Abstract
Significance: When a cutaneous injury occurs, the wound heals via a dynamic series of physiological events, including coagulation, granulation tissue formation, re-epithelialization, and extracellular matrix (ECM) remodeling. The final stage can take many months, yet the new ECM forms a scar that never achieves the flexibility or strength of the original tissue. In certain circumstances, the normal scar is replaced by pathological fibrotic tissue, which results in hypertrophic or keloid scars. These scars cause significant morbidity through physical dysfunction and psychological stress.
Recent Advances and Critical Issues: The cutaneous ECM comprises a complex assortment of proteins that was traditionally thought to simply provide structural integrity and scaffolding characteristics. However, recent findings show that the ECM has multiple functions, including, storage and delivery of growth factors and cytokines, tissue repair and various physiological functions. Abnormal ECM reconstruction during wound healing contributes to the formation of hypertrophic and keloid scars. Whereas adult wounds heal with scarring, the developing foetus has the ability to heal wounds in a scarless fashion by regenerating skin and restoring the normal ECM architecture, strength, and function. Recent studies show that the lack of inflammation in fetal wounds contributes to this perfect healing.
Future Directions: Better understanding of the exact roles of ECM components in scarring will allow us to produce therapeutic agents to prevent hypertrophic and keloid scars. This review will focus on the components of the ECM and their role in both physiological and pathological (hypertrophic and keloid) cutaneous scar formation.

Christopher J. Jackson, PhD
Scope and Significance
This article reviews the extracellular matrix (ECM) and its remodeling during normal cutaneous wound healing and scar formation, and the differential response of the components of the ECM and their role in pathological (hypertrophic and keloid) cutaneous scar formation. This review focuses on the major players involved in the irregular ECM production as being; fibroblasts and their immature counterparts, myofibroblasts; collagens; transforming growth factor (TGF)-β, which controls the production of collagens; proteoglycans and matrix metalloproteinases (MMPs). We also highlight the complexity of interactions occurring in the ECM of skin during (ab) normal scar formation.
Translational Relevance
Abnormal ECM, particularly abnormal collagen remodeling and reorganization, accounts for one of the most important contributing factors to abnormal scarring. Identification of the exact ECM molecules involved in causing abnormal scarring is likely to provide a future treatment target. This may be achieved by promoting the correct balance in collagen ratios or by directly targeting the production of collagen. Overall, a better understanding of the exact roles of ECM components in scarring will help us to produce therapeutic agents to prevent and treat hypertrophic and keloid scars.
Clinical Relevance
There is currently no satisfactory treatment for hypertrophic scars or keloids. This is partly due to not completely understanding the mechanisms underlying these abnormal scars. A better knowledge of the complex interactions could suggest specific elements to target temporally and spatially during wound healing to improve healing process, or enable prevention of abnormal scars.
Wound Healing
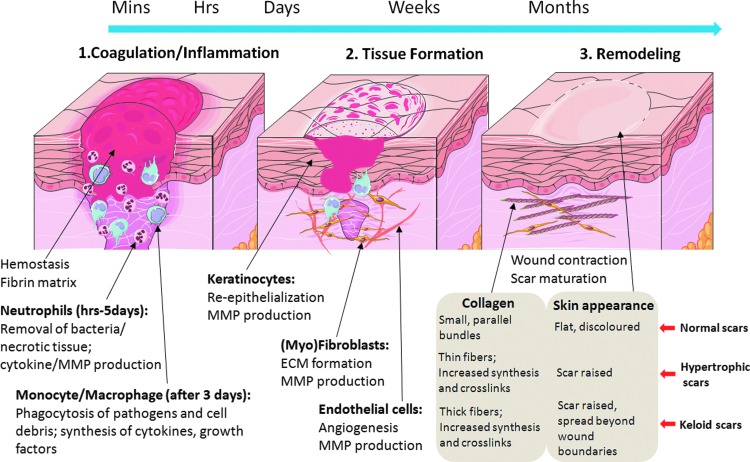
The skin is the largest organ in the human body. It forms an effective barrier between the body and outside environment and protects the body from dehydration and environmental insults. When an injury occurs to the skin, a series of dynamic events begin instantly. Cutaneous wound repair can be divided into a series of overlapping phases, including, inflammatory response, granulation tissue formation, which includes re-epithelialization and angiogenesis, and matrix remodelling.1,2 Figure 1 shows the three classic stages of wound repair. Immediately after injury, the homeostasis process begins and the bleeding is controlled by the aggregation of platelets at the site of injury. The subsequent formation of the fibrin clot stops the bleeding and provides a scaffold for the attachment and proliferation of the cells. The initial inflammatory phase begins at the time of wounding, when activation of the coagulation cascade causes a release of cytokines that stimulate chemotaxis of neutrophils followed by macrophages into the wound for early wound debridement. After 2–3 days, the inflammatory process progresses into the proliferative phase. Fibroblasts are attracted into the wound to synthesize granulation tissue. This granulation tissue is composed of procollagen, elastin, proteoglycans, and hyaluronic acid (HA) and allows ingrowth of new blood vessels that provide nutrition and oxygen to the growing tissue and allow leukocytes to enter the wound site. Keratinocytes provide the major cellular component of the outermost barrier to the environment and serve to restore the barrier function of skin. Once the wound is closed, the immature scar can move on to the final remodelling phase. The ECM molecules laid down during the proliferative phase in a disorganised manner are realigned and cross-linked. This remodelling phase can last up to a year depending on the severity of the wound, which over time gradually contracts to regains its integrity. However, the regained tensile strength in a wound will never approach normal and the maximum a wound can ever achieve is ∼80% of original.3 The following review outlines the involvement of the ECM during the development of scar tissue after cutaneous injury.
Figure 1.
The three classic stages of cutaneous wound healing. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Inflammation, wound healing, and scar formation
The role of inflammation in wound healing is under debate. Many researchers believe that the inflammatory phase is vital for wound healing to proceed; however, there is evidence to suggest otherwise. Whereas normal adult healing results in a fibrous scar, early fetal wounds which have very little, if any, inflammatory response, exhibit scarless healing with complete restoration of the normal skin architecture. Scar formation is exacerbated when inflammation is provoked in fetal wounds, suggesting that the absence of inflammation contributes to the rapid and flawless repair of these wounds.4 Compared to adult dermal wounds, adult oral wounds have substantially lower levels of macrophage, neutrophil, and T cell infiltration and heal rapidly with minimal inflammation and often with minimal scar formation.4 Wounds in the PU.1 null mouse, which is genetically incapable of raising an inflammatory response, heal rapidly with increased vascularity at the wound site and faster reepithelialization of the wound surface, as well as being scarless.5 This is relevant to many chronic wounds which are often associated with excess inflammation and become locked in an inflammatory phase. Recently, our in vitro experiments, in vivo animal models, and human clinical trials have shown that activated protein C (APC) accelerates the healing of chronic wounds, via inhibition of inflammation, stimulation of angiogenesis, regrowth of the dermis, and epidermal regeneration.6–8 To date, the effect of APC on scarring has not been reported.
ECM Components in Skin
Skin is essentially composed of cells (mainly fibroblasts, endothelial cells, and keratinocytes) and ECM. The latter is a dynamic, organized interlocking mesh of many different secreted macromolecules and proteolytic enzymes. The ECM has many functions, including: providing structure, organization, and orientation to cells and tissues; controlling morphogenesis and cellular metabolism by acting as a template for cell migration, proliferation, apoptosis, differentiation, and adhesion; regulate cell activity and function via directly binding to integrins and other cell surface receptors9; act as a reservoir for growth factors and regulate their bioavailability.10 ECM proteins like fibronectin, collagens, proteoglycans, heparin and heparin sulphate, bind many growth factors, such as fibroblast growth factors, TGF-β, vascular endothelial growth factor, epidermal growth factor, and bone morphogenetic proteins. Degradation of ECM proteins by proteolytic enzymes in response to wounds can induce local release of these growth factors from their insoluble anchorage, thereby modulates the process of wound healing. In addition, recent studies also demonstrate that ECM proteins are key components in shaping the stem cell niche to maintain stem cell homeostasis and to direct lineage commitment.11
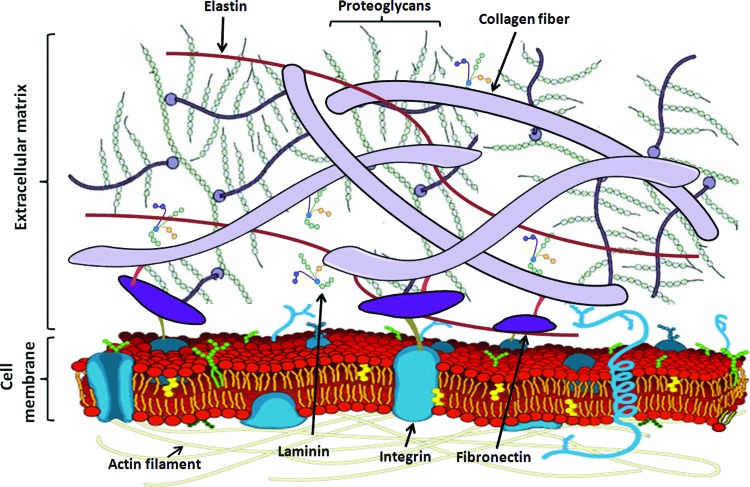
The ECM consists of the structural proteins, collagens, and laminins, elastins and fibronectins to provide flexibility; proteoglycans and hyaluronan to stabilize growth factors and the three-dimensional space by their high water-binding ability and glycoproteins, such as integrins to regulate cell adhesion and signaling between cells and ECM. These molecules interact with each other and the local cells which produce them (Fig. 2). The more important ECM molecules associated with remodeling are discussed briefly below.
Figure 2.
Major extracellular matrix components and their interactions with each other and cell membrane. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Collagens
The most abundant proteins in the ECM are the collagens—a large family of triple helical proteins that are ubiquitously found in the body and conserved in all multicellular animals.12 They have a broad range of functions, including cell adhesion, cell migration, tissue morphogenesis, tissue scaffolding, and tissue repair. Collagens are present in the dermis as fibrillar proteins and not only give structural support to resident cells, but also regulate the resident and inflammatory cell function.
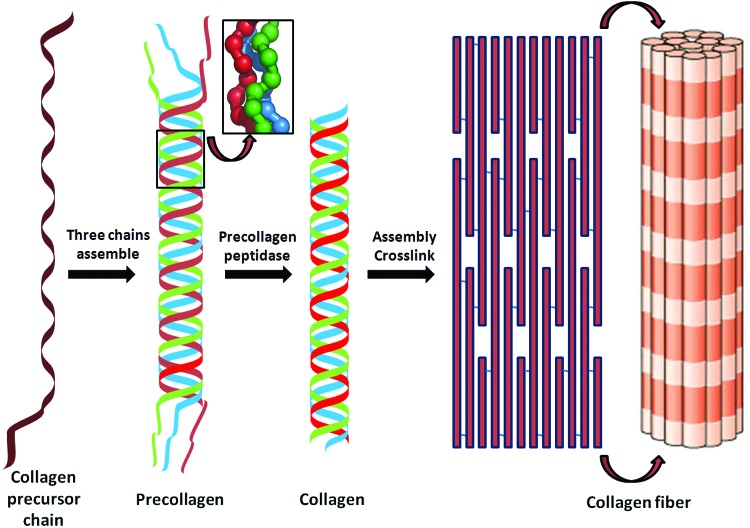
All members of the collagen family consist of three polypeptide α chains which are held together by interchain hydrogen bonds. There are at least 28 different collagens that occur in vertebrates.13 The fibrillar collagens I, III, and V, the fibril associated collagens with interrupted triple helices collagens XII, XIV, XVI, and VI are all expressed in the collagen-rich dermis (Table 1), although their complete functions are still unclear. Fibrillar collagens have enormous tensile strength.14 They are synthesised by cells, particularly fibroblasts, as procollagens containing N- and C-propeptides at each end of a triple helical domain. This synthesis requires specific post-translational enzymes, including intracellular lysine modifications of collagen by lysyl hydroxylases and glycosylation of hydroxylysine by hydroxylysyl galactosyltransferase and galactosylhydroxylysyl glucosyltransferase, extracellular cleavage by a procollagen N-proteinases and the C-proteinases, lysine modifications by lysyl oxidases to form a complex series of cross-links.15,16 Suppressing these enzymes inhibits collagen production and accumulation, which make some of them attractive targets for the development of drugs to treat fibrotic diseases. Cleavage of the C-propeptide is required for fibrillogenesis. The fibril-forming collagens spontaneously aggregate after processing of procollagens into ordered fibrillar structures in vitro. Hydrophobic and electrostatic interactions of collagen monomers are involved in the quarter-staggered arrangement of collagen monomers, which can aggregate into five-stranded fibrils and subsequently into larger fibrils. In the dermis, the fibrils orientate to form a complex network of interlaced basketweave-like fibrils. The molecular arrangement into fibrils is additionally stabilized by the formation of covalent cross-links, which finally contribute to the mechanical resilience of collagen fibrils (Fig. 3).
Table 1.
Collagen distribution and function in skin
| Collagen | % Total | Location | Function | Skin disorders |
|---|---|---|---|---|
| I | 80 | Dermis | Maintains skin structure and tissue integrity | Mutations resulting in osteogenesis imperfecta and Ehlers Danlos syndrome |
| III | 15 | Dermis | Maintains skin structure and integrity | Deficient mice display very severe spontaneous skin wounds and not uniformed the diameters of the collagen fibrils |
| Provide tensility, flexibility and softness | ||||
| Determines the collagen fibril diameter | ||||
| IV | 2–4 | Basement membrane vessels | Separates dermis-epidermis | Mutations resulting in Alport syndrome |
| Supports cells | ||||
| V | <1 | Basement membrane dermis | Bridges and stabilizes the epidermal–dermal interface | Defective product in most cases of Ehlers Danlos syndrome |
| Contributes to epidermis differentiation | Abnormal deposition in dermis correlates with skin thickening | |||
| VI | <1 | Dermis papillary | Provides resistance to tensile stress | Defective linked to atopic dermatitis and trichothiodystrophy with dry, scaly ichthyotic skin |
| May function in the maintaining the barrier function of the skin | ||||
| VII | <1 | Basement membrane | Stabilizes the association of the lower part of the basement membrane to the underlying dermis | Mutation resulting in recessive dystrophic epidermolysis bullosa and epidermolysis bullosa acquisita |
| XIV | <1 | Dermis hair follicles | May act in modulating cell-matrix adhesion | Mature skin with mutation exhibits reduced mechanical properties |
| XVI | <1 | Papillary dermis | Anchores microfibrils to basement membranes | Mutation resulting in epidermolysis bullosa |
| XVII (BP180) | <1 | Basal keratinocytes | Maintains adherence of the epidermis to the basement membrane | Mutation resulting in epidermolysis bullosa, Pemphigoid immunobullous diseases |
Figure 3.
The process of collagen assembly to form collagen fiber. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Fibrillar collagens which form native triple helices are characterized by their resistance to proteases, such as pepsin, trypsin, or chymotrypsin15 and can only be degraded by specific collagenases, especially MMP-1, MMP-8, and MMP-13. After collagenase digestion, gelatin, the remaining product, can be further degraded by the gelatinases, MMP-2 and MMP-9.
Mutations in human collagen genes give rise to numerous connective tissue diseases, including skin disorders. Alport syndrome is an inherited disorder of collagen IV and the classical Elhers-Danlos syndrome is caused by collagen V gene mutations indicated by skin fragility and abnormal wound healing.17 A subset of the skin blistering diseases, epidermolysis bullosa is caused by a collagen VII gene defect.18 See Table 1 for a more complete listing of human skin diseases associated with collagen mutations. Collagen III-deficient mice display very severe spontaneous skin wounds and the diameters of the collagen fibrils of their skin are not uniform; thinner and thicker fibrils are observed compared with normal skin.19
Proteoglycans
Proteoglycans are proteins that are glycosylated. The basic unit comprises a protein core with covalently attached glycosaminoglycan (GAG) chains. In skin, proteoglycans contribute to the overall mechanical properties by connecting to other ECM proteins, attracting water via osmosis to keep the ECM and resident cells hydrated and holding growth factors and cytokines within the ECM. The most abundant proteoglycans in extracts of postnatal human skin are decorin and versican and a catabolic fragment of decorin, termed decorunt.20 Versican appears to colocalize with elastic fibers in skin21 and has been demonstrated to influence cell migration.22 Decorin interacts with collagen and regulates collagen fibril and fiber bundle organization23,24 and is crucial for the robust tensile strength of skin by enhancing the collagen network.23 Versican and decorin show age-related differences in human skin. Degraded versican fragments are in lower abundance in adult skin compared with fetal skin. In contrast, the catabolic fragments of decorin are present in adult skin, but are virtually absent from fetal skin.25
Fibronectin
Fibronectin is a glycoprotein that connects cells with collagen fibers by binding to integrin receptors in the ECM causing reorganization of the cell's cytoskeleton and facilitating cell movement. The functional fibronectin dimer consists of two similar subunits of 220–250 kDa bound by two disulphide bonds. The plasma and cellular forms of fibronectin possess distinct structures and play different roles during tissue repair. The plasma form of fibronectin is incorporated into fibrin clots upon tissue injury and exerts effects on platelet function, which helps mediate homeostasis. Together with fibrin, it provides most of the provisional matrix in dermal wounds, acting to seal the wound and as a scaffold to guide invading leukocytes and endothelial cells during tissue repair.26
Later in the wound-healing response, cellular fibronectin is produced by keratinocytes, endothelial, and dermal (myo)fibroblasts and is abundant in the dermis and in the dermal–epidermal basement membrane region.27–29 It is assembled into a complex three-dimensional fibrillar network on the cell surface, which is vital for establishing and maintaining tissue architecture and for regulating cellular processes, including adhesion, spreading, proliferation, migration, and apoptosis.28 Fibroblasts polarize along the fibronectin fibrils, parallel to the epidermis. The formation of a stable collagen I/III fibrillar network is thought to depend on a pre-existing fibronectin network through a mechanism involving integrins.30 Fibronectin in the wound site is also vital for regulating the neovascularization of granulation tissue during the resolution of tissue injury.26
Other ECM molecules
HA is a high molecular weight polymer consisting of alternative residues of d-glucuronic acid and N-acetylglucosamine, found on the inner surface of the cell membrane. It provides structure and viscosity to the ECM because of its polyanionic and hydrophilic properties. HA regulates cell behavior, including healing and inflammation by interacting with the membrane receptor, CD44.31,32 Unlike other GAGs, HA is not found as a proteoglycan. In the extracellular space, HA causes tissues to resist compression by absorbing water to provide a turgor (swelling) force. It exerts this force particularly in the ECM of load-bearing joints and the interstitium. HA has unique capacity in retaining water and is the key molecule involved in skin moisture.
Elastins provide flexibility to tissues, allowing them to stretch when needed, and then return to their original state. Elastin is arranged in a three-dimensional network that is closely interwoven with collagen fibres.33 Elastins are synthesized by fibroblasts and smooth muscle cells and found abundantly in skin and blood vessels. Elastins are highly insoluble, and tropoelastins are secreted inside a chaperone molecule, which releases the precursor upon contact with a fiber of mature elastin. Cross-linked elastin fibres contribute to the structural integrity of the dermis and regulate various ECM processes, including the recoil and resilience of the skin. In mature skin, the density of the elastin network comprises ∼3–4% of the dry weight of tissue.34
Laminins are cell adhesion molecules that comprise a family of glycoproteins found predominantly in basement membranes of epithelial and endothelial cells and surrounding muscle cells, Schwann cells, and fat cells.35 These proteins are disulfide-linked heterotrimers constituted by the association of three genetically different polypeptides, α, β, and γ chains, which exist in five, four, and three genetically distinct forms, respectively. In vertebrates, at least 16 different isoforms are present. Laminins form networks of web-like structures that remain in close association with cells through interactions with cell surface receptors and resist tensile forces in the basal lamina. They contribute to the ECM structure and influence the cell behavior, such as adhesion, differentiation, migration, phenotype stability, and resistance to anoikis.36 In the skin, laminins support the migration and stable adhesion of keratinocytes and play key regulatory roles in the development of skin appendages and contribute to the pathogenesis of skin cancer.37
ECM Degradation in Skin and Wounds
Matrix metalloproteinases
The breakdown of the ECM is an essential component for wound healing and scar formation. MMPs catalyze the hydrolysis of major ECM molecules, including collagen, elastin, laminin, and fibronectin, as well as the processing of cytokines and growth factors.38 Enzymes within this family can be classified into groups based on their substrate specificity (collagenases, gelatinases, stromelysins, matrilysins and membrane-type MMPs) or their MMP number assigned in temporal order of discovery39 (e.g., Gelatinase A and B are also known as MMP-2 and -9, respectively). The MMP family in vertebrates consists of 25 different enzymes, which share a number of structural and functional similarities. In addition to their matrix-degrading ability, MMPs affect many biological functions by regulating growth factors and their receptors, cytokines and chemokines, and cell surface proteoglycans, and other enzymes.39,40 Collectively, MMPs are able to cleave every ECM component; however, different ECM components are susceptible to proteolysis by different MMPs and not all ECM components are cleaved by every MMP. Fibrillar collagen I, II, and III degradation is mediated by collagenases-1, 2, and 3 (MMP-1, 8, and 13) by proteolysis of the triple helix between Gly775 and Leu776, allowing the collagen chains to unwind. The cleavage fragments rapidly denature and form gelatins that can be degraded by other proteases, such as the gelatinases, MMP-2 and MMP-9. The basement membrane component collagen IV is susceptible to proteolysis by gelatinases, stromelysins, matrilysins, while collagen XVII is degraded by gelatinases.41 Laminin, fibronectin, elastin, or aggrecan can be cleaved by most MMPs. Table 2 lists the MMPs that are expressed and able to degrade collagen and gelatins during cutaneous wound healing.39,42,43 In addition, MMP processing of ECM components can yield bioactive fragments. For example, MMP-2 and MMP-9 expose a cryptic epitope within collagen IV that promotes angiogenesis, while antiangiogenic factors, such as endostatin can be formed from collagen XVIII.43
Table 2.
Matrix metalloproteinases that expressed and degrade collagen during cutaneous wound healing
| Group | MMP | Cells source | Substrates |
|---|---|---|---|
| Collagenases | MMP-1 | Proliferating and migrating keratinocytes fibroblasts | Collagens I, II, III, V, VII, X, XI; gelatines |
| MMP-8 | Neutrophils | Collagens I, II, III, VII, VIII, X | |
| Gelatines | |||
| MMP-13 | Fibroblasts | Collagens I, II, III, IV, IX, X, XIV | |
| Migrating keratinocytes | |||
| Gelatinases | MMP-2 | Fibroblasts | Gelatines |
| Keratinocytes | Collagens III, IV, V, VII, X, XI, XIV | ||
| Endothelial cells | |||
| Macrophages | |||
| MMP-9 | Keratinocytes | Gelatines | |
| Neutrophils | Collagens I, IV, V | ||
| Macrophages | |||
| Endothelial cells | |||
| Stromelysins | MMP-3 | Basal proliferating keratinocytes | Collagens I, III, IV, V, IX, X |
| Fibroblasts | Gelatines | ||
| MMP-10 | Migrating keratinocyte | Collagens I, III, IV, V, IX, X | |
| Fibroblasts | Gelatines | ||
| Metalloelastase | MMP-12 | Macrophages | Collagen IV; gelatines |
| Membrane type MMP | MMP-14 | Migrating keratinocytes | Collagen I, II, III |
| (MT1-MMP) | Gelatines | ||
| Other MMPs | MMP-19 | Keratinocyte | Gelatines |
| Fibroblast | Collagen IV | ||
| Endothelial cells |
MMPs, matrix metalloproteinases.
The expression of MMPs in normal intact skin is very low. Only MMP-7 and MMP-19 are constitutively produced in sweat and sebaceous glands. After skin injury, however, multiple MMPs are produced during the normal healing process, including MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-12, MMP-13, MMP-19, MMP-26, and MMP-28.44,45 MMPs participate in regulatory mechanisms throughout the entire repair process. MMP-1, MMP-7, MMP-9, and MMP-10 are mainly secreted by keratinocytes; whereas MMP-2 is secreted by fibroblasts and MMP-13 by both cell types at comparable levels.45 Inflammatory cells in the wound area are also an important source of MMPs. For example, neutrophils produce MMP-8 and MMP-9. In response to wounding, MMP-1 expression occurs rapidly and peaks in migrating basal keratinocytes at the wound edge at day 1 followed by a gradual decrease, being undetectable at the time of complete re-epithelialization.46 MMP-1 is required in the epidermis to facilitate re-epithelialization by remodelling the basement membrane, promoting cell elongation, and actin cytoskeletal reorganization, and activating extracellular signal-regulated kinase signalling.47 MMP-9 is involved in keratinocyte migration and granulation tissue remodelling, while expression of MMP-2 remains stable throughout healing.48 MMP inhibitors abrogate keratinocyte migration and delay wound healing in vivo.40,49 Fibroblast-mediated wound contraction may be dependent on MMP-3, as MMP-3-deficient fibroblasts have a reduced ability to contract collagen gels and wound healing is delayed in MMP-3 deficient mice.50 MMP-8 is a major collagenase in healing human dermal wounds.51 MMP-8 knock-out mice show prolonged inflammation and a significant delay in wound healing52 and MMP-9 and MMP-13 mutants delay epithelial migration.53,54 MMP-8 can compensate for loss of MMP-13 expression in MMP-13-defficient mice.47,55 MMP-9/-13 double mutants have longer wound-healing delays than the single mutants.53 Mice lacking MMP-14 ultimately result in impaired wound healing,56 possibly through the control of keratinocyte growth factor receptor expression, which is increased in wild type but not in their MMP-14 deficient counterparts.56
In addition, MMPs control inflammation by regulating cytokines and chemokines, by cleaving them to enhance their activity or by degrading them to inhibit their activity.40 Thus, MMPs are key regulators of multiple aspects of tissue repair.
A Disintegrin and Metalloproteinase with Thrombospondin Motifs (ADAMTS)
ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs are secreted zinc metalloproteases with an ancillary domain containing one or more thrombospondin type 1 repeats. This superfamily includes 19 distinct members and is collectively referred to as proteoglycanases participating in proteolysis of the large aggregating proteoglycans aggrecan, versican, and brevican.57 ADAMTS are synthesized as inactive zymogens, activated by the furin cleavage of N-terminal propeptide. They regulate ECM turnover and development58 which is supported by the identification of ADAMTS mutations in human disorders. For example, ADAMTS13 mutations cause inherited thrombotic thrombocytopenic purpura characterized by widespread microvascular thrombosis59; ADAMTS2 mutations induce Ehlers–Danlos syndrome type VIIC with extreme skin fragility, joint laxity, lax skin, umbilical hernia, and blue sclera.60
Tissue inhibitors of MMPs
Among the endogenous inhibitors of MMPs are tissue inhibitors of matrix metalloproteinases (TIMPs), of which there are four family members in mammals, TIMP-1, -2, -3, and -4.39 TIMPs inhibit MMPs in a 1:1 inhibitor to enzyme ratio through interaction of the N-terminal domain of the TIMP molecule with the active site of the MMP. TIMP-1 is synthesized by keratinocytes, fibroblasts, smooth muscle cells, and endothelial cells and especially targets MMP-1. TIMP-1 is present in the epithelium of healing excisional wounds, burn wounds61,62 and in wound fibroblasts, especially around blood vessels.61,62 TIMP-2 is produced by the basal keratinocytes in normally healing wounds and preferentially acts as an effective inhibitor of MMP-2. TIMP-3, which inhibits the activity of MMP-1, -2, -3, -9, and -13, is expressed by macrophage-like cells of the granulation tissue and by endothelial cells. Both TIMP-2 and TIMP-3 are expressed by skin epidermal keratinocytes and fibroblasts. Occasional blood vessels of acute wounds are positive for TIMP-1, -2, and -3. No TIMP-4 expression appears to be present in acute human wounds.61 TIMP-3 also is the most significant endogenous inhibitor of ADAMTS.
ECM Remodelling in Fetal Cutaneous Scarless Healing
There are fundamental differences between fetal and adult wound healing processes, resulting in different outcomes. Adult skin wounds heal with fibrosis and scarring. Unlike normal skin, scar tissue exhibits characteristic features, such as disturbance of the alignment of collagen fibers, excessive dermal fibrosis, disappearance of elastic fiber and appendages, and disruption of skin texture. In contrast, the early developing foetus has the ability to heal wounds by regenerating skin and restoring the normal ECM architecture, strength, and function.63 The mechanisms of fetal wound healing remain largely unknown. Many studies have demonstrated that there are differences in the inflammatory response, cellular mediators, cytokines, growth factors, and ECM modulators and structures between postnatal and fetal wound repair.64 Early in gestation, fetal skin develops rapidly and the ECM forms a loose network allowing migration of cells. The fetal ECM is rich in collagen III and HA. Although collagen I is the predominant component of ECM in both the fetus and the adult, the quantity of collagen in fetal skin is lower than that in adult skin and a ratio of collagen III to collagen I in skin is higher in the fetus than in the adult.65,66 In scarless fetal skin, collagen III comprises ∼30–60% of the total collagen compared to 10–20% in adult skin.66,67 Wounding in this unique setting triggers a complex cascade of tightly controlled events resulting in a scarless phenotype, typically consisting of fine reticular collagen and abundant HA.2
Scarless repair, however, is dependent on gestational age. Fetal rat skin shows that transition from regeneration to scar formation occurs between days 16 (E16) and E19 (term gestation is E21.5) of gestation.68 Wounds made on the E16 gestation in rats are histologically regenerated, but wounds made on the E19 gestation are associated with scarring, although both at E16 and E19 wounds, re-epithelialization was complete by 72 h.68 The exact change that occurs in late gestation to promote scarring is unknown. During late gestation, skin expression of MMP-1 and MMP-14 is doubled, whereas MMP-2 expression increases nearly 50-fold. In both scarless and scarring wounds, upregulation of MMP-1 and MMP-9 occurs. However, the maximal increase in MMP-1 and MMP-9 expression occurs much more rapidly and is much greater in the scarless E16 wounds. MMP-14 expression increases threefold in scarless wounds but is unchanged in scarring wounds. In contrast, TIMP-1 and TIMP-3 expression in E19 scarring wounds increases sixfold and fourfold, respectively. MMP-7 and TIMP-2 expression does not change in response to injury. E16 scarless wounds have greater MMP relative to TIMP expression than E19 scarring wounds. This favors ECM turnover, facilitates migration of fetal cells, and promotes scarless repair.68
Nude mice also heal without scars, which is likely due to their lack of an inflammatory response. In these mice, MMP-9 and MMP-13 show a unique, bimodal pattern of up-regulation during the early and late phases of wound healing.69 High levels of mRNA MMP-9 are present exclusively in the postinjured tissues from nude mice on day 24 after wounding. Consistent with these in vivo observations, dermal fibroblasts cultured from nude mice express higher levels of collagen I and III, MMP-9 and MMP-13 mRNA levels and higher MMP enzyme activity than wild type controls.70 Collectively, these findings suggest that the bimodal pattern of MMP-9 and MMP-13 expression during skin repair process in nude mice could contribute to their ability for scarless healing.69 Further research may reveal novel genes essential to scarless repair that can be manipulated in the adult wound to prevent excessive scar formation. Table 3 lists the changes in the expression of major MMPs and TIMP in normal, hypertrophic, keloid scar and scarless healing skin.
Table 3.
The changes in the expression of main matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase in normal, hypertrophic, keloid scar, and scarless healing skin
| Normal scar | Hypertrophic scar | Keloid scar | Scarless healed skin | |
|---|---|---|---|---|
| MMP-1 | Low | Decreased | Increased | Increased |
| MMP-2 | High | Decreased | Highest | Higher |
| MMP-3 | Low | Increased | Decreased | High |
| MMP-8 | Low | Increased | Decreased | High |
| MMP-9 | Low | Decreased | Low | High |
| MMP-13 | Low | Decreased | Increased | High |
| TIMP-1 | Low | Increased | Increased | Decreased |
| TIMP-2 | Low | Increased | Increased | Decreased |
TIMP, tissue inhibitor of matrix metalloproteinase.
ECM Remodelling in Normal Cutaneous Adult Scars
A normal scar is the body's natural response to repair tissue after injury and does not develop until after the wound has completely healed. ECM composition in the dermis which is relatively stable in healthy adult tissue alters after wound healing. This involves a slow remodeling process whereby loose and highly hydrated ECM that allows cellular invasion and repair is gradually replaced by denser ECM composed mainly of collagen.71 Up to 1 month after injury, skin covering a wound is fragile and can be disrupted by minimal insult and by 6 weeks, ∼50% of its final strength has been attained. During the next 12 months, the scar gradually strengthens, but scar tissue is never as strong as normal, uninjured skin. A visible scar will be present if a wound takes more than 3 or 4 weeks to be re-epithelialized.72 Scar tissue is not only weaker but is functionally deficient. Scars usually have less blood supply and denser ECM which is less resistant to shape change. Sweat glands do not form in scar tissue, which impairs the regulation of body temperature.73 Additionally, scarred skin is less resistant to ultraviolet radiation.74
Collagens
The characteristics of normal scars result from the changes in ECM structure and composition in the dermis. The most significant difference between normal tissue and scar tissue seems to be the orientation of the fibrous matrix.75 In rodents, normal tissue has a reticular collagen pattern, whereas the collagen in scar tissue forms large parallel bundles at approximately right angles to the basement membrane76,77 In humans, instead of a random basketweave formation of the collagen fibers found in normal tissue, in scars the collagen forms cross-links to align in a single direction parallel to the skin, opposite to the rat.78 In addition, there is greater collagen density and larger fiber size in scars compared to normal tissue.75
The collagens comprise the main structural component and comprise the highest protein concentration in the ECM, with 85% of the dermis being collagen. The collagens form a relaxed network of cross-linked long-chain fibers to give the strength and the elasticity of healthy skin and scar tissue. The two dominant types of collagen in wound repair are collagen I and III. In normal skin, collagen fibrils are composed of both collagen I and III with collagen III comprising ∼20% of the total.75 During the early stages of granulation tissue formation, myofibroblasts lay down collagen III, which plays a role in fibrillogenesis and determines the fibril diameter of collagen I. Collagen III expression increases more than the collagen I expression in the early stages of healing, resulting in increased ratio between the two collagen subtypes from 20% up to 50% collagen III.79 During maturation of the scar, the ratio decreases again to normal levels. Thus, increased amounts of collagen III relative to collagen I identifies an immature scar. Table 4 shows main histological differences in collagen between normal, hypertrophic, and keloid scars.
Table 4.
The histological differences in collagen between normal, hypertrophic, and keloid scars*
| Unwounded skin | Normal scar | Hypertrophic Scar | Keloid scar | |
|---|---|---|---|---|
| Collagen orientation to the epithelial surface | Basket weave-like network | Fine, well-organized | Flatter, arranged in a wavy pattern | Collagen I and II fibers lie haphazardly |
| Parallel to epidermis | Parallel to epidermis | Predominantly parallel | Randomly oriented | |
| Collagen bundle thickness | Normal bundle | Small, parallel bundles | Thin collagen thin fibers organized into nodules | Large, thick, closely packed random to epidermis |
| Collagen bundle distance | Smaller bundle distance | Close | Close | Larger bundle distance |
| Collagen synthesis | Normal | Increased than normal skin | Seven times higher than normal | 20 times higher than normal |
| Collagenous-cellular nodules | Absent | Absent | Present | Present |
| Collagen I/III ratio | 5:1 | 6:1 | 6:1 | 17:1 |
| Collagen crosslink | Normal | Lower | High | Twice higher than hypertrophic scar |
Modified from Verhaegen et al.107
(Myo)fibroblasts
The early phases of wound healing involve the formation of a provisional ECM containing fibrin, fibrinogen, and fibronectin. Fibroblasts occupy this matrix and proliferate in response to cytokines produced by neutrophils and macrophages that have migrated into the wound tissue. The fibroblast undergoes phenotypic changes during wound healing, characterized by increased collagen synthesis and contraction but decreased proliferation compared with normal dermal fibroblasts.80 The main function of fibroblasts is to maintain the physical integrity of connective tissue by producing and remodelling the ECM.81 Integrin receptors on the cell surface of fibroblasts provide contact with the surrounding ECM.82 Of the two major fibronectin integrin receptors, αVβ3 and α5β1, the latter is the primary receptor for soluble fibronectin and has a key role in assembling fibronectin into fibrils, though αVβ3 can assemble fibrils in cells that lack α5β1. Collagen recognition is mainly achieved by α1β1 and α2β1 integrins. Binding of α1β1 integrin to collagen I results in an almost complete arrest of collagen synthesis according to a negative feedback regulation mechanism.83
Myofibroblasts are a specialized form of fibroblast responsible for the deposition of a dense, fibrotic collagen matrix. These cells express α-smooth muscle actin (SMA) and play a major role in wound contraction. Their most prominent ECM products are collagens I, III, IV, and V84 and their differentiation is primarily driven by mechanical tension and cytokines, especially TGF-β (see below). During the remodeling phase, myofibroblasts produce decorin, which regulates collagen fibrillogenesis by presenting as a “C”-shaped structure that locates itself between collagen fibrils assuring uniform spatial fibril arrangement.85 Decorin also binds and neutralizes TGF-β, thereby minimizing the stimulatory effects of this cytokine on collagen, fibronectin, and GAG production.86 Eventually, myofibroblasts become surrounded and buried in fibrillar collagen, which may exert adverse effects on the behavior of the cells, such as causing arrest in the G1 phase of their cell cycle.87 Furthermore, degraded collagens promote disassembly of cellular focal adhesions, reducing the ability of myofibroblasts to adhere,88 which, in turn, causes the cells to undergo apoptosis.89 In any case, when the differentiation signals are removed towards the end of the healing phase, myofibroblasts undergo apoptosis, resulting in a collagen-rich, hypocellular scar. Myofibroblasts were not found in wounds created in the mid-gestation period, but they were found in wounds created after the latter portion of the gestation period.90
Transforming growth factor-β
The majority of cells involved in wound healing express TGF-β1, which strongly promotes the chemotaxis of fibroblasts to the site of injury and plays a critical role in fibroblast proliferation and ECM production.91 This growth factor induces the expression of SMA and promotes the synthesis and maturity of collagen I. Although TGF-β1 induces the expression of MMP-2, -9, and -13 in fibroblasts, it actually reduces collagen degradation by inhibiting the expression of MMP-1 and increasing the production of TIMP-1.92,93 There are three isoforms of TGF-α—TGF-β1 and TGF-β2 have the profibrotic properties whereas TGF-β3 is antifibrotic.93 TGF-β1 signalling activates SMAD2 and SMAD3, which use SMAD4 for translocation to the nucleus, whereas TGF-β3 stimulates Smad7. Adjusting the balance of the TGF-β isoform ratio has attracted considerable attention by researchers attempting to correct scar development.91,94 Table 5 lists the differences in TGF-β and (myo) fibroblasts in normal, hypertrophic, and keloid scars.
Table 5.
The differences in transforming growth factor-β and (myo)fibroblasts in normal, hypertrophic, and keloid scars
| Factors | Normal scar | Hypertrophic scar | Keloid scar |
|---|---|---|---|
| TGF-β | ↑TGF-β1 | ↑↑TGF-β1 | ↑↑TGF-β1 |
| ↓TGF-β3/TGF-β1 | ↑TGF-β2 | ↑↑TGF-β2 | |
| ↓↓TGF-β3 | ↓↓TGF-β3 | ||
| ↓↓TGF-β3/TGF-β1 | ↓↓TGF-β3/TGF-β1 | ||
| Fibroblasts | ↑Cell number | ↑↑Cell number | ↑↑Collagen I |
| ↑↑α-SMA | ↑α-SMA | ||
| ↑↑Proliferation | ↑↑Proliferation | ||
| ↓↓Apoptosis | |||
| ↑TGF-β1 | |||
| ↑↑Collagen I | |||
| Myofibroblasts | Absent | Present | Absent |
| MMP/TIMP activity | ↑MMP-1, 2 and 9 | ↓MMP-1, 2 and 9 | ↑↑MMP-2 |
| ↓TIMP-1 | ↑TIMP-1 | ↑TIMP-1 |
TGF, transforming growth factor; SMA, smooth muscle actin; ↑increase; ↓decrease.
Abnormal Cutaneous Scar Types
Formation of abnormal scars present major problems as the contractures can be disfiguring and cause loss of function, limited mobility by scar contraction and stiffness, pruritus, and pain.95 Cosmetic disfigurement caused by scars often leads patients to suffer from psychosocial and social issues, which, in turn, results in a decreased quality of life. Scars are composed of the same ECM molecules as the tissue they replace, but the ratios in scar tissue are different to normal tissue.72 The levels of collagen I and III, fibronectin, and laminin are all increased in raised dermal scar tissue. However, HA and decorin are decreased, and the expression and localization of fibrillin and elastin fibers in the dermis are altered compared with normal skin and scars.96 Scars are classified according to their clinical behavior and appearance. Atrophic scars takes the form of a sunken recess in the skin, which has a pitted appearance and can be caused when underlying structures supporting the skin, such as fat or muscle, are lost and often associated with acne, chickenpox, surgery, or accidents.97 The more severe forms of scarring are frequently referred to as either hypertrophic or keloid scars.
Hypertrophic scars
Hypertrophic scars are raised, erythematous, pruritic, painful, rigid, disfiguring, and a functionally limiting form of dermal fibrosis. They appear as a red lump on the skin raised above the surrounding skin and often develop after thermal or traumatic injury to the deep regions of the skin.77 Clinically, they are identified by excessive dermal fibrosis and scarring resulting from the imbalance between collagen synthesis and degradation during wound healing. Unlike keloids, hypertrophic scars remain within the boundary of the original injury. Hypertrophic scars are characterized by excessive deposition and alterations in morphology of collagen and other ECM proteins.77
Keloids
Keloid scars are red, raised and fibrous and are a more serious form of scarring, because they can grow indefinitely into large, tumorous (although benign) neoplasms.98 They are often distinguished from hypertrophic scars by their growth outside the original wound area. Keloid scars occur most commonly in dark-skinned people and can be caused by numerous lesions, including surgery, accident, acne, or can occur spontaneously. Collagen synthesis in keloids is ∼20 times as great as that in normal unscarred skin and three times as great as in hypertrophic scars.98–100 Abergel et al. showed that not only is collagen production high in hypertrophic scars and keloids, but the ratio of collagen I to III is also high.101 This collagen overproduction can be attributed to the stronger proliferating activity of keloid fibroblasts.102 Aside from high collagen synthesis and proliferation of fibroblasts, fibroblasts in keloids show a rate of fibronectin biosynthesis that is as much as four times as high as that of fibroblasts from normal scars and normal dermis.103,104
ECM Remodelling in Cutaneous Hypertrophic and Keloid Scars
Collagens
Similar to normal scars, the collagen fiber orientation parallel to the epithelial surface is characteristic for mature hypertrophic and keloid scars, rather than the three-dimensional basketweave-like network seen in normal skin.105 However, there are structural and composition differences between normotrophic and abnormal scars, as well as between hypertrophic and keloid scars. Keloids contain large, thick collagen fibers composed of numerous fibrils closely packed together. The fibers are less organized and have less crosslinks than normotrophic scars.100,106,107 Blackburn et al.108 and Ehrlich et al.105 described the presence of abnormally thick collagen bundles in keloid scars and the absence of these in hypertrophic scars. In keloid scars, the distance between collagen bundles was higher and the collagen bundles were more randomly organized in the deep dermis compared with the superficial dermis.107 Linares et al.109 found that collagen bundles in hypertrophic scars were relatively thin when compared with normal skin (Table 4).
The ratio of collagen I/III is altered in keloid tissue (∼17:1) compared to normal scars (∼6:1).107 There is heterogeneity of collagen expression within keloid scar tissue. Fibroblasts from the growing margin of keloid scars show a higher production of collagen I and III and a different collagen I/III ratio compared to fibroblasts isolated from extra- and intralesional keloid biopsy sites.110 The mRNA of collagen I and III is upregulated 20-fold in keloid tissues.111 Agarwal et al.112 recently demonstrated that cartilage oligomeric matrix protein (COMP) functions as organizer of the dermal collagen I network in healthy human skin and COMP deposition is enhanced in the dermis in various fibrotic conditions, including keloids.
Proteoglycans
Proteoglycans influence the physical characteristics of the skin, collagen composition and structure, growth factor activity and cellular behavior.77 The increased turgor of hypertrophic scars is partly attributable to the increase in GAGs.77 The predominant proteoglycan in normal skin is decorin. Hypertrophic scar tissue obtained between 5 and 14 months after injury showed a dramatic reduction in decorin as compared to normal skin.113,114 Decorin inhibits fibroblast proliferation and decreases TGF-β1 production and collagen synthesis in hypertrophic scar fibroblasts.85,115 The low levels of decorin found in the hypertrophic scars therefore, may account for their irregular collagen organization, as well as increased ECM production. In burns, decorin expression is suppressed.113 Myostatin-null mice exhibit delayed skin wound healing through the blockade of TGF-β signaling by decorin116 and recombinant decorin inhibits cell proliferation and downregulates TGF-β1 production in hypertrophic scar fibroblasts.85 Reduced decorin and TGF-β3 in deep dermis lead to hypertrophic scarring.117 Versican is a large proteoglycan with as many as 30 chondroitin sulfate sugar chains, and is produced in sixfold higher quantity in hypertrophic scar. Versican contributes significantly to the rigidity of hypertrophic scar because of its strong hydrophilic properties.77
(Myo)fibroblasts
Fibroblasts from hypertrophic scars behave quite differently to normal fibroblasts. Hypertrophic scar fibroblasts exhibit increased collagen I synthesis, reduced collagenase production and subsequent reduced ability to digest soluble collagen compared to normal fibroblasts.118 Hypertrophic scar tissues have greater numbers of fibroblasts and myofibroblasts than normal skin and normotrophic scars.119 Hypertrophic scars contain excessive microvessels, which are mostly occluded due to the overproliferation and functional regression of endothelial cells induced by myofibroblast hyperactivity and excessive collagen production.120 Myofibroblasts from hypertrophic scars are more resistant to apoptosis compared to normal wound myofibroblasts121 (Table 5). p53, which induces cell cycle arrest and/or apoptotic cell death, has a higher incidence of mutations (exons 5–8) in both keloids and hypertrophic scar fibroblasts.122 These properties of hypertrophic myofibroblasts may play an important role in hypertrophic scar formation.119,121,123 In addition, nuclear factor (NF)-κB is upregulated in keloid fibroblasts and its inhibition suppresses cultured keloid fibroblast proliferation and collagen I production,124,125 suggesting a role for anti-inflammation treatment for excessive scars.
Transforming growth factor-β
When wound repair is completed, the activity of TGF-β1 is normally turned off. Hypertrophic scars and resident fibroblasts show higher expression of TGF-β1 and lower levels of TGF-β3 than normal117,126 (Table 5). TGF-β1 and TGF-β2 mRNA expression is higher in fibroblasts derived from keloids than from hypertrophic scars and normal scars; however, TGF-β3 expression is lower.127 Excessive scar fibroblasts have greater numbers of growth factor receptors and respond more vigorously to growth factors, such as TGF-β.126 The balance between TGF-β1 and TGF-β3 is an important regulator of scar formation. The increase or prolonged activity of TGF-β1 leads to an overproduction and excess deposition of collagen by fibroblasts that often result in hypertrophic scars.128 In an adult rat incisional model, the addition of neutralizing antibody to reduce levels of TGFβ1 and TGFβ2 resulted in reduced collagen content, improved orientation of ECM in the wound, and less scarring compared with controls.129
Matrix metalloproteinases
Decreased levels of MMP-1, MMP-2, MMP-9 and increased levels of TIMP-1 are present in hypertrophic scars and appear to contribute to tissue fibrosis, leading to excessive scars.130,131 There is relatively strong expression of TIMP-1 in hypertrophic scar biopsies in contrast to very low levels of TIMP-1 in normal skin.92 Less synthesis of molecules that promote matrix breakdown (e.g., MMPs) may also explain the lack of scar regression seen in keloids.75 However, there have been reports that the ECM of keloids shows elevated levels of MMPs (Table 4). Neely et al. reported significantly increased MMP-2 activity in keloids and no change in MMP-9 activity.132 When compared to hypertrophic scars, atrophic scars, and donor skin, MMP-2 levels were highest in keloid group with undetectable MMP-9 activity using gelatin zymography analysis.133 In culture, the production of type 1 collagen, MMP-1, MMP-2, and TIMP-1 by keloid fibroblasts was 3-fold, 6-fold, 2.4-fold, and 2-fold greater compared with normal dermal fibroblasts. Adding TGF-β1 to cultured keloid fibroblasts increased the production of MMP-2, decreased MMP-1 and did not change TIMP-1.99 Even though MMPs are increased, they do not degrade the excess collagen in keloid scars. This may be because they are insufficient in quantity to compete with the excessive anabolic signals building up the scar tissue. Alternatively, it may be due to collagen crosslinks, which help determine collagen's susceptibility to MMP cleavage.134 Bone exhibits pyridinoline cross-links which are able to withstand major forces.135 Whereas normal skin collagen does not have these cross-links, hypertrophic and keloid scar collagen does,136 which makes them less susceptible to cleavage by MMPs. The average pyridinoline crosslink content per mole of collagen in keloids is two times higher than in hypertrophic scars.137 Inhibition of this biochemical process to form these rigid cross-links may help prevent abnormal scar formation.75
APC, A Potential Agent to Prevent/Treat Abnormal Scarring?
Recently, our preclinical and clinical studies demonstrate that APC, a natural anticoagulant, promotes wound healing.6–8 Although the effect of APC on cutaneous scarring has not been examined, APC treatment is protective during the development of myocardial fibrosis in mice138 and several unique properties of APC indicate its potential in the prevention of excessive scar formation.
• Acceleration of wound healing. APC treatment results in a faster healing of normal and chronic wound.6–8,139 Prolonged healing process is an important contributing factor for abnormal scaring. Deitch et al.140 reported that the likelihood of development of a hypertrophic scar after an acute burn injury increased when the healing extended beyond 3 weeks. In a pediatric population, a study by Cubison et al.141 showed that the incidence of hypertrophic scar after scald injury is 8% if healing occurred between 10 and 14 days, whereas those taking more than 30 days to heal developed hypertrophic scars in 92% of cases. A recent study also reveals that the overall hypertrophic scar rate rises from 3% to 15% among those patients taking more than 21 days to heal.142
• Inhibition of inflammation: Independent of its effect on coagulation, APC possesses strong anti-inflammatory properties associated with a decrease in proinflammatory cytokines, a reduction of leukocyte recruitment and suppression in the activation of NF-κB.143 Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency.144 Inflammatory response is the leading culprit in forming fibrotic scar (See detailed discussion in “Inflammation, wound healing and scar formation”). Wounds with no or minimum inflammation even in adults repair rapidly and exhibit minimal scarring.4,5
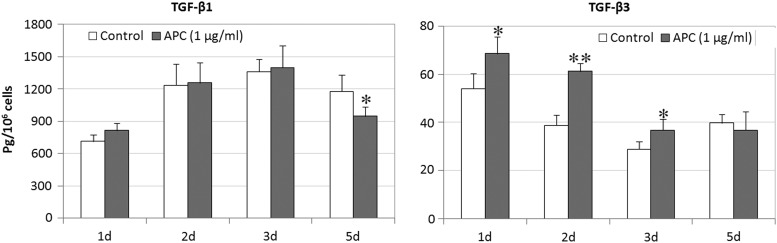
• Modulation of ECM turnover. APC not only increases collagen 1 expression by osteoblasts,145 liver myofibroblasts,146 tenocytes,147 and collagen IV by endothelial cells,148 but also regulates MMP expression/activation with a stimulatory effect on MMP-2 by skin keratinocyte, fibroblasts, and endothelial cells148,149 and an inhibitory effect on MMP-9 by rheumatoid synovial fibroblasts.150 Moreover, our recent data show that APC has the ability to differentially modify the production of TGF-βs (Fig. 4). Skin keratinocytes treated with APC produced more TGF-β3 at day 1, 2, and 3, while had no effect at TGF-β1 at same time and inhibited TGF-β1 at day 5. These affects would assist in preventing excessive scar formation.
Figure 4.
The production of TGF-β1 and TGF-β3 by human keratinocytes treated with APC. Keratinocyte monolayers were treated with APC (1 μg/mL). Culture supernatants were collected at for day 1, 2, 3, and 5 after treatment. TGF-β1 and TGF-β3 in supernatants were detected by enzyme-linked immunosorbent assay. Data are expressed as mean±standard error of the mean from four experiments. APC, activated protein C; TGF, transforming growth factor. *p<0.05, **p<0.01.
Conclusion
Hypertrophic and keloid scars occur as a result of aberrations of physiologic wound healing. By causing pain, pruritus, and contractures, excessive scarring significantly affects the patient's quality of life, both physically and psychologically.100 To date, therapeutic approaches to reduce scarring have been largely unsuccessful. The ECM is important in the maintenance of the structure, function, and signaling of tissues and is actively involved in both cellular and extracellular events that lead to excessive scarring. Targeting components of the ECM during wound repair provides an attractive approach to avoid hypertrophic and keloid scars.
Take Home Messages
• ECM is the name given to the bulk of the tissue in the body, excluding the cells.
• Collagen is one of the major components of the ECM, but there are many more.
• The ECM forms the major component of the skin, along with three major types of cells: keratinocytes, fibroblasts, and endothelial cells.
• After the skin is injured, an organized repair process normally occurs that results in a scar.
• However, the fetus has the remarkable ability to rapidly and completely regenerate skin without scarring or showing any signs of the original injury.
• The reasons why the fetus can heal scarlessly is not fully understood but is thought to involve the ECM.
• In some people, scar formation is exacerbated resulting in disfiguring hypertrophic or keloid scars, for which the ECM plays a major role.
• Targeting factor(s) within the ECM may allow us to prevent the formation of abnormal hypertrophic or keloid scars and improve normal wound healing to mimic that of fetal healing.
• APC has potential as a wound healing treatment to minimize excessive scar formation.
Abbreviations and Acronyms
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- APC
activated protein C
- COMP
cartilage oligomeric matrix protein
- ECM
extracellular matrix
- GAG
glycosaminoglycan
- HA
hyaluronic acid
- MMP
matrix metalloproteinase
- NF
nuclear factor
- SMA
smooth muscle actin
- TGF
transforming growth factor
- TIMP
tissue inhibitor of matrix metalloproteinase
Acknowledgments and Funding Sources
We thank Dr. Margaret Smith and Louise Furphy for critically reviewing the manuscript. Financial support was provided by the Henry Langley Research Fellowship, National Health and Medical Research Council (Australia), and The Lincoln Center.
Author Disclosure and Ghostwriting
CJ Jackson holds a related patent. The authors have no other disclosures relevant to this work. No ghostwriters were used to write this article.
About the Authors
Meilang Xue is a senior postdoctoral research fellow and is head of the Autoimmune/Inflammation Research at the Sutton Research Laboratories, Kolling Institute, University of Sydney. Her research focuses on cutaneous wound healing, matrix metalloproteinase, and inflammation. Christopher J. Jackson is Associate Professor and Director of the Sutton Research Laboratories at the Institute of Bone and Joint Research, Kolling Institute, University of Sydney. He has a strong research interest in wound healing and the proteases responsible for extracellular matrix turnover, the matrix metalloproteinases and serine proteases.
References
- 1.Li J, Chen J, and Kirsner R: Pathophysiology of acute wound healing. Clin Dermatol 2007; 25:9. [DOI] [PubMed] [Google Scholar]
- 2.Reinke JM. and Sorg H: Wound repair and regeneration. Eur Surg Res 2012; 49:35. [DOI] [PubMed] [Google Scholar]
- 3.Schilling JA: Wound healing. Surg Clin North Am 1976; 56:859. [DOI] [PubMed] [Google Scholar]
- 4.Szpaderska AM. and DiPietro LA: Inflammation in surgical wound healing: friend or foe? Surgery 2005; 137:571. [DOI] [PubMed] [Google Scholar]
- 5.Redd MJ, Cooper L, Wood W, Stramer B, and Martin P: Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci 2004; 359:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson CJ, Xue M, Thompson P, Davey RA, Whitmont K, Smith S, Buisson-Legendre N, Sztynda T, Furphy LJ, Cooper A, Sambrook P, and March L: Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair Regen 2005; 13:284. [DOI] [PubMed] [Google Scholar]
- 7.Wijewardena A, Vandervord E, Lajevardi SS, Vandervord J, and Jackson CJ: Combination of activated protein C and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int J Low Extrem Wounds 2011; 10:146. [DOI] [PubMed] [Google Scholar]
- 8.Whitmont K, Reid I, Tritton S, March L, Xue M, Lee M, Fulcher G, Sambrook P, Slobedman E, Cooper A, and Jackson C: Treatment of chronic leg ulcers with topical activated protein C. Arch Dermatol 2008; 144:1479. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez-Bendala J, Inverardi L, and Ricordi C: Regeneration of pancreatic beta-cell mass for the treatment of diabetes. Expert Opin Biol Ther 2012; 12:731. [DOI] [PubMed] [Google Scholar]
- 10.Schultz GS. and Wysocki A: Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009; 17:153. [DOI] [PubMed] [Google Scholar]
- 11.Brizzi MF, Tarone G, and Defilippi P: Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol 2012; 24:645. [DOI] [PubMed] [Google Scholar]
- 12.Boot-Handford RP. and Tuckwell DS: Fibrillar collagen: the key to vertebrate evolution? A tale of molecular incest. Bioessays 2003; 25:142. [DOI] [PubMed] [Google Scholar]
- 13.Myllyharju J. and Kivirikko KI: Collagens and collagen-related diseases. Ann Med 2001; 33:7. [DOI] [PubMed] [Google Scholar]
- 14.Kadler KE, Holmes DF, Trotter JA, and Chapman JA: Collagen fibril formation. Biochem J 1996; 316 (Pt 1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamauchi M. and Sricholpech M: Lysine post-translational modifications of collagen. Essays Biochem 2012; 52:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trackman PC: Diverse biological functions of extracellular collagen processing enzymes. J Cell Biochem 2005; 96:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin P, Teodoro WR, Velosa AP, de Morais J, Carrasco S, Christmann RB, Goldenstein-Schainberg C, Parra ER, Katayama ML, Sotto MN, Capelozzi VL, and Yoshinari NH: Abnormal collagen V deposition in dermis correlates with skin thickening and disease activity in systemic sclerosis. Autoimmun Rev 2012; 11:827. [DOI] [PubMed] [Google Scholar]
- 18.Fine JD, Eady RA, Bauer EA, Bauer JW, Bruckner-Tuderman L, Heagerty A, Hintner H, Hovnanian A, Jonkman MF, Leigh I, McGrath JA, Mellerio JE, Murrell DF, Shimizu H, Uitto J, Vahlquist A, Woodley D, and Zambruno G: The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol 2008; 58:931. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Wu H, Byrne M, Krane S, and Jaenisch R: Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A 1997; 94:1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrino DA, Sorrell JM, and Caplan AI: Age-related changes in the proteoglycans of human skin. Arch Biochem Biophys 2000; 373:91. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein EF, Fisher LW, Li K, LeBaron RG, Tan EM, and Uitto J: Differential expression of the versican and decorin genes in photoaged and sun-protected skin. Comparison by immunohistochemical and northern analyses. Lab Invest 1995; 72:662. [PubMed] [Google Scholar]
- 22.Dutt S, Kleber M, Matasci M, Sommer L, and Zimmermann DR: Versican V0 and V1 guide migratory neural crest cells. J Biol Chem 2006; 281:12123. [DOI] [PubMed] [Google Scholar]
- 23.Brown DC. and Vogel KG: Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix 1989; 9:468. [DOI] [PubMed] [Google Scholar]
- 24.Fleischmajer R, Fisher LW, MacDonald ED, Jacobs L, Jr., Perlish JS, and Termine JD: Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol 1991; 106:82. [DOI] [PubMed] [Google Scholar]
- 25.Carrino DA, Calabro A, Darr AB, Dours-Zimmermann MT, Sandy JD, Zimmermann DR, Sorrell JM, Hascall VC, and Caplan AI: Age-related differences in human skin proteoglycans. Glycobiology 2011; 21:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Hinsbergh VW, Collen A, and Koolwijk P: Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci 2001; 936:426. [DOI] [PubMed] [Google Scholar]
- 27.Clark RA: Fibronectin in the skin. J Invest Dermatol 1983; 81:475. [DOI] [PubMed] [Google Scholar]
- 28.To WS. and Midwood KS: Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 2011; 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Repesh LA, Fitzgerald TJ, and Furcht LT: Fibronectin involvement in granulation tissue and wound healing in rabbits. J Histochem Cytochem 1982; 30:351. [DOI] [PubMed] [Google Scholar]
- 30.Zoppi N, Gardella R, De Paepe A, Barlati S, and Colombi M: Human fibroblasts with mutations in COL5A1 and COL3A1 genes do not organize collagens and fibronectin in the extracellular matrix, down-regulate alpha2beta1 integrin, and recruit alphavbeta3 Instead of alpha5beta1 integrin. J Biol Chem 2004; 279:18157. [DOI] [PubMed] [Google Scholar]
- 31.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, and DeLisser HM: Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 2001; 276:36770. [DOI] [PubMed] [Google Scholar]
- 32.Gao F, Liu Y, He Y, Yang C, Wang Y, Shi X, and Wei G: Hyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesis. Matrix Biol 2010; 29:107. [DOI] [PubMed] [Google Scholar]
- 33.Mithieux SM. and Weiss AS: Elastin. Adv Protein Chem 2005; 70:437. [DOI] [PubMed] [Google Scholar]
- 34.Reddy B, Jow T, and Hantash BM: Bioactive oligopeptides in dermatology: part I. Exp Dermatol 2012; 21:563. [DOI] [PubMed] [Google Scholar]
- 35.Durbeej M: Laminins. Cell Tissue Res 2010; 339:259. [DOI] [PubMed] [Google Scholar]
- 36.Domogatskaya A, Rodin S, and Tryggvason K: Functional diversity of laminins. Annu Rev Cell Dev Biol 2012; 28:523. [DOI] [PubMed] [Google Scholar]
- 37.Sugawara K, Tsuruta D, Ishii M, Jones JC, and Kobayashi H: Laminin-332 and -511 in skin. Exp Dermatol 2008; 17:473. [DOI] [PubMed] [Google Scholar]
- 38.Knapinska A. and Fields GB: Chemical biology for understanding matrix metalloproteinase function. Chembiochem 2012; 13:2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagase H, Visse R, and Murphy G: Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006; 69:562. [DOI] [PubMed] [Google Scholar]
- 40.Xue M, Le NT, and Jackson CJ: Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets 2006; 10:143. [DOI] [PubMed] [Google Scholar]
- 41.Franzke CW, Tasanen K, Schacke H, Zhou Z, Tryggvason K, Mauch C, Zigrino P, Sunnarborg S, Lee DC, Fahrenholz F, and Bruckner-Tuderman L: Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs. EMBO J 2002; 21:5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mott JD. and Werb Z: Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 2004; 16:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loffek S, Schilling O, and Franzke CW: Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J 2011; 38:191. [DOI] [PubMed] [Google Scholar]
- 44.Gill SE. and Parks WC: Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008; 40:1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toriseva M. and Kahari VM: Proteinases in cutaneous wound healing. Cell Mol Life Sci 2009; 66:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, and Parks WC: Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest 1993; 92:2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens LJ. and Page-McCaw A: A secreted MMP is required for reepithelialization during wound healing. Mol Biol Cell 2012; 23:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salo T, Makela M, Kylmaniemi M, Autioharmainen H, and Larjava H: Expression of matrix metalloproteinase-2 and -9 during early human wound healing. LabInvest 1994; 70:176. [PubMed] [Google Scholar]
- 49.Lund LR, Romer J, Bugge TH, Nielsen BS, Frandsen TL, Degen JL, Stephens RW, and Dano K: Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J 1999; 18:4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, and Banda MJ: Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg 1999; 230:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, and Yager DR: Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen 1998; 6:127. [DOI] [PubMed] [Google Scholar]
- 52.Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, Werb Z, Krane SM, Lopez-Otin C, and Puente XS: Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J 2007; 21:2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D'Armiento J, and Okada Y: MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol 2009; 175:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, and Bornstein P: Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol 2009; 28:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartenstein B, Dittrich BT, Stickens D, Heyer B, Vu TH, Teurich S, Schorpp-Kistner M, Werb Z, and Angel P: Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice. J Invest Dermatol 2006; 126:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atkinson JJ, Toennies HM, Holmbeck K, and Senior RM: Membrane type 1 matrix metalloproteinase is necessary for distal airway epithelial repair and keratinocyte growth factor receptor expression after acute injury. Am J Physiol Lung Cell Mol Physiol 2007; 293:L600. [DOI] [PubMed] [Google Scholar]
- 57.Le Goff C. and Cormier-Daire V: The ADAMTS(L) family and human genetic disorders. Hum Mol Genet 2011; 20:R163. [DOI] [PubMed] [Google Scholar]
- 58.Jones GC. and Riley GP: ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res Ther 2005; 7:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, Miyata T, and Fujimura Y: Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci U S A 2002; 99:11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W, Byers PH, Lapiere CM, Prockop DJ, and Nusgens BV: Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet 1999; 65:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaalamo M, Leivo T, and Saarialho-Kere U: Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol 1999; 30:795. [DOI] [PubMed] [Google Scholar]
- 62.Stricklin GP, Li L, Jancic V, Wenczak BA, and Nanney LB: Localization of mRNAs representing collagenase and TIMP in sections of healing human burn wounds. Am J Pathol 1993; 143:1657. [PMC free article] [PubMed] [Google Scholar]
- 63.Samuels P. and Tan AK: Fetal scarless wound healing. J Otolaryngol 1999; 28:296. [PubMed] [Google Scholar]
- 64.Lo DD, Zimmermann AS, Nauta A, Longaker MT, and Lorenz HP: Scarless fetal skin wound healing update. Birth Defects Res C Embryo Today 2012; 96:237. [DOI] [PubMed] [Google Scholar]
- 65.Merkel JR, DiPaolo BR, Hallock GG, and Rice DC: Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med 1988; 187:493. [DOI] [PubMed] [Google Scholar]
- 66.Hallock GG, Rice DC, Merkel JR, and DiPaolo BR: Analysis of collagen content in the fetal wound. Ann Plast Surg 1988; 21:310. [DOI] [PubMed] [Google Scholar]
- 67.Lovvorn HN, 3rd, Cheung DT, Nimni ME, Perelman N, Estes JM, and Adzick NS: Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg 1999; 34:218. [DOI] [PubMed] [Google Scholar]
- 68.Dang CM, Beanes SR, Lee H, Zhang X, Soo C, and Ting K: Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast Reconstr Surg 2003; 111:2273. [DOI] [PubMed] [Google Scholar]
- 69.Gawronska-Kozak B: Scarless skin wound healing in FOXN1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biol 2011; 30:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manuel JA. and Gawronska-Kozak B: Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biol 2006; 25:505. [DOI] [PubMed] [Google Scholar]
- 71.Singer AJ. and Clark RA: Cutaneous wound healing. N Engl J Med 1999; 341:738. [DOI] [PubMed] [Google Scholar]
- 72.Profyris C, Tziotzios C, and Do Vale I: Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J Am Acad Dermatol 2012; 66:1–10; quiz 11–12. [DOI] [PubMed] [Google Scholar]
- 73.Fu XB, Sun TZ, Li XK, and Sheng ZY: Morphological and distribution characteristics of sweat glands in hypertrophic scar and their possible effects on sweat gland regeneration. Chin Med J (Engl) 2005; 118:186. [PubMed] [Google Scholar]
- 74.Rennekampff HO, Busche MN, Knobloch K, and Tenenhaus M: Is UV radiation beneficial in postburn wound healing? Med Hypotheses 2010; 75:436. [DOI] [PubMed] [Google Scholar]
- 75.Wolfram D, Tzankov A, Pulzl P, and Piza-Katzer H: Hypertrophic scars and keloids—a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg 2009; 35:171. [DOI] [PubMed] [Google Scholar]
- 76.Whitby DJ. and Ferguson MW: The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 1991; 112:651. [DOI] [PubMed] [Google Scholar]
- 77.Armour A, Scott PG, and Tredget EE: Cellular and molecular pathology of HTS: basis for treatment. Wound Repair Regen 2007; 15Suppl 1:S6. [DOI] [PubMed] [Google Scholar]
- 78.Ehrlich HP. and Krummel TM: Regulation of wound healing from a connective tissue perspective. Wound Repair Regen 1996; 4:203. [DOI] [PubMed] [Google Scholar]
- 79.Hayakawa T, Hashimoto Y, Myokei Y, Aoyama H, and Izawa Y: Changes in type of collagen during the development of human post-burn hypertrophic scars. Clin Chim Acta 1979; 93:119. [DOI] [PubMed] [Google Scholar]
- 80.Regan MC, Kirk SJ, Wasserkrug HL, and Barbul A: The wound environment as a regulator of fibroblast phenotype. J Surg Res 1991; 50:442. [DOI] [PubMed] [Google Scholar]
- 81.Vedrenne N, Coulomb B, Danigo A, Bonte F, and Desmouliere A: The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol Biol (Paris) 2012; 60:20. [DOI] [PubMed] [Google Scholar]
- 82.Larsen M, Artym VV, Green JA, and Yamada KM: The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 2006; 18:463. [DOI] [PubMed] [Google Scholar]
- 83.Leitinger B: Transmembrane collagen receptors. Annu Rev Cell Dev Biol 2011; 27:265. [DOI] [PubMed] [Google Scholar]
- 84.Klingberg F, Hinz B, and White ES: The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 2013; 229:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Z, Li XJ, Liu Y, Zhang X, Li YY, and Xu WS: Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns 2007; 33:634. [DOI] [PubMed] [Google Scholar]
- 86.Johnson PY, Potter-Perigo S, Gooden MD, Vernon RB, and Wight TN: Decorin synthesized by arterial smooth muscle cells is retained in fibrin gels and modulates fibrin contraction. J Cell Biochem 2007; 101:281. [DOI] [PubMed] [Google Scholar]
- 87.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, and Ross R: Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell 1996; 87:1069. [DOI] [PubMed] [Google Scholar]
- 88.Carragher NO, Levkau B, Ross R, and Raines EW: Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol 1999; 147:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarrazy V, Billet F, Micallef L, Coulomb B, and Desmouliere A: Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen 2011; 19Suppl 1:s10. [DOI] [PubMed] [Google Scholar]
- 90.Estes JM, Vande Berg JS, Adzick NS, MacGillivray TE, Desmouliere A, and Gabbiani G: Phenotypic and functional features of myofibroblasts in sheep fetal wounds. Differentiation 1994; 56:173. [DOI] [PubMed] [Google Scholar]
- 91.Chen MA. and Davidson TM: Scar management: prevention and treatment strategies. Curr Opin Otolaryngol Head Neck Surg 2005; 13:242. [DOI] [PubMed] [Google Scholar]
- 92.Simon F, Bergeron D, Larochelle S, Lopez-Valle CA, Genest H, Armour A, and Moulin VJ: Enhanced secretion of TIMP-1 by human hypertrophic scar keratinocytes could contribute to fibrosis. Burns 2012; 38:421. [DOI] [PubMed] [Google Scholar]
- 93.Huang C, Akaishi S, and Ogawa R: Mechanosignaling pathways in cutaneous scarring. Arch Dermatol Res 2012; 304:589. [DOI] [PubMed] [Google Scholar]
- 94.Leung A, Crombleholme TM, and Keswani SG: Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr 2012; 24:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castoldi E. and Rosing J: APC resistance: biological basis and acquired influences. J Thromb Haemost 2010; 8:445. [DOI] [PubMed] [Google Scholar]
- 96.Sidgwick GP. and Bayat A: Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 2012; 26:141. [DOI] [PubMed] [Google Scholar]
- 97.Plutzky J, Hoskins JA, Long GL, and Crabtree GR: Evolution and organization of the human protein C gene. Proc Natl Acad Sci U S A 1986; 83:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baisch A. and Riedel F: Hyperplastic scars and keloids. Part I: basics and prevention. HNO 2006; 54:893. [DOI] [PubMed] [Google Scholar]
- 99.Fujiwara M, Muragaki Y, and Ooshima A: Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol 2005; 153:295. [DOI] [PubMed] [Google Scholar]
- 100.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, and Jeschke MG: Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 2011; 17:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abergel RP, Chu ML, Bauer EA, and Uitto J: Regulation of collagen gene expression in cutaneous diseases with dermal fibrosis: evidence for pretranslational control. J Invest Dermatol 1987; 88:727. [DOI] [PubMed] [Google Scholar]
- 102.Lim IJ, Phan TT, Tan EK, Nguyen TT, Tran E, Longaker MT, Song C, Lee ST, and Huynh HT: Synchronous activation of ERK and phosphatidylinositol 3-kinase pathways is required for collagen and extracellular matrix production in keloids. J Biol Chem 2003; 278:40851. [DOI] [PubMed] [Google Scholar]
- 103.Babu M, Diegelmann R, and Oliver N: Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol 1989; 9:1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chua AW, Gan SU, Ting Y, Fu Z, Lim CK, Song C, Sabapathy K, and Phan TT: Keloid fibroblasts are more sensitive to Wnt3a treatment in terms of elevated cellular growth and fibronectin expression. J Dermatol Sci 2011; 64:199. [DOI] [PubMed] [Google Scholar]
- 105.Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, Kapanci Y. and Gabbiani G: Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 1994; 145:105. [PMC free article] [PubMed] [Google Scholar]
- 106.Shih B, Garside E, McGrouther DA, and Bayat A: Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen 2010; 18:139. [DOI] [PubMed] [Google Scholar]
- 107.Verhaegen PD, van Zuijlen PP, Pennings NM, van Marle J, Niessen FB, van der Horst CM, and Middelkoop E: Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: an objective histopathological analysis. Wound Repair Regen 2009; 17:649. [DOI] [PubMed] [Google Scholar]
- 108.Blackburn WR. and Cosman B: Histologic basis of keloid and hypertrophic scar differentiation. Clinicopathologic correlation. Arch Pathol 1966; 82:65. [PubMed] [Google Scholar]
- 109.Linares HA, Kischer CW, Dobrkovsky M, and Larson DL: The histiotypic organization of the hypertrophic scar in humans. J Invest Dermatol 1972; 59:323. [DOI] [PubMed] [Google Scholar]
- 110.Syed F, Ahmadi E, Iqbal SA, Singh S, McGrouther DA, and Bayat A: Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol 2011; 164:83. [DOI] [PubMed] [Google Scholar]
- 111.Naitoh M, Hosokawa N, Kubota H, Tanaka T, Shirane H, Sawada M, Nishimura Y, and Nagata K: Upregulation of HSP47 and collagen type III in the dermal fibrotic disease, keloid. Biochem Biophys Res Commun 2001; 280:1316. [DOI] [PubMed] [Google Scholar]
- 112.Agarwal P, Schulz JN, Blumbach K, Andreasson K, Heinegard D, Paulsson M, Mauch C, Eming SA, Eckes B, and Krieg T: Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biol 2013; 32:325–331 [DOI] [PubMed] [Google Scholar]
- 113.Sayani K, Dodd CM, Nedelec B, Shen YJ, Ghahary A, Tredget EE, and Scott PG: Delayed appearance of decorin in healing burn scars. Histopathology 2000; 36:262. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Z, Liu Y, Zhang X, and Xu WS: The content of decorin and its mRNA expression in normal human skin and hyperplastic scars. Zhonghua Shao Shang Za Zhi 2004; 20:76. [PubMed] [Google Scholar]
- 115.van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, Middelkoop E, and Niessen FB: Potential cellular and molecular causes of hypertrophic scar formation. Burns 2009; 35:15. [DOI] [PubMed] [Google Scholar]
- 116.Zhang C, Tan CK, McFarlane C, Sharma M, Tan NS, and Kambadur R: Myostatin-null mice exhibit delayed skin wound healing through the blockade of transforming growth factor-beta signaling by decorin. Am J Physiol Cell Physiol 2012; 302:C1213. [DOI] [PubMed] [Google Scholar]
- 117.Honardoust D, Varkey M, Marcoux Y, Shankowsky HA, and Tredget EE: Reduced decorin, fibromodulin, and transforming growth factor-beta3 in deep dermis leads to hypertrophic scarring. J Burn Care Res 2012; 33:218. [DOI] [PubMed] [Google Scholar]
- 118.Ghahary A, Shen YJ, Nedelec B, Wang R, Scott PG, and Tredget EE: Collagenase production is lower in post-burn hypertrophic scar fibroblasts than in normal fibroblasts and is reduced by insulin-like growth factor-1. J Invest Dermatol 1996; 106:476. [DOI] [PubMed] [Google Scholar]
- 119.Nedelec B, Shankowsky H, Scott PG, Ghahary A, and Tredget EE: Myofibroblasts and apoptosis in human hypertrophic scars: the effect of interferon-alpha2b. Surgery 2001; 130:798. [DOI] [PubMed] [Google Scholar]
- 120.Kischer CW: The microvessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol 1992; 24:281. [PubMed] [Google Scholar]
- 121.Moulin V, Larochelle S, Langlois C, Thibault I, Lopez-Valle CA, and Roy M: Normal skin wound and hypertrophic scar myofibroblasts have differential responses to apoptotic inductors. J Cell Physiol 2004; 198:350. [DOI] [PubMed] [Google Scholar]
- 122.De Felice B, Garbi C, Santoriello M, Santillo A, and Wilson RR: Differential apoptosis markers in human keloids and hypertrophic scars fibroblasts. Mol Cell Biochem 2009; 327:191. [DOI] [PubMed] [Google Scholar]
- 123.Duffield JS, Lupher M, Thannickal VJ, and Wynn TA: Host responses in tissue repair and fibrosis. Annu Rev Pathol 2013; 8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Makino S, Mitsutake N, Nakashima M, Saenko VA, Ohtsuru A, Umezawa K, Tanaka K, Hirano A, and Yamashita S: DHMEQ, a novel NF-kappaB inhibitor, suppresses growth and type I collagen accumulation in keloid fibroblasts. J Dermatol Sci 2008; 51:171. [DOI] [PubMed] [Google Scholar]
- 125.Chalmers E, Cooper P, Forman K, Grimley C, Khair K, Minford A, Morgan M, and Mumford AD: Purpura fulminans: recognition, diagnosis and management. Arch Dis Child 2011; 96:1066. [DOI] [PubMed] [Google Scholar]
- 126.Abdou AG, Maraee AH, Al-Bara AM, and Diab WM: Immunohistochemical expression of TGF-beta1 in keloids and hypertrophic scars. Am J Dermatopathol 2011; 33:84. [DOI] [PubMed] [Google Scholar]
- 127.Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA, and Reitsma PH: Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64. [DOI] [PubMed] [Google Scholar]
- 128.Chalmers RL: The evidence for the role of transforming growth factor-beta in the formation of abnormal scarring. Int Wound J 2011; 8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shah M, Foreman DM, and Ferguson MW: Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet 1992; 339:213. [DOI] [PubMed] [Google Scholar]
- 130.Ulrich D, Ulrich F, Unglaub F, Piatkowski A, and Pallua N: Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg 2010; 63:1015. [DOI] [PubMed] [Google Scholar]
- 131.Eto H, Suga H, Aoi N, Kato H, Doi K, Kuno S, Tabata Y, and Yoshimura K: Therapeutic potential of fibroblast growth factor-2 for hypertrophic scars: upregulation of MMP-1 and HGF expression. Lab Invest 2012; 92:214. [DOI] [PubMed] [Google Scholar]
- 132.Neely AN, Clendening CE, Gardner J, Greenhalgh DG, and Warden GD: Gelatinase activity in keloids and hypertrophic scars. Wound Repair Regen 1999; 7:166. [DOI] [PubMed] [Google Scholar]
- 133.Tanriverdi-Akhisaroglu S, Menderes A, and Oktay G: Matrix metalloproteinase-2 and -9 activities in human keloids, hypertrophic and atrophic scars: a pilot study. Cell Biochem Funct 2009; 27:81. [DOI] [PubMed] [Google Scholar]
- 134.Lauer-Fields JL, Juska D, and Fields GB: Matrix metalloproteinases and collagen catabolism. Biopolymers 2002; 66:19. [DOI] [PubMed] [Google Scholar]
- 135.Knott L. and Bailey AJ: Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone 1998; 22:181. [DOI] [PubMed] [Google Scholar]
- 136.Romanic AM, Adachi E, Hojima Y, Engel J, and Prockop DJ: Polymerization of pNcollagen I and copolymerization of pNcollagen I with collagen I. A kinetic, thermodynamic, and morphologic study. J Biol Chem 1992; 267:22265. [PubMed] [Google Scholar]
- 137.Podobed OV, Prozorovskii NN, Kozlov EA, Tsvetkova TA, Vozdvizhenskii SI, and Del'vig AA: Comparative study of collagen in hypertrophic and keloid cicatrix. Vopr Med Khim 1996; 42:240. [PubMed] [Google Scholar]
- 138.Sopel MJ, Rosin NL, Falkenham AG, Bezuhly M, Esmon CT, Lee TD, Liwski RS, and Legare JF: Treatment with activated protein C (aPC) is protective during the development of myocardial fibrosis: an angiotensin II infusion model in mice. PLoS One 2012; 7:e45663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Julovi SM, Xue M, Dervish S, Sambrook PN, March L, and Jackson CJ: Protease activated receptor-2 mediates activated protein C-induced cutaneous wound healing via inhibition of p38. Am J Pathol 2011; 179:2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deitch EA, Wheelahan TM, Rose MP, Clothier J, and Cotter J: Hypertrophic burn scars: analysis of variables. J Trauma 1983; 23:895. [PubMed] [Google Scholar]
- 141.Cubison TC, Pape SA, and Parkhouse N: Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns 2006; 32:992. [DOI] [PubMed] [Google Scholar]
- 142.Hassan S, Reynolds G, Clarkson J, and Brooks P: Challenging the dogma: relationship between time to healing and formation of hypertrophic scars after burn injuries. J Burn Care Res 2013. PMID: 23877141 [DOI] [PubMed] [Google Scholar]
- 143.Esmon CT: Crosstalk between inflammation and thrombosis. Maturitas 2004; 47:305. [DOI] [PubMed] [Google Scholar]
- 144.Lay AJ, Donahue D, Tsai MJ, and Castellino FJ: Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency. Blood 2007; 109:1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee YJ, Jeong JK, Seol JW, Xue M, Jackson C, and Park SY: Activated protein C differentially regulates both viability and differentiation of osteoblasts mediated by bisphosphonates. Exp Mol Med 2013; 45:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gillibert-Duplantier J, Rullier A, Neaud V, Kisiel W, and Rosenbaum J: Liver myofibroblasts activate protein C and respond to activated protein C. World J Gastroenterol 2010; 16:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xue M, Smith MM, Little CB, Sambrook P, March L, and Jackson CJ: Activated protein C mediates a healing phenotype in cultured tenocytes. J Cell Mol Med 2009; 13:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xue M, Minhas N, Chow SO, Dervish S, Sambrook PN, March L, and Jackson CJ: Endogenous protein C is essential for the functional integrity of human endothelial cells. Cell Mol Life Sci 2010; 67:1537–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xue M, Thompson P, Sambrook PN, March L, and Jackson CJ: Activated protein C stimulates expression of angiogenic factors in human skin cells, angiogenesis in the chick embryo and cutaneous wound healing in rodents. Clin Hemorheol Microcirc 2006; 34:153. [PubMed] [Google Scholar]
- 150.Xue M, March L, Sambrook PN, and Jackson CJ: Differential regulation of matrix metalloproteinase 2 and matrix metalloproteinase 9 by activated protein C: relevance to inflammation in rheumatoid arthritis. Arthritis Rheum 2007; 56:2864. [DOI] [PubMed] [Google Scholar]