Pediatric Pulmonary Hypertension Network (PPHNet), which includes leaders of pediatric pulmonary hypertension programs throughout North America, has heavily discussed this issue,254 and suggested in recent communications256,257 that the Drug Safety Communication was overly conservative in the FDA conclusions about both effectiveness and mortality risk. Furthermore, on deeper examination of the mortality data from STARTS-2 and acknowledging the many limitations of the STARTS trial, PPHNet offered a series of recommendations regarding the use of sildenafil and concluded STARTS-2 to be a failed trial.
On careful re-analysis the group stated that “although children randomized to higher compared with lower sildenafil doses had an unexplained increased mortality, all sildenafil dose groups displayed favorable survival for children with pulmonary arterial hypertension”. Combined with STARTS-1 efficacy results, the long-term survival rates favor use of lower sildenafil doses. After interim STARTS-2 survival data were reviewed, a recommendation was issued to down-titrate all patients remaining in the study to lower sildenafil doses.256,257 Therefore this subject is still controversial and need careful re-evaluation.
The effect of PDE5 inhibitors on the right ventricular function in pulmonary hypertension
PDE5 has been demonstrated to be upregulated in rat right ventricular hypertrophy myocardium, whilst inhibition of PDE5 (with either MY-5445, DA-8159 or sildenafil) significantly increases contractility in the perfused heart (modified Langendorff preparation) and isolated cardiomyocytes. In right ventricular hypertrophy myocardium, but not normal right ventricle, PDE5 inhibition leads to increases in both cGMP and cAMP in right ventricular hypertrophy. However, the abnormal right ventricle attenuated the compensatory development of right ventricular hypertrophy, and pulmonary artery medial wall thickening prevented myocardial fibrosis induced by MCT.55,258–261
These finding were confirmed in surgical specimens from 9 patients, which showed that PDE5 is not expressed in the myocardium of the normal human right ventricle but markedly upregulated in hypertrophied right ventricle myocardium. Further, tissue extracts from the right ventricle of 20 patients also confirmed severity-dependent upregulation of myocytes PDE5 expression in the RV and the impact of this upregulation on myocardial contractility. Thus the ability of PDE5 inhibitors to increase right ventricle inotropy and to decrease RV afterload without significantly affecting systemic hemodynamics makes them ideal for the treatment of diseases affecting the right ventricle, including pulmonary arterial hypertension.55,57
PDE5 inhibitors in left heart failure
Myocardial PDE5 expression and cellular distribution were determined in left ventricular samples from patients with end-stage CHF and normal donors and from mice after transverse aortic constriction induced CHF. Compared with donor human hearts, myocardial PDE5 protein was increased ≈ 4.5-fold in CHF samples, and the increase of myocardial PDE5 expression was significantly correlated with myocardial oxidative stress markers 3′-nitrotyrosine or 4-hydroxynonenal expression. Reducing oxidative stress by treatment with M40401 attenuated cardiomyocyte PDE5 expression. This, with selective inhibition of PDE5, protected the heart against pressure overload-induced left ventricular hypertrophy and congestive heart failure.58,260
Works in failing hearts (tachypacing model), showing PDE5 inhibition, could blunt β-adrenergic–stimulated inotropy.61,262 Takimoto et al.263 reported that sildenafil could both block and reverse maladaptive cardiac remodeling induced by sustained pressure overload using a mouse model (aortic banding). These data suggested that PDE5 may be unregulated in the failing and hypertrophied left ventricle and may down-revaluate itself in normal myocardium.56 Other investigators264–266 demonstrated benefits of PDE5 inhibition on myocardial post ischemic injury and cell survival signaling and showed that PDE5 inhibition enhances ischemia-induced angiogenesis with mobilization of endothelial progenitor cells through a protein kinase G–dependent HIF-1/vascular endothelial growth factor pathway.267 These data suggest that PDE5 inhibition may be a new treatment strategy for cardiac hypertrophy and remodeling and may have a therapeutic potential to treat ischemic cardiovascular diseases.
Lewis et al. showed that in patients with systolic heart failure, sildenafil acts as a selective pulmonary vasodilator during rest and exercise in patients with heart failure and pulmonary hypertension, and improves exercise capacity and quality of life in patients with systolic heart failure and with secondary pulmonary hypertension.268,269 A small double-blind study with 45 heart failure patients (New York Heart Association class II-III) who were randomly assigned to placebo or sildenafil (50 mg three times per day) for 1 year found that sildenafil improves functional capacity and clinical status and that long-term use of sildenafil was well tolerated.270 This study also showed sustained and significant improvement in the left ventricle (diastolic function properties and cardiac geometry), which may suggest that PDE5 inhibitors can be a target therapy for heart failure with preserved ejection fraction or diastolic heart failure. The above finding led to the design of the RELAX trial (PhosphodiesteRasE-5 Inhibition to Improve CLinical Status And EXercise Capacity in Diastolic Heart Failure), which was a multicenter, randomized double-blind, placebo-controlled treatment study designed clinical trial conducted within the National Heart Lung and Blood Institute–sponsored heart failure clinical research network. Its aim was to test the hypothesis that chronic PDE5I (sildenafil 20 mg TID for 12 weeks followed by 60 mg TID for 12 weeks) improves exercise capacity and clinical status in patients with heart failure with preserved ejection fraction (HFpEF).
In this study the investigators adopted the change in VO2 from baseline to 24 weeks as the primary endpoint with change in 6-minute walking distance at 12 and 24 weeks, and the change in a composite clinical score at 24 weeks as secondary endpoints.271 The trial recruited 216 patients (median age 69 years) and 48% were women. However, RELAX was neutral, reporting no benefit of sildenafil compared with placebo in the primary end point or in any secondary functional and structural end points including markers of clinical status. The initial results showed that sildenafil treatment in HFpEF was associated with modest impairment on resting LV contractility despite beneficial effect on the right ventricle function and LV afterload. These offsetting effects on the ventricular vascular function may partially explain the luck of clinical benefit from PDE5i observed in the RELAX trial.272 However, the RELAX study provided many useful clinical observations on the natural history of HFpEF. Clinicians and researchers may argue about the selection of endpoints and interpretation of the data, but this is beyond the scope of this review and more information can be obtained elsewhere.273–276
PDE5 inhibitors in chronic lung diseases
Hypertension associated with chronic obstructive pulmonary diseases (COPD) and interstitial lung disease has also been considered with regards to PDE5 inhibitors. Chronic obstructive lung disease affects the entire lung including the pulmonary vasculature, rather than just the airways.277 Pulmonary hypertension has long been recognized in a subset of patients with obstructive lung diseases. The etiology can be due to hypoxia vasoconstriction leading to permanent medial hypertrophy and remodeling, but other pathological factors may play a part leading to complex interactions including in situ thrombosis, decreased expression of vascular endothelial growth factor and other contributory mechanisms includes inflammation.278,279 Therefore, there may be a case for pharmacologic treatment of pulmonary hypertension in selected patients with advanced COPD and right heart failure. It was thought that PDE5 inhibitors might have a therapeutic potential for chronic airway diseases, in particular due to their effect on the remodeling process. Acute sildenafil use resulted in transient airways dilatation, reduced gas trapping280 and vasodilation.281 However, the main concern with these agents, like with other vasodilators, is perturbation of ventilation–perfusion (V′/Q′) matching. There are some suggestions that sildenafil (and potentially other PDE5 inhibitors) may cause preferential pulmonary vasodilation and improve gas exchange in patients with severe lung fibrosis and secondary pulmonary hypertension, and thus preserve V′/Q′ matching.282 The hypothesis proposes that poorly ventilated areas of the lung would be expected to have lower NO and cGMP levels and so the local effect of PDE5 inhibition would be less than in a well-ventilated lung.283
Initial small observations of six patients suffering from severe COPD suggested some benefit in the use of PDE5 inhibitors, as they demonstrated significantly improved hemodynamic parameters following administration of oral sildenafil. Additionally, the walking distance in the 6-minute walking distances increased significantly in the five patients who accepted a second right heart catheterization.284,285 Nevertheless it will be a challenge for randomized controlled trials to overcome the difficulties of the diagnosis of right ventricular failure and the definition of a relevant primary endpoint in pulmonary hypertensive patients with COPD.286,287 Larger placebo control studies with sildenafil in patients with severe COPD and moderately increased pulmonary arterial pressure a did not improve the results of pulmonary rehabilitation in exercise tolerance.288–290
While these small trials are encouraging, recent larger studies indicate that sildenafil does not have a beneficial effect on exercise capacity in patients with COPD and emphysema without pulmonary hypertension or on the outcomes of respiratory rehabilitation in COPD patients with moderate pulmonary hypertension.288 In addition, some observed that sildenafil significantly worsened gas exchange at rest as well as quality of life.288,291 There is now an ongoing study (TADA-PHiLD trial) which is a multicenter, prospective, randomized, placebo-controlled, double-blind clinical trial that aims to characterize the efficacy and safety profile of PDE5 inhibitors (specifically tadalafil) in patients with chronic obstructive lung disease and moderate to severe pulmonary hypertension.292 Moreover, circulatory exercise impairment remains unstudied, and the low prevalence of this condition might explain why these studies have not yet been performed to date.293
The presence of pulmonary hypertension in idiopathic pulmonary fibrosis patients is associated with poor survival. Based on prior studies, it can be estimated that about 60% of patients with idiopathic pulmonary fibrosis have pulmonary hypertension. Due to the paucity of drugs for this condition, many investigators tried to argue that improving the pulmonary circulation may benefit some of these patients. In a small trial, sildenafil improved the exercise tolerance as measured by 6-minute walking distance in 57% of the patients, which prompted a large, well-controlled trial.294 In fact, a larger study was funded by the National Heart, Lung, and Blood Institute and others and named STEP-IPF (Sildenafil Trial of Exercise Performance in Idiopathic Pulmonary Fibrosis).295 This study was a double-blind, randomized, placebo-controlled trial and included patients with advanced idiopathic pulmonary fibrosis (IPF), and selected for patients with complicating pulmonary hypertension by virtue of the inclusion criterion of a single breath diffusing capacity (DLCO) of < 35% of predicted. The first period consisted of 12 weeks of a double-blind comparison between sildenafil and placebo control.
The primary outcome was the proportion of patients with an increase in the 6-minute walking distance of 20% or more. A total of 180 patients were enrolled in the study. No benefit was shown for sildenafil in the primary outcome, but the presence of some positive secondary outcomes created clinical equipoise for further research. There were small but significant differences in arterial oxygenation, carbon monoxide diffusion capacity, the degree of dyspnea, and quality of life favoring the sildenafil group. Serious adverse events were similar in the two study groups.295 There is good biological and physiological rationale as well as outcome data to support studies addressing PH complicating IPF. There are many elements that are integral to a successful study: a drug that works, administration of the drug at an appropriate dose, a well-targeted patient cohort that is followed long enough to see an effect, and a carefully constructed end point. If all such elements are aligned, a positive study of a pulmonary vasoactive agent might indeed demonstrate an important aspect of IPF to be targeted.296
Conclusion
PDE5 inhibitors have contributed to our knowledge of the pathophysiology of pulmonary vascular disease, and have become the leading oral therapy for pulmonary hypertension worldwide and in particular in the developing word. That is mainly due to the availability and more reasonable cost than other classes of pulmonary hypertension-specific drugs. However, PDE5 inhibitors are not universal and its effects depend on the etiology, as they are most effective in idiopathic and primary pulmonary hypertension. PDE5 inhibitors are proven less effective in other forms of the disease, such as for patients with pulmonary arterial hypertension, even though they have been approved by many authorities for this indication. Similarly, it can be useful in some selected patients with left heart failure (both systolic and diastolic), in selected patients with hypoxia pulmonary hypertension, and in interstitial lung diseases, though these are not indicated yet. Finally, they cannot be universal drugs, even with the same indication and etiology. There is wide clinical variation in patients' responses; this is partly because these drugs can act differently at certain stages of the disease progression. Furthermore, PDE5 inhibitors, like all other currently available specific pulmonary hypertension drugs, have been useful in improving clinical presentation and may delay clinical worsening, but will not cure the condition.
Though most of these drugs are now generic and no longer inspire big clinical trials, their appeal is still high in identification of the right pathology and patient phenotype and genotype that can benefit of this class of drugs. This needs further clinical and basic research for years to come.
References
- 1.Liras S, Bell AS. Phosphodiesterases and Their Inhibitors. John Wiley & Sons; Weinheim, Germany: 2014. p. 238. [Google Scholar]
- 2.Amsallem E, Kasparian C, Haddour G, Boissel J-P, Nony P. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst Rev. 2005;(1):CD002230. doi: 10.1002/14651858.CD002230.pub2. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002230.pub2/pdf/standard. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty AM. Phosphodiesterase 4 inhibitors as novel anti-inflammatory agents. Curr Opin Chem Biol. 1999;3(4):466–473. doi: 10.1016/S1367-5931(99)80068-4. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z, Ducharme Y, Macdonald D, Robichaud A. The next generation of PDE4 inhibitors. Curr Opin Chem Biol. 2001;5(4):432–438. doi: 10.1016/s1367-5931(00)00224-6. [DOI] [PubMed] [Google Scholar]
- 5.Milani E, Nikfar S, Khorasani R, Zamani MJ, Abdollahi M. Reduction of diabetes-induced oxidative stress by phosphodiesterase inhibitors in rats. Comp Biochem Physiol Part C Toxicol Pharmacol. 2005;140(2):251–255. doi: 10.1016/j.cca.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Conti M, Jin S-LC. The molecular biology of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol Biol. 1999;63:1–38. doi: 10.1016/s0079-6603(08)60718-7. [DOI] [PubMed] [Google Scholar]
- 7.Frumkin LR. The pharmacological treatment of pulmonary arterial hypertension. Pharmacol Rev. 2012;64(3):583–620. doi: 10.1124/pr.111.005587. [DOI] [PubMed] [Google Scholar]
- 8.Rotella DP. Phosphodiesterase 5 inhibitors: Current status and potential applications. Nat Rev Drug Discov. 2002;1(9):674–682. doi: 10.1038/nrd893. [DOI] [PubMed] [Google Scholar]
- 9.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn M. Endothelial actions of atrial and B-type natriuretic peptides. Br J Pharmacol. 2012;166(2):522–531. doi: 10.1111/j.1476-5381.2012.01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122(3):216–238. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis SH, Busch JL, Corbin JD. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62(3):525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109(2):159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 15.Tuder R. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009;30(04):376–385. doi: 10.1055/s-0029-1233307. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90(4):1291–1335. doi: 10.1152/physrev.00032.2009. [DOI] [PubMed] [Google Scholar]
- 17.Murad F. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355(19):2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Watson G, Zhao L. Cyclic guanosine monophosphate signalling pathway in pulmonary arterial hypertension. Vascul Pharmacol. 2013;58(3):211–218. doi: 10.1016/j.vph.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Jeon YH, Heo YS, Kim CM, Hyun YL, Lee TG, Ro S, Cho JM. Phosphodiesterase: Overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell Mol Life Sci. 2005;62(11):1198–1220. doi: 10.1007/s00018-005-4533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strada SJ, Uzunov P, Weiss B. Ontogenetic development of a phosphodiesterase activator and the multiple forms of cyclic amp phosphodiesterase of rat brain. J Neurochem. 1974;23(6):1097–1103. doi: 10.1111/j.1471-4159.1974.tb12204.x. [DOI] [PubMed] [Google Scholar]
- 21.Beavo J, Houslay MD. Cyclic Nucleotide Phosphodiesterases: Structure, Regulation, and Drug Action. John Wiley & Sons; San Francisco, CA USA; 1990. [Google Scholar]
- 22.Appleman MM, Thompson WJ, Russell TR. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- 23.Wells JN, Hardman JG. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1976;8:119–143. [PubMed] [Google Scholar]
- 24.Appleman MM, Thompson WJ. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry (Mosc) 1971;10(2):311–316. [PubMed] [Google Scholar]
- 25.Beavo JA. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol Rev. 1995;75(4):725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 26.Conti M. Phosphodiesterases and cyclic nucleotide signaling in endocrine cells. Mol Endocrinol. 2000;14(9):1317–1327. doi: 10.1210/mend.14.9.0534. [DOI] [PubMed] [Google Scholar]
- 27.Manganiello VC, Degerman E. Cyclic nucleotide phosphodiesterases (PDEs): Diverse regulators of cyclic nucleotide signals and inviting molecular targets for novel therapeutic agents. Thromb Haemost. 1999;82(2):407–411. [PubMed] [Google Scholar]
- 28.Soderling SH, Bayuga SJ, Beavo JA. Identification and characterization of a novel family of cyclic nucleotide phosphodiesterases. J Biol Chem. 1998;273(25):15553–15558. doi: 10.1074/jbc.273.25.15553. [DOI] [PubMed] [Google Scholar]
- 29.Surapisitchat J, Beavo JA. Chapter 173 – phosphodiesterase families. In: Bradshaw RA, Dennis EA, editors. Handbook of Cell Signaling. 2nd ed. San Diego: Academic Press; 2010. pp. 1409–1414. Available from: http://www.sciencedirect.com/science/article/pii/B978012374145500173X. [Google Scholar]
- 30.Francis SH, Colbran JL, McAllister-Lucas LM, Corbin JD. Zinc interactions and conserved motifs of the cGMP-binding cGMP-specific phosphodiesterase suggest that it is a zinc hydrolase. J Biol Chem. 1994;269(36):22477–22480. [PubMed] [Google Scholar]
- 31.Sung BJ, Hwang KY, Jeon YH, Lee JI, Heo YS, Kim JH, Moon J, Yoon JM, Hyun YL, Kim E, Eum SJ, Park SY, Lee JO, Lee TG, Ro S, Cho JM. Structure of the catalytic domain of human phosphodiesterase 5 with bound drug molecules. Nature. 2003;425(6953):98–102. doi: 10.1038/nature01914. [DOI] [PubMed] [Google Scholar]
- 32.Zhang KY, Card GL, Suzuki Y, Artis DR, Fong D, Gillette S, Hsieh D, Neiman J, West BL, Zhang C, Milburn MV, Kim SH, Schlessinger J, Bollag G. Glutamine switch mechanism for nucleotide selectivity by phosphodiesterases. Mol Cell. 2004;15(2):279–286. doi: 10.1016/j.molcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Corbin JD, Blount MA, Weeks JL, 2nd, Beasley A, Kuhn KP, Ho YS, Saidi LF, Hurley JH, Kotera J, Francis SH. [3H]Sildenafil binding to phosphodiesterase-5 is specific, kinetically heterogeneous, and stimulated by cGMP. Mol Pharmacol. 2003;63(6):1364–1372. doi: 10.1124/mol.63.6.1364. [DOI] [PubMed] [Google Scholar]
- 34.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol Rev. 2011;91(2):651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 35.Zoraghi R, Corbin JD, Francis SH. Properties and functions of GAF domains in cyclic nucleotide phosphodiesterases and other proteins. Mol Pharmacol. 2004;65(2):267–278. doi: 10.1124/mol.65.2.267. [DOI] [PubMed] [Google Scholar]
- 36.Rybalkin SD, Rybalkina IG, Shimizu-Albergine M, Tang X-B, Beavo JA. PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J. 2003;22(3):469–478. doi: 10.1093/emboj/cdg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corbin JD, Turko Be, IV, asley A, Francis SH. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur J Biochem. 2000;267(9):2760–2767. doi: 10.1046/j.1432-1327.2000.01297.x. [DOI] [PubMed] [Google Scholar]
- 38.Yanaka N, Kotera J, Ohtsuka A, Akatsuka H, Imai Y, Michibata H, Fujishige K, Kawai E, Takebayashi S, Okumura K, Omori K. Expression, structure and chromosomal localization of the human cGMP-binding cGMP-specific phosphodiesterase PDE5A gene. Eur J Biochem. 1998;255(2):391–399. doi: 10.1046/j.1432-1327.1998.2550391.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin C-S, Lau A, Tu R, Lue TF. Expression of three isoforms of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in human penile cavernosum. Biochem Biophys Res Commun. 2000;268(2):628–635. doi: 10.1006/bbrc.2000.2187. [DOI] [PubMed] [Google Scholar]
- 40.Loughney K, Hill TR, Florio VA, Uher L, Rosman GJ, Wolda SL, Jones BA, Howard ML, McAllister-Lucas LM, Sonnenburg WK, Francis SH, Corbin JD, Beavo JA, Ferguson K. Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 3′,5′-cyclic nucleotide phosphodiesterase. Gene. 1998;216(1):139–147. doi: 10.1016/s0378-1119(98)00303-5. [DOI] [PubMed] [Google Scholar]
- 41.Lin C-S. Tissue expression, distribution, and regulation of PDE5. Int J Impot Res. 2004;16(S1):S8–10. doi: 10.1038/sj.ijir.3901207. [DOI] [PubMed] [Google Scholar]
- 42.Lin C-S, Lau A, Tu R, Lue TF. Identification of three alternative first exons and an intronic promoter of human PDE5A gene. Biochem Biophys Res Commun. 2000;268(2):596–602. doi: 10.1006/bbrc.2000.2186. [DOI] [PubMed] [Google Scholar]
- 43.Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83(5, Suppl 1):3–12. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 44.Sly MK, Eberhart RC, Prager MD. Anti-platelet action of nitric oxide and selective phosphodiesterase inhibitors. Shock. 1997;8:115–118. doi: 10.1097/00024382-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Berkels R, Klotz T, Sticht G, Englemann U, Klaus W. Modulation of human platelet aggregation by the phosphodiesterase type 5 inhibitor sildenafil. J Cardiovasc Pharmacol. 2001;37(4):413–421. doi: 10.1097/00005344-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Pannbacker RG, Fleischman DE, Reed DW. Cyclic nucleotide phosphodiesterase: High activity in a mammalian photoreceptor. Science. 1972;175(4023):757–758. doi: 10.1126/science.175.4023.757. [DOI] [PubMed] [Google Scholar]
- 47.Baehr W, Devlin MJ, Applebury ML. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979;254(22):11669–11677. [PubMed] [Google Scholar]
- 48.Lincoln TM, Hall CL, Park CR, Corbin JD. Guanosine 3′: 5′-cyclic monophosphate binding proteins in rat tissues. Proc Natl Acad Sci. 1976;73(8):2559–2563. doi: 10.1073/pnas.73.8.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coquil JF, Franks DJ, Wells JN, Dupuis M, Hamet P. Characteristics of a new binding protein distinct from the kinase for guanosine 3′:5′-monophosphate in rat platelets. Biochim Biophys Acta. 1980;631(1):148–165. doi: 10.1016/0304-4165(80)90063-x. [DOI] [PubMed] [Google Scholar]
- 50.Beavo JA, Hansen RS, Harrison SA, Hurwitz RL, Martins TJ, Mumby MC. Identification and properties of cyclic nucleotide phosphodiesterases. Mol Cell Endocrinol. 1982;28(3):387–410. doi: 10.1016/0303-7207(82)90135-6. [DOI] [PubMed] [Google Scholar]
- 51.Manganiello VC, Murata T, Taira M, Belfrage P, Degerman E. Diversity in cyclic nucleotide phosphodiesterase isoenzyme families. Arch Biochem Biophys. 1995;322(1):1–13. doi: 10.1006/abbi.1995.1429. [DOI] [PubMed] [Google Scholar]
- 52.Lin C-S, Lin G, Xin Z-C, Lue TF. Expression, distribution and regulation of phosphodiesterase 5. Curr Pharm Des. 2006;12(27):3439–3457. doi: 10.2174/138161206778343064. [DOI] [PubMed] [Google Scholar]
- 53.Corbin JD, Beasley A, Blount MA, Francis SH. High lung PDE5: A strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun. 2005;334(3):930–938. doi: 10.1016/j.bbrc.2005.06.183. [DOI] [PubMed] [Google Scholar]
- 54.Wharton J, Strange JW, Møller GM, Growcott EJ, Ren X, Franklyn AP, Phillips SC, Wilkins MR. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005;172(1):105–113. doi: 10.1164/rccm.200411-1587OC. [DOI] [PubMed] [Google Scholar]
- 55.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116(3):238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 56.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119(3):408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shan X, Quaile MP, Monk JK, French B, Cappola TP, Margulies KB. Differential expression of PDE5 in failing and nonfailing human myocardium. Circ Heart Fail. 2012;5(1):79–86. doi: 10.1161/CIRCHEARTFAILURE.111.961706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, dos Remedios C, Pritzker M, Hall JL, Garry DJ, Chen Y. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation. 2010;121(13):1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gebska MA, Stevenson BK, Hemnes AR, Bivalacqua TJ, Haile A, Hesketh GG, Murray CI, Zaiman AL, Halushka MK, Krongkaew N, Strong TD, Cooke CA, El-Haddad H, Tuder RM, Berkowitz DE, Champion HC. Phosphodiesterase-5A (PDE5A) is localized to the endothelial caveolae and modulates NOS3 activity. Cardiovasc Res. 2011;90(2):353–363. doi: 10.1093/cvr/cvq410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ, Kass DA. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96(1):100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 61.Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA. Compartmentalization of cardiac β-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115(16):2159–2167. doi: 10.1161/CIRCULATIONAHA.106.643536. [DOI] [PubMed] [Google Scholar]
- 62.Mullershausen F, Friebe A, Feil R, Thompson WJ, Hofmann F, Koesling D. Direct activation of PDE5 by cGMP long-term effects within NO/cGMP signaling. J Cell Biol. 2003;160(5):719–727. doi: 10.1083/jcb.200211041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biswas KH, Visweswariah SS. Distinct allostery induced in the cyclic GMP-binding, cyclic GMP-specific phosphodiesterase (PDE5) by cyclic gmp, sildenafil, and metal ions. J Biol Chem. 2011;286(10):8545–8554. doi: 10.1074/jbc.M110.193185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rybalkina IG, Tang X-B, Rybalkin SD. Multiple affinity states of cGMP-specific phosphodiesterase for sildenafil inhibition defined by cGMP-dependent and cGMP-independent mechanisms. Mol Pharmacol. 2010;77(4):670–677. doi: 10.1124/mol.109.062299. [DOI] [PubMed] [Google Scholar]
- 65.Corbin JD, Zoraghi R, Francis SH. Allosteric-site and catalytic-site ligand effects on PDE5 functions are associated with distinct changes in physical form of the enzyme. Cell Signal. 2009;21(12):1768–1774. doi: 10.1016/j.cellsig.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bessay EP, Zoraghi R, Blount MA, Grimes KA, Beasley A, Francis SH, Corbin JD. Phosphorylation of phosphodiesterase-5 is promoted by a conformational change induced by sildenafil, vardenafil, or tadalafil. Front Biosci. 2007;12:1899–1910. doi: 10.2741/2196. [DOI] [PubMed] [Google Scholar]
- 67.Wang H, Liu Y, Huai Q, Cai J, Zoraghi R, Francis SH, Corbin JD, Robinson H, Xin Z, Lin G, Ke H. Multiple conformations of phosphodiesterase-5: Implications for enzyme function and drug development. J Biol Chem. 2006;281(30):21469–21479. doi: 10.1074/jbc.M512527200. [DOI] [PubMed] [Google Scholar]
- 68.Francis SH, Zoraghi R, Kotera J, Ke H, Bessay EP, Blount MA. Phosphodiesterase 5: Molecular characteristics relating to structure function and regulation. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 69.Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem. 2002;277(5):3310–3317. doi: 10.1074/jbc.M106562200. [DOI] [PubMed] [Google Scholar]
- 70.Chaumais M-C, Perrin S, Sitbon O, Simonneau G, Humbert M, Montani D. Pharmacokinetic evaluation of sildenafil as a pulmonary hypertension treatment. Expert Opin Drug Metab Toxicol. 2013;9(9):1193–1205. doi: 10.1517/17425255.2013.804063. [DOI] [PubMed] [Google Scholar]
- 71.Broughton BJ, Chaplen P, Knowles P, Lunt E, Pain DL, Wooldridge KRH, Ford R, Marshall S, Walker JL, Maxwell DR. New inhibitor of reagin-mediated anaphylaxis. Nature. 1974;251:650–652. doi: 10.1038/251650a0. doi:10.1038/251650a0. [DOI] [PubMed] [Google Scholar]
- 72.Kukovetz WR, Holzmann S, Wurm A, Pöch G. Evidence for cyclic GMP-mediated relaxant effects of nitro-compounds in coronary smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1979;310(2):129–138. doi: 10.1007/BF00500277. [DOI] [PubMed] [Google Scholar]
- 73.Gibson A. Phosphodiesterase 5 inhibitors and nitrergic transmission—from zaprinast to sildenafil. Eur J Pharmacol. 2001;411(1-2):1–10. doi: 10.1016/s0014-2999(00)00824-4. [DOI] [PubMed] [Google Scholar]
- 74.Bruzziches R, Francomano D, Gareri P, Lenzi A, Aversa A. An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin Pharmacother. 2013;14(10):1333–1344. doi: 10.1517/14656566.2013.799665. [DOI] [PubMed] [Google Scholar]
- 75.Bell AS, Brown D, Terrett NK. Pyrazolopyrimidinone antianginal agents. 1993 US5250534 A. [Google Scholar]
- 76.Campbell S. Science, art and drug discovery: A personal perspective. Clin Sci. 2000;99:255–260. [PubMed] [Google Scholar]
- 77.Naylor AM. Endogenous neurotransmitters mediating penile erection. Br J Urol. 1998;81(3):424–431. doi: 10.1046/j.1464-410x.1998.00553.x. [DOI] [PubMed] [Google Scholar]
- 78.Rotella DP. Tadalafil lilly ICOS. Curr Opin Investig Drugs Lond Engl. 2003;4(1):60–65. [PubMed] [Google Scholar]
- 79.Coleman CI, Carabino JM, Vergara CM. Fei Wang.Vardenafil. Formulary. 2003;38(3):131. [Google Scholar]
- 80.Salem EA, Kendirci M, Hellstrom WJG. Udenafil, a long-acting PDE5 inhibitor for erectile dysfunction. Curr Opin Investig Drugs Lond Engl. 2006;7(7):661–669. [PubMed] [Google Scholar]
- 81.Burke RM, Evans JD. Avanafil for treatment of erectile dysfunction: Review of its potential. Vasc Health Risk Manag. 2012;8:517–523. doi: 10.2147/VHRM.S26712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paick JS, Ahn TY, Choi HK, Chung WS, Kim JJ, Kim SC, Kim SW, Lee SW, Min KS, Moon KH, Park JK, Park K, Park NC, Suh JK, Yang DY, Jung HG. Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med. 2008;5(11):2672–2680. doi: 10.1111/j.1743-6109.2008.00945.x. [DOI] [PubMed] [Google Scholar]
- 83.Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8(2):47–52. [PubMed] [Google Scholar]
- 84.Jeremy JY, Ballard SA, Naylor AM, Miller MAW, Angelini GD. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol. 1997;79(6):958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 85.Shamloul R, Ghanem H. Erectile dysfunction. The Lancet. 2013;381(9861):153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 86.Blount MA, Beasley A, Zoraghi R, Sekhar KR, Bessay EP, Francis SH, Corbin JD. Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol Pharmacol. 2004;66(1):144–152. doi: 10.1124/mol.66.1.144. [DOI] [PubMed] [Google Scholar]
- 87.Gresser U, Gleiter CH. Erectile dysfunction: Comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil–review of the literature. Eur J Med Res. 2002;7(10):435–446. [PubMed] [Google Scholar]
- 88.Saenz de Tejada I, Angulo J, Cuevas P, Fernández A, Moncada I, Allona A, Lledó E, Körschen HG, Niewöhner U, Haning H, Pages E, Bischoff E. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int J Impot Res. 2001;13(5):282–290. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122(1):88–95. doi: 10.1161/CIRCULATIONAHA.110.944603. [DOI] [PubMed] [Google Scholar]
- 90.Burgess G, Hoogkamer H, Collings L, Dingemanse J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. Eur J Clin Pharmacol. 2008;64(1):43–50. doi: 10.1007/s00228-007-0408-z. [DOI] [PubMed] [Google Scholar]
- 91.Wrishko RE, Dingemanse J, Yu A, Darstein C, Phillips DL, Mitchell MI. Pharmacokinetic interaction between tadalafil and bosentan in healthy male subjects. J Clin Pharmacol. 2008;48(5):610–618. doi: 10.1177/0091270008315315. [DOI] [PubMed] [Google Scholar]
- 92.Muirhead GJ, Wulff MB, Fielding A, Kleinermans D, Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000;50(2):99–107. doi: 10.1046/j.1365-2125.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garraffo R, Lavrut T, Ferrando S, Durant J, Rouyrre N, MacGregor TR, Sabo JP, Dellamonica P. Effect of tipranavir/ritonavir combination on the pharmacokinetics of tadalafil in healthy volunteers. J Clin Pharmacol. 2011;51(7):1071–1078. doi: 10.1177/0091270010379808. [DOI] [PubMed] [Google Scholar]
- 94.Loulergue P, Gaillard R, Mir O. Interaction involving tadalafil and CYP3A4 inhibition by ritonavir. Scand J Infect Dis. 2011;43(3):239–240. doi: 10.3109/00365548.2010.526139. [DOI] [PubMed] [Google Scholar]
- 95. LEVITRA [Internet]. Available from: http://www.levitra.com/.
- 96. CIALIS [Internet]. Available from: http://pi.lilly.com/us/cialis-pi.pdf.
- 97.Chinello P, Cicalini S, Pichini S, Pacifici R, Tempestilli M, Petrosillo N. Sildenafil plasma concentrations in two HIV patients with pulmonary hypertension treated with ritonavir-boosted protease inhibitors. Curr HIV Res. 2012;10(2):162–164. doi: 10.2174/157016212799937263. [DOI] [PubMed] [Google Scholar]
- 98.Prickaerts J, Steinbusch HWM, Smits JFM, de Vente J. Possible role of nitric oxide-cyclic GMP pathway in object recognition memory: Effects of 7-nitroindazole and zaprinast. Eur J Pharmacol. 1997;337(2-3):125–136. doi: 10.1016/s0014-2999(97)01301-0. [DOI] [PubMed] [Google Scholar]
- 99.Evgenov OV, Ichinose F, Evgenov NV, Gnoth MJ, Falkowski GE, Chang Y, Bloch KD, Zapol WM. Soluble guanylate cyclase activator reverses acute pulmonary hypertension and augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs. Circulation. 2004;110(15):2253–2259. doi: 10.1161/01.CIR.0000144469.01521.8A. [DOI] [PubMed] [Google Scholar]
- 100.Ichinose F, Adrie C, Hurford WE, Zapol WM. Prolonged pulmonary vasodilator action of inhaled nitric oxide by zaprinast in awake lambs. J Appl Physiol. 1995;78(4):1288–1295. doi: 10.1152/jappl.1995.78.4.1288. [DOI] [PubMed] [Google Scholar]
- 101.Thusu KG, Morin FC, 3rd, Russell JA, Steinhorn RH. The cGMP phosphodiesterase inhibitor zaprinast enhances the effect of nitric oxide. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1605–1610. doi: 10.1164/ajrccm.152.5.7582302. [DOI] [PubMed] [Google Scholar]
- 102.Ziegler JW, Ivy DD, Wiggins JW, Kinsella JP, Clarke WR, Abman SH. Effects of dipyridamole and inhaled nitric oxide in pediatric patients with pulmonary hypertension. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1388–1395. doi: 10.1164/ajrccm.158.5.9710117. [DOI] [PubMed] [Google Scholar]
- 103.Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, Mirrakhimov MM, Aldashev A, Wilkins MR. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104(4):424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- 104.Sylvester JT, Shimoda LA, Aaronson PI, Ward JPT. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92(1):367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Euler USv, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand. 1946;12(4):301–320. [Google Scholar]
- 106.Rabinovitch M, Gamble W, Nadas AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: Hemodynamic and structural features. Am J Physiol. 1979;236(6):H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- 107.Alexander AF. The bovine lung: Normal vascular histology and vascular lesions in high mountain disease. Respiration. 1962;19(6):528–542. doi: 10.1159/000192262. [DOI] [PubMed] [Google Scholar]
- 108.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107(25):3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 109.Maclean MR, Johnston ED, Mcculloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: Changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283(2):619–624. [PubMed] [Google Scholar]
- 110.Hanasato N, Oka M, Muramatsu M, Nishino M, Adachi H, Fukuchi Y. E-4010, a selective phosphodiesterase 5 inhibitor, attenuates hypoxic pulmonary hypertension in rats. Am J Physiol-Lung Cell Mol Physiol. 1999;277(2):L225–L232. doi: 10.1152/ajplung.1999.277.2.L225. [DOI] [PubMed] [Google Scholar]
- 111.Weimann J, Ullrich R, Hromi J, Fujino Y, Clark MW, Bloch KD, Zapol WM. Sildenafil is a pulmonary vasodilator in awake lambs with acute pulmonary hypertension. Anesthesiology. 2000;92(6):1702–1712. doi: 10.1097/00000542-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 112.Ichinose F, Adrie C, Hurford WE, Bloch KD, Zapol WM. Selective pulmonary vasodilation induced by aerosolized zaprinast. Anesthesiology. 1998;88(2):410–416. doi: 10.1097/00000542-199802000-00020. [DOI] [PubMed] [Google Scholar]
- 113.McMAHON TJ, Ignarro LJ, Kadowitz PJ. Influence of zaprinast on vascular tone and vasodilator responses in the cat pulmonary vascular bed. J Appl Physiol. 1993;74:1704–1711. doi: 10.1152/jappl.1993.74.4.1704. [DOI] [PubMed] [Google Scholar]
- 114.Kouyoumdjian C, Adnot S, Levame M, Eddahibi S, Bousbaa H, Raffestin B. Continuous inhalation of nitric oxide protects against development of pulmonary hypertension in chronically hypoxic rats. J Clin Invest. 1994;94(2):578. doi: 10.1172/JCI117372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kirsch M, Kemp-Harper B, Weissmann N, Grimminger F, Schmidt HHHW. Sildenafil in hypoxic pulmonary hypertension potentiates a compensatory up-regulation of NO-cGMP signaling. FASEB J. 2008;22(1):30–40. doi: 10.1096/fj.06-7526com. [DOI] [PubMed] [Google Scholar]
- 116.Sauzeau V, Rolli-Derkinderen M, Lehoux S, Loirand G, Pacaud P. Sildenafil prevents change in RhoA expression induced by chronic hypoxia in rat pulmonary artery. Circ Res. 2003;93(7):630–637. doi: 10.1161/01.RES.0000093220.90027.D9. [DOI] [PubMed] [Google Scholar]
- 117.Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guérin P, Sagan C, Pacaud P, Loirand G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol. 2005;146(7):1010–1018. doi: 10.1038/sj.bjp.0706408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pauvert O, Bonnet S, Rousseau E, Marthan R, Savineau JP. Sildenafil alters calcium signaling and vascular tone in pulmonary arteries from chronically hypoxic rats. Am J Physiol – Lung Cell Mol Physiol. 2004;287(3):L577–L583. doi: 10.1152/ajplung.00449.2003. [DOI] [PubMed] [Google Scholar]
- 119.Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, Ermert L, Ermert M, Weissmann N, Rose F, Guenther A, Walmrath D, Seeger W, Grimminger F. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;169(1):39–45. doi: 10.1164/rccm.200302-282OC. [DOI] [PubMed] [Google Scholar]
- 120.Liu H, Liu Z, Guan Q. Oral sildenafil prevents and reverses the development of pulmonary hypertension in monocrotaline-treated rats. Interact Cardiovasc Thorac Surg. 2007;6(5):608–613. doi: 10.1510/icvts.2006.147033. [DOI] [PubMed] [Google Scholar]
- 121.Sawamura F, Kato M, Fujita K, Nakazawa T, Beardsworth A. Tadalafil, a long-acting inhibitor of PDE5, improves pulmonary hemodynamics and survival rate of monocrotaline-induced pulmonary artery hypertension in rats. J Pharmacol Sci. 2009;111(3):235–243. doi: 10.1254/jphs.09110fp. [DOI] [PubMed] [Google Scholar]
- 122.Fan YF, Zhang R, Jiang X, Wen L, Wu DC, Liu D, Yuan P, Wang YL, Jing ZC. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc Res. 2013;99(3):395–403. doi: 10.1093/cvr/cvt109. [DOI] [PubMed] [Google Scholar]
- 123.Yen CH, Leu S, Lin YC, Kao YH, Chang LT, Chua S, Fu M, Wu CJ, Sun CK, Yip HK. Sildenafil limits monocrotaline-induced pulmonary hypertension in rats through suppression of pulmonary vascular remodeling. J Cardiovasc Pharmacol. 2010;55(6):574–584. doi: 10.1097/FJC.0b013e3181d9f5f4. [DOI] [PubMed] [Google Scholar]
- 124.Bogdan S, Seferian A, Totoescu A, Dumitrache-Rujinski S, Ceausu M, Coman C, Ardelean CM, Dorobantu M, Bogdan M. Sildenafil reduces inflammation and prevents pulmonary arterial remodeling of the monocrotaline – induced disease in the wistar rats. Maedica (Buchar) 2012;7(2):109–116. [PMC free article] [PubMed] [Google Scholar]
- 125.Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: From angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5(8):689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prasad S, Wilkinson J, Gatzoulis MA. Sildenafil in primary pulmonary hypertension. N Engl J Med. 2000;343(18):1342. doi: 10.1056/NEJM200011023431814. [DOI] [PubMed] [Google Scholar]
- 127.Wilkens H, Guth A, König J, Forestier N, Cremers B, Hennen B, Böhm M, Sybrecht GW. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation. 2001;104(11):1218–1222. doi: 10.1161/hc3601.096826. [DOI] [PubMed] [Google Scholar]
- 128.Ghofrani HA, Wiedemann R, Rose F, Olschewski H, Schermuly RT, Weissmann N, Seeger W, Grimminger F. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002;136(7):515–522. doi: 10.7326/0003-4819-136-7-200204020-00008. [DOI] [PubMed] [Google Scholar]
- 129.Ss K, B D. Chronic oral sildenafil therapy in severe pulmonary artery hypertension. Indian Heart J. 2001;54(4):404–409. [PubMed] [Google Scholar]
- 130.Sastry BK, Narasimhan C, Reddy NK, Anand B, Prakash GS, Raju PR, Kumar DN. A study of clinical efficacy of sildenafil in patients with primary pulmonary hypertension. Indian Heart J. 2001;54(4):410–414. [PubMed] [Google Scholar]
- 131.Sastry BKS, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension: A randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol. 2004;43(7):1149–1153. doi: 10.1016/j.jacc.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 132.Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension comparison with inhaled nitric oxide. Circulation. 2002;105(20):2398–2403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- 133.Michelakis ED, Tymchak W, Noga M, Webster L, Wu XC, Lien D, Wang SH, Modry D, Archer SL. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108(17):2066–2069. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- 134.Lepore JJ, Maroo A, Pereira NL, Ginns LC, Dec GW, Zapol WM, Bloch KD, Semigran MJ. Effect of sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am J Cardiol. 2002;90(6):677–680. doi: 10.1016/s0002-9149(02)02586-9. [DOI] [PubMed] [Google Scholar]
- 135.Singh B, Gupta R, Punj V, Ghose T, Sapra R, Grover DN, Kaul U. Sildenafil in the management of primary pulmonary hypertension. Indian Heart J. 2002;54(3):297–300. [PubMed] [Google Scholar]
- 136.Zimmermann GS, von Wulffen W, Huppmann P, Meis T, Ihle F, Geiseler J, Leuchte HH, Tufman A, Behr J, Neurohr C. Haemodynamic changes in pulmonary hypertension in patients with interstitial lung disease treated with PDE-5 inhibitors. Respirology. 2014;19(5):700–706. doi: 10.1111/resp.12294. [DOI] [PubMed] [Google Scholar]
- 137.Watanabe H, Ohashi K, Takeuchi K, Yamashita K, Yokoyama T, Tran QK, Satoh H, Terada H, Ohashi H, Hayashi H. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther. 2002;71(5):398–402. doi: 10.1067/mcp.2002.123554. [DOI] [PubMed] [Google Scholar]
- 138.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G, Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 139.Moncada I, Jara J, Subirá D, Castaño I, Hernández C. Efficacy of sildenafil citrate at 12 hours after dosing: Re-exploring the therapeutic window. Eur Urol. 2004;46(3):357–361. doi: 10.1016/j.eururo.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 140.Rubin LJ, Badesch DB, Fleming TR, Galiè N, Simonneau G, Ghofrani HA, Oakes M, Layton G, Serdarevic-Pehar M, McLaughlin VV, Barst RJ. SUPER-2 Study Group Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: The SUPER-2 study. Chest. 2011;140(5):1274–1283. doi: 10.1378/chest.10-0969. [DOI] [PubMed] [Google Scholar]
- 141.Affuso F, Palmieri EA, Di Conza P, Guardasole V, Fazio S. Tadalafil improves quality of life and exercise tolerance in idiopathic pulmonary arterial hypertension. Int J Cardiol. 2006;108(3):429–431. doi: 10.1016/j.ijcard.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 142.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ, Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 143.Oudiz RJ, Brundage BH, Galiè N, Ghofrani HA, Simonneau G, Botros FT, Chan M, Beardsworth A, Barst RJ, PHIRST Study Group Tadalafil for the treatment of pulmonary arterial hypertension: A double-blind 52-week uncontrolled extension study. J Am Coll Cardiol. 2012;60(8):768–774. doi: 10.1016/j.jacc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 144.Frantz RP, Durst L, Burger CD, Oudiz RJ, Bourge RC, Franco V, Waxman AB, McDevitt S, Walker S. Conversion from sildenafil to tadalafil: Results from the sildenafil to tadalafil in pulmonary arterial hypertension (SITAR) study. J Cardiovasc Pharmacol Ther. 2014;19(6):550–557. doi: 10.1177/1074248414528066. [DOI] [PubMed] [Google Scholar]
- 145.Archer SL, Michelakis ED. An evidence-based approach to the management of pulmonary arterial hypertension. Curr Opin Cardiol. 2006;21(4):385–392. doi: 10.1097/01.hco.0000231410.07426.9b. [DOI] [PubMed] [Google Scholar]
- 146.O'Callaghan D, Gaine SP. Combination therapy and new types of agents for pulmonary arterial hypertension. Clin Chest Med. 2007;28(1):169–185. doi: 10.1016/j.ccm.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 147.Kawut SM, Horn EM, Berekashvili KK, Garofano RP, Goldsmith RL, Widlitz AC, Rosenzweig EB, Kerstein D, Barst RJ. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95(2):199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 148.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: Updated accp evidence-based clinical practice guidelines. Chest. 2007;131(6):1917–1928. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 149.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 150.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30(20):2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 151.Galiè N, Negro L, Simonneau G. The use of combination therapy in pulmonary arterial hypertension: New developments. Eur Respir Rev. 2009;18(113):148–153. doi: 10.1183/09059180.00003809. [DOI] [PubMed] [Google Scholar]
- 152.McGoon MD, Miller DP. REVEAL: A contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21(123):8–18. doi: 10.1183/09059180.00008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stiebellehner L, Petkov V, Vonbank K, Funk G, Schenk P, Ziesche R, Block LH. Long-term treatment with oral sildenafil in addition to continuous IV epoprostenol in patients with pulmonary arterial hypertension. Chest. 2003;123(4):1293–1295. doi: 10.1378/chest.123.4.1293. [DOI] [PubMed] [Google Scholar]
- 154.Hoeper M, Faulenbach C, Golpon H, Winkler J, Welte T, Niedermeyer J. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J. 2004;24(6):1007–1010. doi: 10.1183/09031936.04.00051104. [DOI] [PubMed] [Google Scholar]
- 155.Mathai SC, Girgis RE, Fisher MR, Champion HC, Housten-Harris T, Zaiman A, Hassoun PM. Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension. Eur Respir J. 2007;29(3):469–475. doi: 10.1183/09031936.00081706. [DOI] [PubMed] [Google Scholar]
- 156.Porhownik NR, Al-Sharif H, Bshouty Z. Addition of sildenafil in patients with pulmonary arterial hypertension with inadequate response to bosentan monotherapy. Can Respir J J Can Thorac Soc. 2008;15(8):427–430. doi: 10.1155/2008/897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gruenig E, Michelakis E, Vachiéry JL, Vizza CD, Meyer FJ, Doelberg M, Bach D, Dingemanse J, Galiè N. Acute hemodynamic effects of single-dose sildenafil when added to established bosentan therapy in patients with pulmonary arterial hypertension: Results of the COMPASS-1 study. J Clin Pharmacol. 2009;49(11):1343–1352. doi: 10.1177/0091270009341182. [DOI] [PubMed] [Google Scholar]
- 158.Bendayan D, Shitrit D, Kramer MR. Combination therapy with prostacyclin and tadalafil for severe pulmonary arterial hypertension: A pilot study. Respirology. 2008;13(6):916–918. doi: 10.1111/j.1440-1843.2007.01176.x. [DOI] [PubMed] [Google Scholar]
- 159.Zhuang Y, Jiang B, Gao H, Zhao W. Randomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertension. Hypertens Res. 2014;37(6):507–512. doi: 10.1038/hr.2014.28. [DOI] [PubMed] [Google Scholar]
- 160.Hirashiki A, Kondo T, Murohara T. Combination therapy adding tadalafil to existing ambrisentan in patients with pulmonary arterial hypertension. Hypertens Res. 2014;37(6):488–489. doi: 10.1038/hr.2014.37. [DOI] [PubMed] [Google Scholar]
- 161.Barst RJ, Oudiz RJ, Beardsworth A, Brundage BH, Simonneau G, Ghofrani HA, Sundin DP, Galiè N, Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group Tadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30(6):632–643. doi: 10.1016/j.healun.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 162.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH, Rubin LJ. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174(11):1257–1263. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 163.Humbert M, Barst RJ, Robbins IM, Channick RN, Galiè N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24(3):353–359. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 164.Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch DB, PACES Study Group Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: A randomized trial. Ann Intern Med. 2008;149(8):521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 165.Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost A, Engel P, Kramer MR, Serdarevic-Pehar M, Layton GR, Sitbon O, Badesch DB, PACES Study Group Long-term sildenafil added to intravenous epoprostenol in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2014;33(7):689–697. doi: 10.1016/j.healun.2014.02.019. Available from: http://www.sciencedirect.com/science/article/pii/S1053249814009978. [DOI] [PubMed] [Google Scholar]
- 166.Barst R. How has epoprostenol changed the outcome for patients with pulmonary arterial hypertension? Int J Clin Pract. 2010;64:23–32. doi: 10.1111/j.1742-1241.2010.02525.x. [DOI] [PubMed] [Google Scholar]
- 167.Gomberg-Maitland M, McLaughlin V, Gulati M, Rich S. Efficacy and safety of sildenafil added to treprostinil in pulmonary hypertension. Am J Cardiol. 2005;96(9):1334–1336. doi: 10.1016/j.amjcard.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 168.Buckley MS, Staib RL, Wicks LM. Combination therapy in the management of pulmonary arterial hypertension. Int J Clin Pract. 2013;67:13–23. doi: 10.1111/ijcp.12136. [DOI] [PubMed] [Google Scholar]
- 169.Lunze K, Gilbert N, Mebus S, Miera O, Fehske W, Uhlemann F, Mühler EG, Ewert P, Lange PE, Berger F, Schulze-Neick I. First experience with an oral combination therapy using bosentan and sildenafil for pulmonary arterial hypertension. Eur J Clin Invest. 2006;36:32–38. doi: 10.1111/j.1365-2362.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 170.Torres F, Gupta H, Soto F, Park M, Frey N, Murali S, Benza R. Safety and efficacy of bosentan in combination with sildenafil in pulmonary arterial hypertension: The COMPASS-3 study. Eur Respir J. 2011;38(Suppl 55):409. [Google Scholar]
- 171. COMPASS-2 press release [Internet]. Available from: http://www1.actelion.com/en/our-company/news-and-events/index.page?newsId = 1769001.
- 172.Spence R, Mandagere A, Harrison B, Dufton C, Boinpally R. No clinically relevant pharmacokinetic and safety interactions of ambrisentan in combination with tadalafil in healthy volunteers. J Pharm Sci. 2009;98(12):4962–4974. doi: 10.1002/jps.21789. [DOI] [PubMed] [Google Scholar]
- 173. NIH. Study of ambrisentan and phosphodiesterase type-5 Inhibitor (PDE-5i) to treat pulmonary arterial hypertension [Internet]. Available from: www.clinicaltrials.gov/ct2/show/NCT00617305.
- 174. ATHENA-1 [Internet]. Available from: http://www.phaonlineuniv.org/ResourceLibrary/Resource.cfm?ItemNumber = 3756.
- 175. AMBITION [Internet]. Available from: www.clinicaltrials.gov/ct2/show/NCT01178073.
- 176.Kylhammar D, Persson L, Hesselstrand R, Rådegran G. Prognosis and response to first-line single and combination therapy in pulmonary arterial hypertension. Scand Cardiovasc J. 2014;48(4):223–233. doi: 10.3109/14017431.2014.931595. [DOI] [PubMed] [Google Scholar]
- 177.Collins FS, Orringer EP. Pulmonary hypertension and cor pulmonale in the sickle hemoglobinopathies. Am J Med. 1982;73(6):814–821. doi: 10.1016/0002-9343(82)90763-x. [DOI] [PubMed] [Google Scholar]
- 178.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: Cardiac catheterization results and survival. Blood. 2003;101(4):1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 179.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39(1):112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 180.Simonneau G, Parent F. Pulmonary hypertension in patients with sickle cell disease: not so frequent but so different. Eur Respir J. 2012;39(1):3–4. doi: 10.1183/09031936.00119811. [DOI] [PubMed] [Google Scholar]
- 181.Aliyu ZY, Kato GJ, Taylor J, 6th, Babadoko A, Mamman AI, Gordeuk VR, Gladwin MT. Sickle cell disease and pulmonary hypertension in Africa: A global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol. 2008;83(1):63–70. doi: 10.1002/ajh.21057. [DOI] [PubMed] [Google Scholar]
- 182.Gladwin MT, Kato GJ. Cardiopulmonary complications of sickle cell disease: Role of nitric oxide and hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2005:51–57. doi: 10.1182/asheducation-2005.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 184.Machado RF, Gladwin MT. Pulmonary hypertension in hemolytic disorders: Pulmonary vascular disease: The global perspective. Chest. 2010;137(6_suppl):30S–38S. doi: 10.1378/chest.09-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schnog JJ, Jager EH, van der Dijs FP, Duits AJ, Moshage H, Muskiet FD, Muskiet FA. Evidence for a metabolic shift of arginine metabolism in sickle cell disease. Ann Hematol. 2004;83(6):371–375. doi: 10.1007/s00277-004-0856-9. [DOI] [PubMed] [Google Scholar]
- 186.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 187.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116(5):687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 189.Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, Hunter L, Coles W, Nichols J, Hunter C, Sachdev V, Castro O, Gladwin MT. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130(3):445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Derchi G, Forni GL, Formisano F, Cappellini MD, Galanello R, D'Ascola G, Bina P, Magnano C, Lamagna M. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90(4):452–458. [PubMed] [Google Scholar]
- 191.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307(12):1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mehari A, Alam S, Tian X, Cuttica MJ, Barnett CF, Miles G, Xu D, Seamon C, Adams-Graves P, Castro OL, Minniti CP, Sachdev V, Taylor JG, 6th, Kato GJ, Machado RF. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187(8):840–847. doi: 10.1164/rccm.201207-1222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 194.Gladwin MT, Barst RJ, Gibbs JS, Hildesheim M, Sachdev V, Nouraie M, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, Novelli E, Girgis RE, Morris CR, Berman Rosenzweig E, Badesch DB, Lanzkron S, Castro OL, Taylor JG, 6th, Goldsmith JC, Kato GJ, Gordeuk VR, Machado RF, walk-PHaSST Investigators and Patients Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PloS One. 2014;9(7):e99489. doi: 10.1371/journal.pone.0099489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Goldsmith JC, Woolson R, Gordeuk VR, Gibbs S, Little JA, Kato GJ, Schraufnagel DE, Krishnamurti L, Girgis R, Morris CR, Berman-Rosenzweig E, Badesch DB, Waclawiw MA, Gladwin MT. Evaluation of sildenafil therapy for patients with sickle cell disease and increased tricuspid regurgitant velocity: Preliminary results of the Walk-PHaSST trial. B16. Pulmonary Arterial Hypertension: From Assessing Risk to Therapeutic Results. 2010:A2514. doi:10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A2514. [Google Scholar]
- 196.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, Gibbs JS, Little JA, Schraufnagel DE, Krishnamurti L, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Onyekwere O, Castro OL, Sachdev V, Waclawiw MA, Woolson R, Goldsmith JC, Gladwin MT, walk-PHaSST Investigators and Patients Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118(4):855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zuber JP, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, Nuesch R, Magenta L, Ledergerber B, Jenni R, Speich R, Opravil M, Swiss HIV Cohort Study Group Pulmonary arterial hypertension related to HIV infection: Improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004;38(8):1178–1185. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]
- 198.Wong AR, Rasool AHG, Abidin NZ, Noor AR, Quah BS. Sildenafil as treatment for human immunodeficiency virus-related pulmonary hypertension in a child. J Paediatr Child Health. 2006;42(3):147–148. doi: 10.1111/j.1440-1754.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 199.Degano B, Guillaume M, Savale L, Montani D, Jaïs X, Yaici A, Le Pavec J, Humbert M, Simonneau G, Sitbon O. HIV-associated pulmonary arterial hypertension: Survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24(1):67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 200.Degano B, Valmary S, Sitbon O, Humbert M.Pulmonary arterial hypertension and HIV and other viral infections Humbert M, Souza R, Simonneau G.Pulmonary Vascular Disorders. Prog Respir Res Vol 41Basel: Karger; 2012105–112. Available from: http://content.karger.com/ProdukteDB/produkte.asp?Aktion=ShowAbstractBuch&ArtikelNr=334362&ProduktNr=255594 [Google Scholar]
- 201.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 202.Ghofrani HA, Distler O, Gerhardt F, Gorenflo M, Grünig E, Haefeli WE, Held M, Hoeper MM, Kähler CM, Kaemmerer H, Klose H, Köllner V, Kopp B, Mebus S, Meyer A, Miera O, Pittrow D, Riemekasten G, Rosenkranz S, Schranz D, Voswinckel R, Olschewski H. Treatment of pulmonary arterial hypertension (PAH): Updated recommendations of the cologne consensus conference 2011. Int J Cardiol. 2011;154(Suppl 1):S20–S33. doi: 10.1016/S0167-5273(11)70490-9. [DOI] [PubMed] [Google Scholar]
- 203.Schumacher YO, Zdebik A, Huonker M, Kreisel W. Sildenafil in HIV-related pulmonary hypertension. AIDS. 2001;15(13):1747–1748. doi: 10.1097/00002030-200109070-00026. [DOI] [PubMed] [Google Scholar]
- 204.D'Alto M, Romeo E, Argiento P, Sarubbi B, Santoro G, Grimaldi N, Correra A, Scognamiglio G, Russo MG, Calabrò R. Bosentan-sildenafil association in patients with congenital heart disease-related pulmonary arterial hypertension and Eisenmenger physiology. Int J Cardiol. 2012;155(3):378–382. doi: 10.1016/j.ijcard.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 205.Singh TP, Rohit M, Grover A, Malhotra S, Vijayvergiya R. A randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J. 2006;151(4):851e1–851e5. doi: 10.1016/j.ahj.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 206.Mukhopadhyay S, Sharma M, Ramakrishnan S, Yusuf J, Gupta MD, Bhamri N, Trehan V, Tyagi S. Phosphodiesterase-5 inhibitor in eisenmenger syndrome: A preliminary observational study. Circulation. 2006;114(17):1807–1810. doi: 10.1161/CIRCULATIONAHA.105.603001. [DOI] [PubMed] [Google Scholar]
- 207.Chau EMC, Fan KYY, Chow WH. Effects of chronic sildenafil in patients with Eisenmenger syndrome versus idiopathic pulmonary arterial hypertension. Int J Cardiol. 2007;120(3):301–305. doi: 10.1016/j.ijcard.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 208.Lu XL, Xiong CM, Shan GL, Zhu XY, Wu BX, Wu GH, Liu ZH, Ni XH, Cheng XS, Gu Q, Zhao ZH, Zhang DZ, Li WM, Zhang C, Tian HY, Guo YJ, Guo T, Liu HM, Zhang WJ, Gu H, Huang SA, Chen JY, Wu WF, Huang K, Li JJ, He JG. Impact of sildenafil therapy on pulmonary arterial hypertension in adults with congenital heart disease. Cardiovasc Ther. 2010;28(6):350–355. doi: 10.1111/j.1755-5922.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- 209.Sun YJ, Yang T, Zeng WJ, Gu Q, Ni XH, Zhao ZH, Liu ZH, Xiong CM, He JG. Impact of sildenafil on survival of patients with Eisenmenger syndrome. J Clin Pharmacol. 2013;53(6):611–618. doi: 10.1002/jcph.78. [DOI] [PubMed] [Google Scholar]
- 210.Mukhopadhyay S, Nathani S, Yusuf J, Shrimal D, Tyagi S. Clinical efficacy of phosphodiesterase-5 inhibitor tadalafil in Eisenmenger syndrome-a randomized, placebo-controlled, double-blind crossover study. Congenit Heart Dis. 2011;6(5):424–431. doi: 10.1111/j.1747-0803.2011.00561.x. [DOI] [PubMed] [Google Scholar]
- 211.Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD, Wernovsky G, Paridon SM, Rychik J. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: A randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011;123(11):1185–1193. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Manes A, Palazzini M, Leci E, Reggiani MLB, Branzi A, Galiè N. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: A comparison between clinical subgroups. Eur Heart J. 2014;35(11):716–724. doi: 10.1093/eurheartj/eht072. [DOI] [PubMed] [Google Scholar]
- 213.Okyay K, Cemri M, Boyac B, Yalcn R, Cengel A. Use of long-term combined therapy with inhaled iloprost and oral sildenafil in an adult patient with Eisenmenger syndrome. Cardiol Rev. 2005;13(6):312–314. doi: 10.1097/01.crd.0000181618.29506.1e. [DOI] [PubMed] [Google Scholar]
- 214.Iversen K, Jensen AS, Jensen TV, Vejlstrup NG, Søndergaard L. Combination therapy with bosentan and sildenafil in Eisenmenger syndrome: A randomized, placebo-controlled, double-blinded trial. Eur Heart J. 2010;31(9):1124–1131. doi: 10.1093/eurheartj/ehq011. [DOI] [PubMed] [Google Scholar]
- 215.Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2011;183(12):1605–1613. doi: 10.1164/rccm.201011-1854CI. [DOI] [PubMed] [Google Scholar]
- 216.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345(20):1465–1472. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- 217.De Jesus Perez VA, Zamanian RT, Jais X, D'Armini AM, Jansa P. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2011;2011(364):1677–1678. doi: 10.1056/NEJMc1102390. [DOI] [PubMed] [Google Scholar]
- 218.Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113(16):2011–2020. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- 219.Ghofrani HA, Schermuly RT, Rose F, Wiedemann R, Kohstall MG, Kreckel A, Olschewski H, Weissmann N, Enke B, Ghofrani S, Seeger W, Grimminger F. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2003;167(8):1139–1141. doi: 10.1164/rccm.200210-1157BC. [DOI] [PubMed] [Google Scholar]
- 220.Reichenberger F, Voswinckel R, Enke B, Rutsch M, El Fechtali E, Schmehl T, Olschewski H, Schermuly R, Weissmann N, Ghofrani HA, Grimminger F, Mayer E, Seeger W. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2007;30(5):922–927. doi: 10.1183/09031936.00039007. [DOI] [PubMed] [Google Scholar]
- 221.Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon E, Toshner MR, Sheares KK, Hughes R, Morrell NW, Pepke-Zaba J. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest. 2008;134(2):229–236. doi: 10.1378/chest.07-2681. [DOI] [PubMed] [Google Scholar]
- 222.Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C, CHEST-1 Study Group Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 223.Hsu AR, Barnholt KE, Grundmann NK, Lin JH, McCallum SW, Friedlander AL. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol. 2006;100(6):2031–2040. doi: 10.1152/japplphysiol.00806.2005. [DOI] [PubMed] [Google Scholar]
- 224.Richalet JP, Gratadour P, Robach P, Pham I, Déchaux M, Joncquiert-Latarjet A, Mollard P, Brugniaux J, Cornolo J. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am J Respir Crit Care Med. 2005;171(3):275–281. doi: 10.1164/rccm.200406-804OC. [DOI] [PubMed] [Google Scholar]
- 225.Aldashev AA, Kojonazarov BK, Amatov TA, Sooronbaev TM, Mirrakhimov MM, Morrell NW, Wharton J, Wilkins MR. Phosphodiesterase type 5 and high altitude pulmonary hypertension. Thorax. 2005;60(8):683–687. doi: 10.1136/thx.2005.041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ghofrani HA, Reichenberger F, Kohstall MG, Mrosek EH, Seeger T, Olschewski H, Seeger W, Grimminger F. Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mount Everest base camp: A randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med. 2004;141(3):169–177. doi: 10.7326/0003-4819-141-3-200408030-00005. [DOI] [PubMed] [Google Scholar]
- 227.Reichenberger F, Kohstall MG, Seeger T, Olschewski H, Grimminger F, Seeger W, Ghofrani HA. Effect of sildenafil on hypoxia-induced changes in pulmonary circulation and right ventricular function. Respir Physiol Neurobiol. 2007;159(2):196–201. doi: 10.1016/j.resp.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 228.Bates MG, Thompson AA, Baillie JK, Sutherland AI, Irving JB, Hirani N, Webb DJ. Sildenafil citrate for the prevention of high altitude hypoxic pulmonary hypertension: Double blind, randomized, placebo-controlled trial. High Alt Med Biol. 2011;12(3):207–214. doi: 10.1089/ham.2011.0007. [DOI] [PubMed] [Google Scholar]
- 229.Faoro V, Lamotte M, Deboeck G, Pavelescu A, Huez S, Guenard H, Martinot JB, Naeije R. Effects of sildenafil on exercise capacity in hypoxic normal subjects. High Alt Med Biol. 2007;8(2):155–163. doi: 10.1089/ham.2007.1058. [DOI] [PubMed] [Google Scholar]
- 230.Maggiorini M, Brunner-La Rocca HP, Peth S, Fischler M, Böhm T, Bernheim A, Kiencke S, Bloch KE, Dehnert C, Naeije R, Lehmann T, Bärtsch P, Mairbäurl H. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: A randomized trial. Ann Intern Med. 2006;145(7):497–506. doi: 10.7326/0003-4819-145-7-200610030-00007. [DOI] [PubMed] [Google Scholar]
- 231.Xu Y, Liu Y, Liu J, Qian G. Meta-analysis of clinical efficacy of sildenafil, a phosphodiesterase type-5 inhibitor on high altitude hypoxia and its complications. High Alt Med Biol. 2014;15(1):46–51. doi: 10.1089/ham.2013.1110. [DOI] [PubMed] [Google Scholar]
- 232.Travadi JN, Patole SK. Phosphodiesterase inhibitors for persistent pulmonary hypertension of the newborn: A review. Pediatr Pulmonol. 2003;36(6):529–535. doi: 10.1002/ppul.10389. [DOI] [PubMed] [Google Scholar]
- 233.Scipioni A, Giorgi M, Nuccetelli V, Stefanini S. Immunohistochemical localisation of PDE5 in rat lung during pre- and postnatal development. J Biomed Biotechnol. 2009;2009:1–7. doi: 10.1155/2009/932961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jaillard S, Larrue B, Deruelle P, Delelis A, Rakza T, Butrous G, Storme L. Effects of phosphodiesterase 5 inhibitor on pulmonary vascular reactivity in the fetal lamb. Ann Thorac Surg. 2006;81(3):935–942. doi: 10.1016/j.athoracsur.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 235.Larrue B, Jaillard S, Lorthioir M, Roubliova X, Butrous G, Rakza T, Warembourg H, Storme L. Pulmonary vascular effects of sildenafil on the development of chronic pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2005;288(6):L1193–L1200. doi: 10.1152/ajplung.00405.2004. [DOI] [PubMed] [Google Scholar]
- 236.Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91(1):307–310. doi: 10.1097/00000542-199907000-00041. [DOI] [PubMed] [Google Scholar]
- 237.Bigatello LM, Hess D, Dennehy KC, Medoff BD, Hurford WE. Sildenafil can increase the response to inhaled nitric oxide. Anesthesiology. 2000;92(6):1827. doi: 10.1097/00000542-200006000-00044. [DOI] [PubMed] [Google Scholar]
- 238.Miller OI, Tang SF, Keech A, Pigott NB, Beller E, Celermajer DS. Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: A randomised double-blind study. Lancet. 2000;356(9240):1464–1469. doi: 10.1016/S0140-6736(00)02869-5. [DOI] [PubMed] [Google Scholar]
- 239.Abrams D, Schulze-Neick I, Magee AG. Sildenafil as a selective pulmonary vasodilator in childhood primary pulmonary hypertension. Heart. 2000;84(2):e4. doi: 10.1136/heart.84.2.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral Sildenafil in infants with persistent pulmonary hypertension of the newborn: A pilot randomized blinded study. Pediatrics. 2006;117(4):1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 241.Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, Wessel DL. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009;155(6):841–847. doi: 10.1016/j.jpeds.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 242.Steinhorn RH, Kinsella JP, Butrous G, Dilleen M, Oakes M, Wessel DL. Abstract 2768: Open-Label, Multicentre, Pharmacokinetic Study of IV Sildenafil in the Treatment of Neonates With Persistent Pulmonary Hypertension of the Newborn (PPHN) Circulation. 2007;116(16 Suppl):II_614. [Google Scholar]
- 243.Schulze-Neick I, Hartenstein P, Li J, Stiller B, Nagdyman N, Hübler M, Butrous G, Petros A, Lange P, Redington AN. Intravenous sildenafil is a potent pulmonary vasodilator in children with congenital heart disease. Circulation. 2003;108(10 suppl 1):II167–II173. doi: 10.1161/01.cir.0000087384.76615.60. [DOI] [PubMed] [Google Scholar]
- 244.Fraisse A, Butrous G, Taylor M, Oakes M, Dilleen M, Wessel D. Intravenous sildenafil for postoperative pulmonary hypertension in children with congenital heart disease. Intensive Care Med. 2011;37(3):502–509. doi: 10.1007/s00134-010-2065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tunks RD, Barker PCA, Benjamin DK, Cohen-Wolkowiez M, Fleming GA, Laughon M, Li JS, Hill KD. Sildenafil exposure and hemodynamic effect after fontan surgery. Pediatr Crit Care Med. 2014;15(1):28–34. doi: 10.1097/PCC.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Douwes JM, Roofthooft MTR, Loon RLEV, Ploegstra M-J, Bartelds B, Hillege HL, Berger RM. Sildenafil add-on therapy in paediatric pulmonary arterial hypertension, experiences of a national referral centre. Heart. 2014;100(3):224–230. doi: 10.1136/heartjnl-2013-304895. [DOI] [PubMed] [Google Scholar]
- 247.van Loon RL, Roofthooft MT, van Osch-Gevers M, Delhaas T, Strengers JL, Blom NA, Backx A, Berger RM. Clinical characterization of pediatric pulmonary hypertension: Complex presentation and diagnosis. J Pediatr. 2009;155(2):176–82e1. doi: 10.1016/j.jpeds.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 248.Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, Haworth SG, Ivy D, Lopes AA, Raj JU, Sandoval J, Stenmark K, Adatia I. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: Report from the PVRI pediatric taskforce. Panama Pulm Circ. 2011;1(2):286–298. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lammers AE, Adatia I, Cerro MJ, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, Ivy D, Lopes AA, Raj JU, Sandoval J, Stenmark K, Haworth SG. Functional classification of pulmonary hypertension in children: Report from the PVRI pediatric taskforce. Panama Pulm Circ. 2011;1(2):280–285. doi: 10.4103/2045-8932.83445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Barst RJ, Ertel SI, Beghetti M, Ivy DD. Pulmonary arterial hypertension: A comparison between children and adults. Eur Respir J. 2011;37(3):665–677. doi: 10.1183/09031936.00056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Humpl T, Reyes JT, Holtby H, Stephens D, Adatia I. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005;111(24):3274–3280. doi: 10.1161/CIRCULATIONAHA.104.473371. [DOI] [PubMed] [Google Scholar]
- 252.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, Sastry BK, Pulido T, Layton GR, Serdarevic-Pehar M, Wessel DL. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125(2):324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 253.Barst RJ, Beghetti M, Pulido T, Layton G, Konourina I, Zhang M, Ivy DD, STARTS-2 Investigators STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation. 2014;129(19):1914–1923. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 254.Abman SH, Kinsella JP, Rosenzweig EB, Krishnan U, Kulik T, Mullen M, Wessel DL, Steinhorn R, Adatia I, Hanna B, Feinstein J, Fineman J, Raj U, Humpl T, Pediatric Pulmonary Hypertension Network (PPHNet) Implications of the U.S. food and drug administration warning against the use of sildenafil for the treatment of pediatric pulmonary hypertension. Am J Respir Crit Care Med. 2013;187(6):572–575. doi: 10.1164/rccm.201210-1928PP. [DOI] [PubMed] [Google Scholar]
- 255. US Food and Drug Administration. FDA Drug Safety Communication: FDA recommends against use of Revatio (sildenafil) in children with pulmonary hypertension [Internet]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm317123.htm.
- 256.McElhinney DB. A new START for sildenafil in pediatric pulmonary hypertension: Reframing the dose-survival relationship in the STARTS-2 trial. Circulation. 2014;129(19):1905–1908. doi: 10.1161/CIRCULATIONAHA.114.009584. [DOI] [PubMed] [Google Scholar]
- 257.Barst RJ, Beghetti M, Pulido T, Layton G, Konourina I, Zhang M, Ivy DD, STARTS-2 Investigators STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation. 2014;129(19):1914–1923. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 258.Kang KK, Ahn GJ, Sohn YS, Ahn BO, Kim WB. DA-8159, a new PDE5 inhibitor, attenuates the development of compensatory right ventricular hypertrophy in a rat model of pulmonary hypertension. J Int Med Res. 2003;31(6):517–528. doi: 10.1177/147323000303100608. [DOI] [PubMed] [Google Scholar]
- 259.Nagendran J, Sutendra G, Paterson I, Champion HC, Webster L, Chiu B, Haromy A, Rebeyka IM, Ross DB, Michelakis ED. Endothelin axis is upregulated in human and rat right ventricular hypertrophy. Circ Res. 2013;112(2):347–354. doi: 10.1161/CIRCRESAHA.111.300448. [DOI] [PubMed] [Google Scholar]
- 260.Xie Y-P, Chen B, Sanders P, Guo A, Li Y, Zimmerman K, Wang LC, Weiss RM, Grumbach IM, Anderson ME, Song LS. Sildenafil prevents and reverses transverse-tubule remodeling and Ca2+ handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension. 2012;59(2):355–362. doi: 10.1161/HYPERTENSIONAHA.111.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Friedberg MK, Redington AN. Right versus left ventricular failure differences, similarities, and interactions. Circulation. 2014;129(9):1033–1044. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
- 262.Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15(10):1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 263.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 264.Kukreja RC, Salloum F, Das A, Ockaili R, Yin C, Bremer YA, Fisher PW, Wittkamp M, Hawkins J, Chou E, Kukreja AK, Wang X, Marwaha VR, Xi L. Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol. 2005;42(5-6):219–232. doi: 10.1016/j.vph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 265.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92(6):595–597. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 266.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280(13):12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 267.Sahara M, Sata M, Morita T, Nakajima T, Hirata Y, Nagai R. A phosphodiesterase-5 inhibitor vardenafil enhances angiogenesis through a protein kinase G-dependent hypoxia-inducible factor-1/vascular endothelial growth factor pathway. Arterioscler Thromb Vasc Biol. 2010;30(7):1315–1324. doi: 10.1161/ATVBAHA.109.201327. [DOI] [PubMed] [Google Scholar]
- 268.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115(1):59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 269.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116(14):1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 270.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4(1):8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 271.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E, Heart Failure Clinical Research Network PhosphdiesteRasE-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (RELAX) trial: Rationale and design. Circ Heart Fail. 2012;5(5):653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Borlaug B, Lewis G, McNulty S, Semigran M, LeWinter M, Chen H, Lin G, Anstrom K, Velazquez E, Shah M, Margulies K, Redfield M. Diverging effects of sildenafil on ventricular-vascular function in HFPEF. J Am Coll Cardiol. 2014;63(12_S) Available from: http://dx.doi.org/10.1016/S0735-1097(14)60759-2. [Google Scholar]
- 273.Sharma K, Kass DA. Heart failure with preserved ejection fraction mechanisms, clinical features, and therapies. Circ Res. 2014;115(1):79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Melenovsky V, Hwang S-J, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu193. pii: ehu193. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mohammed SF, Borlaug BA, McNulty S, Lewis GD, Lin G, Zakeri R, Semigran MJ, LeWinter M, Hernandez AF, Braunwald E, Redfield MM. Resting ventricular-vascular function and exercise capacity in heart failure with preserved ejection fraction: A RELAX trial ancillary study. Circ Heart Fail. 2014;7(4):580–589. doi: 10.1161/CIRCHEARTFAILURE.114.001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zakeri R, Borlaug BA, McNulty SE, Mohammed SF, Lewis GD, Semigran MJ, Deswal A, LeWinter M, Hernandez AF, Braunwald E, Redfield MM. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction A RELAX trial ancillary study. Circ Heart Fail. 2014;7(1):123–130. doi: 10.1161/CIRCHEARTFAILURE.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Minai OA, Chaouat A, Adnot S. Pulmonary hypertension in COPD: Epidemiology, significance, and management pulmonary vascular disease: The global perspective. Chest. 2010;137(6_suppl):39S–51S. doi: 10.1378/chest.10-0087. [DOI] [PubMed] [Google Scholar]
- 278.Voelkel NF, Cool CD. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(46 suppl):28s–32s. doi: 10.1183/09031936.03.00000503. [DOI] [PubMed] [Google Scholar]
- 279.Barberà JA. Chronic obstructive pulmonary disease: A Disease of the endothelium? Am J Respir Crit Care Med. 2013;188(1):5–7. doi: 10.1164/rccm.201305-0857ED. [DOI] [PubMed] [Google Scholar]
- 280.Goudie A, Hopkinson P, Anderson W, Lipworth B, Struthers A. The effects of sildenafil on lung function in COPD. Eur Respir J. 2012;40(Suppl 56):P2188. [Google Scholar]
- 281.Wang T, Liu Y, Chen L, Wang X, Hu XR, Feng YL, Liu DS, Xu D, Duan YP, Lin J, Ou XM, Wen FQ. Effect of sildenafil on acrolein-induced airway inflammation and mucus production in rats. Eur Respir J. 2009;33(5):1122–1132. doi: 10.1183/09031936.00055908. [DOI] [PubMed] [Google Scholar]
- 282.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: A randomised controlled trial. Lancet. 2002;360(9337):895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 283.Amen EM, Becker E-M, Truebel H. Analysis of V/Q-matching—a safety biomarker in pulmonary drug development? Biomarkers. 2011;16(S1):S5–10. doi: 10.3109/1354750X.2011.585243. [DOI] [PubMed] [Google Scholar]
- 284.Alp S, Skrygan M, Schmidt WE, Bastian A. Sildenafil improves hemodynamic parameters in COPD—an investigation of six patients. Pulm Pharmacol Ther. 2006;19(6):386–390. doi: 10.1016/j.pupt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 285.Madden BP, Allenby M, Loke T-K, Sheth A. A potential role for sildenafil in the management of pulmonary hypertension in patients with parenchymal lung disease. Vascul Pharmacol. 2006;44(5):372–376. doi: 10.1016/j.vph.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 286.Naeije R, Boerrigter BG. Pulmonary hypertension at exercise in COPD: Does it matter? Eur Respir J. 2013;41(5):1002–1004. doi: 10.1183/09031936.00173512. [DOI] [PubMed] [Google Scholar]
- 287.Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):20–22. doi: 10.1513/pats.200407-037MS. [DOI] [PubMed] [Google Scholar]
- 288.Blanco I, Santos S, Gea J, Güell R, Torres F, Gimeno-Santos E, Rodriguez DA, Vilaró J, Gómez B, Roca J, Barberà JA. Sildenafil to improve respiratory rehabilitation outcomes in COPD: A controlled trial. Eur Respir J. 2013;42(4):982–992. doi: 10.1183/09031936.00176312. [DOI] [PubMed] [Google Scholar]
- 289.Goudie AR, Lipworth BJ, Hopkinson PJ, Wei L, Struthers AD. Tadalafil in patients with chronic obstructive pulmonary disease: A randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2014;2(4):293–300. doi: 10.1016/S2213-2600(14)70013-X. [DOI] [PubMed] [Google Scholar]
- 290.Holverda S, Rietema H, Bogaard HJ, Westerhof N, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Acute effects of sildenafil on exercise pulmonary hemodynamics and capacity in patients with COPD. Pulm Pharmacol Ther. 2008;21(3):558–564. doi: 10.1016/j.pupt.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 291.Lederer DJ, Bartels MN, Schluger NW, Brogan F, Jellen P, Thomashow BM, Kawut SM. Sildenafil for chronic obstructive pulmonary disease: A randomized crossover trial. COPD. 2012;9(3):268–275. doi: 10.3109/15412555.2011.651180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Maron BA, Goldstein RH, Rounds SI, Shapiro S, Jankowich M, Garshick E, Moy ML, Gagnon D, Choudhary G. Study design and rationale for investigating phosphodiesterase type 5 inhibition for the treatment of pulmonary hypertension due to chronic obstructive lung disease: The TADA-PHiLD (TADAlafil for pulmonary hypertension associated with chronic obstructive lung disease) trial. Pulm Circ. 2013;3(4):889–897. doi: 10.1086/674759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hurdman J, Condliffe R, Elliot CA, Swift A, Rajaram S, Davies C, Hill C, Hamilton N, Armstrong IJ, Billings C, Pollard L, Wild JM, Lawrie A, Lawson R, Sabroe I, Kiely DG. Pulmonary hypertension in COPD: Results from the ASPIRE registry. Eur Respir J. 2013;41(6):1292–1301. doi: 10.1183/09031936.00079512. [DOI] [PubMed] [Google Scholar]
- 294.Collard HR, Anstrom KJ, Schwarz MI, Zisman DA. SIldenafil improves walk distance in idiopathic pulmonary fibrosis. Chest. 2007;131(3):897–899. doi: 10.1378/chest.06-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Nathan SD, King CS. Treatment of pulmonary hypertension in idiopathic pulmonary fibrosis: Shortfall in efficacy or trial design? Drug Des Devel Ther. 2014;8:875–885. doi: 10.2147/DDDT.S64907. [DOI] [PMC free article] [PubMed] [Google Scholar]