Abstract
Treatment of cardiovascular diseases remains challenging considering the limited regeneration capacity of the heart muscle. Developments of reprogramming strategies to create in vitro and in vivo cardiomyocytes have been the focus point of a considerable amount of research in the past decades. The choice of cells to employ, the state-of-the-art methods for different reprogramming strategies, and their promises and future challenges before clinical entry, are all discussed here.
Introduction
Each somatic cell is tightly programmed to perform a very specific function, and has the appropriate structure and intracellular components to perform its functions. During fetal life there is a certain degree of plasticity with cells being reprogrammed to a different type of cell during the process of organogenesis1. This process of “natural” reprogramming stops after birth. Strategies to induce reprograming of somatic cells after birth started in 1958, with the work of Gurdon and colleagues in Cambridge, UK, using nuclear transfer in frogs2. Slow progress in the field continued until it was realized twenty years ago that the technique can be used for tissue regeneration as well as for producing in vitro models of disease for drug testing and genetic manipulations. This resulted in a massive expansion in the field with the publication of thousands of papers related to this topic. Several strategies have been developed for reprogramming which include nuclear transfer, and forced expression of one or more transcription factors or microRNA, to produce pluripotent cells followed by strategies to induce differentiation to the desired cell type (indirect reprograming)3. More recently, strategies to reprogram cells from one somatic cell type to another, without passing through the pluripotent stage (direct reprogramming) has been developed.4,5 We here describe the evolution of the different types of reprogramming with particular reference to the heart, as well as work done at QCRC.
Adult stem cells and reprogramming into cardiomyocytes
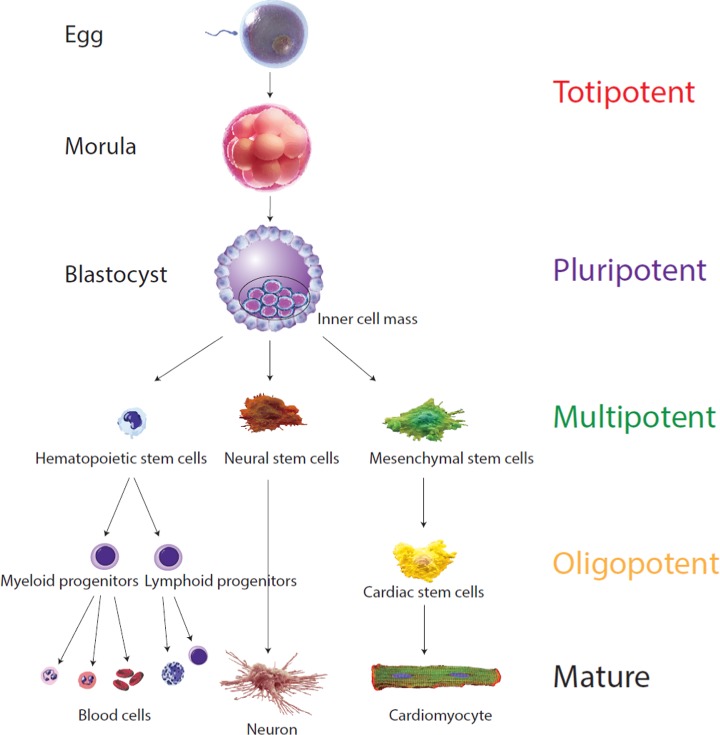
Stem cells are unspecialized cells with potentially unlimited proliferation attributes (self-renewal) and the capacity to differentiate into specialized cell types.6 These cells, though, can be further classified into subtypes of stem cells according to how many specialized cell types they can differentiate into, often called their “potency” or “differentiation potential” (Figure 1). From “totipotent” in the fertilized egg, cells specialize along embryo development and only “multipotent”, “oligopotent” and “unipotent” can be found in adults. These adult stem cells, however, all maintain the property of self-renewal and a certain differentiation capacity. The feasibility of cell therapy has been investigated in several of these adult stem cell populations.7–11 First reported in 1999,12 adult stem cells such as bone marrow mesenchymal stem cells (BM-MSCs), for which the possibility of autologous stem cell isolation has long been known, were shown to be reprogrammable into cardiomyocytes (CMs). Since that time, colossal efforts have been made to employ MSCs (in particular BM-MSCs) in heart failure clinical application, and there was a focus on improving in vitro or in vivo differentiation of MSCs into CMs. Thus, the use of bone marrow cells (BMCs) for treating myocardial infarction and heart failure have been reported in a large number of clinical trials.13 However, conflicting results, limited in vitro and in vivo reprogramming of human MSCs into CMs and the limited clinical benefits obtained, have led to research on other adult stem cell types such as cardiac stem cells.14–18
Figure 1.
Different cells' “potency”. The “potency” of a cell is defined by the number of cell types it has the capacity to differentiate into. The fertilized egg is “totipotent”, cells having the potential to develop into an entire organism and therefore possesses the totality of potentials. This totipotent cell will divide in human for 4 days retaining this full capacity until a blastocyst develops, where these cells acquire some specialization. The cells from the inner cell mass cannot develop anymore into an entire organism, as they are unable to form the placenta but can still differentiate into all cell types within the organism. They are therefore qualified as “pluripotent”. Pluripotent cells will further multiply and acquire more specialization. The resulting “multipotent” cells retain the capacity to differentiate into various cell types. They are already specialized into ectoderm, endoderm or mesoderm. Finally, cells are considered “oligopotent” when they can only differentiate into very limited cell types (adapted from Ref.180).
Within the heart, different populations of cardiac stem cells (CSCs) have been extensively described and isolated based on extracellular marker expression or isolation processes.19,20 We can quote five main types of CSCs: cardiac c-kit+ cells (defined by Lin- c-kit+ markers), cardiac Sca-1+ progenitor cells (defined by Sca-1 expression), side-population cells (defined by their capacity to efflux Hoechst dye when analyzed in flow cytometry), cardiosphere-derived cells (CDCs) (defined by their capacity to form a sphere by tissue explanting technology) and genetically engineered cells such as Isl1-expressing cells. Among these five CSCs type described, only two populations of CSCs (c-kit+ and CDCs) have been escalated to phase I clinical trials, yet the clinical benefit following implantation of the c-kit+ CSCs has been challenged recently.21–23 The outcomes of these trials at phase I only delineate the safety and tolerability of transplantation of those cells but present limitations on understanding the benefits for patients means that further clinical trials will be approached cautiously.
Facing the limited benefits observed using these adult stem cells, pluripotent stem cells (PSCs) (embryonic stem cells (ESCs) and induced-PSCs (iPSCs)) retained therefore the most appealing potential for cardiac regenerative medicine. Since the first demonstration of feasibility in 1985 on murine ESCs, much effort has focused on establishing reliable and efficient differentiation strategies to produce CMs from PSCs. Presently, various cytokines and small molecules are capable of improving CM differentiation from PSCs with nearly 90% efficiency. As discussed later in this review, CMs can be derived from PSCs in a step-wise manner via sequential treatment with cytokines or small molecules.24,25 Both types of PSCs present advantages and challenges regarding clinical applicability. Abundant data from preclinical studies have demonstrated the safety, feasibility, and efficacy of these cells, justifying the careful entry of PSCs based therapies into clinical trials.26,27
Reprogramming
Indirect reprogramming
iPSCs
The derivation and clinical use of human ESCs are limited by ethical problems and immunological barriers respectively. To circumvent these issues, a sizable number of researchers have dedicated considerable effort to deriving PSCs from adult, non-pluripotent somatic cells, which display the same characteristics as ESCs. This was first achieved in mice in 2006, and in humans in 2007, by Yamanaka et al. The first iPSCs were generated following retroviral transfection of adult fibroblasts with 4 transcription factors previously known to be involved in the maintenance of pluripotency (Oct3/4, Sox2, Klf4, and c-Myc, the OSKM factors).28 Yamanaka discovered that the iPSCs he generated are similar to ESCs by their common expression of stem cell genes and proteins, open epigenetic configuration, growth capacities, embryoid body and the ability to form teratomas, and most importantly having similar potency and differentiation capacities. Hence, Yamanaka's work has transformed our understanding of genetic reprogramming of somatic cells to a pluripotent state, and set the ground for considerable work aimed at bringing this technology to clinical applications. Since this breakthrough, multiple alternative approaches and technologies have been developed to generate hiPSCs. The OKSM factors can be replaced by an alternative set of genes (Oct3/4, Sox2, Nanog, and Lin28,29,30), avoiding the use of the oncogene c-Myc. The concerns of genomic instability due to viral delivery and integration of those factors have also been circumvented by the development of non-viral, non-integrative approaches.31–44 Common hiPSCs culture methods utilize a feeder layer of inactivated MEFs to support self-renewal of hiPSCs and to maintain their undifferentiated state. However, the use of MEFs exposes the cultured hiPSCs to animal contaminants. As reported for hESCs, autologous skin fibroblasts isolated from the same donor/patient can efficiently replace the MEFs.45,46 Additionally, Matrigel-coated surfaces, which is a gelatinous protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells, is also commonly used.47 However, the use of Matrigel may entail a risk of contamination with animal pathogens and can be substituted by vitronectin and synthemax®.48 hiPSCs have also been generated from various tissues including skin fibroblasts,28,49–51 keratinocytes,52 neural stem cells,53 melanocytes,54 cord blood cells,55,56 peripheral blood cells57 and adipocytes.58 This progress made possible the development of clinical grade strategies to generate autologous iPSCs with potential for clinical use.
Very recently, a highly controversial publication by Obokata et al. sent shock waves throughout the scientific community. The publication, rapidly withdrawn, stated that “IPS-like” cells could also be derived without introduction or delivery of any transcription factors. This controversial approach, dubbed “stimulus-triggered acquisition of pluripotency” (STAP), stated that when cells are subjected to a strong external stimulus such as transient low-pH, somatic cells (from neonatal mice) could be reprogrammed into a pluripotent state. Even if unclear, the proposed mechanism of the phenomenon was that the stimulation resulted in a decrease of DNA methylation in the regulatory regions of pluripotent transcription factors and markers. This mechanism was recently successfully achieved by Lee et al.,59 demonstrating that external stimulation (via TLR3 ligand) activating the cells' inflammatory pathway, led to global changes in the expression of epigenetic modifiers, which augmented chromatin remodeling and nuclear reprogramming. Although the “STAP” study could not be confirmed to date and raised numerous ethical and scientific suspicions by other groups, it has nevertheless elevated the possibility of iPSCs derivation without genetic modification and strengthens more than ever iPSCs into the race for clinical application.
Alternative approaches: Nuclear Transfer Embryonic Stem Cells
Somatic cell nuclear transfer embryonic stem cells (SCNT-ESCs) are cells that are obtained by substituting the oocyte genome with a somatic cell donor genome.60 Once artificially activated, embryonic development begins. As the embryo reaches the blastocyst stage, and as is typical in embryonic stem cell derivation, the inner cell mass gives rise to patient-specific pluripotent stem cells (Figure 2). This method involves various chemicals such as caffeine, fetal bovine serum, puromycin, kinase inhibitor 6-DMAP, HDAC inhibitor scriptaid, and calcium depletion, which all intersect at different stages during development of SCNT-ESCs.
Figure 2.
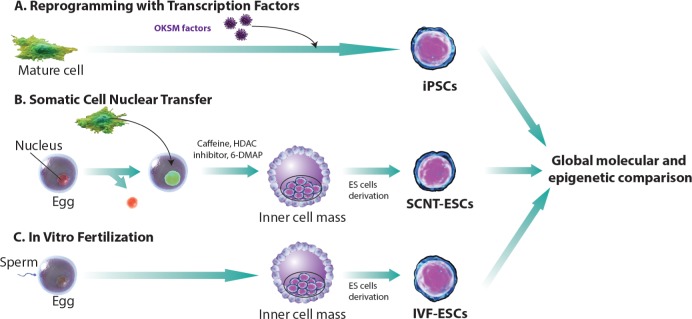
Comparing techniques for generating stem cells. There are three distinct ways to derive pluripotent stem cells in vitro. (A) iPS cell derivation is achieved via conversion of somatic cells with the addition of a cocktail of transcription factors, originally described as “Yamanaka factors”. (B) Tradtional embryonic stem cell derivation can be augmented by replacing the nucleus of an egg with a nucleus from a somatic cell at an early stage, as the cells mature and form the blastocyst. The inner cell mass (ICM) is isolated to form Nuclear Transfer embryonic stem (NT ES) cells. (C) In vitro fertilization is performed and ES cells are derived when reaching the blastocyte level to obtain IVF-ESCs (adapted from Ref.61).
Given the recent successes in the derivation of patient-specific pluripotent stem cells via somatic cell nuclear transfer, groups have begun to decipher the epigenetic differences at the mechanistic level between SCNT-ES and iPSCs. Understanding the molecular determinants for these two cell types should further elucidate methodologies that will improve current limitations of pluripotent cell derivation for both in vitro disease modeling and cellular replacement strategies. Ma et al. performed this much-needed comprehensive experiment that profiles the molecular characteristics of iPSCs and SCNT-ESCs. These experiments compared and contrasted iPSCs, SCNT-ESCs and in vitro fertilization (IVF) produced ESCs. They demonstrated that iPSCs derived with transcription factor-based methodologies displayed inadequate epigenetic reprogramming. While the same somatic cells derived via SCNT were epigenetically and transcriptionally similar to control IVF-ESCs, there is residual DNA methylation in cells derived by transcription factor-based reprogramming. It is interesting to note that genome-wide microarray-based DNA methylation techniques demonstrated that iPSCs, at a frequency eightfold higher than SCNT-ESCs, contain somatic patterns of CpG methylation, which is consistent with previous studies demonstrating that iPSCs retain an “epigenetic memory” of the somatic cells they are derived from.61,62 Hence, SCNT-ESCs are better at retuning genomics of reprogramming more faithfully than iPSCs. Taken together, these subsequent data highlight the importance of investing more in studies that will lend insight into improving current reprogramming paradigms. Methodologies of reprogramming will have to take into account reprogramming factors upstream of pluripotency. One place to look in order to identify possible strategies will be the ooplasm which may possibly contain processes that demethylate the somatic genome more faithfully compared with the more subdued or passive demethylation process during transcription factor-based reprogramming. In any case, the development of hiPSCs has emerged as a promising source of PSCs for tissue engineering, cell-based therapies, novel drug screening, and disease modeling. The drastic improvement of hiPSCs reprogramming63,64 has reduced the time and cost of iPSCs generation and has fostered even more the enthusiasm toward PSCs use in cellular therapy. Beside the improvement of hiPSCs generation, major improvements have been made in the differentiation, purification, and maturation of iPSCs derived CMs, precluding iPSCs cardiac clinical use.
Cardiomyocyte generation from PSCs
Lessons from developmental biology
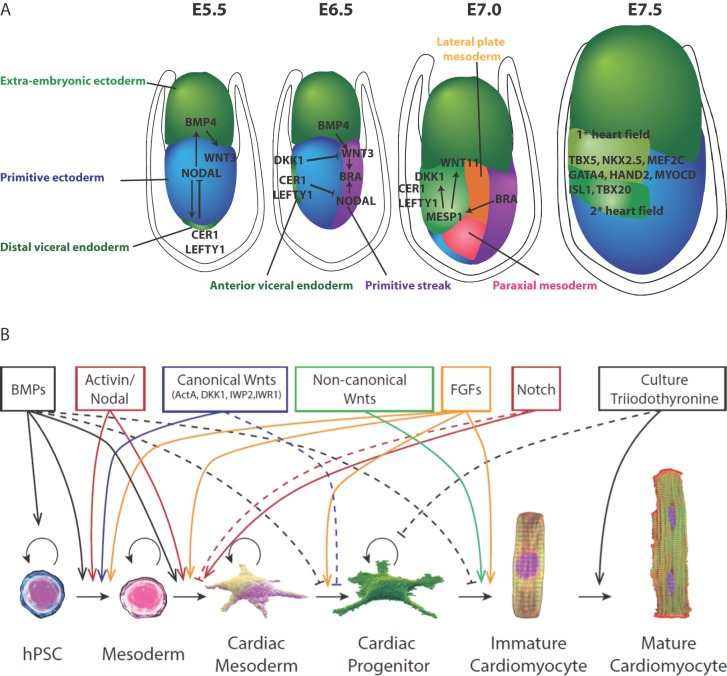
In order to achieve cardiac lineage reprogramming and to derive efficient differentiation protocols, investigators took lessons from developmental biology and the mechanisms of cardiac commitment in early embryos. Gastrulation begins with mesoderm induction through Nodal signaling in the primitive ectoderm. Nodal, a cytokine belonging to the TGF-β superfamily, plays a crucial role in the formation of the primitive streak and germ layers. As gastrulation proceeds, cardiac progenitor cells are among the first cell lineages to be established from mesoderm cells emerging from the primitive streak. These cells express mesoderm gene inducers and transcription factors such as BMP4, Wnt3, Brachyury T, and MESP1.65–67 The latter acts as a key regulator of cardiovascular lineage commitment and drives expression of various transcription factors including NKx2.5, Gata4, Mef2c, and Tbx5 for cardiac differentiation and maturation.68,69 MESP-1 also represses early mesoderm induction through direct inhibition of Wnt and Nodal signaling pathways by DKK1 and CER170,71 (Figure 3A). Accordingly, induction of cardiac differentiation of human PSCs can be initiated by sequential stimulation with specific recombinant growth factors such as basic fibroblast growth factor (bFGF), BMP4, Wnt3, and Activin A, followed by addition of DKK1 or other Wnt inhibitors.72–74 Further cytokines have been reported to increase the differentiation efficiency such as the vascular endothelial growth factor (VEGF),74 Noggin75 (BMP antagonist), and IWR-1/IWP-276 (inhibitors of Wnt) among others. Taken together, these findings have led to the establishment of efficient protocols allowing the production of CMs from PSCs with yields close to 90%.16,25 Nevertheless, much effort is still needed to improve purification and maturation of the derived CMs.77,78
Figure 3.
Lesson from embryology and factors involved in hPSC cardiac differentiation. (A) During mouse embryonic development at E5.5 gastrulation occurs by the formation of the primitive streak. An epithelial to mesenchymal transition (EMT) of anterior primitive ectoderm allows cells to move laterally between primitive ectoderm and visceral endoderm. At E6.5 cells located proximally to the primitive ectoderm go on to form the extraembryonic mesoderm. Cells adjacent to this zone form heart, blood and mesoderm derivatives. The most distal portion of the primitive streak gives rise to endoderm cells. At E7.0 the lateral plate mesoderm is formed which delaminates to form two layers. Cardiac mesoderm goes on to form the first heart field (FHF) laterally and more ventrally the second heart field (SHF) is formed both by coordinated expression of Dkk1, MESP1, Nodal and WNT signaling. Other cardiogenic signals, such as BMP and FGF, activate cardiac-specific transcription factors such as Nkx2.5, GATA4, HAND2, which coordinate to move both heart fields to the midline. Whereby at E7.5, the FHF progenitors form the heart tube which later contributes to the left ventricle. SHF progenitors join with the CMs of the FHF which leads to the rightward looping of the cardiac tube which eventually progresses towards formation of cardiac chambers. (B) Schematic representation of a family of factors reported to trigger progression from pluripotent state to CM (adapted from Ref.24).
Improvement of CMs differentiation
Many strategies have been proposed to improve the differentiation and maturation of iPSCs derived CMs. In particular, hypoxic culture conditions,79,80 knockdown of selected genes,81 and forced aggregation of iPSCs-derived EBs in a chemically defined medium82 have shown promising results.
Further, a high-throughput screening system has been established to identify small molecules that promote the differentiation of CMs from iPSCs. Among these, vitamin C,83 cyclosporine A84 (immunosuppressant), and triiodothryonine85 (T3, thyroid hormone) showed a marked induction of cardiac differentiation. Of note, the exogenous expression of human Apolipoprotein-A186 or induction of the Wnt/β-catenin signaling pathway87 have been shown to enhance cardiac differentiation and maturation of hESCs and hiPSCs. These cardiogenic effects are thought to be mediated by the BMP4/SMAD signaling pathway. A recent publication of highly reproducible and efficient differentiation protocols, via temporal modulation of canonical Wnt signaling (90% of differentiation),16,25 sets the path for clinically scalable applications (Figure 3B).
Aside from all the different iPSCs to CMs differentiation protocols that are being considered, a crucial parameter influencing the differentiation potential is the cellular origin of the iPSCs.88 This is likely to be due to the “epigenetic memory” of iPSCs, which manifests as differential gene expression and variable differentiation potential.88–90 In brief, the two major factors determining iPSCs differentiation to CMs are the starting cell population and the cardio-inductive growth factors used.
Purification and enrichment of iPSCs derived CMs
Following differentiation, even if great yields are obtained, only pure CMs could potentially be used clinically. The iPSCs derived CMs therefore need to be purified and enriched before potential implantation to avoid risks of teratoma formation or inappropriate tissue engraftment. Frequently used methods include manual dissection of spontaneously beating CMs using a pulled-glass micropipette,91 cell separation based on density gradient,92 and fluorescence-activated cell sorting (FACS).93,94 Physical enrichment by manual dissection or density gradient separation has limited success due to their low yield. The FACS technique relies on a positive selection of CMs cells that are phenotypically different from other cells. This can be realized by tagging cardiac-specific proteins with fluorescent antibodies followed by a subsequent step of detection and sorting using a FACS machine. Hence, a panel of surface markers is being used for the enrichment of CMs. This panel includes the following surface markers: CD166,95 vascular endothelial growth factor 2 (VEGFR2) and platelet-derived growth factor-α (PDGFR-α),17 elastin microfibril interface 2 (EMILIN2),96 signal regulatory protein-α (SIRPA-α),97 and vascular cell adhesion protein1 (VCAM1).97,98 A major limitation for this approach is the lack of specific CMs surface markers that could specifically identify and select only cardiac cells from a pool of differentiating/undifferentiating cells.99 Therefore, genetically modified hESCs lines have been developed to select the terminally differentiated CMs based on the expression of a luminescent reporter gene (e.g the green fluorescent protein, GFP) coupled to the regulatory sequence of a cardiac-specific gene like MYH6,100 Nkx2.5,98 myosin light chain 2V (MLC2V),101 and insulin gene enhancer protein 1 (ISL1).102 The expression level of the reporter fluorescent proteins reflects the transcriptional activity of the attached cardiac gene. Although the latter method showed a marked CMs purification and enrichment, they obviously cannot be used on a larger scale or for clinical applications. Interestingly, Hattori et al. reported that a fluorescent dye that labels mitochondria may serve as a selective marker of hESCs/hiPSCs derived CMs. The authors claimed that a significant enrichment of CMs with a high degree of purity (>99%) could be obtained by FACS with this dye.93
Purification and enrichment of PSCs derived CMs is an important limiting step for cell-based therapy for cardiac regeneration. Though different protocols for purification and enrichment of CMs have been proposed, their efficiency is controversial. An efficient method of purification should be fast, specific, and scalable with no genetic modifications. Such a method would greatly enhance the potential of iPSCs derived CMs in the cardiology clinic.
Maturation of iPSCs-derived CMs
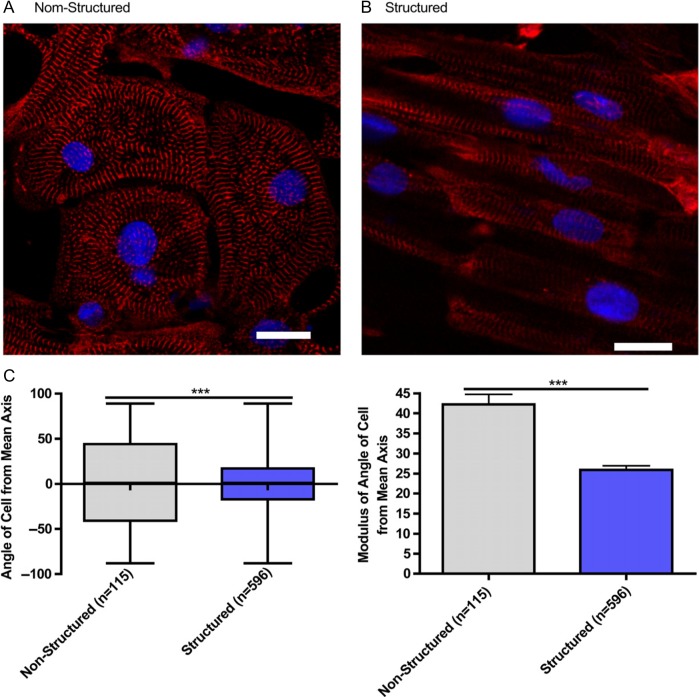
Beyond the differentiation protocol improvements, much work focused on the maturation of PSCs derived CMs. Indeed, the maturity of generated CMs remains a critical bottleneck. For instance, despite the fact that iPSCs derived CMs present most of the characteristics of adult CMs (self-induced action potential, proteins/genes expression), the generated cells lack maturity for the most part (non-expression of mlc2v contractile protein, lack of organization, absence of M-band etc) and more closely resemble embryonic CMs than adult CMs.103 A remarkable publication by Murry and colleagues104 showed that implantation of a large number of human embryonic stem cell-derived CMs in non-human primates following heart failure could lead to re-muscularization of infarcted heart and even electromechanical junctions with surrounding muscle, but even over a three month period, cells failed to completely mature, and the non-human primate ultimately experienced arrhythmic complications. Long term culture of PSC derived CMs has been shown to improve the maturity of differentiating cells, but remains of restricted interest for potential clinical applications.105 Our group and others have previously demonstrated that a forced alignment of the cells improves the maturity of the CMs generated78,106 (Figure 4). The mechanical and electrical stimulation of derived CMs has also been proven to favor maturation of cells toward an adult phenotype.107,108 Besides, the use of scaffolds for maturation of CMs may lead to their potential interest for clinical use.
Figure 4.
Microgrooved culture substrates' effect on calcium cycling of cardiac myocytes derived from human-induced pluripotent stem cells. Representative immunofluorescence of iPSC-CM cultured on unstructured polydimethylsiloxane (A) and microgrooved polydimethylsiloxane (B), Red - sarcomeric a-actin, Blue - DAPI, scale bar 20 mm.Quantification of cell alignment iPSC-CM on structured and unstructured constructs (C). (Adapted from Ref.78)
IPSCs and targeted gene therapy
While iPSCs propose to circumvent the autologous hurdle of cell therapy, this technique cannot be used straight away for patients presenting disorders linked to genetic abnormalities. The ability to generate gene targeted mutations or manipulations in iPSCs has opened new areas of research in dissecting multifaceted genetic interactions. It has become increasingly easy to utilize genome engineering techniques which involve site-specific nucleases acting as DNA scissors that introduce double strand breaks, allowing expression of nucleotide alterations, knockin reporters, and small insertions or deletions to study loss of function mutations.
The possibility of using iPSCs for targeted gene therapy reflects further their promising therapeutic potential. Hockemeyer et al. demonstrated in 2011 the possibility of genetic engineering of hESCs and hiPSCs using TALE nuclease.109 Reversion of disease mutation was further demonstrated in hPSCs by various groups utilizing CRISPR-Cas and TALENs.110–114 For example, Jessup et al. showed that overexpression of SERCA2a via gene therapy can improve the contractility of iPSCs derived CMs of dilated cardiomyopathy patients.115 Jiang et al. demonstrated that the allele specific silencing of a dominant mutation could suppress hypertrophic cardiomyopathy condition in mice.116 Subsequently, other groups have introduced conversion of normal genes to disease models by genome editing, targeting specific mutations to mimic known diseases and syndromes in hPSCs.117,118 Gonzalez et. al., have even combined two gene editing tools, TALEN and CRISPR/Cas systems, developing a more efficient platform which can rapidly and simultaneously introduce multiple gene alterations along with stage specific inducible genetic alterations which they named the iCRISPR platform.119 Thus, the possibility to use the high proliferative capacity of iPSCs would enable the expansion of clonal populations of genetically modified iPSCs ex vivo followed by their subsequent differentiation and selection of the “corrected” cells for transplantation.120 This approach would therefore resolve the issue of non-specific targeting, which is one of the drawbacks of conventional gene therapy.
This genetic editing could also be of great interest for clinical applications as previous studies have shown that even autologous mouse iPSCs are rejected by an immune response.121 Perhaps the combination of this new wave of technology platforms for engineering genetic alterations and better understanding reprogramming may circumvent strategies that will have to be employed to produce iPSCs which need to evade immune rejection in the therapeutic context.
Immunogenicity of iPSCs
Recently, human ESCs have been used in phase-I clinical trials to treat acute spinal cord injury122 and Stargardt's macular dystrophy.123 In preclinical studies, they have also been directed to differentiate into CMs for in vivo studies.104,124,125 hESCs derived CMs were demonstrated to reduce LV remodeling and promote LV systolic function in rat hearts following myocardial infarction.124,125 More recently, hESCs derived CMs were also shown to generate extensive vascularized cardiac muscle in the infarcted hearts of non-human primates.104 Despite the advantage of using hESCs to generate large numbers of human CMs for clinical transplantation, these cells are non-self and therefore contribute to immune rejection unless with courses of immunosuppression. Similar to human ESCs, human iPSCs would have a better potential in cell replacement therapy by virtue of their “autologous” nature; however, in any transplantation setting, the histocompatibility of iPSCs must be considered before transplantation.
The immunogenicity of human iPSCs has not been carefully examined and whether they are truly “autologous” remains controversial. In one study, teratomas derived from syngeneic murine ESCs were accepted while those derived from syngeneic murine iPSCs were rejected by activated T cells, suggesting that syngeneic iPSCs are more immunogenic upon transplantation compared with their syngeneic ESC counterparts.126 Later, Abe et al. showed that teratomas derived from integration-free, syngeneic murine iPSCs did not trigger immune rejection and had the same rate of success following transplantation compared with their syngeneic ESC counterparts.127 In addition, Boyd et al. also demonstrated that tissues of the three embryonic germ layers (endothelial cells, hepatocytes and neuronal cells) derived from syngeneic murine iPSCs did not trigger immune rejection following transplantation.128 More recently, Wu et al. have shown that syngeneic murine iPSCs were rejected but their differentiated progeny such as endothelial cells were accepted following intramuscular injection.129
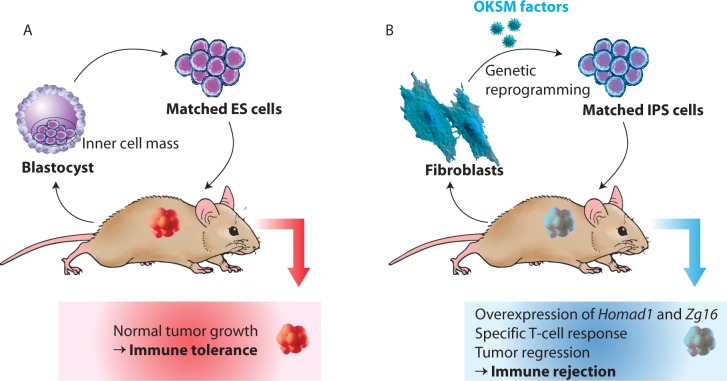
We are still unsure why syngeneic iPSCs were accepted in some transplantation settings but not in others. It could be due to expression of non-self-proteins during the reprogramming process. Xu et al. discovered that syngeneic murine iPSCs express minor antigens such as Zg16, Hormad1, and Retn, which are not normally found in murine ESCs126 (Figure 5). Indeed, forced expression of Zg16 and Hormad1 could induce tertoma regression. Overexpression of these minor antigens in some iPSC lines is believed to be a result of mutations in the coding sequences of iPSCs130 or the epigenetic difference between iPSCs and ESCs.131 Moreover, the viral vectors carrying pluripotency-inducing transgenes for reprogramming could also trigger antiviral immunity or anti-DNA antibody production. Therefore, the use of nonviral and integration-free methods for reprogramming could be a safer option to prevent immune surveillance. For instance, we could use modified mRNAs which have been shown to induce iPSC reprogramming with very high efficiency and minimal immune recognition. Nevertheless, more work is still needed to examine whether human iPSCs are truly “autologous” and whether the degree of immunogenicity would be regained following cardiomyocyte differentiation for transplantation.132
Figure 5.
Immunogenicity of induced pluripotent stem cells. (A) Zhao et al. Performed transplantation studies assessed by injections of embryonic stem cells from the same genetic background found that ES cell-based transplants formed normal tumor growth. Hence, autologous ES cell transplantation evades immune system rejection. (B) Conversely, autologous iPS cells, derived from the same fetal fibroblasts, exhibited immunological rejection upon transplantation whilst in the same genetic background (adapted from Ref.181).
Direct reprogramming
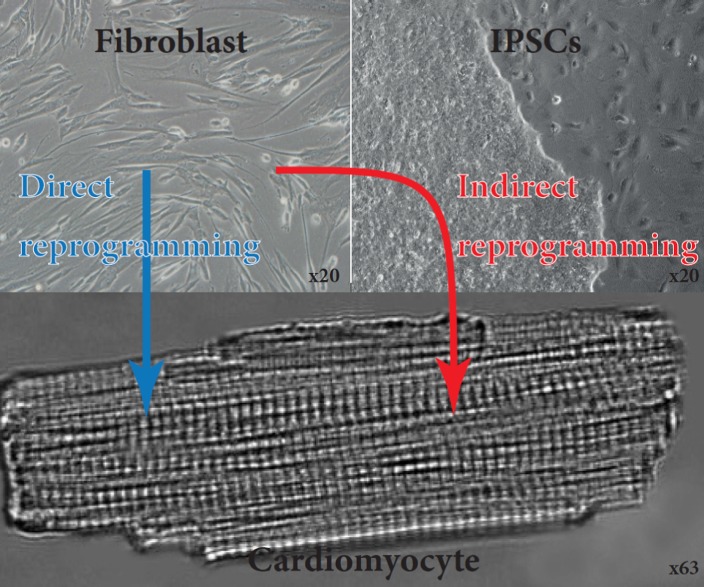
Both types of PSCs (ESCs and iPSCs), cultured or induced, prior to CMs differentiation, trigger the risk of teratoma formation, inappropriate tissue engraftment and immune rejection when later implanted into the patient. This issue remains one of the major drawbacks of these approaches in clinical trials. Even if new strategies and drugs are tested specifically to eliminate undifferentiated cells and therefore their tumorigenic risk, the path remains long before a potential clinical application.133 Therefore, development of strategies avoiding the pluripotent stage raised great enthusiasm. The publication of direct genetic reprogramming approaches (known as “trans-differentiation” or “direct conversion”) brought exciting perspectives for cardiac cellular regeneration. This genetic modification allows the direct conversion of a terminally differentiated cell type into so-called induced cardiomyocytes (iCMs) (Figure 6).
Figure 6.
Generation of functional cardiomyocytes by direct and indirect reprogramming of fibroblasts. Fibroblasts derived from skin biopsy from a patient. These cells were then reprogrammed using OSKM factors towards induced pluripotent stem cells. These are then directed to differentiate towards CMs. Fibroblasts can also be directly reprogrammed towards CMs.
Reprogramming of fibroblast toward cardiac fate
The use of transcription factors (TFs) to induce the expression of specialized genes and therefore the reprogramming of adult fibroblast was first demonstrated by Weintraub et al. in 1987 using the transcription factor (myogenic regulatory factor) MyoD to induce skeletal muscle cells trans-differentiation.134,135 Subsequently, the identification of only a single factor driving cardiac fate has long been elusive. Yamanaka's seminal discovery that necessitates co-transfection of multiple factors simultaneously to reprogram fibroblasts into iPSCs, has led to subsequent studies to identify a cohort of TFs essential for direct cardiac reprogramming.3 The first demonstration of fibroblasts reprogramming into CMs was published in 2010 by Ieda et al.5 Working on neonatal and adult mouse fibroblasts, they originally tested 14 central TFs for cardiac differentiation and serially eliminated factors to finally establish that at least 3 TFs were necessary for CM trans-differentiation.5 They demonstrated that transduction of the following TFs GATA4 (member of the GATA family of zinc finger transcription factors), Mef2C (Myocyte-specific enhancer factor 2C) and Tbx5 (T-box transcription factor 5) (the three of them are now commonly referred as the GMT factors), successfully converted 15% of mouse cardiac fibroblast into α myosin heavy chain (α-MHC) expressing cells, out of which 20% also expressed cardiac troponin T (cTnT). They described these cells as “cardiomyocyte-like” cells. This first demonstration was later scrutinized when Chen et al. published that using the same GMT factors in adult fibroblast, no expression of α-MHC or NKX2.5 (Homeobox protein NKX2.5 often used as an early cardiac differentiation marker98) could be detected.136,137 However, feasibility is now confirmed by different groups,138–141 but a broad range of efficiency is reported using the GMT factors. The heterogeneous nature of reported results could in part be explained by the variation in genetic background of mice used, the fibroblasts isolation protocols, the culture methods, the promoter employed for expression of the TFs as well as the type of virus used (lentivirus, retrovirus) and their respective efficiency of transduction.
The direct reprogramming of mouse fibroblast was also later performed using alternative combination or additional TFs. Indeed, Song et al. showed that the addition of a basic helix-loop-helix transcription factor Hand2 to the GMT factors could increase the percentage of cells co-expressing α-MHC and cTnT.138 More recently, Addis et al. showed that the addition of Hand2 and NKX2.5 (used here as a transcription factor to induce differentiation) could increase by >50 fold the cardiac reprogramming of fibroblasts.139 Similarly, Christoforou et al. showed that the addition of the following TFs Mesp1, MYOCD (Myocardin), Smarcd3 (Baf60c) and Srf (serum response factor) could also enhance the efficiency of GMT factors.140 Protze et al. demonstrated that the replacement of Gata4 by MyoCD gave more cardiac gene expression rate compared to GMT factors.141 Alternatively, Jayawardena et al. demonstrated that a combination of miRNA with addition of JAK (Janus protein tyrosine kinase 1) inhibior 1 (to inhibit the JAK/STAT pathway) could also successfully convert fibroblast into iCMs.142 It is now accepted that in appropriate conditions, iCMs can be derived from mouse fibroblasts.143–145 The efficiency of those various combinations remains difficult to compare since various promoters, fibroblasts and viral deliveries were employed. In addition, the analysis of physiological parameters like calcium oscillation and spontaneous action potential were not performed in all of these studies. The evaluation of the efficiency should give greater consideration to these physiological parameters over cardiac genes expression. This in vitro demonstration in mouse still remained to be translated into human. The first report in August 2012 by Islas et al. demonstrated that neonatal foreskin fibroblast could be reprogrammed into cardiac progenitors using the transcription factors MESp1 and ETS2.146 However, it was only early this year that Nam et al. revealed the possibility of reprogramming adult cardiac or dermal fibroblast into cardiac fate using the optimized combination of GMT factors with Hand2, MYOCD and two microRNAs miR-1, and miR-133.147 The evidence presented confirms that the expression of proteins and microRNAs produces cells that display calcium transients, sarcomere-like structures, and spontaneous contractility. This indicates that human fibroblasts are amenable to reprogramming by forced expression of cardiac-specific transcription factors with muscle-specific microRNAs in order to produce cardiac-like myocytes. Further work in elucidating the exact mechanisms and role of these factors and microRNAs will represent a possible step toward the clinic and its therapeutic applicability. Confirmation of these results was done by Wada et al. a few months later, where they document that the addition of Mesp1 and MyoCD induced reprogramming of cardiac fibroblast into iCMs presenting cardiac gene expression, Ca2+ oscillation and action potential amenable to synchronous beating when co-cultured with murine CMs.148 A few months later, Fu et al. reported that fibroblast from human neonatal skin or fetal heart could be reprogrammed using GMT factors with the addition of ESRRG and MESP1. This resulted in global cardiac gene-expression and phenotypic shifts.149 The demonstration of feasibility on human fibroblasts encourages further investigation towards this approach.
What kind of fibroblasts should be targeted?
The in vitro demonstration of direct fibroblast reprogramming previously discussed was performed on embryonic, neonatal, adult tail-tip, skin and cardiac fibroblasts.5,141,142 Fibroblasts have long been considered as of uniform cell type across different tissues.150,151 Morphologically they are flat, spindle-shaped cells with multiple processes emanating from the main cell body. They can be defined as mesenchymal cells producing extracellular matrix (ECM) components like collagens and fibronectin.152 This simple morphological definition highlighted a mistaken identity with mesenchymal stromal/stem cells (MSCs).153 This view has now been challenged by the demonstration of phenotypic heterogeneity of fibroblast from different tissues in different conditions.151 This heterogeneity poses an active area of investigation for direct reprogramming methods to focus on the most appropriate fibroblasts. But which fibroblast populations are the most appropriate to focus on? To answer this question, we might first need to address the question of the different strategies that can be foreseen using this direct reprogramming technique. Indeed, two types of therapy (summarized in Figure 7) can be envisioned using direct reprogramming.
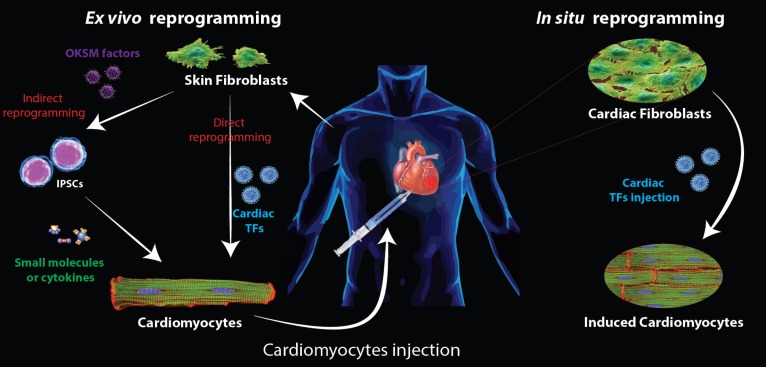
Figure 7.
Schematic representation of heart repair strategies using reprogramming technologies Two strategies to repair post myocardial infarction are envisioned using in vitro reprogramming techniques starting from skin fibroblast. First fibroblasts are reprogrammed into iPSCs before being differentiated into CMs using small molecules or cytokines. The second approach consists of a direct reprogramming of the skin fibroblast into iCMs. In both cases, the CMs produced in vitro have to be injected into the patient. When considering in situ reprogramming, the transcription factors (carried by viruses) are directly delivered to the patient and TFs will be expressed in cardiac fibroblasts, reprogramming them directly into iCMs. OKSM factors: Oct4, Sox2, Klf4, c-Myc.
The first strategy would be a cell-replacement based therapy, where functional iCMs are generated in vitro and then injected to the site of injury. This approach relies on the same concepts developed with a strategy using PSCs. Advantages of this alternative approach in lieu of PSCs are the avoidance of potential teratoma formation and an increase in kinetics that would be gained from avoiding a PSCs intermediate state. In this case, the most promising autologous cells would most probably be, as for iPSCs, the use of adult skin fibroblasts. Similarly the choice for derivation of iPSCs from skin fibroblasts is driven by the easy access to skin fibroblast cells for personalized medicine with autologous cell-based treatment. The possibility of an in situ reprogramming approach is the most appealing aspect in direct reprogramming. In this case, using viral delivery of TFs and specific targeting, the fibroblasts would be reprogrammed directly in place. This would present the considerable advantage of replacing the lost contractile tissues without any in vitro manipulations and hazardous cell injection. In this case, cardiac fibroblasts would represent the target of choice. A first obvious reason to justify this choice comes from the composition of human hearts. Indeed, cardiac fibroblasts represent the largest cell population of the heart (∼50–60% of cells), while CMs represent only 40% of cells.154 A second characteristic that makes cardiac fibroblasts a target of choice for direct reprogramming is their ideal location. Indeed, after infarction, lost CMs are slowly replaced by fibrotic tissues caused by fibroblast invasion. Therefore, these fibroblasts are ideally placed, with ideal homing abilities to be converted in iCMs to reconstitute the lost contractility. However, it is still to be proven that these fibroblasts invading the post-myocardial infarction area are, as normal heart fibroblasts, prone to cardiac reprogramming.
Long believed to be simple “gap filler” and matrix producer, the perception of cardiac fibroblasts is now considerably evolved. Fibroblasts are now known to play an important active role in heart function, facilitating the intercellular communication between myocytes and endothelial cells and even between fibroblasts themselves.155 Moreover, they have been shown to affect the electrophysiological properties. A direct interaction between myocytes and fibroblasts occurs via gap junction connexins (Cx40, Cx43 and Cx45) to facilitate the electrical conduction in the heart.150,151,155,156 They also express cadherin (notably N and T-cadherin) which play a critical role in heart development and function.157 This in situ strategy is the spearhead of direct reprogramming strategy, particularly since proof of concept has been achieved.
In situ reprogramming promises in animal models
The successful in vitro trans-differentiation led rapidly to attempts at direct in situ reprogramming. To date, five studies demonstrated the feasibility of such a technique in murine models.138,142,158–160 In three of the mouse models, the specific expression of the TFs in fibroblasts was ascertained by the use of cre-recombination under the control of a fibroblasts-specific promoter like fibroblast specific protein 1 (FSP1), periostin or transcription factor 21 (Tcf21) promoters. This approach ensured the targeted conversion of non-myocyte fibroblast into iCMs and avoided analysis of ectopic activation of TFs in neighboring CMs. In their rat model, Mathison et al. chose an over expression system using CMV promoter which nevertheless reported overall ejection fraction improvement.159
The demonstration of effective reprogramming into functional cardiac-like myocytes with evidence of electrical coupling and modest but significant improvement of cardiac function was ultimately reported. These trials are a great demonstration of proof of principle that cardiac fibroblasts can be reprogrammed into CMs-like cells in their native environment which is shaping the way towards a blueprint for future clinical translation. Regardless of the modest improvement observed, the data suggest that optimization of the TFs cocktails or co-injection with various pro-angiogenic molecules and fibroblast-activating peptide should further be pursued.158,159
Sinoatrial cells direct reprogramming
In mice and rats, cellular transplantation experiments using PSCs derived CMs can improve function after myocardial injury. However, two critical bottlenecks related to its maturation and electrophysiological behavior remain unresolved. First, current understanding of the relationship between cardiac-specific genes and functionally distinct cardiac myocytes (nodal-like, atrial-like, and ventricle-like) is poorly understood. Secondly, the electromechanical integration and arrhythmogenic potential of these PSCs derived CMs need to be further defined. Previous demonstration of direct and indirect reprogramming into CMs almost systematically produced mixed atrial, ventricular and nodal-like CMs. A little more than a year ago, Kapoor et al. paved the way for a different type of cardiac direct reprogramming. They elegantly demonstrated in vitro that the transfer into ventricular CMs of Tbx18 (a critical gene for early sinoatrial node (SAN) cells specification) could convert ventricular CMs in 2 to 4 days into cells displaying “spontaneous electrical firing, physiologically indistinguishable from that of SAN cells”. They also demonstrated in their publication that this approach could be used in situ as they established in guinea-pig that focal Tbx18 gene transfer could correct bradycardy phenotype.161 In a more recent publication this year, Josowitz et al., using bacterial artificial chromosome (BAC) reporter constructs, were able to identify and purify hPSC-derived atrial-like CMs based on sarcolepin expression. This allowed for delineating cells via flow cytometry, whereby cells with high fluorescence specifically expressing atrial-specific gene sarcolipin (SLN) were isolated. These cells exhibited atrial specific calcium handling and electrophysiological characteristics.162 These sets of data should glean methodologies that will pave the way for more concrete specific CM subtype generation as well as for developing ways to study chamber-specific pathologies and lineage development.
Importance of the niche
We previously discussed that reprogramming and differentiation is based on transcription factors and cytokines/small molecules treatments attempting to mimic naturally occurring development. However, such simple culture conditions often fail to give cells the full environment necessary for appropriate differentiation, leading to partial reprogramming and immature differentiation. Much research focused on recreating this more complex and multifactorial environment, or “niche”, in which the cells are naturally placed. We previously discussed that maturation of PSCs derived CMs is more efficient when cells are subjected to an electro-mechanical stimulating environment as encountered in vivo. Besides, complex molecular stimulus which can be provided by the niche is hardly reproducible artificially. For instance, direct differentiation of MSCs into CMs is often unsuccessful or at least inefficient in human.163–165 However, an acquisition of CM features is observed when MSCs are simply co-cultured with neonatal CMs, revealing a complex autocrin/paracrine system of CMs.166–168 Similarly, clinical trial using MSCs injection following ischemic heart failure demonstrated modest benefit.13 It is now accepted that, rather than direct differentiation of these MSCs into CMs, it is the paracrine effect of the MSCs and their influence on the microenvironment that drive those beneficial effects by stimulating endogenous cardiac progenitors and fibroblasts.13,169–172 Fioret et al. recently demonstrated that endothelial cells are also a key component of the cardiac ventricular myocyte niche.173 The extracellular matrix, and more particularly its content in metalloprotein MMP9, has also recently been demonstrated as an essential component that can influence the differentiation of hESCs into CMs.174 Sandler et al. demonstrated recently that direct reprogramming of endothelial cells into hematopoietic stem cells was made possible when using vascular cells as a feeder to recreate the vascular niche.175 Transferring this approach into direct cardiac reprogramming or the possibility of using feeders to recreate a more “natural” niche for PSCs cardiac differentiation is certainly a promising area of research.176
Future challenges
After the first retinal cells derived from iPSCs were transplanted into a woman with eye disease earlier this year,177 reprogramming into iPSCs before differentiation into CMs seems to be the most advanced area of research for cardiac cellular therapy. However, many problems remain to be addressed before that stage can be reached with cardiomyocytes. Firstly, even if differentiation efficiency has been achieved recently, the purification of genetically unmodified cardiomyocytes needs to be worked out. Even if teratoma formation encountered with PSCs is avoided and time gain is anticipated, the path remains long before application of PSCs becomes a clinical reality for cardiac injuries. The cell maturity prior to implantation definitely needs to be improved. The potential immune-rejection of cells, even autologous, needs to be further studied and anticipated. Beyond these capital points, an optimization of cell delivery with potential tissue engineering and scaffolds has to be addressed, as this could affect the maturity, the engraftment capacity, the electro-mechanical coupling and the survival of the cells. Nevertheless, the subsequent milestones achieved over the past decades make PSCs clinical use for cardiac regeneration more promising than ever, and efforts should be pursued in that direction.
As exciting as direct reprogramming is, this approach could be even more challenging. When considered for primary in vitro differentiation before cell-based therapy, one might consider that differentiation of PSCs into CMs is a more advanced field. The same hurdles encountered with PSCs derived CMs will have to be ruled out before clinical translation of directly reprogrammed cells can be envisioned. Besides, even at the in vitro level, it remains difficult to compare the various TFs cocktails reported and future work should attempt to compare those cocktails side by side. Also, despite successful expression of cardiac markers, not all studies could demonstrate functionality of the iCMs141] and the focus should now be on functionality (analyzing spontaneous contractility or induced action potential measurements) rather than on single gene expression. Similarly, characterization of iCMs subtype (atrial, ventricular or nodal) and their maturity needs to be addressed and fine-tuned. The efficiency of differentiation also remains to be improved in order to reach clinical levels and close the gap with PSCs differentiation efficiency. The possibility of an in situ direct reprogramming represents an exciting blueprint for this technique. Even if some of the drawbacks previously discussed were avoided, a large number remain. Besides, in contrast to problems encountered in gene therapies, other efforts need to be made to optimize direct reprogramming before any treatment can be envisioned. Indeed, the first lesson from mice models could be that the cocktail of TFs used in the human context might need to be validated and consolidated for humans. In lieu of mouse studies, human reprogramming towards CMs has been less straightforward. Successful direct reprogramming of human cells had initially been discovered in making functionally immature neurons178,179 with lower efficiencies than in mouse. Therefore, it seems that human cells are typically more resistant and require activation of innate immunity for efficient reprogramming.59 It is prudent to speculate that human reprogramming requires more acute control of regulatory elements to convert cells towards a different cell fate lineage. Early larger mammalian animal models would deliver more certainty regarding the validity of this approach in humans. Finally, specific delivery of TFs needs to be optimized and validated for clinical use by drug dosing and toxicology testing. Optimization of the promoter used (allowing for the gene expression to be turned off after differentiation) also needs to be performed, and similarly to PSCs derived CMs cell-based therapy, the maturity of the cells achieved in situ needs to be validated.
Acknowledgements
This work was funded by Qatar Foundation JSREP Grant 3-021-3-012 and Qatar Foundation research program. We are grateful to Dr. Despina Sanoudou, Dr. Demetrios Arvanitis and Dr. Youness Ait Mou for providing us with their help and cardiomyocytes images. We are also grateful to Dr. Christopher Rao for authorizing us to use his previously published figure in Ref. 78. The contents of this review are solely the responsibility of the authors and do not necessarily represent the official views of the Qatar National Research Fund.
References
- 1.Lancaster MA, Knoblich JA. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 2.Michael Fischberg JBG, Tom R. Elsdale nuclear transplantation in Xenopus laevis. Nature. 1958;(181):424. [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slack JM. Stem cells in epithelial tissues. Science. 2000;287(5457):1431–1433. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- 7.Menasche P, Hagège AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT, Marolleau JP. Myoblast transplantation for heart failure. Lancet. 2001;357(9252):279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 8.Alaiti MA, Ishikawa M, Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res. 2010;156(3):112–129. doi: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Jiang M, He B, Zhang Q, Ge H, Zang MH, Han ZH, Liu JP, Li JH, Zhang Q, Li HB, Jin Y, He Q, Gong XR, Yin XY. Randomized controlled trials on the therapeutic effects of adult progenitor cells for myocardial infarction: Meta-analysis. Expert Opin Biol Ther. 2010;10(5):667–680. doi: 10.1517/14712591003716437. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 11.Bai X, Alt E. Myocardial regeneration potential of adipose tissue-derived stem cells. Biochem Biophys Res Commun. 2010;401(3):321–326. doi: 10.1016/j.bbrc.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raynaud CM, Yacoub MH. Clinical trials of bone marrow derived cells for ischemic heart failure. Time to move on? TIME, SWISS-AMI, CELLWAVE, POSEIDON and C-CURE. Glob Cardiol Sci Pract. 2013;2013(3):207–211. doi: 10.5339/gcsp.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M, Millard RW, Ashraf M. Role of GATA-4 in differentiation and survival of bone marrow mesenchymal stem cells. Prog Mol Biol Transl Sci. 2012;111:217–241. doi: 10.1016/B978-0-12-398459-3.00010-1. [DOI] [PubMed] [Google Scholar]
- 15.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S. Cardiogenic differentiation of mesenchymal stem cells on elastomeric poly (glycerol sebacate)/collagen core/shell fibers. World J Cardiol. 2013;5(3):28–41. doi: 10.4330/wjc.v5.i3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burridge PW, Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L, Zambidis ET. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6(4):e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Wei H, Tan G, Manasi QS, Kong G, Yong P, Koh C, Ooi TH, Lim SY, Wong P, Gan SU, Shim W. One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res. 2012;9(2):87–100. doi: 10.1016/j.scr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Tang YL, Wang YJ, Chen LJ, Pan YH, Zhang L, Weintraub NL. Cardiac-derived stem cell-based therapy for heart failure: Progress and clinical applications. Exp Biol Med (Maywood) 2013;238(3):294–300. doi: 10.1177/1535370213477982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barile L, Messina E, Giacomello A, Marbán E. Endogenous cardiac stem cells. Prog Cardiovasc Dis. 2007;50(1):31–48. doi: 10.1016/j.pcad.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 21.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Lancet E. Expression of concern: The SCIPIO trial. Lancet. 2014;383(9925):1279. doi: 10.1016/S0140-6736(14)60608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Notice of retraction. Circulation. 2014;129(16):e466. doi: 10.1161/CIR.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10(1):16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical WNT signaling. Proc Natl Acad Sci U S A. 2012;109(27):E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 31.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Izpisúa Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460(7259):1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460(7259):1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho HJ, Lee CS, Kwon YW, Paek JS, Lee SH, Hur J, Lee EJ, Roh TY, Chu IS, Leem SH, Kim Y, Kang HJ, Park YB, Kim HS. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood. 2010;116(3):386–395. doi: 10.1182/blood-2010-02-269589. [DOI] [PubMed] [Google Scholar]
- 38.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27(5):459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang M, Chen Z, Hu S, Jia F, Li Z, Hoyt G, Robbins RC, Kay MA, Wu JC. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation. 2009;120(11 Suppl):S230–S237. doi: 10.1161/CIRCULATIONAHA.108.841155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kay MA, C Y, He ZY, Chen A. A robust system for production of minicircle DNA vectors. Nat Biotechnol. 2010;28(12):1287–1289. doi: 10.1038/nbt.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He E, Yue CY, Simeon F, Zhou LH, Too HP, Tam KC. Polyplex formation between four-arm poly(ethylene oxide)-b-poly(2-(diethylamino)ethyl methacrylate) and plasmid DNA in gene delivery. J Biomed Mater Res A. 2009;91(3):708–718. doi: 10.1002/jbm.a.32255. [DOI] [PubMed] [Google Scholar]
- 42.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 43.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9(2):113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, Morrisey EE. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20(9):933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 46.Unger C, Felldin U, Nordenskjöld A, Dilber MS, Hovatta O. Derivation of human skin fibroblast lines for feeder cells of human embryonic stem cells. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc01c07s5. Chapter 1: Unit 1C 7. [DOI] [PubMed] [Google Scholar]
- 47.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3(8):637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 48.Goh PA, Caxaria S, Casper C, Rosales C, Warner TT, Coffey PJ, Nathwani AC. A systematic evaluation of integration free reprogramming methods for deriving clinically relevant patient specific induced pluripotent stem (iPS) cells. PLoS One. 2013;8(11):e81622. doi: 10.1371/journal.pone.0081622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 50.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105(8):2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gai H, Leung EL, Costantino PD, Aguila JR, Nguyen DM, Fink LM, Ward DC, Ma Y. Generation and characterization of functional cardiomyocytes using induced pluripotent stem cells derived from human fibroblasts. Cell Biol Int. 2009;33(11):1184–1193. doi: 10.1016/j.cellbi.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Aasen T, Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia G, Edel M, Boué S, Belmonte JC. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 53.Kim JB, Greber B, Araúzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Schöler HR. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461(7264):649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 54.Utikal J, Maherali N, Kulalert W, Hochedlinger K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J Cell Sci. 2009;122(Pt 19):3502–3510. doi: 10.1242/jcs.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodríguez-Pizà I, Vassena R, Raya A, Boué S, Barrero MJ, Corbella BA, Torrabadella M, Veiga A. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5(4):353–357. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, Zweigerdt R, Gruh I, Meyer J, Wagner S, Maier LS, Han DW, Glage S, Miller K, Fischer P, Schöler HR, Martin U. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5(4):434–441. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 57.Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, Kim K, Miller JD, Ng K, Daley GQ. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113(22):5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, Wu JC. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106(37):15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151(3):547–558. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, Paull D, Nestor MW, Freeby M, Greenberg E, Goland RS, Leibel RL, Solomon SL, Benvenisty N, Sauer MV, Egli D. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510(7506):533–536. doi: 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- 61.Krupalnik V, Hanna JH. Stem cells: The quest for the perfect reprogrammed cell. Nature. 2014;511(7508):160–162. doi: 10.1038/nature13515. [DOI] [PubMed] [Google Scholar]
- 62.Ma H, Morey R, O'Neil RC, He Y, Daughtry B, Schultz MD, Hariharan M, Nery JR, Castanon R, Sabatini K, Thiagarajan RD, Tachibana M, Kang E, Tippner-Hedges R, Ahmed R, Gutierrez NM, Van Dyken C, Polat A, Sugawara A, Sparman M, Gokhale S, Amato P, Wolf DP, Ecker JR, Laurent LC, Mitalipov S. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511(7508):177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, Mansour AA, Caspi I, Krupalnik V, Zerbib M, Maza I, Mor N, Baran D, Weinberger L, Jaitin DA, Lara-Astiaso D, Blecher-Gonen R, Shipony Z, Mukamel Z, Hagai T, Gilad S, Amann-Zalcenstein D, Tanay A, Amit I, Novershtern N, Hanna JH. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502(7469):65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- 64.Obokata H, Wakayama T, Sasai Y, Kojima K, Vacanti MP, Niwa H, Yamato M, Vacanti CA. Stimulus-triggered fate conversion of somatic cells into pluripotency. Nature. 2014;505(7485):641–647. doi: 10.1038/nature12968. [DOI] [PubMed] [Google Scholar]
- 65.Arnold SJ, Robertson EJ. Robertson, making a commitment: Cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10(2):91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 66.Costello I, Pimeisl IM, Dräger S, Bikoff EK, Robertson EJ, Arnold SJ. The T-box transcription factor eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nat Cell Biol. 2011;13(9):1084–1091. doi: 10.1038/ncb2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.David R, Jarsch VB, Schwarz F, Nathan P, Gegg M, Lickert H, Franz WM. Induction of MesP1 by Brachyury(T) generates the common multipotent cardiovascular stem cell. Cardiovasc Res. 2011;92(1):115–122. doi: 10.1093/cvr/cvr158. [DOI] [PubMed] [Google Scholar]
- 68.Lindsley RC, Gill JG, Murphy TL, Langer EM, Cai M, Mashayekhi M, Wang W, Niwa N, Nerbonne JM, Kyba M, Murphy KM. Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs. Cell Stem Cell. 2008;3(1):55–68. doi: 10.1016/j.stem.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bondue A, Blanpain C. Mesp1: A key regulator of cardiovascular lineage commitment. Circ Res. 2010;107(12):1414–1427. doi: 10.1161/CIRCRESAHA.110.227058. [DOI] [PubMed] [Google Scholar]
- 70.Katoh M, Katoh M. CER1 is a common target of WNT and NODAL signaling pathways in human embryonic stem cells. Int J Mol Med. 2006;17(5):795–799. [PubMed] [Google Scholar]
- 71.Gadue P, Huber TL, Paddison PJ, Keller GM. WNT and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(45):16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 73.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical WNT/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135(17):2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- 74.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 75.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23(5):607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 76.Ren Y, Lee MY, Schliffke S, Paavola J, Amos PJ, Ge X, Ye M, Zhu S, Senyei G, Lum L, Ehrlich BE, Qyang Y. Small molecule WNT inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol. 2011;51(3):280–287. doi: 10.1016/j.yjmcc.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, Yamashita JK. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One. 2011;6(8):e23657. doi: 10.1371/journal.pone.0023657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rao C, Prodromakis T, Kolker L, Chaudhry UA, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, Yacoub MH, Athanasiou T, Terracciano CM. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials. 2013;34(10):2399–2411. doi: 10.1016/j.biomaterials.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ Res. 2012;111(3):344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157(3):565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamanaka S, Zahanich I, Wersto RP, Boheler KR. Enhanced proliferation of monolayer cultures of embryonic stem (ES) cell-derived cardiomyocytes following acute loss of retinoblastoma. PLoS One. 2008;3(12):e3896. doi: 10.1371/journal.pone.0003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burridge PW, Anderson D, Priddle H, Barbadillo Muñoz MD, Chamberlain S, Allegrucci C, Young LE, Denning C. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25(4):929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 83.Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, Chen K, Li Y, Liu X, Xu J, Zhang S, Li F, He W, Labuda K, Song Y, Peterbauer A, Wolbank S, Redl H, Zhong M, Cai D, Zeng L, Pei D. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6(1):71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Fujiwara M, Yan P, Otsuji TG, Narazaki G, Uosaki H, Fukushima H, Kuwahara K, Harada M, Matsuda H, Matsuoka S, Okita K, Takahashi K, Nakagawa M, Ikeda T, Sakata R, Mummery CL, Nakatsuji N, Yamanaka S, Nakao K, Yamashita JK. Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with cyclosporin-A. PLoS One. 2011;6(2):e16734. doi: 10.1371/journal.pone.0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee YK, Ng KM, Chan YC, Lai WH, Au KW, Ho CY, Wong LY, Lau CP, Tse HF, Siu CW. Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway. Mol Endocrinol. 2010;24(9):1728–1736. doi: 10.1210/me.2010-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ng KM, Lee YK, Lai WH, Chan YC, Fung ML, Tse HF, Siu CW. Exogenous expression of human apoA-I enhances cardiac differentiation of pluripotent stem cells. PLoS One. 1978;6(5):e19787. doi: 10.1371/journal.pone.0019787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous WNT/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5(6):e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu H, Yi BA, Wu H, Bock C, Gu H, Lui KO, Park JH, Shao Y, Riley AK, Domian IJ, Hu E, Willette R, Lepore J, Meissner A, Wang Z, Chien KR. Highly efficient derivation of ventricular cardiomyocytes from induced pluripotent stem cells with a distinct epigenetic signature. Cell Res. 2012;22(1):142–154. doi: 10.1038/cr.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28(8):848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108(3):407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu C, Police S, Hassanipour M, Gold JD. Cardiac bodies: A novel culture method for enrichment of cardiomyocytes derived from human embryonic stem cells. Stem Cells Dev. 2006;15(5):631–639. doi: 10.1089/scd.2006.15.631. [DOI] [PubMed] [Google Scholar]
- 93.Hattori F, Chen H, Yamashita H, Tohyama S, Satoh YS, Yuasa S, Li W, Yamakawa H, Tanaka T, Onitsuka T, Shimoji K, Ohno Y, Egashira T, Kaneda R, Murata M, Hidaka K, Morisaki T, Sasaki E, Suzuki T, Sano M, Makino S, Oikawa S, Fukuda K. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7(1):61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 94.Kahler DJ, Ahmad FS, Ritz A, Hua H, Moroziewicz DN, Sproul AA, Dusenberry CR, Shang L, Paull D, Zimmer M, Weiss KA, Egli D, Noggle SA. Improved methods for reprogramming human dermal fibroblasts using fluorescence activated cell sorting. PLoS One. 2013;8(3):e59867. doi: 10.1371/journal.pone.0059867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rust W, Balakrishnan T, Zweigerdt R. Cardiomyocyte enrichment from human embryonic stem cell cultures by selection of ALCAM surface expression. Regen Med. 2009;4(2):225–237. doi: 10.2217/17460751.4.2.225. [DOI] [PubMed] [Google Scholar]
- 96.Van Hoof D, Dormeyer W, Braam SR, Passier R, Monshouwer-Kloots J, Ward-van Oostwaard D, Heck AJ, Krijgsveld J, Mummery CL. Identification of cell surface proteins for antibody-based selection of human embryonic stem cell-derived cardiomyocytes. J Proteome Res. 2010;9(3):1610–1618. doi: 10.1021/pr901138a. [DOI] [PubMed] [Google Scholar]
- 97.Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29(11):1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elliott DA, Braam SR, Koutsis K, Ng ES, Jenny R, Lagerqvist EL, Biben C, Hatzistavrou T, Hirst CE, Yu QC, Skelton RJ, Ward-van Oostwaard D, Lim SM, Khammy O, Li X, Hawes SM, Davis RP, Goulburn AL, Passier R, Prall OW, Haynes JM, Pouton CW, Kaye DM, Mummery CL, Elefanty AG, Stanley EG. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011;8(12):1037–1040. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 99.Harding SE, Ali NN, Brito-Martins M, Gorelik J. The human embryonic stem cell-derived cardiomyocyte as a pharmacological model. Pharmacol Ther. 2007;113(2):341–353. doi: 10.1016/j.pharmthera.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 100.Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol Ther. 2007;15(11):2027–2036. doi: 10.1038/sj.mt.6300303. [DOI] [PubMed] [Google Scholar]
- 101.Huber I, Itzhaki I, Caspi O, Arbel G, Tzukerman M, Gepstein A, Habib M, Yankelson L, Kehat I, Gepstein L. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 2007;21(10):2551–2563. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]
- 102.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460(7251):113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 103.Ivashchenko CY, Pipes GC, Lozinskaya IM, Lin Z, Xiaoping X, Needle S, Grygielko ET, Hu E, Toomey JR, Lepore JJ, Willette RN. Human-induced pluripotent stem cell-derived cardiomyocytes exhibit temporal changes in phenotype. Am J Physiol Heart Circ Physiol. 2013;305(6):H913–H922. doi: 10.1152/ajpheart.00819.2012. [DOI] [PubMed] [Google Scholar]
- 104.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J. 2013;77(5):1307–1314. doi: 10.1253/circj.cj-12-0987. [DOI] [PubMed] [Google Scholar]
- 106.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34(23):5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Massé S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M. Biowire: A platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10(8):781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinelli V, Cellot G, Toma FM, Long CS, Caldwell JH, Zentilin L, Giacca M, Turco A, Prato M, Ballerini L, Mestroni L. Carbon nanotubes instruct physiological growth and functionally mature syncytia: Nongenetic engineering of cardiac myocytes. ACS Nano. 2013;7(7):5746–5756. doi: 10.1021/nn4002193. [DOI] [PubMed] [Google Scholar]
- 109.Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, Zeitler B, Cherone JM, Meng X, Hinkley SJ, Rebar EJ, Gregory PD, Urnov FD, Jaenisch R. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29(8):731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soldner F, Laganière J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S, Zhang L, Guschin D, Fong LK, Vu BJ, Meng X, Urnov FD, Rebar EJ, Gregory PD, Zhang HS, Jaenisch R. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146(2):318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park CY, Kim J, Kweon J, Son JS, Lee JS, Yoo JE, Cho SR, Kim JH, Kim JS, Kim DW. Targeted inversion and reversion of the blood coagulation factor 8 gene in human iPS cells using TALENs. Proc Natl Acad Sci U S A. 2014;111(25):9253–9258. doi: 10.1073/pnas.1323941111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsunaga T, Yamashita JK. Single-step generation of gene knockout-rescue system in pluripotent stem cells by promoter insertion with CRISPR/Cas9. Biochem Biophys Res Commun. 2014;444(2):158–163. doi: 10.1016/j.bbrc.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 113.Ma N, Liao B, Zhang H, Wang L, Shan Y, Xue Y, Huang K, Chen S, Zhou X, Chen Y, Pei D, Pan G. Transcription activator-like effector nuclease (TALEN)-mediated gene correction in integration-free beta-thalassemia induced pluripotent stem cells. J Biol Chem. 2013;288(48):34671–34679. doi: 10.1074/jbc.M113.496174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yoshimi K, Kaneko T, Voigt B, Mashimo T. Allele-specific genome editing and correction of disease-associated phenotypes in rats using the CRISPR-CAS platform. Nat Commun. 2014;5:4240. doi: 10.1038/ncomms5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124(3):304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang J, Wakimoto H, Seidman JG, Seidman CE. Allele-specific silencing of mutant Myh6 transcripts in mice suppresses hypertrophic cardiomyopathy. Science. 2013;342(6154):111–114. doi: 10.1126/science.1236921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horii T, Tamura D, Morita S, Kimura M, Hatada I. Generation of an ICF syndrome model by efficient genome editing of human induced pluripotent stem cells using the CRISPR system. Int J Mol Sci. 1977;14(10):4–81. doi: 10.3390/ijms141019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tay FC, Tan WK, Goh SL, Ramachandra CJ, Lau CH, Zhu H, Chen C, Du S, Phang RZ, Shahbazi M, Fan W, Wang S. Targeted transgene insertion into the AAVS1 locus driven by baculoviral vector-mediated zinc finger nuclease expression in human-induced pluripotent stem cells. J Gene Med. 2013;15(10):384–395. doi: 10.1002/jgm.2745. [DOI] [PubMed] [Google Scholar]
- 119.Gonzalez F, Zhu Z, Shi ZD, Lelli K, Verma N, Li QV, Huangfu D. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell. 2014;15(2):215–226. doi: 10.1016/j.stem.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4(130):130ra47. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Effie Apostolou KH. Hochedlinger_ips cells under attack.pdf. [Google Scholar]
- 122.Lebkowski J. GRNOPC1: The world's first embryonic stem cell-derived therapy. Interview with Jane Lebkowski. Regen Med. 2011;6(6 Suppl):11–13. doi: 10.2217/rme.11.77. [DOI] [PubMed] [Google Scholar]
- 123.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379(9817):713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 124.Cai J, Yi FF, Yang XC, Lin GS, Jiang H, Wang T, Xia Z. Transplantation of embryonic stem cell-derived cardiomyocytes improves cardiac function in infarcted rat hearts. Cytotherapy. 2007;9(3):283–291. doi: 10.1080/14653240701247838. [DOI] [PubMed] [Google Scholar]
- 125.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50(19):1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 126.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474(7350):212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 127.Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, Abe M. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 128.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12(4):407–412. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 129.de.Almeida PE, Meyer EH, Kooreman NG, Diecke S, Dey D, Sanchez-Freire V, Hu S, Ebert A, Odegaard J, Mordwinkin NM, Brouwer TP, Lo D, Montoro DT, Longaker MT, Negrin RS, Wu JC. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat Commun. 2014;5:3903. doi: 10.1038/ncomms4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465(7295):175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ben-David U, Nudel N, Benvenisty N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun. 2013;4:1992. doi: 10.1038/ncomms2992. [DOI] [PubMed] [Google Scholar]
- 134.Lattanzi L, Salvatori G, Coletta M, Sonnino C, Cusella De Angelis MG, Gioglio L, Murry CE, Kelly R, Ferrari G, Molinaro M, Crescenzi M, Mavilio F, Cossu G. High efficiency myogenic conversion of human fibroblasts by adenoviral vector-mediated Myod gene transfer. An alternative strategy for ex vivo gene therapy of primary myopathies. J Clin Invest. 1998;101(10):2119–2128. doi: 10.1172/JCI1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 136.Yoshida Y, Yamanaka S. Labor pains of new technology: Direct cardiac reprogramming. Circ Res. 2012;111(1):3–4. doi: 10.1161/CIRCRESAHA.112.271445. [DOI] [PubMed] [Google Scholar]
- 137.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, Wu JC, Milan D, Wu SM. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111(1):50–55. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, Gearhart JD. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J Mol Cell Cardiol. 2013;60:97–106. doi: 10.1016/j.yjmcc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Christoforou N, Chellappan M, Adler AF, Kirkton RD, Wu T, Addis RC, Bursac N, Leong KW. Transcription factors MYOCD, SRF, Mesp1 and SMARCD3 enhance the cardio-inducing effect of GATA4, TBX5, and MEF2C during direct cellular reprogramming. PLoS One. 2013;8(5):e63577. doi: 10.1371/journal.pone.0063577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012;53(3):323–332. doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 142.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110(11):1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ieda M. Heart regeneration using reprogramming technology. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89(3):118–128. doi: 10.2183/pjab.89.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Murry CE, Pu WT. Reprogramming fibroblasts into cardiomyocytes. N Engl J Med. 2011;364(2):177–178. doi: 10.1056/NEJMcibr1013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Addis RC, Epstein JA. Induced regeneration–the progress and promise of direct reprogramming for heart repair. Nat Med. 2013;19(7):829–836. doi: 10.1038/nm.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Islas JF, Liu Y, Weng KC, Robertson MJ, Zhang S, Prejusa A, Harger J, Tikhomirova D, Chopra M, Iyer D, Mercola M, Oshima RG, Willerson JT, Potaman VN, Schwartz RJ. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci U S A. 2012;109(32):13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, Olson EN. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A. 2013;110(14):5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci U S A. 2013;110(31):12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1(3):235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: Friend or foe? Am J Physiol Heart Circ Physiol. 2006;291(3):H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 151.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: The renaissance cell. Circ Res. 2009;105(12):1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kanekar S, Borg TK, Terracio L, Carver W. Modulation of heart fibroblast migration and collagen gel contraction by IGF-I. Cell Adhes Commun. 2000;7(6):513–523. doi: 10.3109/15419060009040308. [DOI] [PubMed] [Google Scholar]
- 153.Hematti P. Mesenchymal stromal cells and fibroblasts: A case of mistaken identity? Cytotherapy. 2012;14(5):516–521. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- 154.Nag AC. Study of non-muscle cells of the adult mammalian heart: A fine structural analysis and distribution. Cytobios. 1980;28(109):41–61. [PubMed] [Google Scholar]
- 155.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: Structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94(6):828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 156.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93(5):421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 157.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, Müller W, Chien KR. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97(2):189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 158.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mathison M, Gersch RP, Nasser A, Lilo S, Korman M, Fourman M, Hackett N, Shroyer K, Yang J, Ma Y, Crystal RG, Rosengart TK. In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor. J Am Heart Assoc. 2012;1(6):e005652. doi: 10.1161/JAHA.112.005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, Wada R, Katsumata Y, Kaneda R, Nakade K, Kurihara C, Obata Y, Miyake K, Fukuda K, Ieda M. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111(9):1147–1156. doi: 10.1161/CIRCRESAHA.112.271148. [DOI] [PubMed] [Google Scholar]
- 161.Kapoor N, Liang W, Marbán E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31(1):54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Josowitz R, Lu J, Falce C, D'Souza SL, Wu M, Cohen N, Dubois NC, Zhao Y, Sobie EA, Fishman GI, Gelb BD. Identification and purification of human induced pluripotent stem cell-derived atrial-like cardiomyocytes based on sarcolipin expression. PLoS One. 2014;9(7):e101316. doi: 10.1371/journal.pone.0101316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mohanty S, Bose S, Jain KG, Bhargava B, Airan B. TGFbeta1 contributes to cardiomyogenic-like differentiation of human bone marrow mesenchymal stem cells. Int J Cardiol. 2013;163(1):93–99. doi: 10.1016/j.ijcard.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 164.Shinmura D, Togashi I, Miyoshi S, Nishiyama N, Hida N, Tsuji H, Tsuruta H, Segawa K, Tsukada Y, Ogawa S, Umezawa A. Pretreatment of human mesenchymal stem cells with pioglitazone improved efficiency of cardiomyogenic transdifferentiation and cardiac function. Stem Cells. 2011;29(2):357–366. doi: 10.1002/stem.574. [DOI] [PubMed] [Google Scholar]
- 165.Raynaud CM, Halabi N, Elliott DA, Pasquier J, Elefanty AG, Stanley EG, Rafii A. Human embryonic stem cell derived mesenchymal progenitors express cardiac markers but do not form contractile cardiomyocytes. PLoS One. 2013;8(1):e54524. doi: 10.1371/journal.pone.0054524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109(1):74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 167.Koninckx R, Hensen K, Daniëls A, Moreels M, Lambrichts I, Jongen H, Clijsters C, Mees U, Steels P, Hendrikx M, Rummens JL. Human bone marrow stem cells co-cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy. 2009;11(6):778–792. doi: 10.3109/14653240902988818. [DOI] [PubMed] [Google Scholar]
- 168.Xie XJ, Wang JA, Cao J, Zhang X. Differentiation of bone marrow mesenchymal stem cells induced by myocardial medium under hypoxic conditions. Acta Pharmacol Sin. 2006;27(9):1153–1158. doi: 10.1111/j.1745-7254.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 169.Ismail S, O'Brien T, Barry F. 188 the cardioprotective effect of MSC secreted protein in an in vitro model of myocardial injury: The mechanistic insight. Heart. 2014;100(Suppl 3):A105. [Google Scholar]
- 170.Galie PA, Stegemann JP. Injection of mesenchymal stromal cells into a mechanically stimulated in vitro model of cardiac fibrosis has paracrine effects on resident fibroblasts. Cytotherapy. 2014;16(7):906–914. doi: 10.1016/j.jcyt.2014.01.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mao Q, Lin CX, Liang XL, Gao JS, Xu B. Mesenchymal stem cells overexpressing integrin-linked kinase attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. Mol Med Rep. 2013;7(5):1617–1623. doi: 10.3892/mmr.2013.1348. [DOI] [PubMed] [Google Scholar]
- 172.Xiang MX, He AN, Wang JA, Gui C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J Zhejiang Univ Sci B. 2009;10(8):619–624. doi: 10.1631/jzus.B0920153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fioret BA, Heimfeld JD, Paik DT, Hatzopoulos AK. Endothelial cells contribute to generation of adult ventricular myocytes during cardiac homeostasis. Cell Rep. 2014;8(1):229–241. doi: 10.1016/j.celrep.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mishra PK, Kuypers NJ, Singh SR, Leiberh ND, Chavali V, Tyagi SC. Cardiac stem cell niche, MMP9, and culture and differentiation of embryonic stem cells. Methods Mol Biol. 2013;1035:153–163. doi: 10.1007/978-1-62703-508-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sandler VM, Lis R, Liu Y, Kedem A, James D, Elemento O, Butler JM, Scandura JM, Rafii S. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511(7509):312–318. doi: 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Balmer GM, Riley PR. Harnessing the potential of adult cardiac stem cells: Lessons from haematopoiesis, the embryo and the niche. J Cardiovasc Transl Res. 2012;5(5):631–640. doi: 10.1007/s12265-012-9386-3. [DOI] [PubMed] [Google Scholar]
- 177.Japanese woman is first recipient of next-generation stem cells [Google Scholar]
- 178.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, Moreno H, Abeliovich A. Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons. Cell. 2011;146(3):359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 180.Abraham I. Stem cell potency. 2011 [Google Scholar]
- 181.Apostolou E, Hochedlinger K. Stem cells: iPS cells under attack. Nature. 2011;474(7350):165–166. doi: 10.1038/474165a. [DOI] [PubMed] [Google Scholar]