Abstract
Human milk provides crucial nutrition and immunologic protection for infants. When a mother's own milk is unavailable, donated human milk, pasteurized to destroy bacteria and viruses, is a lifesaving replacement. Flash-heat pasteurization is a simple, low-cost, and commonly used method to make milk safe, but currently there is no system to monitor milk temperature, which challenges quality control. FoneAstra, a smartphone-based mobile pasteurization monitor, removes this barrier by guiding users through pasteurization and documenting consistent and safe practice. This study evaluated FoneAstra's efficacy as a quality control system, particularly in resource-limited settings, by comparing bacterial growth in donor milk flash-heated with and without the device at a neonatal intensive care unit in Durban, South Africa.
Materials and Methods: For 100 samples of donor milk, one aliquot each of prepasteurized milk, milk flash-heated without FoneAstra, and milk pasteurized with FoneAstra was cultured on routine agar for bacterial growth. Isolated bacteria were identified and enumerated.
Results: In total, 300 samples (three from each donor sample) were analyzed. Bacterial growth was found in 86 of the 100 samples before any pasteurization and one of the 100 postpasteurized samples without FoneAstra. None of the samples pasteurized using FoneAstra showed bacterial growth.
Conclusions: Both pasteurization methods were safe and effective. FoneAstra, however, provides the additional benefits of user-guided temperature monitoring and data tracking. By improving quality assurance and standardizing the pasteurization process, FoneAstra can support wide-scale implementation of human milk banks in resource-limited settings, increasing access and saving lives.
Introduction
Human breastmilk is considered a pillar of child survival and is the optimal source of nutrition and immunologic protection for all infants.1 It is of even greater importance to vulnerable infants, including those who are preterm, low-birth-weight, severely malnourished, human immunodeficiency virus (HIV)-infected or -exposed, or orphaned. Human milk banks (HMBs) provide safe donated human milk to these babies.
HMBs have been shown to improve infant health and survival and reduce the cost burden on healthcare systems.2–6 Preterm and low-birth-weight infants in particular are susceptible to hospital-acquired infections, most commonly necrotizing enterocolitis (NEC).2 A systematic review and meta-analysis concluded that donor breastmilk compared with formula milk is associated with a lower risk of NEC or sepsis among preterm or low-birth-weight infants by up to 31%.7,8 In light of the impact that human milk can have on infant health outcomes, the World Health Organization has called for countries to investigate the safe use of donor milk through HMBs for vulnerable infants as a risk-reduction strategy.9
South Africa is one of only 12 countries where infant mortality is rising. It also has one of the world's lowest rates of exclusive breastfeeding (8% for babies <6 months of age).3 In response, South Africa's infant feeding policy was changed in August 2011 to promote exclusive breastfeeding as the primary feeding strategy for all South African mothers, including those living with HIV.3 The Ministry of Health also recommends that all hospitals with neonatal intensive care units (NICUs) establish HMBs to provide donor milk to infants when the mother cannot breastfeed, is severely ill, or has died.
Human milk is a complex, nonsterile fluid. It contains numerous commensal bacteria, which colonize the infant's intestine and protect against overcolonization with other, pathogenic bacteria. Human milk can also contain pathogenic bacteria, such as Staphylococcus aureus and Escherichia coli. If milk with these bacteria is fed to a mother's own infant, the presence of antibodies and bacteriostatic components generally prevent it from harming that child.10,11 However, donated milk with such bacteria can present a danger if consumed by other infants. For this reason and because human milk can contain viruses or other contaminants, international standards require milk to be pasteurized to destroy bacteria and other pathogens.
Although pasteurization and quality control are crucial to HMB safety and milk quality assurance, they can place a burden on small HMBs or those in low-resource settings. Low-cost systems for quality assurance are vital to the safe scale-up of HMBs worldwide.
The criteria for safe donor milk, as specified by the United Kingdom's National Institute for Health and Care Excellence, are prepasteurization counts per milliliter of <1×105 colony-forming units (cfu) for total viable organisms, <1×104 cfu of Enterobacteriaceae, or 104 cfu for S. aureus.12 The current gold standard for pasteurizing breastmilk is the use of a commercial-grade pasteurizer, which uses the Holder pasteurization method.12 The high cost of these pasteurizers is often a barrier to the establishment of HMBs.13
Flash-heat pasteurization is a simple, low-cost method recently proposed for use in HMBs.14 It was designed to approximate commercial high-temperature, short-time heat treatments, which use specialized, expensive equipment to heat milk to 72°C for 15 seconds. High-temperature, short-time methods are considered to be superior because they can kill pathogens while retaining more of the protective elements of human milk than other pasteurization methods.11,15,16 Flash-heat uses common household utensils (a sauce pan and a glass jar) to achieve a similar process. Previously, it was adapted to allow HIV-positive mothers to pasteurize their own milk, in their own homes, to help prevent mother-to-child transmission of HIV.17 Flash-heat was previously shown to eliminate pathogenic and nonpathogenic bacteria, as well as to inactivate both cell-free and cell-associated HIV-116 in breastmilk18,19 without diminishing the bacteriostatic activity of breastmilk,10 and the treated breastmilk can be stored for 8 hours without refrigeration without a significant increase of bacteria.11
Although flash-heating has been proven safe in laboratory and field tests, healthcare workers in low-resource settings find it challenging to monitor milk temperatures. Fear of over- or underheating breastmilk undermines workers' confidence in the method. Additionally, it is crucial for health workers to discern and record whether milk has reached the appropriate temperature (72°C), but limited monitoring and quality control at HMBs using flash-heat have made it difficult for advocates to promote its use.
To address this challenge, Chaudhri et al.13 developed FoneAstra, a simple, low-cost, smartphone-based temperature monitoring system to improve the safety and consistency of pasteurization. FoneAstra monitors the temperature of milk at each step of flash-heating and provides continuous audiovisual feedback to guide workers through the pasteurization process. It also sends temperature and time data to a central server, where it can be used to generate reports or archived (see www.hmbasa.org.za/links.html for more information).
The objective of this study was to evaluate FoneAstra's safety and effectiveness as a quality control system, particularly in resource-limited settings, by comparing bacterial growth in donor milk flash-heated with and without the system in a South African hospital.
Materials and Methods
The study was conducted at the King Edward VIII Hospital (KEH) NICU in Durban, South Africa. Breastmilk was obtained from donors who were HIV-negative breastfeeding mothers. These mothers were screened as donor milk providers according to the South African standard human milk banking guidelines.20 As part of routine HMB practice, all mothers gave written consent that samples of their breastmilk could be tested for bacterial contamination and measured for nutritional quality. The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (approval number BE009/12).
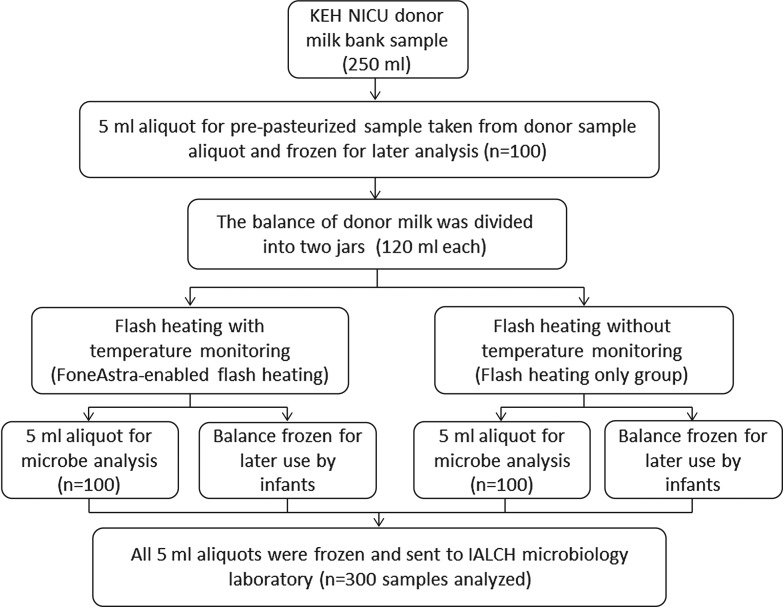
The KEH NICU donor milk bank collected 100 portions of donor milk between June 2012 and November 2012 from mothers in the KEH NICU (n=30), from another nearby NICU (n=36), and from mothers in the community who donated milk to the hospital (n=34). Figure 1 describes the sampling and collection process performed on each sample (portion) of donor milk.
FIG. 1.
Flow diagram showing sample handling and allocation from each sample of donor milk (n=100). IALCH, Inkosi Albert Luthuli Central Hospital; KEH NICU, King Edward VIII Hospital neonatal intensive care unit.
Pasteurization methods
Traditional flash-heating without FoneAstra
A sample of donor human milk (120 mL) in an uncovered glass food jar was placed in approximately 450 mL of water in a 1.5-L pan. The water and milk were heated together over a low-cost induction stove purchased in South Africa until the water reached a rolling boil. The jar was removed from the water, and a 5-mL aliquot was extracted, placed in a sterile tube, and frozen for later microbiological analysis.
Flash-heating with the FoneAstra pasteurization monitoring system
A sample of donor human milk (120 mL) was placed in an uncovered glass food-grade jar. Additionally, the FoneAstra temperature probe was placed into a jar with 120 mL of water, providing a temperature control while avoiding contact with donor milk. FoneAstra is configured to correct the water temperature readings to align with milk temperatures, similar to automated pasteurization system water controls. Both jars were placed in a customized stand and then into approximately 450 mL of water in the same 1.5-L pan used for the traditional flash-heating. FoneAstra's software program continuously monitored and stored the temperature of milk as it was being heated. After the milk reached 72°C for 15 seconds, a message on the cell phone prompted the user to remove the milk from the hot water bath and cool it by placing it in a cold water bath. The pasteurization process was considered complete when the temperature reached 25°C in the water control jar. The jar of breastmilk was then removed from the cold water bath, and a 5-mL aliquot was extracted, placed in a sterile tube, and frozen for later microbiological analysis.
Laboratory methodology and data collection
Frozen samples were transported in batches to the National Health Laboratory Services Microbiology Laboratory at the Inkosi Albert Luthuli Central Hospital in Durban for microbiological analysis. Samples were defrosted at room temperature for 30–60 minutes before processing as follows:
A sterilized 10-μL precooled loop was immersed in the breastmilk. The contents of the loop were transferred aseptically onto a colistin–nalidixic acid plate and streaked out. The process was repeated aseptically for a MacConkey's agar plate. The colistin–nalidixic acid agar plates were incubated at 37°C in a CO2 incubator for 18 hours, whereas the MacConkey's agar plates were incubated at 37°C aerobically for 24 hours. Any growth on the colistin–nalidixic acid plates was indicative of Gram-positive bacteria, and growth on the MacConkey's agar plates was indicative of Gram-negative bacteria. A semiquantitative count was performed on all positive cultures, which, if required, were then further identified using conventional laboratory tests. Colony counts were recorded as follows: 1–10 colonies, equivalent to <104 organisms/mL; 11–100 colonies, equivalent to 104–105 organisms/mL; and >100 colonies, equivalent to >105 organisms/mL. The culture results were interpreted, taking into account the bacterial count and number of morphological microbial types present. S. aureus and coagulase-negative Staphylococcus were differentiated using mannitol salt agar and DNA plates. A cefoxitin (30-μg) disk was used to differentiate between a susceptible S. aureus and a methicillin-resistant S. aureus. The Gram-negative organisms were identified using API® 20E, API 20NE, or Vitek® 2 machine, using an identification card.
Results
Prepasteurized samples
Bacterial growth was found in 86 of the 100 prepasteurized samples (86%). Overall, 138 organisms were identified; 94 were Gram-positive (68%) and the remaining 44 were Gram-negative organisms (32%).
Seven of the prepasteurized samples had >105 cfu/mL of total viable microorganisms. According to international guidelines,12 this level of contamination is unacceptably high; in many HMBs in high-resource countries this milk would be discarded.
The organism most commonly isolated in the prepasteurized samples was Staphylococcus species. A Staphylococcus species was present in 76 (55.1%) of the 86 samples; in 49 (57%) it was the only organism found. Additional findings are in Table 1.
Table 1.
Gram-Positive Organisms Isolated in Prepasteurized Samples
| Organism | Number of samples | Percentage of total organisms isolated |
|---|---|---|
| Staphylococcus species | 76 | 55.1 |
| S. aureus | 5 | 3.6 |
| E. faecalis | 9 | 6.5 |
| α-Hemolytic streptococci | 4 | 2.9 |
Various Gram-negative organisms, including environmental Gram-negative organisms, were also isolated. Small numbers of non–lactose fermenters were isolated in 2% of total samples; neither of the identification systems (API or Vitek 2 machine) could provide confirmatory identifications (Table 2).
Table 2.
Gram-Negative Organisms Isolated in Prepasteurized Samples
| Organism | Number of samples | Percentage of samples with isolated organisms |
|---|---|---|
| Total Pseudomonas species | 8 | 5.8 |
| P. aeruginosa | 6 | 4.3 |
| P. putida | 2 | 1.5 |
| Total K. pneumoniae | 5 | 3.6 |
| Total E. coli | 1 | 0.7 |
| Total Enterobacter species | 8 | 5.8 |
| E. cloacae | 7 | 5.1 |
| E. asburiae | 1 | 0.7 |
| Total S. marcescens | 8 | 5.8 |
| Total B. diminuta | 1 | 0.7 |
| Total environmental organisms | 13 | 9.4 |
| S. maltophilia | 2 | 1.4 |
| Acinetobacter species | 8 | 5.8 |
| S. paucimobilis | 1 | 0.7 |
| Non–lactose fermenters (not identified by API or Vitek machine) | 2 | 1.4 |
More than two organisms were found in 33 (38%) of the prepasteurized samples. Candida species was identified in one sample (0.7%). This sample had a mixed culture containing Stenotrophomonas maltophilia, Sphingomonas paucimobilis, and Brevundimonas diminuta.
Postpasteurization samples
Among the 100 samples pasteurized using the flash-heat method without the FoneAstra system, Staphylococcus species was identified in one (1%) postpasteurized sample, with the growth of <104 cfu/mL. The matching prepasteurized sample had Streptococcus viridans, Staphylococcus species, and Enterococcus faecalis, with total growth of >105 cfu/mL.
Among the 100 samples pasteurized using the flash-heat method with the FoneAstra system, no bacterial growth was observed.
Discussion
Previous studies7,8,21 have documented the impact of donor human milk on improving neonatal health outcomes. This study explored a simplified process for pasteurization to enable increased access of safe donor milk. The results of this study suggest that the FoneAstra pasteurization monitor is a safe, effective tool for flash-heat human milk pasteurization. Flash-heat pasteurization with FoneAstra completely destroyed all bacteria identified in prepasteurized samples. This study did not assess impact on neonatal health from use of FoneAstra-pasteurized donor milk.
One sample from traditional flash-heat pasteurization still contained Staphylococcus species. Investigation into this contamination revealed that it was the first sample processed by a new technician, suggesting that incorrectly performed pasteurization may have left some bacteria alive. This detail, as well as the presence of a potential pathogen, highlights the importance of temperature monitoring and the unique role FoneAstra, as a simple, user-friendly system, can play in increasing milk safety in any setting.
This study also found that 86% of prepasteurization samples had bacteria and that 38% of these had more than one organism. These findings are in agreement with the literature,22–24 demonstrating that expressed human milk can contain a wide variety of microbes. It has been shown that human milk from healthy women contains approximately 103–104 cfu/mL, representing a continuous source of potential commensal bacteria for the infant intestine.22 The exact mechanism by which bacteria reach the mammary gland continues to spark debate; the presence of microorganisms in human milk could represent pathogenic organisms as well as breast skin commensals or the infant's oral microbiota. It is assumed that infants acquire bacteria from the maternal intestinal and vaginal microbiota during birth and transfer these bacteria from the mouth to the breast skin and from there to the mammary gland during breastfeeding.22
Staphylococcus species, the most prevalent Gram-positive organism isolated, and α-hemolytic streptococci are considered to be commensal organisms in breastmilk. Several potential pathogens were identified in the prepasteurized milk samples, including S. aureus, E. faecalis, non–lactose fermenters, and Gram-negative organisms. Although some of the isolated Gram-negative organisms are considered to be environmental contaminants, often found in water, soil, and hospital surfaces, these organisms do have the potential to cause disease in humans.
There is considerable debate as to whether HMBs in resource-limited settings should conduct microbiological analyses of prepasteurization samples, given the large number of bacteria present in these samples and the cost of enumerating and identifying the organisms. In our 100 prepasteurization samples, 7% would have had to be discarded because they had >105 cfu/mL of total organisms. An additional 12% and 4% of samples, respectively, would have been discarded for contamination with Enterobacteriaceae (>105 cfu/mL of coliforms) and S. aureus (>104 cfu/mL). Using these criteria, 23% of the samples would have been discarded. This is a very large number, especially in South Africa, where high HIV prevalence rates (estimated at 12.3%)25 have resulted in a small pool of eligible donors. Therefore in South Africa, as in other countries, it is undesirable to discard so many samples when accurate, controlled pasteurization can render milk completely safe. For this reason HMBs in South Africa, following new Ministry of Health guidelines (in finalization as of August 2014), will soon only request microbiological analyses postpasteurization. Extra precautions and training are still needed to minimize contamination from environmental sources, encourage donor mothers to practice meticulous hand washing and general hygiene, and ensure that HMBs sterilize collection containers and breast pumps.
Conclusions
When a mother's milk isn't available, donor milk from HMBs can give vulnerable babies lifesaving nutrition and immunity. Without consistent and effective pasteurization, however, bacteria and pathogens in this milk can be harmful or even deadly to the very children it is meant to help.
Although HMBs successfully use flash-heat pasteurization to remove contaminants in milk, the method can be challenging to perform consistently, leaving babies at risk. Furthermore, current quality control systems can be expensive and time consuming, hindering the expansion of desperately needed HMBs, particularly in resource-limited settings. Ensuring that donor human milk is consistently safe and available worldwide requires reliable, low-cost pasteurization monitoring and reporting systems. FoneAstra answers this need by providing a portable, inexpensive, easy-to-use tool to guide and monitor the flash-heat process.
Today, there is a growing international call to safely expand HMBs to improve newborn care. Low-cost, trustworthy pasteurization monitoring systems are essential to protect and improve the lives of vulnerable infants worldwide.
Acknowledgments
The authors would like to acknowledge and thank the following: the human milk donors who provide their breastmilk for infants in need, and who provided samples for this research; Nosipho Dludla and Dr. Aletta Motene, managers of the KEH NICU HMB, for their assistance with obtaining human milk samples for the study, and Eshana Panday of the National Health Laboratory Service, for microbiological analyses of donor breastmilk samples; Penny Reimers and Elizabeth Simons, for their assistance with the pasteurization of donor breastmilk samples; Lenore Spies and the Nutrition Department of the KwaZulu-Natal Department of Health, for their cooperation and helpful comments throughout the study; and The College of Health Sciences, University of KwaZulu-Natal, for a Master scholarship granted to Mageshree Naicker for the study. We also thank the many funders who graciously supported this work: the Bill & Melinda Gates Foundation, through the Grand Challenge Explorations Initiative; the University of Washington Department of Computer Science and Engineering; private foundations and individuals of the PATH Health Innovation Portfolio for donations; and the National Science Foundation, for research grant 11S-1111433.
Disclosure Statement
No competing financial interests exist.
All authors contributed toward the planning and design of the study. M.N. supervised all microbiological culture work and analyzed the data. M.N. and A.C. wrote the first draft of the article; the other authors contributed editing and approved the final manuscript.
References
- 1.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: A pooled analysis. Lancet 2000;355:451–455 [PubMed] [Google Scholar]
- 2.Wight NE. Donor human milk versus formula for preventing necrotising enterocolitis in preterm infants: Systematic review. J Pediatr 2003;143:137–138 [PubMed] [Google Scholar]
- 3.South Africa: Policy Turnaround on Breastfeeding. IRIN News. August26, 2011. Available at www.irinnews.org/report/93600/south-africa-policy-turnaround-on-breastfeeding (accessed January27, 2015)
- 4.Wight NE. Donor human milk for preterm infants. J Perinatol 2001;21:249–254 [DOI] [PubMed] [Google Scholar]
- 5.Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: Systematic review and meta-analysis. Arch Dis Child Fetal Neonatal 2007;92:F169–F175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams AF, Kingdon CC, Weaver G. Banking for the future: Investing in human milk. Arch Dis Child Fetal Neonatal 2007;92:F158–F159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley MA, Henderson G, Anthony MY, et al. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2007;(4):CD002971. [DOI] [PubMed] [Google Scholar]
- 8.Updegrove K. Necrotizing enterocolitis: The evidence for use of human milk in prevention and treatment. J Hum Lact 2004;20:335–339 [DOI] [PubMed] [Google Scholar]
- 9.Arnold LD. Global health policies that support the use of banked donor human milk: A human rights issue. Int Breastfeed J 2006;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chantry CJ, Israel-Ballard K, Moldoveanu Z, et al. Effect of flash-heat treatment on immunoglobulins in breast milk. J Acquir Immune Defic Syndr 2009;51:264–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Israel-Ballard K, Coutsoudis A, Chantry CJ, et al. Bacterial safety of flash-heated and unheated expressed breastmilk during storage. J Trop Pediatr 2006;52:399–405 [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence (NICE). Donor Milk Banks: The Operation of Donor Milk Bank Services. NICE Clinical Guideline 93. February2010. Available at www.nice.org.uk/guidance/cg93 (accessed January27, 2015) [PubMed]
- 13.Chaudri RV, Kaza J, Palludan J, et al. A system for safe flash-heat pasteurization of human breast milk. Presented at the 5th ACM Workshop on Networked Systems for Developing Regions, 2011, Bethesda, MD Available at http://dl.acm.org/citation.cfm?id=2442887 (accessed January27, 2015) [Google Scholar]
- 14.Coutsoudis I, Adhikari M, Nair N, et al. Feasibility and safety of setting up a donor breastmilk bank in a neonatal prem unit in a resource-limited setting: An observational, longitudinal cohort study. BMC Public Health 2011;11:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israel-Ballard KA, Abrams BF, Coutsoudis A, et al. Vitamin content of breast milk from HIV-1-infected mothers before and after flash-heat treatment. J Acquir Immune Defic Syndr 2008;48:444–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Israel-Ballard K, Chantry C, Dewey K, et al. Viral, nutritional, and bacterial safety of flash-heated and Oretoria-pasteurized breast milk to prevent mother-to-child transmission of HIV in resource-poor countries: A pilot study. J Acquir Immune Defic Syndr 2005;40:175–181 [DOI] [PubMed] [Google Scholar]
- 17.Chantry CJ, Young SL, Rennie W, et al. Feasibility of using flash-heated breastmilk as an infant feeding option for HIV-exposed, uninfected infants after 6 months of age in urban Tanzania. J Acquir Immune Defic Syndr 2012;60:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Israel-Ballard K, Donovan R, Chantry C, et al. Flash-heat inactivation of HIV-1 in human milk: A potential method to reduce postnatal transmission in developing countries. J Acquir Immune Defic Syndr 2007;45:318–323 [DOI] [PubMed] [Google Scholar]
- 19.Volk ML, Hanson CV, Israel-Ballard K, et al. Inactivation of cell-associated and cell-free HIV-1 by flash-heat treatment of breast milk. J Acquir Immune Defic Syndr 2010;53:665–666 [DOI] [PubMed] [Google Scholar]
- 20.Human Milk Banking Association of South Africa. Guidelines for the Operation of a Donor Human Milk Bank in South Africa: Best Practice for the Collection, Storage and Handling of Human Milk. Human Milk Banking Association of South Africa, Durban, South Africa, compiled 2008, updated 2011. Available at http://hmbasa.org.za/HMBASA%20guidelines.pdf (accessed January27, 2015) [Google Scholar]
- 21.Bertino E, Arslanoglu S, Martano C, et al. Biological, nutritional and clinical aspects of feeding preterm infants with human milk. J Biol Regul Homeost Agents 2012;26(3 Suppl):9–13 [PubMed] [Google Scholar]
- 22.Jeurink PV, van Bergenhenegouwen J, Jimenez E, et al. Human milk: A source of more life than we imagine. Benef Microbes 2013;4:17–30 [DOI] [PubMed] [Google Scholar]
- 23.Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics 2012;129:950–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin R, Langa S, Reviriego C, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 2003;143:754–758 [DOI] [PubMed] [Google Scholar]
- 25.van der Linde I. Human Sciences Research Council. Plenary Session 3, 20 June 2013. HIV/AIDS in South Africa: At Last the Glass Is Half Full. Available at www.hsrc.ac.za/en/media-briefs/hiv-aids-stis-and-tb/plenary-session-3-20-june-2013-hiv-aids-in-south-africa-at-last-the-glass-is-half-full (accessed September22, 2014)