Abstract
Cardiovascular diseases are the leading cause of death worldwide. Thrombosis, the formation of blood clot (thrombus) in the circulatory system obstructing the blood flow, is one of the main causes behind various ischemic arterial syndromes such as ischemic stroke and myocardial infarction, as well as vein syndromes such as deep vein thrombosis, and consequently, pulmonary emboli. Several thrombolytic agents have been developed for treating thrombosis, the most common being tissue plasminogen activator (tPA), administrated systemically or locally via IV infusion directly proximal to the thrombus, with the aim of restoring and improving the blood flow. TPA triggers the dissolution of thrombi by inducing the conversion of plasminogen to protease plasmin followed by fibrin digestion that eventually leads to clot lysis. Although tPA provides powerful thrombolytic activity, it has many shortcomings, including poor pharmacokinetic profiles, impairment of the reestablishment of normal coronary flow, and impairment of hemostasis, leading to life-threatening bleeding consequences. The bleeding consequence is ascribed to the ability of tPA to circulate throughout the body and therefore can lysis all blood clots in the circulation system, even the good ones that prevent the bleeding and promote injury repair. This review provides an overview of the different delivery approaches for tPA including: liposomes, ultrasound-triggered thrombolysis, anti-fibrin antibody-targeted tPA, camouflaged-tPA, tpA-loaded microcarriers, and nano-modulated delivery approaches.
Keywords: thrombus, tissue plasminogen activator, controlled delivery, clot busting
Introduction
Hemostasis is a multifactorial state that ensures efficient blood flow through peripheral vascular districts. It is affected by the characteristics of blood vessel walls, platelets, the fibrinolytic system, and the coagulation pathway, which are all intimately related (Figure 1). All these factors function normally to produce an equilibrium between antithrombotic and prothrombotic factors.1,2 Any misbalance in this equilibrium will lead to thrombosis: the formation of a blood clot inside a vessel, causing its occlusion or stenosis, and leading to various clinical presentations, depending on the occluded vessel. For instance, cerebrovascular thrombosis will lead to stroke and coronary artery thrombosis will lead to ischemic heart diseases (angina or myocardial infarction).
Figure 1.
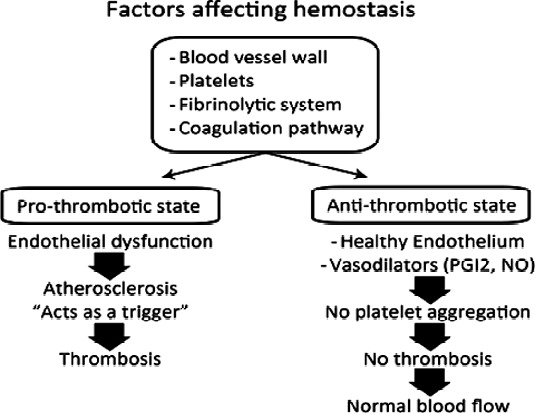
Factors affecting hemostasis.
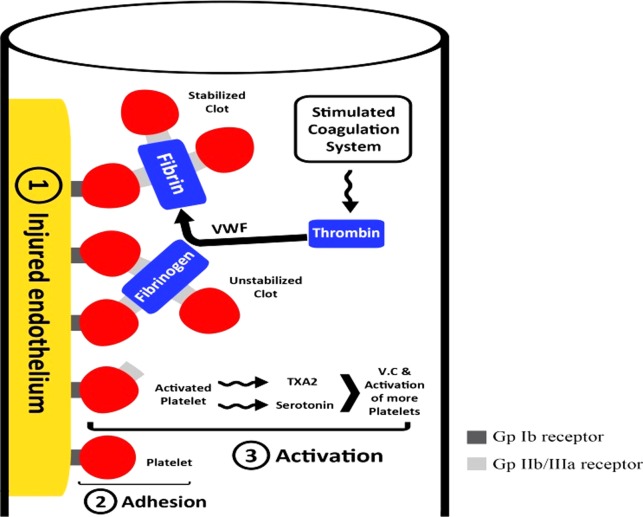
Thrombosis takes place in two stages, primary hemostasis, and secondary hemostasis (Figure 2).3 Primary hemostasis is initiated by platelets' adherence to the damaged vascular endothelium, in a complex involving multiple platelet membrane receptors, to form a platelet plug.4 Secondary hemostasis includes the activation of the coagulation system, which finally leads to conversion of fibrinogen into fibrin to form a hemostatic clot.5 Platelet adhesion to the endothelium via the GP Ib receptor and von Willebrand factor (VWF) is followed by platelet activation (in the form of shape changes in the platelet, release of thromboxane A2, serotonin and other agents, and expression of GP IIb/ IIIa receptors on platelets' surface). The final step is binding of fibrinogen and VWF to the activated GP IIb/ IIIa receptors of two platelets, creating a growing aggregate.3,6
Figure 2.
An illustration of the stages of thrombosis.
As the primary and secondary hemostasis phases are dynamically interactive, the plasma coagulation system is activated at the time of formation of the platelet plug. Coagulation is often been represented as two independent pathways (extrinsic and intrinsic) that converge into a single interrelated system or pathway, with thrombin generation as the end point of the cascade.3,7 Thrombin converts fibrinogen into fibrin, and it also activates factor XIII, leading to stabilization of the fibrin clot. In addition, it is a potent stimulant of platelet aggregation.8 As a result, thrombin generation is a key goal in the management of thrombosis.
In spite of the relatively large number of available drugs, tissue plasminogen activator (tPA) is still the main and the primary thrombolytic agent used in the treatment of established thrombus in myocardial infarction (MI) and pulmonary embolism.9–11 Tissue plasminogen factor is one of the physiological plasminogen activators which can be used clinically. This family also includes streptokinase (SK) and urokinase (UK). These agents catalyze the hydrolysis of plasminogen at the Arg561–Val562 bond, which results in the formation of active plasmin. The plasmin then acts by degrading the insoluble fibrin clot, which forms the nucleus of the thrombus.12–14 However, tPA has a short half-life ( < 5 min), which necessitates its administration in a large dose (1 mg/kg) to have the desired effect. This can lead to the degradation of clotting factors and hemorrhage.15,16 Although a lower bolus dose can be used, followed by prolonged infusion, it is not the ideal manner in which to optimize the benefit of, and minimize side effects from, tPA.
In order to minimize the risk of bleeding, many strategies have been developed to offer local thrombolytic action of tPA, or in other words, targeting of tPA. In this review, we are going to discuss the most common delivery approaches of tPA such as liposomes, ultrasound-triggered thrombolysis, anti-fibrin antibody-targeted tPA, camouflaged-tPA, tpA-loaded microcarriers, and nano-modulated delivery approaches.
Controlled Delivery Approaches for tPA
Liposomes
Liposomes are vesicular drug delivery systems that consist of lipid bilayer arrays.17,18 Each lipid layer consists of two parts, hydrophilic and hydrophobic. The hydrophilic parts are directed towards the aqueous phase, while the hydrophobic segments are directed towards each other.19 This structure gives liposomes the opportunity to encapsulate both water-soluble and water-insoluble bioactive materials.20 Liposomes are highly biocompatible with low immunogenicity,21 and they are also characterized by their efficient encapsulation of drugs. This separation from external conditions leads to a decrease in side effects resulting from nonspecific actions, such as bleeding in the case of plasminogen activators.22
Liposomes have been investigated as a drug delivery system for tPA (Figure 3).23–25 Heeremans and his team have proved that tPA-loaded liposomes have higher anti-thrombolytic effect as compared to the free tPA.24 The main drawback of liposomes, however, is their relatively rapid rate of clearance from the circulatory system, due to phagocytosis by the reticuloendothelial system (RES), resulting in their short half-life.26
Figure 3.
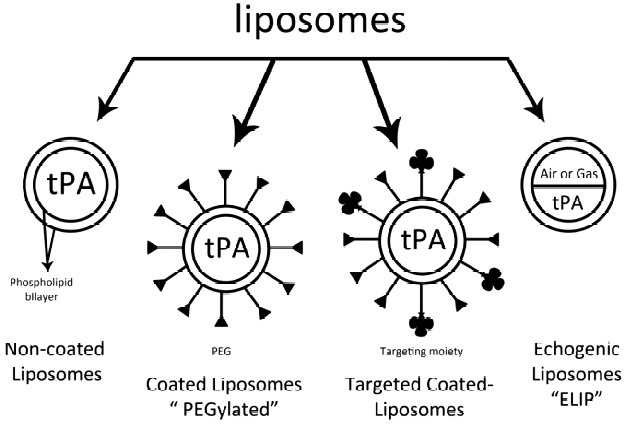
Different investigated types of liposomes-loaded tPA.
This shortcoming has been overcome via chemical modifications of the surface of the liposome with substances such as polyethylene glycol (PEG) in a process called PEGylation, leading to a significant decrease in the uptake of liposomes by RES.27,28
PEG has several advantages, including high water solubility and low cytotoxicity. In addition, the PEGylated-liposomes or vesicles demonstrate high stability due to steric repulsion, which prevents the fusion and disruption of the vesicles.29 Moreover, liposomal modification with PEG widens the applicability of liposomes by enhancing their circulation time, in addition to improving their targeting capabilities.30
Ji-Young Kim et al. for instance, used PEGylated liposomes to prolong circulation of tPA.25 In their study, encapsulation of tPA into conventional liposomes (EPCL) and PEGylated-liposomes (EPC-PEGL) prolonged the half-life of tPA by 16- and 21-fold, respectively, compared with free tPA. The half-life of free tPA was prolonged from about 5.87 minutes in the terminal phase, to 50.03 min and 132.62 min for EPCL and EPC-PEGL, respectively.
In another study,31 Absar and his co-workers encapsulated tPA into PEGylated and non-PEGylated liposomes decorated with the peptide sequence (CQQHHLGGAKQAGDV) of fibrinogen gamma-chain that has the affinity to bind with the GPIIb/IIIa receptors expressed on activated platelets. This decoration enhanced the liposomal affinity to bind with activated platelets. Consequently, the half-life of tPA has extended from 7 minutes (for free tPA) to 103 and 141 minutes in the case of non-PEGylated and PEGylated liposomes, respectively.
Another advantage of liposomes is the ability to encapsulate gas and fluid to form an echogenic liposome (ELIP). These echogenic liposomes have been utilized as ultrasound contrast agents to assist in ultrasound-enhanced thrombolysis.32
Ultrasound-enhanced thrombolysis
Ultrasound has been found beneficial in thrombolysis since the 1970s,33,34 and can be used either alone,35–37 or as the trigger component of a drug delivery system for tPA.38–40
Francis41 has proposed that ultrasound can enhance thrombolysis via two different approaches, mechanical fragmentation of the clot or by enhancing enzymatic fibrinolysis by increasing the enzyme transport to thrombus via static42 and perfusion43 systems.
Recently, Everbach and Francis attributed the effect of ultrasound to the growth and/ or collapse of micro-bubbles within the clot, followed by the occurrence of two pathways. In the first pathway, the formed bubbles are enlarged and their diameters exceed that of the pores of the fibrin lattice surrounding them, leading to stretching of the clot fibers. In the other pathway, the bubbles may collapse in a violent way, producing acoustic emissions and inertial cavitations, and consequently altering the structure of clot fibers. Both pathways lead to same result; producing new binding sites for the fibrinolytic enzyme, in addition to a stirring force produced by micro-streaming around the bubbles, which increases the chance of the fibrinolytic enzyme coming in contact with the fibrin strands.44
This assumption was in agreement the suggestion of Nyborg and Ziskin in 1985, where they attributed the action of ultrasound to three main mechanisms: acoustic streaming, cavitation and a thermal effect.45 To explain the process more clearly, Alexandrov stated that “if you put sugar in a water cup, the sugar promptly goes down to the bottom, and it will take some time for it to dissolve completely due to absence of water motion. Upon stirring water with a spoon, sugar dissolves much faster. In a similar fashion, the enzyme spends a long time to reach the goal because of the stagnant flow near occlusion”.46
In spite of the various advantages of ultrasound, using it alone has some drawbacks. For instance, it causes vessel wall damage41 and high intensity ultrasound can break the clot into smaller particles, causing embolization.41,47
In addition, it was discovered, based on both in-vitro48 and in-vivo49 studies, that ultrasound-induced thrombolysis may cause activation of platelets, leading to re-occlusion. As a result, ultrasound use has been restricted as a trigger for releasing tPA.
Kudo and his group were the first to report using ultrasound as a non-invasive approach to increase the efficiency of systemic tPA.50–52 With the aid of a canine model, they found that continuous application of transcutaneous ultrasound at frequency of 200 kHz enhanced tPA-induced thrombolysis.50–52 Many subsequent experimental studies, either using ultrasound with tPA alone53,54 or, more recently, in the presence of various contrast agents,39,40,55,56 have shown that ultrasound increased the efficiency of tPA.
Based on the promising results of these experimental studies,53–56 a number of clinical studies have been carried out which demonstrated the enhanced efficacy of ultrasound-based thrombolysis in stroke patients.57–60
Alexandrov and his team used diagnostic ultrasound to improve thrombolysis by tPA.57,58 They used a 2-MHz transcranial Doppler (TCD) in patients with acute ischemic stroke due to occlusion of middle cerebral artery (MCA), and the TCD was applied in a continuous manner. They observed that patients monitored with TCD during clinical-course treatment with systemic tPA revealed early recanalization and dramatic recovery.57 Afterwards, this observation was tested through a multicenter clinical trial, CLOTBUST (Combined Lysis of Thrombus in Brain ischemia using transcranial Ultrasound and Systemic tPA).58 The study involved two groups of patients, each group consisting of 63 patients, where the target group received continuous ultrasound (tPA+TCD) and the control group received placebo (tPA alone). The results showed that 83% of the target group showed recanalization (46% complete and 27% partial) versus 50% (17% complete and 33% partial) for the control group. In addition, 3.8 % of both groups demonstrated symptomatic intracerebral hemorrhage.58 As a result, it was postulated, by Alexandrov et al. that continuous monitoring by TCD might have augmented the thrombolytic effect of tPA by exposing more clot surface to tPA. Exposing more surface of the clot surface was attributed to pressure gradients formed at the clot site by the ultrasonic energy emitted from TCD, where this pressure leads to tPA molecules being forced and lodged into the clot.57
In another clinical trial, Eggers et al.60 used diagnostic ultrasound to improve the thrombolytic effect of tPA. A total of 25 patients were used in the study; a target group of 11 patients received duplex monitoring and tPA, while the control group, consisting of 14 patients, received tPA alone. The results showed better recanalization and neurological outcome after 3 months in the target group. However, the intracerebral hemorrhage rate was higher in the target group. These results were not considered sufficient to judge the effect of transcranial duplex due to the small number of patients investigated in the study.60
In another clinical trial using therapeutic low-frequency ultrasound, Daffertshofer et al.61 found that low-frequency (300 KHz) ultrasound led to a considerable increase in the rate of symptomatic intracerebral hemorrhage, up to 36%, and consequently, the TRUMBI trial (TRanscranial low-frequency Ultrasound-Mediated thrombolysis in Brain Ischemia) was terminated. As a conclusion, and surprisingly, it has been found that lower ultrasound frequencies (in kilohertz) are causing higher rates of intracerebral hemorrhage,61 while, the diagnostic frequencies (in megahertz) did not and are safe enough to be used in humans.58,60
For further improvement of ultrasound enhanced-thrombolysis, the concept of using contrast agents for better imaging and delivery of thrombolytic agents was tested. Micro-bubbles and echogenic liposomes were two principle examples of these utilized contrast agents.
Micro-bubbles
Micro-bubbles (MBs) are tiny gas- or air-filled microspheres and were first used as contrast agents for imaging due to their acoustic characteristics.62–64 In diagnostic ultrasonograpghy, MBs create acoustic impedance that is higher than that of red blood cells,65 giving them the ability to send stronger echoes and leading to better reflection. The mechanism by which the MBs enhanced ultrasound-accelerated thrombolysis was attributed to stable and inertial cavitation as these MBs act as nuclei for cavitation decreasing the amount of energy required for the cavitation.66,67 Stable cavitation leads to oscillations of MBs, resulting in micro-streaming, and therefore, erosion of clot surface which enhances the penetration of the clot by fibrinolytic enzymes.44 Inertial cavitation is induced by increasing the acoustic power on MBs, leading to an explosion which emits the absorbed energy.68,69 The effect of MBs on thrombolysis depends on many factors, such as bubble size, concentration of the MBs in the clot area, and the stability of the bubbles in blood stream.67 Being air-filled and encapsulated by a weak shell, this leads the first generation of MBs to be cleared rapidly from systemic circulation due to their low stability. In addition, their relatively large size reduced their ability to cross into lung circulation and get into the thrombus.70,71 As a result, a second generation of MBs was introduced by filling the MBs with a high molecular weight gas in addition to encapsulation of the MBs by phospholipids72 – galactose in the case of levovist, or thin shell albumin in the case of Albunex.67,68
Before being introduced to the clinical trials, the effect of MBs on enhancing ultrasound-based thrombolysis was tested by many experimental studies.73–75 In one of these clinical trials, Molina et al.67 evaluated 111 patients with acute stroke caused by MCA occlusion. Patients were divided into 3 groups. The first group included 38 patients who received tPA plus continuous two-hour TCD monitoring plus galactose-based MBs (tPA+US+MBs). The second group included 37 patients who received tPA with continuous two-hour TCD monitoring (tPA+US). Finally, the third group consisted of 36 patients receiving tPA with placebo monitoring (tPA alone). It was found that complete recanalization was significantly higher in the first group (54.5%) than in the second (40.8%) and third (23.9%) groups. Furthermore, 2.6 % of the first group, 2.7% of the second, and 5.5% of the third group showed symptomatic intracranial hemorrhage. These results were in agreement with the results obtained by Viguier and his group.73
In another pilot study, Alexandrov et al. tested perflutren-lipid microspheres in patients with acute ischemic stroke.74 Fifteen patients were divided into two groups; target group (12 patients), and a control group (3 patients). The target group received tPA with perflutren-lipid microspheres in addition to two-hour continuous TCD monitoring (tPA+MBs+TCD), while the control group received tPA with monitoring by TCD only (tPA+TCD). The results demonstrated that within 2 hours after tPA bolus, 50% of the target group showed complete recanalization (6/12 patients), and 33% showed partial recanalization (4/12) while 2 patients showed no recanalization (17%). No patients in the control group showed complete recanalization.
Both studies by Alexandrov et al.74 and Molina et al.67 are considered as an extension for the CLOTBUST study,58 introducing the concept of testing contrast agents in the form of MBs. Table 1 summarizes and compares the results of CLOTBUST58 with Molina's study,67 and Alexandrov's study.74
Table 1.
A summary of the progress of ultrasound-enhanced thrombolysis through using micro-bubbles (MBs), and comparing the studies outcome including both recanalization and symptomatic intracerebral hemorrhage (SICH).
| CLOTBUST58 | Molina's study67 | Alexandrov's study74 | |||||
| Target group | Control group | Target group | Control group | Target group | Control group | ||
| Number of subjects | 63 | 63 | 38 | 73 | 12 | 3 | |
| Treatment | tPA+ TCD | tPA alone | tPA+TCD+MBs | tPA+TCD | tPA | tPA+TCD+MBs | tPA+TCD |
| Complete recanalization after 2 hrs from tPA administration | 29/63 (46%) | 11/63(18%) | 54.5% | 40.8% | 23.9% | 6/12 (50%) | 0% |
| SICH; In the active treatment group (target) | 3.8% | 2.6% | 0% | ||||
The second example of contrast agents is echogenic liposomes (ELIP). Echogenic liposomes are multifunctional liposomes, phospholipid-bilayer encapsulated vesicles, which can be used as contrast agents for sonography, and at the same time, as a drug delivery system.19,75
On encapsulating a gas in a liposome, the gas acts as a hydrophobic drug and stays trapped between the two monolayers of the lipid-bilayer of the liposome.76 The overall entrapment efficiency tPA into the liposomes was about 50%. Of that 50%, around 35% of the loaded tPA were associated with the lipid bilayer and only 15% were encapsulated within the liposome.77 Therefore, the term ‘tPA-loaded echogenic liposome’ refers to the total of the tPA associated with the lipid shell, in addition to the part encapsulated in the aqueous phase.23
The exposure of the liposomes to ultrasound induces the disruption of the lipid shell and hence the release of the drug.19 As a result, ELIP loaded with tPA (t-ELIP) acts as a targeted drug delivery system by increasing the concentration of tPA in the area of the thrombus, leading to a decrease in the required high systemic dose of tPA, and consequently lowers the possibilities of hemorrhage. The gas encapsulated into t-ELIP will exert a cavitation-related mechanism, as explained earlier, leading to more lytic effect against the thrombus.78–80 In a study aimed at evaluation of the effect of exposing tPA-loaded ELIP to ultrasound in thrombolysis, Laing et al. found that ELIP-induced thrombolysis improved by 49.5% when ultrasound was added to the treatment protocol.77 This was in agreement with what Shaw and his team found when they compared the thrombolytic efficacy of tPA alone with that of tPA with ultrasound (120 kHz), and t-ELIP with t-ELIP exposed to ultrasound.81 It was found that ultrasound enhanced the efficacy of both tPA (from 31% alone to 71% with ultrasound) and t-ELIP (from 48% alone to 89% with ultrasound) and the study confirmed that t-ELIP can be used as an effective delivery system for tPA.81
In conclusion, contrast agents can enhance ultrasound-induced thrombolysis. Further modifications of MBs and echogenic liposomes, as well as the trials of targeting MBs82,83 and echogenic liposomes,84 may lead to the introduction of new efficient thrombolytic strategies that could be applied at the clinical level.
Anti-fibrin antibody targeting method
One of the main strategies of targeting tPA, or enhancing the local effect of tPA on thrombus, is the use of anti-fibrin antibody targeting.85,86 Yang and his co-workers designed an approach87–90 and called it “ATTEMPTS” (Antibody Targeted Triggered Electrically Modified Prodrug Type Strategy). It was designed to deliver tPA to the clot site in an inactive form, followed by triggering its activation locally, and consequently reducing the risk of bleeding.
This system depends on tight reversible electrostatic interaction between the targeting moiety in the form of an anionic heparin molecule (Hep) conjugated to an anti-fibrin antibody (Ab), and the drug moiety in the form of a tPA molecule decorated by a cationic peptide.
The antibody plays a vital role in targeting the system to the site of thrombus. The heparin-antibody conjugate blocks the active site of tPA, inhibiting its anti-fibrinolytic effect. Then, to activate this system, protamine sulfate is administered as an antagonist for heparin and triggers the dissociation of the Hep-antibody complex, resulting in the release of tPA from the inhibitory action of this complex.
This approach was tested experimentally in-vitro and in-vivo, and did not elicit any considerable degradation of the coagulation factors when compared to free tPA.87,91
In 2005, Yang and his group presented a new strategy to overcome the problem of random results of modification tPA with cationic peptide.90 They used a genetic engineering approach of site-directed mutagenesis to develop an inherent region with surface expressing positive charges to trigger binding with heparin.90 This led to a safe strategy for enhancing thrombolysis.
Camouflaged-tPA delivery approach
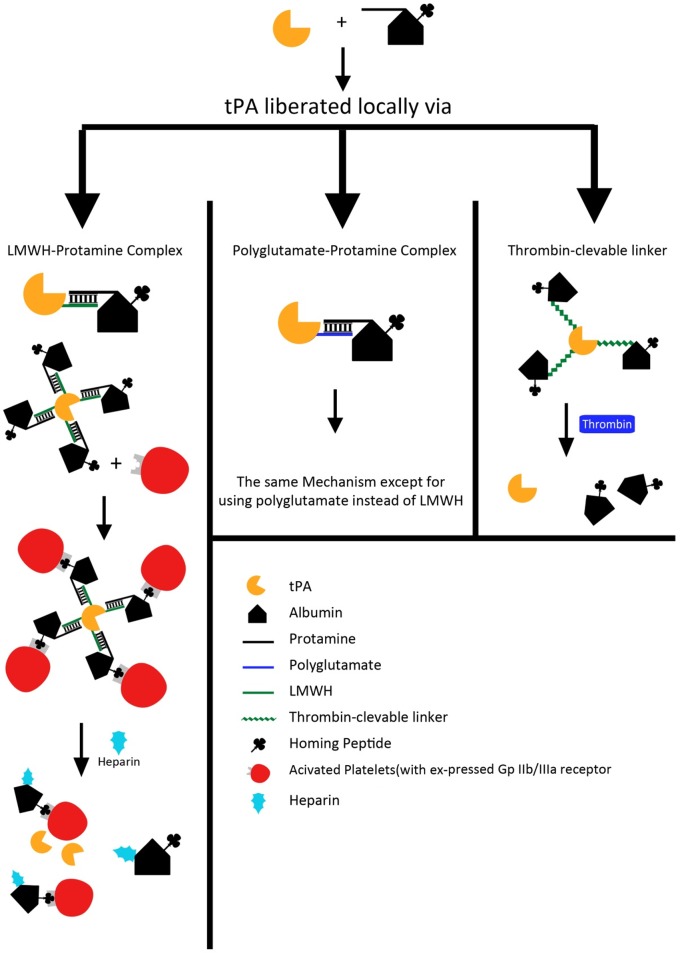
In a number of studies,92–94 Absar et al. tried to introduce an efficient delivery system for tPA. Camouflaged-tPA was produced by conjugating tPA with low molecular weight heparin (LMWH) followed by complexion of this conjugate with albumin-protamine.92 The administration of heparin with tPA led to 71% clot lysis, which is more effective than tPA alone (52% clot lysis). However, using heparin with tPA increased the bleeding risk, which led to a two-fold increase in activated partial thromboplastin time (aPTT). In a contrary situation, albumin-camouflaged heparin triggered strategy achieved a similar clot lysis activity (70%) compared to tPA plus heparin administration, but with no prolongation of aPTT after 1 hour of treatment. This indicates that the camouflaged-tPA can be applied for targeted thrombolysis with a reduced risk of hemorrhage.92 Based on the fact that LMWH is a therapeutically active molecule, Absar et al. in another study93, have tested a relatively inert negatively-charged compound to synthesize oligoanion-modified tPA to avoid any potential side effects. The tPA was conjugated to polyglutamate, and separately the human serum albumin (HSA) was conjugated to protamine. The conjugation of tPA with polyglutamate forms a reversible electrostatic complex that could be disrupted by negatively charged heparin via competitive binding. The electrostatic complex formation between polyglutamate and protamine can be reversed by heparin also. It was found that camouflaged-tPA heparin triggered delivery system demonstrated higher activity than the un-camouflaged tPA, where this higher activity may be attributed to the protection of camouflaged tPA from its macromolecular inhibitors. In another modification for the camouflaged-tPA delivery system, Absar and his colleagues94 have conjugated tPA with HSA via a thrombin-cleavable peptide or linker (GFPRGFPAGGC). In addition, the surface of albumin was decorated by the peptide sequence (CQQHHLGGAKQAGDV) of fibrinogen gamma-chain that has the affinity to bind with GPIIb/IIIa receptors, which are expressed on activated platelets. This conjugate showed an activity of 25%, which increased to about 86% of that of native tPA when the conjugate was incubated with thrombin. This approach can introduce an efficient delivery system for tPA working in an on/off triggered manner (Figure 4).
Figure 4.
An illustration of the various camouflaged-tPA delivery approaches. Adopted with modification from Absar et al.92–94
Microcarriers delivery system
Microcarriers (MC) have many advantages, such as offering a large volume for encapsulating drugs, and the possibility of co-encapsulation of magnetic nanomaterials to facilitate the control of microspheres against vascular flow.95 Torno and Kaminski compared the lysis effect of free tPA with that of tPA combined with magnetic microcarriers (MMC), and with that of tPA combined with MMC upon exposure to external magnetic field (MF). They also examined the lysis effect of tPA combined with MMC, exposed to both MF and ultrasound (US). They found that under static and no-flow conditions, thrombolysis efficiency improved by 1.7 and 2.7 fold for red and white clots, respectively in the case of (tPA+MMC+MF). Whereas under flow conditions, they reported a two-fold increase in lysis with a significant reduction in recanalization time by 7-fold. Furthermore, in the case of (tPA+MMC+MF+US), a maximal lysis efficiency of almost 98% was achieved for both red and white clots.96 Later, Kaminski et al.95 developed a new magnetic-targeting delivery system via encapsulating tPA in magnetic poly (lactic acid)-poly(ethylene glycol) (PLA-PEG) microcarriers. They reported that this delivery system has increased the overall activity of tPA by preserving its concentration up to 74 ug/ml, which is significantly higher than the concentration needed for efficient clot lysis (1–4 ug/ml).95
Nano-modulated delivery approaches
One of the first nano-modulated approaches is the use of tPA-loaded magnetic nanoparticles (MNPs). MNPs offer a good drug delivery system because of their composition and tailorability. MNPs are usually composed of a core made of iron oxide, Fe3O4, and a polymer coating. The core is responsible for the supermagnetic characteristics, which can be controlled by applying an external magnetic field, while the polymer coating is beneficial in increasing the stability and inhibiting the particles aggregation.97,98 In addition, being composed of iron oxide, the MNPs demonstrate very limited toxicity and high biocompatibility making their use safe and popular.99,100
In this context, Maet et al. studied the efficacy of magnetic targeted delivery of recombinant tPA (r-tPA) in a rat embolic model.101 A blood clot was produced in vitro and then injected into the iliac artery. Then, a permanent magnet was placed above the left iliac artery in order to manipulate the MNPs. It was found from the study that the intra-arterial infusion of the r-tPA significantly reversed the iliac flow within 15 minutes. Moreover, retention of MNPs against the hemodynamic dragging force in the iliac artery of the rat occurred. On the other hand, the MNPs retention was very limited in the absence of the magnetic field.
In another study using the same embolic rat model, Ma and his team have developed polyacrylic acid-coated MNPs to target r-tPA.102,103 Polyacrylic acid (PAA) was used in this study to stabilize MNPs because it produces electrostatic and steric repulsion preventing the particles aggregation. The study showed that static magnetic field produced by the external magnet with PAA-MNP-r-tPA didn't produce reversal in hemodynamics. On the other hand, applying the magnetic field periodically in an on/off way did the job, indicating that thrombolysis is achieved by mechanical dragging force created by movement of MNPs under the effect of external magnetic field. In conclusion, moving the external magnetic field led to suspension of MNPs and enhancing the penetration of r-tPA into the thrombus.102
In accordance with Ma's results,101–103 Chen et al.99 used chitosan coated-MNPs to deliver tPA. Chitosan is a hydrophilic polysaccharide obtained from chitin which is naturally extracted from the shells of shrimps and crabs. Chitosan has many beneficial characteristics, such as excellent biodegradability and low toxicity.104 Chen et al.99 found that clot lysis time was reduced when using chitosan-MNP-tPA in the presence of an applied external magnetic field by 58% compared to other runs without magnetic targeting, or by 53% compared to free tPA at the same dose of tPA (0.1 mg/ml).
The concept of on/off triggering of thrombolysis has been utilized in several approaches,92–94,102 and a novel ultrasound-responsive nano-delivery system for tPA has been developed.38,105,106 This system is composed of tPA complexed with cationic gelatin and PEG-gelatin. This composition depends on the electrostatic interaction between tPA, with its negative zeta potential, and a positively charged cationic gelatin leading to inactivation of tPA. Upon exposure of this composite to ultrasound, the tPA regains its activity. Upon administration of the delivery system without ultrasound, none of the animals showed recanalization. In the case of free tPA, however, half of the animals showed recanalization.38
Uesugi et al. modified this system by adding zinc ions to the complex of tPA and gelatin.105 It was found that zinc ions modified the resulting complex through coordinate and ionic bonds leading to greater suppression of tPA activity. Exposing this modified system to ultrasound at 1 MHz and 0.75 W/cm2 for 5 minutes, the system showed a 2-fold increase in tPA activity compared to the original level.105
Kawata and Uesgui et al. evaluated this modified system in vivo,106 where they found that the plasma tPA activity, determined immediately after injection of tPA-loaded nanoparticles, was almost 71.4% lower than after administration of the same dose of free tPA. On the other hand, the activity of nanoparticles recovered to a similar level to free tPA after being exposed to transcutaneous ultrasound for 5 minutes.
After 40 minutes the plasma tPA activity produced by nanoparticles after being exposed to ultrasound reached a level higher than that produced by free tPA.106 This approach gives hope for a safe drug delivery system for tPA, leading to reducing the risk of bleeding resulting from using high doses of free tPA.
Future prospective
Nanomedicine potentially opens up novel delivery approaches and new methods of targeting tPA to decrease the risk of bleeding. Shear-activated nanotherapeutics (SA-NTs) represent one of these novel approaches in controlled delivery and targeting of tPA.107 The mechanism of SA-NT relies on the fact that there is difference between normal blood vessels and stenosed or thrombosed one. As a result SA-NTs can be targeted to the site of thrombus in a size similar to that of platelets. Upon exposure to high local shear stress, they break up into nanosized particles. In addition, they are safe to be used as they are rapidly cleared (80% clearance in 5 minutes).107
Nanomedicine also allows for the development of “stealth” technology in which tPA is encapsulated in gelatin nanoparticles complexed with zinc ions.38,105,106 This opens up the possibility of developing protein therapeutics in vascular diseases, another promising field.
References
- 1.Schussheim AE, Fuster V. Thrombosis, antithrombotic agents, and the antithrombotic approach in cardiac disease. Prog Cardiovasc Dis. 1997;40(3):205–238. doi: 10.1016/s0033-0620(97)80035-7. [DOI] [PubMed] [Google Scholar]
- 2.Abbate R, Cioni G, Ricci I, Miranda M, Gori AM. Thrombosis and acute coronary syndrome. Thromb Res. 2012;129(3):235–240. doi: 10.1016/j.thromres.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Canon CP, Fuster V. Thrombogenesis, antithrombotic, and thrombolytic therapy. In: Fuster V, Alexander RW, O'Rourke R, editors. Hurst's The Heart. 10th ed. New York: McGraw Hill; 2001. pp. 1373–1386. [Google Scholar]
- 4.Eisenberg PR, Ghigliotti G. Platelet-dependent and procoagulant mechanisms in arterial thrombosis. Int J Cardiol. 1999;68(Suppl 1):S3–S10. doi: 10.1016/s0167-5273(98)00284-8. [DOI] [PubMed] [Google Scholar]
- 5.Verstraete M. Biology and chemistry of thrombosis. In: Haber E, Braunwald E, editors. Thrombolysis: Basic Contributions and Clinical Progress. Vol. 1. St Louis, MO: CV Mosby Year Book; 1991. p. 3. [Google Scholar]
- 6.Coller BS. The role of platelets in arterial thrombosis and the rationale for blockade of platelet GPIIb/IIIa receptors as antithrombotic therapy. Eur Heart J. 1995;16(Suppl L):11–15. doi: 10.1093/eurheartj/16.suppl_l.11. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen MC, Pride YB, Michael Gibson C. Chapter 36 Anticoagulation: Antithrombin therapy. In: Jeremias A, Brown DL, editors. Cardiac Intensive Care. Philadelphia, PA: ELSEVIER; 2010. pp. 443–451. [Google Scholar]
- 8.Colman RW, Marder VJ, Salzman EW, Hirsh J. Overview of hemostasis. In: Colman RW, Hirsh J, Marder VJ, Salzman EW, editors. Hemostasis and Thrombosis; Basic Principles and Clinical Practice. 3rd ed. Philadelphia, PA: JB Lippincott; 1994. pp. 3–18. [Google Scholar]
- 9.Kleindorfer D, Lindsell C, Brass L, Koroshetz W, Broderick J. National US estimates of recombinant tissue plasminogen activator use. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 10.Machado L, Sazonova I, Kozak A, Wiley D, El-Remessy A, Ergul A, Hess D, Waller J, Fagan S. Minocycline and tissue-type plasminogen activator for stroke: Assessment of interaction potential. Stroke. 2009;40:3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BJ. Thrombolysis in acute myocardial infarction: Analysis of studies comparing accelerated t-PA and streptokinase. J Accid Emerg Med. 1999;16(6):407–411. doi: 10.1136/emj.16.6.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baruah DB, Dash RN, Chaudhari MR, Kadam SS. Plasminogen activators: A comparison. Vascul Pharmacol. 2006;44:1–9. doi: 10.1016/j.vph.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Maizel AS, Bookstein JJ. Streptokinase, urokinase, and tissue plasminogen activator: Pharmacokinetics, relative advantages, and methods for maximizing rates and consistency of lysis. Cardiovasc Intervent Radiol. 1986;9:234–244. doi: 10.1007/BF02577952. [DOI] [PubMed] [Google Scholar]
- 14.Vyas SP, Vaidya B. Targeted delivery of thrombolytic agents: Role of integrin receptors. Exp Opin Drug Deliv. 2009;6:499–508. doi: 10.1517/17425240902878002. [DOI] [PubMed] [Google Scholar]
- 15.Huber K, Runge MS, Bode C, Gulba D. Thrombolytic therapy in acute myocardial infarction. Ann Hematol. 1996;73:S29–S38. [PubMed] [Google Scholar]
- 16.Stricker RB, Wong D, Shiu DT, Reyes PT, Shuman MA. Activation of plasminogen by tissue plasminogen activator on normal and thromb-asthenic platelets: Effects on surface proteins and platelet aggregation. Blood. 1986;68:275–280. [PubMed] [Google Scholar]
- 17.Vaidya B, Nayak MK, Dash D, Agrawal GP, Vyas SP. Development and characterization of site specific target sensitive liposomes for the delivery of thrombolytic agents. Int J Pharm. 2011;403(1-2):254–261. doi: 10.1016/j.ijpharm.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Allison SD. Liposomal drug delivery. J Infus Nurs. 2007;30(2):89–95. doi: 10.1097/01.NAN.0000264712.26219.67. quiz 120. [DOI] [PubMed] [Google Scholar]
- 19.Huang SL. Liposomes in ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60(10):1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Nii T, Ishii F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int J Pharm. 2005;298(1):198–205. doi: 10.1016/j.ijpharm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Psarros C, Lee R, Margaritis M, Antoniades C. Nanomedicine for the prevention, treatment and imaging of atherosclerosis. Nanomedicine. 2012;8(Suppl 1):S59–S68. doi: 10.1016/j.nano.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Torchilin VP. Targeting of drugs and drug carriers within the cardiovascular system. Adv Drug Deliv Rev. 1995;17:75–101. [Google Scholar]
- 23.Heeremans JL, Gerritsen HR, Meusen SP, Mijnheer FW, Gangaram Panday RS, Prevost R, Kluft C, Crommelin DJ. The preparation of tissue-type plasminogen activator (t-PA) containing liposomes: Entrapment efficiency and ultracentrifugation damage. J Drug Target. 1995;3(4):301–310. doi: 10.3109/10611869509015959. [DOI] [PubMed] [Google Scholar]
- 24.Heeremans JL, Prevost R, Bekkers ME, Los P, Emeis JJ, Kluft C, Crommelin DJ. Thrombolytic treatment with tissue-type plasminogen activator (t-PA) containing liposomes in rabbits: A comparison with free t-PA. Thromb Haemost. 1995;73:488–494. [PubMed] [Google Scholar]
- 25.Kim JY, Kim JK, Park JS, Byun Y, Kim CK. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials. 2009;30(29):5751–5765. doi: 10.1016/j.biomaterials.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Christian DA, Cai S, Bowen DM, Kim Y, Pajerowski JD, Discher DE. Polymersome carriers: From self-assembly to siRNA and protein therapeutics. Eur J Pharm Biopharm. 2009;71:463–474. doi: 10.1016/j.ejpb.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 28.Bergstrand N. Liposomes for drug delivery: From physico-chemical studies to applications (PhD thesis) Sweden: Uppsala University; 2003. [Google Scholar]
- 29.Nikolova AN, Jones MN. Effect of grafted PEG-2000 on the size and permeability of vesicles. Biochim Biophys Acta. 1996;1304:120–128. doi: 10.1016/s0005-2760(96)00112-9. [DOI] [PubMed] [Google Scholar]
- 30.Levchenko TS, Hartner WC, Torchilin VP. Methodist Debakey Cardiovasc J. 2012;8(1):36–41. doi: 10.14797/mdcj-8-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Absar S, Nahar K, Kwon YM, Ahsan F. Thrombus-targeted nanocarrier attenuates bleeding complications associated with conventional thrombolytic therapy. Pharm Res. 2013;30(6):1663–1676. doi: 10.1007/s11095-013-1011-x. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya B, Agrawal GP, Vyas SP. Functionalized carriers for the improved delivery of plasminogen activators. Int J Pharm. 2012;424(1-2):1–11. doi: 10.1016/j.ijpharm.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 33.Trübestein G, Engel C, Etzel F, Sobbe A, Cremer H, Stumpff U. Thrombolysis by ultrasound. Clin Sci Mol Med. 1976;3:697–698. doi: 10.1042/cs051697s. [DOI] [PubMed] [Google Scholar]
- 34.Tachibana K, Tachibana S. Ultrasonic vibration for boosting fibrinolytic effects of urokinase in vivo. Thromb Haemost. 1981;46:211. [Google Scholar]
- 35.Cintas P, Le Traon AP, Larrue V. High rate of recanalization of middle cerebral artery occlusion during 2-MHz transcranial color-coded Doppler continuous monitoring without thrombolytic drug. Stroke. 2002;33:626–628. doi: 10.1161/hs0202.103073. [DOI] [PubMed] [Google Scholar]
- 36.Rosenschein U, Bernstein JJ, DiSegni E, Kaplinsky E, Bernheim J, Rozenzsajn LA. Experimental ultrasonic angioplasty: Disruption of atherosclerotic plaques and thrombi in vitro and arterial recanalization in vivo. J Am Coll Cardiol. 1990;15:711–717. doi: 10.1016/0735-1097(90)90651-5. [DOI] [PubMed] [Google Scholar]
- 37.Ariani M, Fishbein MC, Chae JS, Sadeghi H, DonMichael A, Dubin SB, Siegel RJ. Dissolution of peripheral arterial thrombi by ultrasound. Circulation. 1991;84:1680–1688. doi: 10.1161/01.cir.84.4.1680. [DOI] [PubMed] [Google Scholar]
- 38.Uesugi Y, Kawata H, Jo J, Saito Y, Tabata Y. An ultrasound-responsive nano delivery system of tissue-type plasminogen activator for thrombolytic therapy. J Control Release. 2010;147:269–277. doi: 10.1016/j.jconrel.2010.07.127. [DOI] [PubMed] [Google Scholar]
- 39.Laing ST, Moody MR, Kim H, Smulevitz B, Huang SL, Holland CK, McPherson DD, Klegerman ME. Thrombolytic efficacy of tissue plasminogen activator-loaded echogenic liposomes in a rabbit thrombus model. Thromb Res. 2012;130:629–635. doi: 10.1016/j.thromres.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hitchcock KE, Caudell DN, Sutton,M JT, Klegerman E, Vela D, Pyne-Geithman GJ, Abruzzo T, Cyr PE, Geng YJ, McPherson DD, Holland CK. Ultrasound-enhanced delivery of targeted echogenic liposomes in a novel ex vivo mouse aorta model. J Control Release. 2010;144:288–295. doi: 10.1016/j.jconrel.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francis CW. Ultrasound-enhanced thrombolysis. Echocardiography. 2001;18(3):239–246. doi: 10.1046/j.1540-8175.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- 42.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqi F, Blinc A, Braaten J, Francis CW. Ultrasound increases flow through fibrin gels. Thromb Haemost. 1995;73:495–498. [PubMed] [Google Scholar]
- 44.Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol. 2000;26(7):1153–1160. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 45.Nyborg WL, Ziskin MC.Biological Effects of Ultrasound NewYork: Churchill Livingstone; 1985, P 1–33. [Google Scholar]
- 46.Alexandrov AV. Ultrasound enhanced thrombolysis for stroke. Int J Stroke. 2006;1(1):26–29. doi: 10.1111/j.1747-4949.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- 47.Rosenschein U, Frimerman A, Laniado S, Miller HI. Study of the mechanism of ultrasound angioplasty from human thrombi and bovine aorta. Am J Cardiol. 1994;74(12):1263–1266. doi: 10.1016/0002-9149(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 48.Williams AR, Chater BV, Allen KA, Sherwood MR, Sanderson JH. Release of beta-thromboglobulin from human platelets by therapeutic intensities of ultrasound. Br J Haematol. 1978;40(1):133–142. doi: 10.1111/j.1365-2141.1978.tb03647.x. [DOI] [PubMed] [Google Scholar]
- 49.Kornowski R, Meltzer RS, Chernine A, Vered Z, Battler A. Does external ultrasound accelerate thrombolysis? Results from a rabbit model. Circulation. 1994;89(1):339–344. doi: 10.1161/01.cir.89.1.339. [DOI] [PubMed] [Google Scholar]
- 50.Kudo S, Furuhata H, Hara M, Maie K, Hamano K, Okamura T. Noninvasive thrombolysis with ultrasound. (abstract) Circulation. 1989;80(suppl. I):I–345. [Google Scholar]
- 51.Kudo S. Thrombolysis with ultrasound effect. Tokyo Jikeikai Med J. 1989;104:1005–1012. [Google Scholar]
- 52.Hamano K, Fujinaga T, Muto M, Yoshizawa S, Kudo S, Hara M, Okamura T, Furuhata H. Thrombolysis by transcutaneous ultrasonic irradiation. (abstract) Circulation. 1990;82(suppl III):III–309. [Google Scholar]
- 53.Kimura M, Iijima S, Kobayashi K, Furuhata H. Evaluation of the thrombolytic effect of tissue-type plasminogen activator with ultrasound irradiation: In vitro experiment involving assay of the fibrin degradation products from the clot. Biol Pharm Bull. 1994;17:126–130. doi: 10.1248/bpb.17.126. [DOI] [PubMed] [Google Scholar]
- 54.Akiyama M, Ishibashi T, Yamada T, Furuhata H. Low-frequency ultrasound penetrates the cranium and enhances thrombolysis in vitro. Neurosurgery. 1998;43:828–832. doi: 10.1097/00006123-199810000-00062. [DOI] [PubMed] [Google Scholar]
- 55.Porter TR. The utilization of ultrasound and microbubbles for therapy in acute coronary syndromes. Cardiovascular Res. 2009;83:636–642. doi: 10.1093/cvr/cvp206. [DOI] [PubMed] [Google Scholar]
- 56.Porter TR, Kricsfeld D, Lof J, Everbach EC, Xie F. Effectiveness of transcranial and transthoracic ultrasound and microbubbles in dissolving intravascular thrombi. J Ultrasound Med. 2001;20(12):1313–1325. doi: 10.7863/jum.2001.20.12.1313. [DOI] [PubMed] [Google Scholar]
- 57.Alexandrov AV, Demchuk AM, Felberg RA, Christou I, Barber PA, Burgin WS, Malkoff M, Wojner AW, Grotta JC. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored by 2-MHz transcranial Doppler monitoring. Stroke. 2000;31:610–614. doi: 10.1161/01.str.31.3.610. [DOI] [PubMed] [Google Scholar]
- 58.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moyé LA, Hill MD, Wojner AW, CLOTBUST Investigators Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 59.Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, Alvarez-Sabin J. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- 60.Eggers J, Koch B, Meyer K, Konig I, Seidel G. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53:797–800. doi: 10.1002/ana.10590. [DOI] [PubMed] [Google Scholar]
- 61.Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: Increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator. Stroke. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 62.De Jong N, Ten Cate FJ. Principles and recent developments in ultrasound contrast agents. Ultrasonics. 1991;29:324–330. doi: 10.1016/0041-624x(91)90030-c. [DOI] [PubMed] [Google Scholar]
- 63.Feinstein SB, Shah PM. Advances in contrast two-dimensional echocardiography. Cardiovasc Clin. 1986;17:95–102. [PubMed] [Google Scholar]
- 64.Burns PN. Ultrasound contrast agents in radiological diagnosis. Radiol Med (Torino) 1994;87:71–82. [PubMed] [Google Scholar]
- 65.Moehring MA, Klepper JR. Pulse Doppler ultrasound detection, characterization and size estimation of emboli in flowing blood. IEEE Trans Biomed Eng. 1994;41:35–44. doi: 10.1109/10.277269. [DOI] [PubMed] [Google Scholar]
- 66.Becker A, Marxer E, Brüßler J, Sophia Hoormann A, Kuhnt D, Bakowsky U, Nimsky C. Ultrasound active nanoscaled lipid formulations for thrombus lysis. Eur J Pharm Biopharm. 2011;77(3):424–429. doi: 10.1016/j.ejpb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabín J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37(2):425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 68.Dijkmans PA, Juffermans LJ, Musters RJ, van Wamel A, ten Cate FJ, van Gilst W, Visser CA, de Jong N, Kamp O. Microbubbles and ultrasound: From diagnosis to therapy. Eur J Echocardiogr. 2004;5(4):245–256. doi: 10.1016/j.euje.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Morgan KE, Allen JS, Dayton PA, Chomas JE, Klibaov AL, Ferrara KW. Experimental and theoretical evaluation of microbubble behavior: Effect of transmitted phase and bubble size. IEEE Trans Ultrason Ferroelectr Freq Control. 2000;47(6):1494–1509. doi: 10.1109/58.883539. [DOI] [PubMed] [Google Scholar]
- 70.Alter J, Sennoga CA, Lopes DM, Eckersley RJ, Wells DJ. Microbubble stability is a major determinant of the efficiency of ultrasound and microbubble mediated in vivo gene transfer. Ultrasound Med Biol. 2009;35(6):976–984. doi: 10.1016/j.ultrasmedbio.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 71.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Sidhu PS, Allan PL, Cattin F, Cosgrove DO, Davies AH, Do DD, Karakagil S, Langholz J, Legemate DA, Martegani A, Llull JB, Pezzoli C, Spinazzi A. Diagnostic efficacy of SonoVue®, a second generation contrast agent, in the assessment of extracranial carotid or peripheral arteries using colour and spectral Doppler ultrasound: A multicentre study. Br J Radiol. 2006;79:44–51. doi: 10.1259/bjr/23954854. [DOI] [PubMed] [Google Scholar]
- 73.Viguier A, Petit R, Rigal M, Cintas P, Larrue V. Continuous monitoring of middle cerebral artery recanalization with transcranial color-coded sonography and Levovist. J Thromb Thrombolysis. 2005;19:55–59. doi: 10.1007/s11239-005-0940-6. [DOI] [PubMed] [Google Scholar]
- 74.Alexandrov AV, Mikulik R, Ribo M, Sharma VK, Lao AY, Tsivgoulis G, Sugg RM, Barreto A, Sierzenski P, Malkoff MD, Grotta JC. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke. 2008;39:1464–1469. doi: 10.1161/STROKEAHA.107.505727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith DA, Vaidya SS, Kopechek JA, Huang SL, Klegerman ME, McPherson DD, Holland CK. Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med Biol. 2010;36(1):145–157. doi: 10.1016/j.ultrasmedbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang SL, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochim Biophys Acta. 2004;1665:134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119(6):777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith DAB, Porter TM, Martinez J, Huang S, MacDonald RC, McPherson DD, Holland CK. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med Biol. 2007;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 79.Smith DAB, Vaidya S, Kopechek JA, Hitchcock KE, Huang SL, McPherson DD, Holland CK. Echogenic liposomes loaded with recombinant tissue-type plasminogen activator (rt-PA) for image-guided, ultrasound-triggered drug release. J Acoust Soc Am. 2007;122:3007. [Google Scholar]
- 80.Gore JM, Sloan M, Price TR, Young Randall AM, Bovill E, Collen D, Forman S, Knatterud GL, Sopko G, Terrin ML. Intracerebral hemorrhage, cerebral infarction, and subdural hematoma after acute myocardial infarction and thrombolytic therapy in the thrombolysis in myocardial infarction study. Thrombolysis in myocardial infarction, Phase II, pilot and clinical trial. Circulation. 1991;83:448–459. doi: 10.1161/01.cir.83.2.448. [DOI] [PubMed] [Google Scholar]
- 81.Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res. 2009;124(3):306–310. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martina D, Alleiımann E, Bettinger T, Bussat P, Lassus A, Pochon S, Schneider M. Grafting of abciximab to a microbubble-based ultrasound contrast agent for targeting to platelets expressing GP IIb/IIIa—characterization and in vitro testing. Eur J Pharm Biopharm. 2008;68:555–564. doi: 10.1016/j.ejpb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Mu Y, Li L, Ayoufu G. Experimental study of the preparation of targeted microbubble contrast agents carrying urokinase and RGDS. Ultrasonics. 2009;49:676–681. doi: 10.1016/j.ultras.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Klegerman ME, Zou Y, Mcpherson DD. Fibrin targeting of echogenic liposomes with inactivated tissue plasminogen activator. J Liposome Res. 2008:95–112. doi: 10.1080/08982100802118482. [DOI] [PubMed] [Google Scholar]
- 85.Runge MS, Bode C, Matsueda GR, Haber E. Antibody-enhanced thrombolysis: Targeting of tissue plasminogen activator in vivo. Proc Natl Acad Sci U S A. 1987;84(21):7659–7662. doi: 10.1073/pnas.84.21.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marsh JN, Senpan A, Hu G, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. Fibrin-targeted perfluorocarbon nanoparticles for targeted thrombolysis. Nanomedicine (Lond) 2007;2(4):533–543. doi: 10.2217/17435889.2.4.533. [DOI] [PubMed] [Google Scholar]
- 87.Liang J-F, Li Y, Connell ME, Yang VC. Synthesis and characterization of positively charged tPA as a prodrug using a heparin/protamine-based drug delivery system. AAPS Pharmsci. 2000;2(1):E7. doi: 10.1208/ps020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang J-F, Li Y, Song H, Park Y-J, Naik SS, Yang VC. ATTEMPTS: A heparin/protamine-based delivery system for enzyme drugs. J Control Release. 2002;78:67–79. doi: 10.1016/s0168-3659(01)00484-9. [DOI] [PubMed] [Google Scholar]
- 89.Park Y-J, Liang J-F, Song H, Li Y, Naik SS, Yang VC. ATTEMPTS: A heparin/protamine-based triggered release system for the delivery of enzyme drugs without associated side-effects. Adv Drug Delivery Rev. 2003;55:251–265. doi: 10.1016/s0169-409x(02)00181-3. [DOI] [PubMed] [Google Scholar]
- 90.Yang VC, Naik SS, Song H, Dombkowski AA, Crippen G, Liang JF. Construction and characterization of a t-PA mutant for use in ATTEMPTS: A drug delivery system for achieving targeted thrombolysis. J Control Release. 2005;110(1):164–176. doi: 10.1016/j.jconrel.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 91.Naik SS, Liang J-F, Park Y-J, Lee W-K, Yang VC. Application of ATTEMPTS for drug delivery. J Contr Rel. 2005;10:34–45. doi: 10.1016/j.jconrel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 92.Absar S, Choi S, Yang VC, Kwon YM. Heparin-triggered release of camouflaged tissue plasminogen activator for targeted thrombolysis. J Control Release. 2012;157(1):46–54. doi: 10.1016/j.jconrel.2011.09.060. [DOI] [PubMed] [Google Scholar]
- 93.Absar S, Choi S, Ahsan F, Cobos E, Yang VC, Kwon YM. Preparation and characterization of anionic oligopeptide-modified tissue plasminogen activator for triggered delivery: An approach for localized thrombolysis. Thromb Res. 2013;131(3):e91–e99. doi: 10.1016/j.thromres.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 94.Absar S, Kwon YM, Ahsan F. Bio-responsive delivery of tissue plasminogen activator for localized thrombolysis. J Control Release. 2014;177:42–50. doi: 10.1016/j.jconrel.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 95.Kaminski MD, Xie Y, Mertz CJ, Finck MR, Chen H, Rosengart AJ. Encapsulation and release of plasminogen activator from biodegradable magnetic microcarriers. Eur J Pharm Sci. 2008;35(1-2):96–103. doi: 10.1016/j.ejps.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 96.Torno MD, Kaminski MD, Xie Y, Meyers RE, Mertz CJ, Liu X, O'Brien WD, Jr, Rosengart AJ. Improvement of in vitro thrombolysis employing magnetically-guided microspheres. Thrombo Res. 2008;121:799–811. doi: 10.1016/j.thromres.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 97.Dobson J. Magnetic nanoparticles for drug delivery. Drug Dev Res. 2006;67:55–60. [Google Scholar]
- 98.Horak D, Babic M, Mackova H, Benes MJ. Preparation and properties of magnetic nano- and microsized particles for biological and environmental separations. J Sep Sci. 2007;30(1):1751–1772. doi: 10.1002/jssc.200700088. [DOI] [PubMed] [Google Scholar]
- 99.Chen JP, Yang PC, Ma Y-H, Wu T. Characterization of chitosan magnetic nanoparticles for in situ delivery of tissue plasminogen activator. Carbohy Polym. 2011;84:364–372. [Google Scholar]
- 100.Kempe M, Kempe H, Snowball I, Wallén R, Arza CR, Götberg M, Olsson T. The use of magnetite nanoparticles for implant-assisted magnetic drug targeting in thrombolytic therapy. Biomaterials. 2010;31(36):9499–9510. doi: 10.1016/j.biomaterials.2010.07.107. [DOI] [PubMed] [Google Scholar]
- 101.Ma Y-H, Hsu Y-W, Chang Y-J, Hua M-Y, Chen J-P, Wu T. Intra-arterial application of magnetic nanoparticles for targeted thrombolytic therapy: A rat embolic model. J Magn Magn Mater. 2007;311:342–346. [Google Scholar]
- 102.Ma YH, Wu SY, Wu T, Chang YJ, Hua MY, Chen JP. Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials. 2009;30(19):3343–3351. doi: 10.1016/j.biomaterials.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 103.Lin CL, Lee CF, Chiu WY. Preparation and properties of poly(acrylic acid) oligomer stabilized superparamagnetic ferrofluid. J Colloid Interface Sci. 2005;291(2):411–420. doi: 10.1016/j.jcis.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 104.Ravi Kumar MNV. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27. [Google Scholar]
- 105.Uesugi Y, Kawata H, Saito Y, Tabata Y. Ultrasound-responsive thrombus treatment with zinc-stabilized gelatin nano-complexes of tissue-type plasminogen activator. J Drug Target. 2012;20(3):224–234. doi: 10.3109/1061186X.2011.633259. [DOI] [PubMed] [Google Scholar]
- 106.Kawata H, Uesugi Y, Soeda T, Takemoto Y, Sung JH, Umaki K, Kato K, Ogiwara K, Nogami K, Ishigami K, Horii M, Uemura S, Shima M, Tabata Y, Saito Y. A new drug delivery system for intravenous coronary thrombolysis with thrombus targeting and stealth activity recoverable by ultrasound. J Am Coll Cardiol. 2012;60(24):2550–2557. doi: 10.1016/j.jacc.2012.08.1008. [DOI] [PubMed] [Google Scholar]
- 107.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, Feldman CL, Wagner DD, Ingber DE. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337(6095):738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]