Abstract
Significance: Proteoglycans have a distinct spatial localization in normal skin and are essential for the correct structural development, organization, hydration, and functional properties of this tissue. The extracellular matrix (ECM) is no longer considered to be just an inert supportive material but is a source of directive, spatial and temporal, contextual information to the cells via components such as the proteoglycans. There is a pressing need to improve our understanding of how these important molecules functionally interact with other matrix structures, cells and cellular mediators in normal skin and during wound healing.
Recent Advances: New antibodies to glycosaminoglycan side chain components of skin proteoglycans have facilitated the elucidation of detailed localization patterns within skin. Other studies have revealed important proliferative activities of proteinase-generated fragments of proteoglycans and other ECM components (matricryptins). Knockout mice have further established the functional importance of skin proteoglycans in the assembly and homeostasis of the normal skin ECM.
Critical Issues: Our comprehension of the molecular and structural complexity of skin as a complex, dynamic, constantly renewing, layered connective tissue is incomplete. The impact of changes in proteoglycans on skin pathology and the wound healing process is recognized as an important area of pathobiology and is an area of intense investigation.
Future Directions: Advanced technology is allowing the development of new artificial skins. Recent knowledge on skin proteoglycans can be used to incorporate these molecules into useful adjunct therapies for wound healing and for maintenance of optimal tissue homeostasis in aging skin.

Margaret Mary Smith, PhD

James Melrose, PhD
Introduction
Scope and significance
The scope of this review is to detail the complexity and localization of proteoglycans in skin. These structurally diverse molecules are now recognized as important in the development, function, metabolism, damage (whether by aging, ultraviolet [UV] irradiation, or injury), and healing of this tissue.
Translational relevance
Proteoglycans support the hydration of the extracellular matrix (ECM) of normal skin, providing resilience, viscoelasticity, and a cushioned environment conducive to cellular function and development. Proteoglycans also act in supportive scaffolding roles as struts and connectors, which aid in the proper alignment of fibrous and elastic components in skin. Many proteoglycans have the ability to sequester and control the bioavailability of growth factors in the ECM surrounding cells. These growth factors stimulate cell populations in skin that orchestrate the normal turnover and repair.
Clinical relevance
There is a critical need to recapitulate the normal intricately organized ECM of healthy young skin after injury. Armed with a greater knowledge of normal skin composition, structural organization, and the functional properties of its constituent proteoglycans, we will better understand deviations in these components that occur in aged and damaged skin, where healing may be slower, incomplete, and/or aberrant (fibrosis/scarring). This will lead to new treatments aimed at altering the content of certain proteoglycan components of the skin ECM to enhance repair and, ultimately, scarless wound healing.
Proteoglycans
Proteoglycans are glycosylated molecules where one or more specific glycosaminoglycan (GAG) and/or O- and N-linked oligosaccharides are attached to a core protein. The GAGs are usually sulfated; chondroitin sulfate/dermatan sulfate (CS/DS), keratan sulfate (KS), and heparan sulfate (HS)/heparin are the most common.1 GAG chain length, degree, and position of sulfation and degree of epimerization greatly vary, (1) between different proteoglycans, (2) on the same proteoglycan at different sites, and (3) between the same proteoglycans in different tissues. These variations in GAG attachments are of both functional and developmental significance. Chondroitin 4-O-sulfation is required for proper CS localization and modulation of signaling pathways in tissue morphogenesis and emerging biological roles in mammalian development.2,3 Detailed structural analyses on HS and heparin indicate these molecules are important in information storage and transfer.4
The complexity of these sugar-protein structures suggests new facets to an old paradigm in developmental biology, with the emergence of the sugar code and realization that dynamic changes in HS produce a characteristic (nonrandom) heparanome for cells.1 GAGs can interact with many bioactive binding partners to trigger cell signaling, proliferation, ECM production, and differentiation, underscoring their importance in developmental processes.5 Proteoglycans can be classified on the basis of the type of GAG chain they possess and by their tissue location, with a clear distinction between those that reside in the ECM and those that are cell-associated. ECM proteoglycans are usually substituted with CS, DS, and/or KS; cell-associated proteoglycans are more commonly substituted with HS. Most work conducted on growth factor/morphogen interactivity with GAGs has centered on HS and DS.2 Heparin is a component of the intracellular proteoglycan of mast cells; serglycin6 and similar proteoglycans are synthesized by monocytes/macrophages, T-lymphocytes, and endothelial cells. Often these molecules contain oversulfated chondroitins in addition to HS.
Skin composition
As the largest organ in the body, skin is also one of the most dynamic and complex of organs, with a constant renewal of both ECM and cells throughout life. The varied population of resident cell types throughout the well-defined layers of skin (epidermis and dermis), include epithelial, fibroblasts, keratinocytes, vascular endothelial, lymphatic endothelial, melanocytes, and nerve cells, each of which are capable of producing a unique set of ECM proteoglycans. The main cell types of the epidermis and the dermis are the keratinocyte and the fibroblast respectively. The cellular complexity of skin is further increased during healing responses with cells including mast cells, monocytes/macrophages, polymorphonuclear leucocytes, and cytotoxic T-lymphocytes migrating into the tissue as a response to infection.
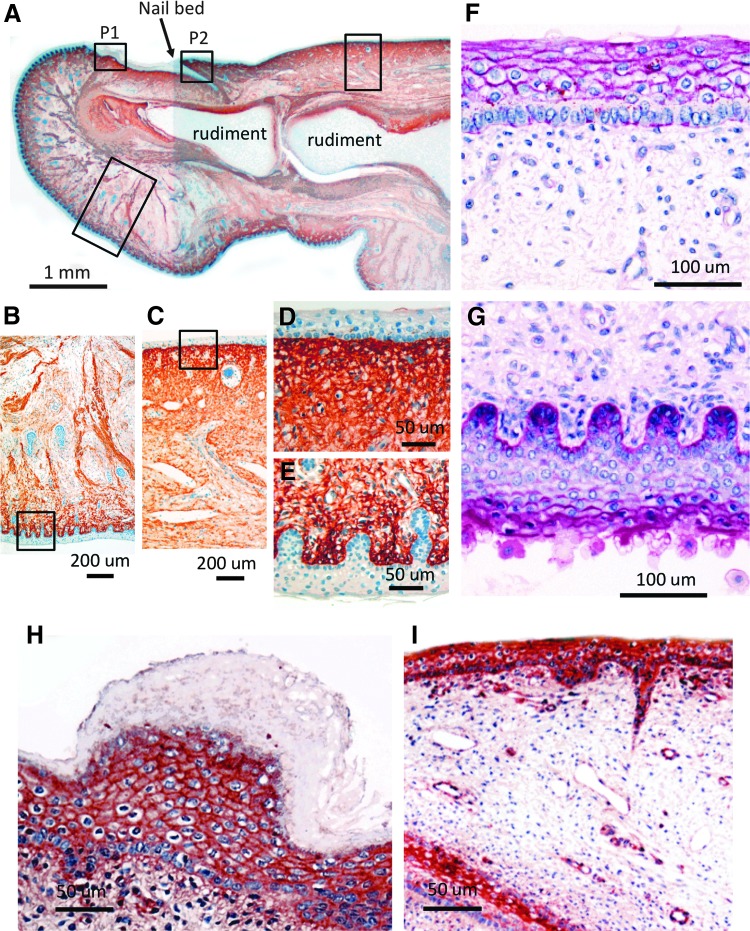
The macrostructure of normal skin heavily depends on the three-dimensional (3D) organization of the constituent collagen fibers. The most prevalent ECM structural component in skin is collagen type I, shown localized to the dermis in fetal tissue in Figure 1A–E. Normal healthy collagenous matrix alignment requires certain proteoglycans for both initial formation and maintenance. The proteoglycan composition and structural complexity of skin ECM layers will thus be a product of the metabolism (both synthesis and turnover) of the tissue's current and previous cell populations.
Figure 1.
Immunolocalization of type I collagen (A–E), periodic acid-Schiff-positive glycosaminoglycans (GAGs) (F, G), and perlecan (H, I) in vertical sections of human fetal big toe (14 weeks gestation). The boxed areas in photosegment (A) represent selected areas of the underside and upper-surface of the big toe presented at higher magnification in (B, E, G) and (C, D, F, H, I) respectively. The central cartilage rudiments of the most distal toe joint and the nail bed are labeled for orientation of the specimen. The specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, and then 4 μm microtome sections were adhered to positively charged microscope slides. Type I collagen was immunolocalized using a mouse monoclonal antibody (2 μg/mL; Clone I-8H5 from MP Biomedicals) overnight at 4°C. Primary antibody localization was visualized using a biotinylated-anti-mouse IgG-conjugated secondary antibody using diaminobenzidene as chromogen with 10 min color development.216 Type I collagen was prominently immunolocalized in the dermis but not the epidermis and clearly demarcated these tissues.216,217 Periodic acid-Schiff-positive staining of the upper (F) and underside of skin (G) visualized GAG localized at the epidermal–dermal junction and pericellularly in the epidermis.218–220 The boxed areas labeled P1 and P2 in (A) represent areas where perlecan immunolocalization is also depicted at higher magnification in (H, I). Monoclonal antibody A7L6 to perlecan domain IV was used for immunolocalization using NovaRED as chromogen.216,217 Perlecan is a prominent proteoglycan of the epidermis and epidermal–dermal junction and the nail bed but is largely absent from the keratinized tissue overlying the nail bed in (H). Scale bars 1 mm in (A), 200 μm in (B, C) 50 μm in (D, E, H, I), and 100 μm in (F, G). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Proteoglycans are also localized in other structures within skin. Hair follicles consist of a hair shaft sprouting from differentiated keratinocytes within an inner and outer root sheath located in the epidermis and a dermal papilla and connective tissue sheath below, in the dermis. Each of these separately developed tissues is rich in a variety of different ECM proteoglycans.7
Extracellular Proteoglycans
Most of the proteoglycans expressed in tissue ECM through the body are also present in skin (Table 1; Fig. 1F, G). These include large, aggregating, space-filling, water-retaining proteoglycans, particularly versican, and the small leucine-rich repeat proteoglycans (SLRPs) decorin, biglycan, lumican, keratocan, and fibromodulin. HS-containing proteoglycans, such as perlecan and the syndecans (SDCs), have also been localized to specific sites in hair follicles and to the dermis during the growth phase.
Table 1.
Skin proteoglycans
| Proteoglycan | Core-protein (kDa) | GAG | Tissue/cellular location |
|---|---|---|---|
| Extracellular proteoglycans | |||
| Versican Vo isoform | 373 | CS | ECM, widespread distribution in epidermis, dermis, basal muscle, and vasculature and elastic networks in skina,10 |
| Versican V1 isoform | 265 | CS | |
| Versican V2 isoform | 180 | CS | |
| Versican V3 isoform | 72 | GAG absent | |
| Aggrecan | CS/KS | Absent from normal skin; accumulates in scar tissue | |
| Perlecan | 467 | HS, varies with cell typeb | Ubiquitous cell associated pericellular and ECM component, basement membrane basal epidermis/dermis40,191,223 |
| Decorin | 36 | CS/DS | ECM, collagen fibril associated201 |
| Biglycan | 38 | CS/DS | ECM and cell associated201 |
| Fibromodulin | 42 | KS | ECM, collagen fibril associated201 |
| Lumican | 38 | KS | ECM, collagen fibril associated176, 177, 201 |
| Keratocan | 37 | KS | ECM75 |
| Cell-associated proteoglycans | |||
| GPC-1 | 56 | HS | Epidermis, keratinocyte pericellular matrix107 |
| SDC-1 | 33 | HS, CS/DS | Epidermis/dermis, pericellular component of keratinocytes, fibroblasts also expressed by capillary endothelial and glandular cells187 |
| SDC-2 | 23 | HS | |
| SDC-4 | 22 | HS | |
| NG2/CSPG-4 | 251 | CS | Transmembrane81 |
| Intracellular proteoglycans | |||
| Serglycin | 10–19 | HS,C6S,CS-B, CS-E, DS | Intracellular proteoglycan of secretory granules in mast cells, endothelial cells, neutrophils, cytotoxic T-lymphocytes115,192 |
| Part time proteoglycan co-receptors | |||
| CD44 (includes epican) | 37–81c | CS/DS or HSd | Transmembrane HA receptor, some variants identified in inflammatory and autoimmune skin conditionse,143,224 |
| Betaglycan | 110 | HS/CS | Transmembrane, type III TGF-β coreceptor225 |
| Endoglin | 68 dimer | HS/CS | TGF-β co-receptor, modulates TGF-β signaling136,137,226 |
Versican exists as four isoforms, however, it is not fully known how their localizations vary in skin.
Of the perlecans that have been isolated and characterized for specific cell types, endothelial cells produce the archetypal form of perlecan, which only contains HS substitution; smooth muscle cells produce a hybrid for containing C4S and HS while keratinocytes are unique in the proteoglycan field producing a form of perlecan containing HS, CS, and KS.
Variable core protein sizes reflects the 19 variant forms identified.
HS substitution identified in CD44 variant epiphycan.
CD44s, CD44v3, CD44v6, and CD44v7 have been identified in the epidermis/dermis of normal skin and in cutaneous lupus erythematosus, dermatomyositis, scleroderma, and scleromyxedema.
GAG, glycosaminoglycan; GPC, glypican; CS, chondroitin sulfate; DS, dermatan sulfate; ECM, extracellular matrix; HA, hyaluronan; HS, heparan sulfate; KS, keratan sulfate; NG2, nerve-glial antigen-2; CSPG, chondroitin sulfate proteoglycan; SDC, syndecan; TGF-β, transforming growth factor-β.
Versican
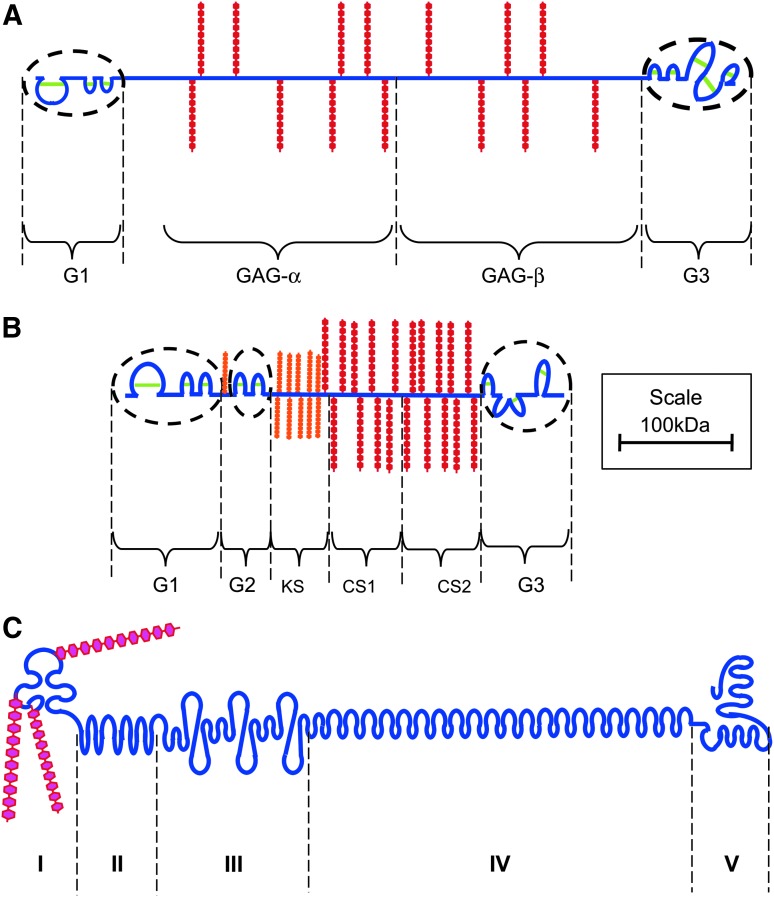
Versican is so named from its multiple protein-binding motifs and its versatility of function. The versican gene, originally cloned from fibroblasts, encodes a chondroitin sulfate proteoglycan (CSPG),8 which is structurally related to the other large space-filling proteoglycan, aggrecan, possessing terminal domains analogous to the aggrecan G1 and G3 regions (Fig. 2). Versican does not contain an interglobular domain (IGD) or G2 region like aggrecan and its central GAG-attachment regions (GAGα and GAGβ) also differ in amino acid sequence and GAG attachments. The human versican gene (VCAN) is composed of 15 exons, with alternative splicing occurring in exons encoding the GAG-attachment region.9 This generates four VCAN mRNAs. The presence of both the GAGα and GAGβ regions gives rise to the V0 form of versican, the presence of only the GAGβ region gives rise to the V1 form, the presence of only the GAGα region gives rise to the V2 form, and the absence of both the GAGα and GAGβ regions gives rise to the V3 form. We can detect both the GAGα and GAGβ regions in young healthy mouse skin (Fig. 3), which agrees with the presence of V0 and V1 isoforms; a recent study was unable to detect V2 and V3 in adult human skin.10 The V1 isoform of versican is the most common form found in the ECM of many tissues and can be cleaved by aggrecanases (a disintegrin and metalloproteinase with thrombospondin motifs [ADAMTS]-1, -4, -5, and -9)11 and matrix metalloproteinases (MMPs).12
Figure 2.
Structural depiction of the major extracellular matrix proteoglycans of skin versican (A), aggrecan (B), perlecan (C). The disulfide stabilized (green) globular domains of versican and aggrecan are circled, and domains I–V of perlecan are in bolded capitals. Core proteins are depicted in blue, chondroitin sulfate (CS) side chains in red, keratan sulfate (KS) in orange, and heparan sulfate (HS) chains in cerise. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 3.
Localization of versican GAGα (A) and GAGβ (B) splice variants in formalin-fixed paraffin-mounted sections of back skin from 12-week-old C57BL/6 wild-type mice. The immunolocalizations were undertaken using rabbit polyclonal antibodies to the GAGα (amino acids 535–598) and GAGβ (amino acids 1,360–1,439) core protein domains of mouse versican216,221 purchased from Chemicon through Invitrogen and visualized with the Dako Envision rabbit detection system (NovaRED chromogen). An isotype negative control for versican GAGα is given in (C). The surface (S) and underlying muscle (M) are marked. Arrows indicate the pericellular localization of versican. Scale bar=100 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Versican does not have a KS attachment region on the core protein and, compared to aggrecan, contains significantly fewer CS chains that are more sparsely distributed along a 400-kDa core protein. Like aggrecan, the G1 region of versican is a functional hyaluronan (HA)-binding region. Versican, together with HA, a variable molecular weight nonsulfated linear GAG, forms macro-aggregates that are important for skin hydration and viscoelasticity.13 Highly hydrated versican-HA aggregates yield looser matrices than aggrecan-HA aggregates and are hence more conducive to cellular migration, an important property for wound healing. Versican is a major component of blood vessels in skin and associated with elastic networks through G3-mediated interactions with fibulin-1,14 a component of elastic microfibrils. As with aggrecan, the lectin domain within the G3 region of versican also has the ability to interact with tenascins and matrilin-1 and -3, enabling stabilization of extended collagen-HA-versican networks within tissues.15 These networks are presumed to be important for sensing of the mechanical microenvironment at the local level, allowing these mechanosensory processes to regulate tissue metabolism.15
Versican not only acts as a structural entity but it also influences cellular function through G3-mediated interactions with epidermal growth factor (EGF) receptors. Versican can promote mammary tumor and melanoma progression16,17 and is overexpressed in cutaneous malignant melanoma.18 Aggrecanase-cleaved fragments of versican regulate the regression of ECM after apoptosis in embryonic interdigital webs.11
Aggrecan
Aggrecan is the major proteoglycan in articular cartilage and can form extremely large, link protein-stabilized aggregates with HA. It has a core protein of 250 kDa, three IGDs (G1, G2, and G3) and specific CS and KS-binding domains (Fig. 2). Aggrecan is normally a minor constituent of skin (Table 1), however it can accumulate in this tissue in mice when ADAMTS-5 (aggrecanase) activity is ablated.19 This accumulation occurs in the pericellular matrix of progenitor fibroblasts, perhaps preventing them from maturing sufficiently to assist in wound healing. Aggrecan gene (ACAN) expression is also elevated during keloid scarring (see Proteoglycans in Wound Healing section). Aggrecan is absent from the dermis of normal adult skin but can be seen in the connective tissue sheath and under the hair matrix in follicles.7
Antitumor and antiangiogenic agents based on the G1 domain of aggrecan and versican have been developed and are examples of antagonistic matricryptins. Neovastat (AE-941) has undergone phase I/II clinical trials in plaque psoriasis;20 Metastatin from cartilage aggrecan21 and a 42-mer amino acid peptide based on Metastatin's HA-binding motif (BH-P)22 potently inhibit the proliferation and colony-forming capability of B16 melanoma cells by activating caspase-3 and -8 pathways to selectively trigger apoptosis only in these cells.
Perlecan
Perlecan (heparin sulfate proteoglycan 2 [HSPG2]) is a basement membrane proteoglycan named after its “string of pearls” appearance under electron microscopy. The perlecan core protein is large (467 kDa) and contains five domains. Endothelial cells produce perlecan containing three HS chains in domain-I, smooth muscle cell, and chondrocyte; perlecan has some HS chains replaced by CS, while keratinocytes produce a form of perlecan containing KS, CS, and HS.23 Although perlecan has a ubiquitous pericellular distribution in fetal and adult skin, it is a particularly prominent component of the human fetal epidermis and less abundant in fetal dermis (Fig. 1H, I). Based on the temporal distribution of perlecan in other tissues (meniscus and cartilage),24,25 we deduce that the amount of perlecan will be diminished in adult compared with fetal skin; however, this has yet to be confirmed.
Each of the five core protein domains of perlecan display homology to growth factors, protein modules involved in lipid metabolism, cell adhesion, and matrix assembly/stabilization.26 The HS chains mediate growth factor and morphogen interactions that are extremely important during organ development. Perlecan interacts with fibroblast growth factors (FGF)-1, -2, -7, -9, and -18, platelet-derived growth factor (PDGF), vascular endothelial cell growth factor (VEGF), hepatocyte growth factor, bone morphogenetic protein (BMP)-1, -2, -4, and -7, hedgehog, Wnt, and Activin A to promote cell proliferation, differentiation, and ECM production27 and with laminin, fibronectin, thrombospondin, α5β1, and α2β1 integrin to promote cell attachment and recruitment in development and tissue remodeling. Perlecan also interacts with proline/arginine-rich and leucine-rich repeat protein (PRELP), von Willebrand factor A domain-related protein, types IV, VI, XIII, and XVIII collagen, fibrillin-1 and -2, nidogen-1 and -2, latent transforming growth factor (TGF) beta-binding protein (LTBP)-1 and -2, fibulin-2, and tropoelastin to promote and stabilize ECM assembly.26,28,29 Perlecan is a low affinity FGF co-receptor participating in cell signaling to drive cell proliferation and ECM production.30 Recent studies have also shown perlecan-HS interactions are important in fibrillin and elastin assembly28,29,31 and ECM stabilization when located on dermal elastic fibers in skin.32
Studies in Hspg2 exon 3 null mice33 reveal the specific role perlecan-HS chains have in skeletal development. Healing of skin lesions in these knockout mice was significantly delayed with impaired angiogenesis.34 Little is known of how perlecan is processed in skin; this could potentially be through alternative splicing and/or proteolytic processing. Mast cells contain multiple, truncated forms of perlecan,35 some of which have known matrikine functions. Tolloid-like metalloproteinase (BMP-1) cleaves between the LG2 and LG3 domains of domain V, releasing an antiangiogenic peptide termed endorepellin,36–38 another matricryptin. Endorepellin interacts with α2β1 integrin and disrupts endothelial-basement membrane interactions critical for the stabilization of tube formation. This forms the basis of its antiangiogenic activity, contrasting with native perlecan, which promotes cellular attachment and vasculogenesis.36–38
Endothelial cell perlecan is also susceptible to cleavage in domains IV and V on digestion with MMP-1, -3, and plasmin.39 A recent study examining the supramolecular organization of the epidermis–dermis basement membrane of normal skin revealed a composite structure composed of independent laminin 332 and type IV collagen networks held together by perlecan aggregates at strategic points in a spot-welding type manner.40 Perlecan-deficient keratinocytes assemble a very thin and poorly organized epidermis due to premature apoptosis and a failure to lay down a normal stratified epidermal layer.41 Perlecan regulates the survival and terminal differentiation of keratinocytes in the epidermis; thus, deficient levels are detrimental to the integrity of the basement membrane.41
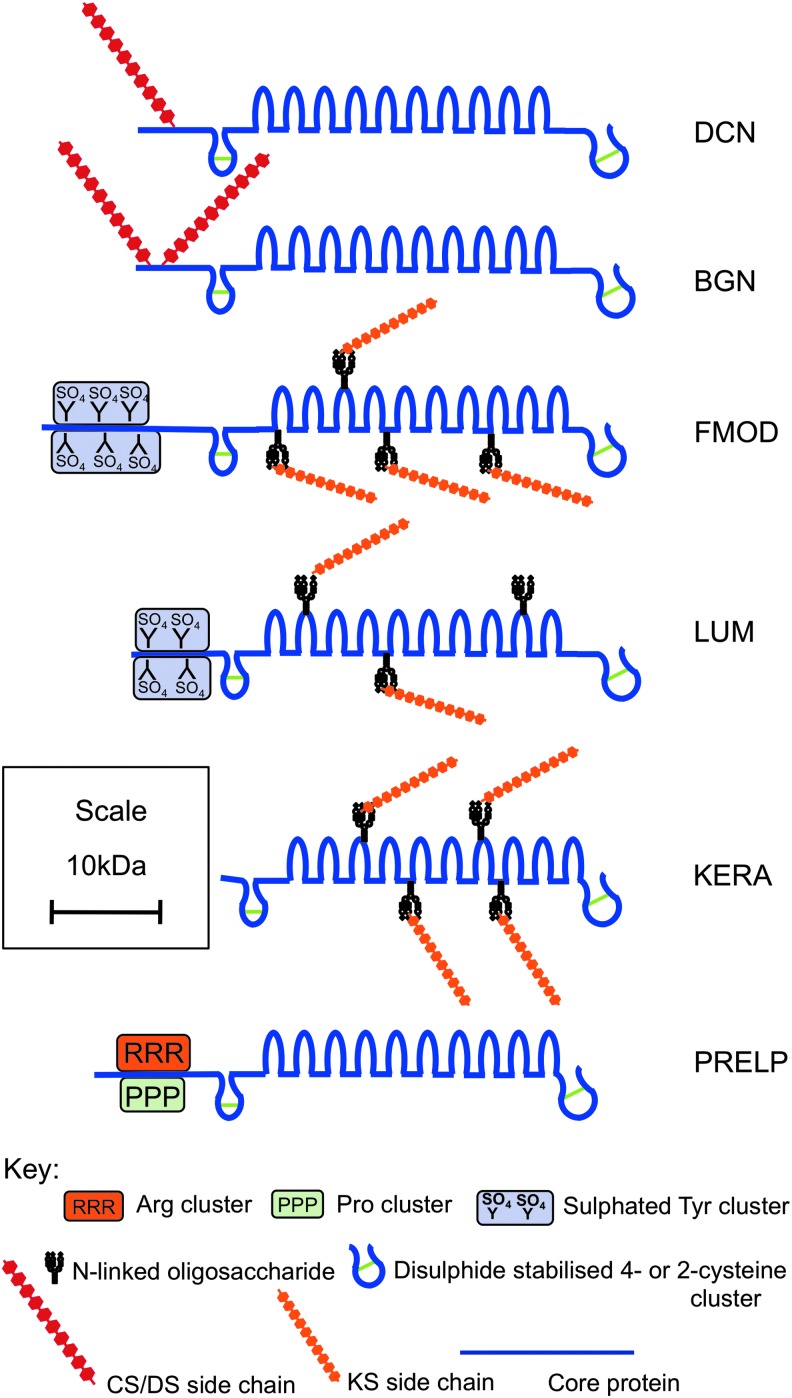
The SLRPs
The SLRPs contain multiple leucine-rich repeat (LRR) motifs42 and are categorized into subfamilies on the basis of gene organization, LRR number, GAG type, and structural organization.43 The SLRPs are important not only as components of most ECM structures but are also responsible for development, organization, maintenance, and remodeling of the ECM during injury and repair.43,44 The comparative content of SLRPs in the ECM of different canine tissues establishes skin (with cartilage and cornea) as one of the highest localizations for these proteoglycans.45 SLRPs in skin include decorin, biglycan, lumican, fibromodulin, keratocan, and nonglycanated proline arginine-rich protein (PRELP, prolargin) (Fig. 4). The CS/DS attachment sites in human decorin and biglycan are within the extreme amino terminus of their core.43 Lumican and fibromodulin contain four N-linked oligosaccharide chains within their central LRR regions that may be modified to KS. The mature core proteins of decorin and biglycan have 14 and 21 amino acid pro-regions removed.46 Small N-terminal signal peptides are also removed from fibromodulin and lumican to form their mature core proteins. Fibromodulin and lumican also contain sulfated tyrosine residues clustered at their N-termini in the mature protein, contributing to their anionic nature.47,48 Many of the SLRPs interact with the TGF-β isoforms (TGF-β1, TGF-β2, and TGF-β3), controlling their bioavailability in skin. SLRPs are therefore important in fibrosis, where excessive TGF-β activity is evident. All the SLRPs also interact with collagen VI, XII, and XIV, fibronectin and elastin, EGF, insulin growth factor (IGF), and tumor necrosis factor (TNF)-α.
Figure 4.
Structural depiction of members of the small leucine-rich repeat proteoglycan family that have been identified in skin: decorin (DCN), biglycan (BGN), fibromodulin (FMOD), lumican (LUM), keratocan (KERA), and prolargin (PRELP). Core proteins are depicted in blue, CS side chains in red, KS in orange. Amino terminal arginine, proline, and sulfated tyrosine clusters are also indicated. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Decorin
Decorin is so-named as it “decorates” the surface of collagen fibrils. The decorin gene (DCN) encodes a 36-kDa core protein, containing a single CS or DS chain.49 Decorin interacts with the “d” and “e” bands of collagen type I fibrils, fibronectin, C1q, EGF receptor, TGF-β, and thrombospondin.50 Decorin has important regulatory functions in collagen fibrillogenesis,51 fibrosis in vivo, and bioavailability of TGF-β.50 The regions of decorin that interact with collagen are located within LRR regions at dissimilar sites to those involved in its interaction with TGF-β. Molecular modeling shows decorin accommodates a single collagen molecule within its concave face52,53; this surface localization of decorin may protect the collagen molecules from proteolysis by collagenases.54 The decorin core protein is cleaved by MMPs in vitro,55,56 by three isoforms of BMP-157 and by membrane type-1 matrix metalloproteinase (MT1-MMP).58 Proteinase-cleaved fragments of decorin, particularly from LRR regions, can activate EGF receptor-mediated receptor phosphorylation and result in anti-proliferative (quiescence) effects.59
Decorin is expressed by cultured dermal keratinocytes and detected in the suprabasal layers of the epidermis. It modulates the formation of ECM in skin, where the protein and its mRNA are present in high concentrations in the reticular but not papillary dermis.60 Postnatal skin also contains decorin with shorter GAG chains and catabolic fragments of decorin.10 DCN knockout mice have fragile skin and abnormally large and irregularly shaped collagen fibrils, reminiscent of those observed in Ehlers–Danlos Syndrome.51 DS epimerase 1 (Dse) knockout mice have shorter DS changes on decorin and consequently larger diameter skin collagen fibrils.61 Decorin is thus important for the formation of uniform correctly sized collagen fibrils during development, healing, and chronic inflammation, although the exact mechanisms of its interactions with collagen molecules are unknown.
Biglycan
The biglycan gene (BGN) encodes a 38-kDa core protein, with two CS/DS chains, although nonglycanated forms of biglycan have also been detected. Pro-biglycan is cleaved by BMP-146 and mature biglycan by MMPs.56 Biglycan interacts with the lattice-forming type VI collagen, which form chondron basket-like structures around cells and interact with BMPs and TGF-β in the initiation of the inflammatory response during tissue stress. In skin, biglycan is localized to the ECM of the epidermis and the basement membranes and connective tissue sheath of the hair follicle.7 Biglycan has emerging roles as a signaling molecule with an extensive repertoire of molecular interactions with growth factors and receptors that regulate cell growth, morphogenesis, and immunity.44
Fibromodulin
The fibromodulin gene (FMOD) encodes a 42-kDa core protein, is KS-substituted and shares significant sequence homology with decorin and biglycan.62 It contains four N-linked oligosaccharide sites within the LRR domains that can be substituted with KS. Fibromodulin also contains a number of N-terminal sulfated tyrosine residues that facilitate interaction with clusters of basic residues in a variety of bioactive, heparin-binding proteins.47,48 Nonglycanated forms of fibromodulin and lumican can accumulate in tissues due to an age-dependant decline in KS synthesis.63 Fibromodulin interacts with types I and II collagen fibrils, inhibiting fibrillogenesis in vitro.64 It also interacts with TGF-β and C1q, thus having roles in TGF-β sequestration and bioregulation.44,50 FMOD is expressed in human epidermal keratinocytes in vitro and in human epidermis in vivo.65 The fragile skin of FMOD-knockout mice demonstrate the importance of fibromodulin to collagen assembly and stability in the ECM of this tissue,66 although detailed mechanistics are unknown. When bound to collagen, fibromodulin is susceptible to cleavage by MMP-13, which removes the N-terminal sulfated tyrosine cluster. However, soluble fibromodulin is not susceptible to cleavage by MMP-13, neither is it degraded by MMP-2, -8, and -9.67
Lumican
The lumican gene (LUM) encodes a 38-kDa core protein,68 that has four N-linked sites within the LRR domain that can be substituted with KS. Lumican interacts with similar partners as fibromodulin, and has similar roles in collagen fibril formation. Both SLRPs have homologous sequences in the five to seven LRRs that compete for collagen binding69 and they bind to the same regions of type I collagen fibrils.70 Conversely, despite significant similarities between lumican and decorin, they have dissimilar binding sites on type I collagen71 and independently modulate collagen fibrillogenesis.72 Like other SLRP members, lumican is also susceptible to degradation by MMPs and it can also be cleaved by MT1-MMP.73 Lumican is expressed by melanoma cells but not by normal melanocytes and was thus suggested as a marker of malignant melanoma.74
Keratocan
Keratocan is a 60–70-kDa KS substituted member of the SLRP proteoglycan family with a widespread distribution in cornea, cartilage, tendon, sclera, aorta, lung, skeletal muscle, and skin.75 Corneal keratocan has a 37-kDa core protein that contains three to four KS chains with attachment points in the LRR region; skin keratocan is less sulfated.75 Keratocan shares 35% amino acid identity with fibromodulin and lumican but only 10–12% homology with decorin or biglycan. Its expression is downregulated by FGF-2- and TGF-β1-activation of JNK signaling pathway in activated corneal keratocytes during corneal stromal healing76 so we can speculate it has a similar role in skin.
Proline/arginine-rich and leucine-rich repeat protein
PRELP (prolargin) is a 55-kDa nonglycanated SLRP so classified on the basis of its sequence and general structural organization.77 It contains N terminal clusters of arginine and proline with anchoring roles in basement membranes.78,79 The N-terminus of PRELP binds to the heparin and the HS of perlecan and acts as a basement membrane anchor and/or linking module to other collagenous networks in skin.80
Cell-Associated Proteoglycans
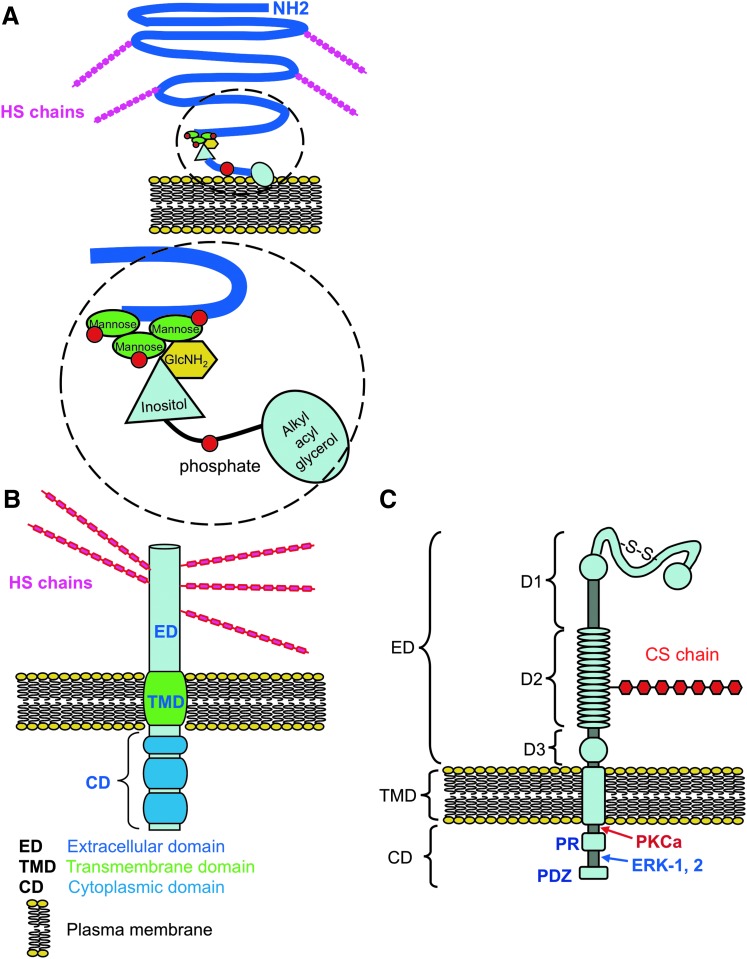
Nerve-glial antigen-2/CSPG-4
CSPG-4, also known as high molecular weight melanoma associated antigen in humans and nerve-glial antigen-2 (NG2) in rodents, is a transmembrane CSPG that is expressed by immature progenitor cells including oligodendrocyte, chondroblasts/osteoblasts, myofibroblasts, smooth muscle cells, pericytes, interfollicular epidermal, and hair follicle cells.81,82 CSPG-4 occurs as a 250-kDa glycoprotein, a 450-kDa C4S-proteoglycan, or as a nonglycanated form (Fig. 5C).83,84 The CS side chain of CSPG-4 facilitate interactions with α4β1 integrin and fibronectin, influences its cell surface distribution, and has roles in the activation of pro-MMP2 by transmembrane MMPs. This ability to influence integrin and MMP activation associates CSPG-4 with the regulation of cellular migration and invasion in skin and implicates this proteoglycan as a molecular target in the prevention of melanoma spread.85,86 CSPG-4 may participate in cell signaling both directly, as a co-receptor with another receptor tyrosine kinase, or by association with cytoplasmic kinases such as focal adhesion kinase or extracellular regulated kinase (ERK)-1, -2. The core protein of CSPG-4 binds FGF-1 and PDGF and presents these mediators to their cognate receptors in situ.87 The central nonglobular domain of CSPG-4 binds to collagen V and VI88,89 and other ECM molecules, facilitating cellular attachment and ECM stabilization. Binding of CSPG-4 to ECM components also induces cytoskeletal reorganization conducive to cell spreading and migration.90,91
Figure 5.
Structural organization of cell surface proteoglycans identified in skin, glypican (GPC) (A), syndecan (SDC) (B), and nerve-glial antigen-2 (NG2)/chondroitin sulfate proteoglycan (CSPG)-4 (C). Core proteins are depicted in blue, with extracellular (ED), transmembrane (TMD), and cytoplasmic (CD) domains indicated. CS side chains are depicted in NG2 in red and HS chains in SDC and GPC in cerise; the lipid bilayer of the plasma membrane is depicted in yellow. Organization of the extracellular sub domains D1, D2, and D3 of NG2 proteoglycan are also indicated; D1 contains two laminin G type domains, D2 contains a single CS side chain. Although not all forms of NG2 are glycanated, this region of NG2 interacts with type V, VI collagen, growth factors, integrins, and matrix metalloproteinases (MMPs). Two MMP cleavage sites have been demonstrated in the D3 domain, which result in shedding of the extracellular portion of NG2. Protein kinase C alpha (PKCα) and extracellular regulated kinase (ERK)-1, -2 interactive regions in the CD where tyrosine phosphorylation occurs during cell signaling are indicated. GPC contains multiple HS chains in the ED. Detail of the GPC core protein attachment region to the phospholipid bilayer of the plasma membrane by a triphospho-mannose-glucosamine-inositol-glycerophosphate linkage are indicated in the exploded region in (C). Modified from O'Connell and Weeraratna222 and Price et al.84 with permission. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In early postnatal mouse skin, NG2 is expressed in the dermis, outer root sheath of hair follicles, and basal keratinocytes of the epidermis. 81 In adult skin, NG2 proteoglycan is most prominently expressed by stem cells in the hair follicles and regulated by TGF-β. In NG2 knockout mice, the epidermis is very thin compared with wild-type mice due to the reduced proliferation of the basal keratinocytes in the epidermis, providing clues as to the likely role of this proteoglycan in skin development, homeostasis, and its possible role in melanoma progression.81,84
The glypican family
The glypicans (GPCs) are a family of HS proteoglycans, which are anchored to the cell-surface via a covalent linkage to glycosylphosphatidylinositol (GPI) (Fig. 5A). Six GPC family members that share 17–67% identity have so far been identified. The HS chains on GPC are attached to a C-terminal region near the GPI anchor point at the cell membrane. These HS chains bind growth factors and morphogens and modify cell signaling pathways, cellular proliferation, and matrix production.104–106 GPC-1 has been identified in skeletal and smooth muscle epidermis and hair folllicles in rodents but not in the dermis.107 Neither GPC-2,108 a major neural tissue proteoglycan, nor GPC-3, -4, or -5109 are expressed in skin. GPC-1 has been immunolocalized to the pericellular matrix of keratinocytes in normal skin and shown to be an intracellular proteoglycan of epidermal cells at the edge of chronic ulcers.95
The syndican family
The SDCs are transmembrane HS-proteoglycans that carry three to five HS and CS/DS chains. They facilitate interactions with a large variety of ligands, including FGF, VEGF, and TGF-β family members. They are thus involved in wound healing, inflammation, cell adhesion, and vascular biology.92–94 All SDCs have an N-terminal signal peptide, an ectodomain, a membrane-anchoring hydrophobic transmembrane region, and a C-terminal cytoplasmic domain (CD) (Fig. 5B). The SDCs are differentially expressed in skin; SDC-1 is highly expressed by keratinocytes, fibroblasts, and epithelial cells.95 SDC-1 mediates cell interactions with the ECM, cell attachment and migration, and responses to growth factors. The interaction of SDC-1 with laminin 322 is essential for keratinocyte migration96 and is consistent with reduced migration of keratinocytes in SDC1 knockout mice and enhanced TGF-β signaling.97 Changes in SDC-1 and SDC-4 expression correlate with the progression of skin carcinogenesis and the metastatic potential of melanoma.98,99 SDC1 expression is reduced in basal cell, squamous and metastatic human skin cancers and has been linked to malignant conversion in skin carcinogenesis.99 SDC1 expression is elevated in psoriatic epidermis.100
SDC-2 is also expressed by keratinocytes and endothelial cells and by dermal fibroblasts and glandular cells. In normal skin, SDC-2 is confined to the keratinocytes of the superficial epidermis where it is an intracellular proteoglycan. SDC-2 influences the migratory potential of melanoma cells101 and SDC-4 affects the migration of adult dermal fibroblasts.102 SDC-4 is pericellularly expressed by keratinocytes in normal skin, weakly by fibroblasts, and appears in capillaries. SDC-4 has been associated with leg ulcers.103
Intracellular Proteoglycans
Serglycin
Serglycin is an intracellular proteoglycan produced by endothelial cells, mast cells, and lymphocytes. Serglycin is so named from the high proportion of repeating serine and glycine residues in its core protein. It is localized to the α-secretory granules of platelets and mast cells, where it binds and controls the activity of various mediators including factor-4 in platelets, serotonin, dopamine, or histamine in mast cells or proteases like tryptase and chymase.110,111 Serglycin also localizes neutrophil elastase to the azurophilic granules of neutrophils,112 and granzyme-B in cytotoxic T-lymphocytes.113,114 Mast cells show significant heterogeneity depending on tissue location. In rodents, mast cells are classified as mucosal or connective tissue types, with the former primarily residing in the intestinal mucosa and the latter residing in skin, peritoneum and elsewhere. The GAG substitution on serglycin isolated from mast cell subclasses is quite variable. Serglycin is decorated with CS chains in the secretory granules of circulating basophils, whereas in resident mast cells it is decorated with heparin.110 Endothelial cell serglycin in blood vessels of dermatomyositic and discoid lupus erthythamotosus skin are highly substituted with C6S.115 Mast cell numbers dramatically increase in response to infection where proteases are released from the secretory granules at specific tissue sites to inactivate invading organisms. Serglycin has important roles to play in inflammatory, allergic, and immune reactions in skin.116–119 Extracellular release of serglycin may result in interactions with chemokines produced by inflammatory cells; therefore, mast cell heparin may regulate eosinophil recruitment by binding to the chemokine CCL11 thus protecting it from proteolytic degradation in situ.119
Cell-Surface Co-Receptors and “Part Time” HS Proteoglycans
Some cell-surface co-receptors are also termed “part time” CS–HS proteoglycans, since the proteins do not always have a GAG chain attached, thus nonglycanated forms of these proteoglycans have also been identified. These include endoglin, betaglycan, and CD44 variants 3–10, and 8–10. Keratinocytes also produce a variant HS-substituted CD44 (epican).120–122
Endoglin
Endoglin is a glycoprotein/proteoglycan co-receptor expressed on endothelial cells, macrophages, keratinocytes, and stromal cells in the epidermis/dermis and bulb region of hair follicles and sweat glands.122 It is upregulated under inflammatory conditions and in skin lesions where endothelial cell proliferation is prevalent. Endoglin is a 68-kDa, dimeric, type 1 integral membrane protein containing extracellular, transmembrane, and CDs.123–125 Mouse and porcine endoglin share 71% and 96% sequence identity respectively with human endoglin and almost identical transmembrane and CDs.126,127 Human, porcine, and mouse endoglin also display similarities in their extracellular domains to the type III TGF-β receptor (TβRIII) betaglycan, but mouse and porcine endoglin do not contain an Asp-Gly-Arg cell receptor interactive motif.126 Endoglin, like betaglycan, is a TGF-β co-receptor that modulates TGF-β signaling in endothelial cells and macrophages.128,129 Unlike betaglycan, which binds all TGF-β isoforms with high affinity, endoglin binds TGF-β1 and -β3 in the presence of TβRII but does not bind TGF-β2.128,129 Although endoglin is not a signaling receptor, it has been shown to associate with TGF-β signaling receptors (TβRII, activin receptor-like kinase [ALK]1 and ALK5) and to modulate their phosphorylation status and downstream Smad-dependent and independent signaling in endothelial cells.130
Endoglin is expressed in normal and psoriatic skin; its levels are significantly elevated in psoriatic skin where it appears to have a role in the extravasation of peripheral blood mononuclear cells via the endothelium.131 Endoglin is also significantly upregulated on dermal blood vessels in scleroderma132 and primary malignant melanomas.133 Endoglin controls the expression of ALK1 and ALK5 by endothelial cells and regulates endothelial cells by upregulating TGF-β/ALK1 cell signaling.134 Endoglin counteracts TGF-β-mediated suppression of CD4(+) T cell proliferation by induction of Smad-independent signaling via ERK.135 Endoglin has a profibrotic role in scleroderma fibroblasts promoting TGF-β1/ALK1/Smad1 cell signaling and collagen production,136 however, results have been variable with reports of both positive and negative enhancement of TGF-β effects in fibrosis.137 Endoglin is essential for angiogenesis with its expression upregulated in inflammation and may have regulatory roles in transendothelial leucocyte trafficking.138
Betaglycan
Betaglycan/TβRIII is a 300-kDa membrane anchored cell-surface CS/HS proteoglycan that binds to several members of the TGF-β superfamily via its core protein, and FGF-2 via its HS chains. Betaglycan presents TGF-β to the type II TGF-β signaling receptor serine threonine kinase to effect cell signaling.139 Betaglycan is not directly involved in TGF-β signal transduction, but by binding all TGF-β isoforms at the cell surface140 with relatively high affinity, it acts as a positive regulator of TGF-β access to signaling receptors. Soluble betaglycan can also bind TGF-β in free solution, which anatagonizes TGF-β signaling through TβRI/TβRII, since the betaglycan-TGF-β complex cannot now bind to the membrane-bound betaglycan TGF-β type III co-receptor.139 This may regulate tissue fibrosis through excessive TGF-β activity. TGF-β is localized to the hair follicle bulb, the putative stem cell niche, in young skin.141
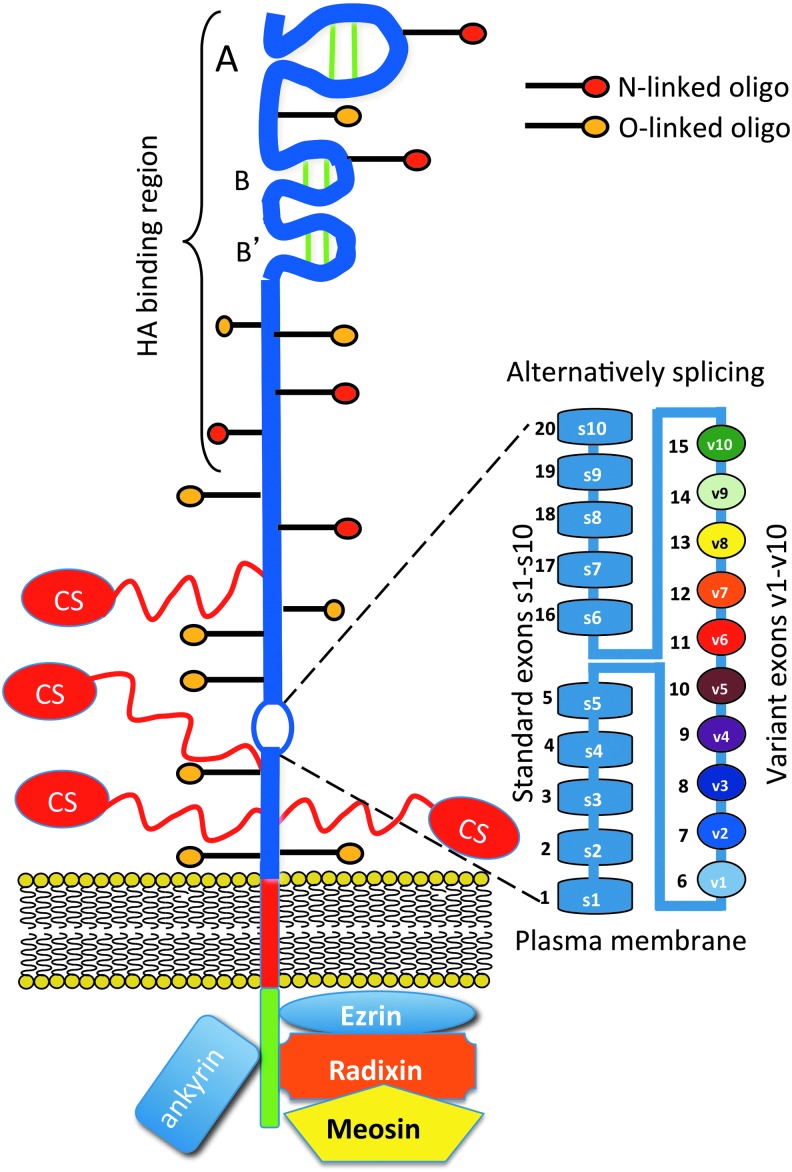
CD44/epican
CD44 (Pgp-1, ECMRIII) has a widespread distribution, with most cells expressing the CD44s standard form of 363 amino acids and a predicted molecular weight of 37 kDa. The apparent molecular mass of CD44s protein estimated by gel electrophoresis is ∼80 kDa. The higher molecular weight value than predicted from the number of amino acids encoded for CD44 can be accounted for by post-translational modifications including N- and O-linked glycosylation, CS, HS, and sialic acid substitution. Epican, the largest variant form of CD44 containing peptides from all variant exons, is substituted with HS and has a molecular weight in excess of 200 kDa. A schematic of the structural organization of CD44 is provided in Figure 6. Some epithelial cells also express a larger isoform (CD44E)142 with insertion of v8–v10 exons at the alternative splice site. CD44 is the major HA receptor in skin,143 however, in the alternatively spliced CD44v3, HS is substituted for CS and this form of CD44 can additionally bind FGF-2 and EGF. In normal skin, CD44 is expressed by eccrine (sweat) gland cells,143 where its distribution is asymmetric, with intense dermal but little luminal staining. In skin undergoing inflammation or neoplastic attack, CD44 expression can be widespread on keratinocytes and infiltrating lymphocytes.144,145
Figure 6.
Schematic depiction of the structural organization of CD44. The extracellular region of the core protein is depicted in blue with the amino terminal disulphide stabilized A, B, and B′ loops that form the hyaluronan (HA)-binding region indicated. Areas of putative CS side chain, N- and O- linked oligosaccharide substitution are also indicated. The standard CD44s is encoded by exons s1-s10 and alternative splicing of CD variants by exons v1–v10 inserted between s5 and s6. The transmembrane and CDs are also shown and cytoskeletal interactive proteins that have roles in cell signaling. Modified from Goodison et al.147 with permission. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
CD44 mediates cell interaction with HA, cell aggregation, migration, proliferation, and activation; cell–cell and cell–substrate adhesion; endocytosis of HA; and assembly of pericellular matrices, for example, between HA and versican. The importance of CD44 to skin homeostasis is immediately apparent in CD44 antisense transgenic mice where defective accumulation of HA accompanies severe disturbances in the basal keratinocytes, which display aberrations in their normal mitogen and growth factor-mediated proliferative responses, decrease in skin elasticity and hydration, and impaired responses to local inflammation and tissue repair.145 This strongly supports an important role for HA and CD44 in skin physiology and tissue repair. CD44 is also a direct and functional target of the micro RNA miR-34a, which targets CD44 in prostate cancer stem cells; knockdown of CD44 inhibits tumor invasion.146
CD44 is a “part time” proteoglycan, as not all variants of CD44 are glycosylated. CD44 is a highly variable cell surface receptor, and different cell types synthesize CD44 of considerable structural diversity. Alternative splicing of variant exons and post-translational modifications in the glycosylation pattern of CD44 generates a diverse collection of CD44 isoforms (Fig. 6). Three broad size categories of CD44 molecules have been identified, (1) a predominant category synthesized by most cells of hemopoietic origin in a 80–90-kDa size range, (2) epithelial CD44 of a size range of 110–160 kDa, and (3) a proteoglycan form of CD44 substituted with CS chains of relatively large size range>200 kDa.147 Keratinocytes also synthesize a 250-kDa form of CD44 substituted with HS chains.120,121
Patients with malignant melanomas have significantly lower serum levels of soluble CD44.148 As soluble recombinant CD44v6 disrupted the interaction of cellular CD44 with HA, inhibited tumor formation, and halted metastasis;149 it may have a positive clinical outcome for melanoma patients.
Summary of Proteoglycan Localization in Normal Skin
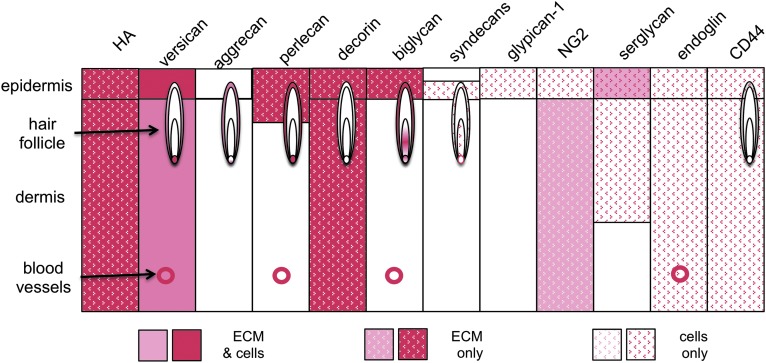
Figure 7 is a summary schematic of the diverse and discrete localizations of the various proteoglycans identified in skin where information is available in the medical literature. None of the proteoglycans studied are ubiquitous throughout the ECM and cells of skin tissue, with the possible exception of HA. This summary is by no means complete; further study is required to more fully define the complex distribution of proteoglycans in skin tissues.
Figure 7.
Summary schematic of known proteoglycan localizations in normal human skin. Variations in staining density are relative within the section for each individual proteoglycan and not between proteoglycans. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The Effect of Age, Injury, and Disease on Proteoglycans in Skin
Proteoglycans in wound healing
Wound healing is a dynamic, highly ordered, and tightly regulated process comprised of inflammatory, proliferative, remodeling, and maturation phases.150–152 These healing processes are driven by cellular responses to ECM changes and are optimal in fetal skin and impaired in both aged and diseased skin. Wound healing becomes much slower with age but, after menopause, scarring is also reduced, most likely due to loss of estrogen-induced TGF-β release from fibroblasts.153 There is no documented correlation between the speed of a wound repair and the amount of scarring that occurs. TGF-β plays a major role in normal skin in both the differentiation of progenitor cell populations in the follicle bulb niche and in skin homeostasis. Recruitment of inflammatory cells is essential for normal wound healing to induce EGF, TGF-β1, and FGF release at the wound site, where granulation tissue fills the defect site as a temporary containment measure. These growth factors induce focal recruitment, maturation, and proliferation of fibroblasts, fibrocytes, keratinocytes, and myofibroblasts that lay down collagen, proteoglycans, and other ECM components at the damage site during the proliferative phase of wound repair with the original fibrin clot being replaced with a more mechanically stable matrix containing collagen, fibronectin, and HA.
Although not strictly a proteoglycan, HA is nevertheless an abundant GAG component of skin and of considerable functional importance. The cell regulatory properties of HA in skin are attributable to its participation in signal transduction events through cell surface and intracellular HA receptors such as CD44, receptor for HA mediated motility, lymphatic vessel endothelial cell HA receptor (LYVE)-1, Cdc-37, and P-32.154 Such interactions can inhibit or stimulate cellular proliferation, cellular migration, and angiogenesis (and consequently tumorogenesis). Many of these cellular effects are dependent on the size of the HA. Large HA (>500 kDa) is anti-inflammatory and antiangiogenic, whereas small molecular weight HA (≤500 kDa) or HA oligosaccharides act in an antagonistic manner to block or counteract effects induced by high molecular weight HA.154,155 For example, HA oligosaccharides induce angiogenesis and promote wound repair in skin.156 In the early inflammatory phase of wound repair, abundant HA acts as a promoter of inflammation, which is crucial to the wound-healing process.157,158 In wound granulation tissue, HA promotes both cell migration into the provisional wound matrix and cell proliferation.144 Granulation tissue is highly inflammatory but is stabilized by the moderating action of large molecular weight HA on inflammation.144 HA is also a free-radical scavenger and protects cells from free-radical damage, an important attribute in the context of tissue repair.159 HA regulates keratinocyte proliferation in response to extracellular stimuli in skin.144 In normal skin, HA is found in relative high concentrations in the basal layer of the epidermis where proliferating keratinocytes are found.159 Its suggested role here is in the re-epithelization process: it serves as an integral part of the ECM of basal keratinocytes and has roles in maintenance of an accessible epidermal extracellular space. This allows a hydrated matrix conducive to migration of keratinocytes and nutrients.144 In wound healing, HA and CD44 are expressed at the wound margins, correlating with the influx of migrating keratinocytes that actively promote tissue repair.144,145 This HA-containing matrix is slowly resorbed and replaced by a stronger ECM during tissue remodeling at later stages of wound repair. HA oligosaccharides also stimulate a number of cell types to produce elevated levels of active MMPs, which contribute to the metastatic spread of tumors in skin.160,161
Fibrosis occurs due to a dysregulation of the wound-healing process either at the proliferative or remodeling phase, with in some cases, an irritant persisting in the tissue that drives these processes.162 Fibroblasts (myofibroblasts) are the major biosynthetic cell type that lay down ECM in wound repair; fibrosis can occur in any tissue where this process occurs. There is now a greater appreciation of the contributions of matrix components and soluble mediators in skin remodeling.150,163,164 The controlled release of growth factors and cellular mediators (chemokines and cytokines) sequestered by proteoglycans at the wound site actively recruit inflammatory cells that begin the process of controlling the injury and healing the tissue damage. Tight regulation of chemokine bioavailability is critical to control the magnitude of the inflammatory response to tissue injury and an atypical chemokine receptor (D6) recently identified has critical regulatory roles to play in this process.165
Major differences have long been noted in the healing response of fetal and adult skin.153,166–170 It is not known why some fetal wounds heal without scarring while wound repair in mature skin usually leaves a percentage of abnormal tissue, although TGF-β3, or the ratio of TGF-β3 to TGF-β1, may be important.171 These growth factors stimulate ECM production and inhibit ECM catabolism but also attract macrophages, neutrophils, and fibroblasts. The stimulation of HA synthesis by TGF-β3 and the resulting prolonged abundance of this GAG has been linked to scarless fetal wound healing.172 In addition, high levels of HA are present in scarlessly healed wounds of immunodeficient mice.173 Fibromodulin is upregulated and decorin is downregulated in fetal wounds.153 Hypertrophic scars have less decorin174 and more versican and biglycan than normal scars.175 As mentioned earlier, versican-HA complexes may help hydrate the tissue during the repair processes,13 aggrecan may hinder cell migration to the wound,19 and SDCs are important for growth factor signaling during this process.92 Reduced decorin, fibromodulin, and TGF-β3 in the deep dermis leads to postburn hypertrophic scarring.176,177 Delayed wound closure occurred both in FMOD knockout mice associated with an increase in TGF-β3178 and in LUM knockout mice with a delay in TGF-β1 expression.179 Modulation of SLRP binding of TGF-β isoforms may be a key area for future accelerated and/or scarless wound-healing therapies. SDC-1 expression is transiently upregulated in the epidermis upon tissue injury.180 In keratinocytes, loss of SDC-1 delays wound healing, reduces cell migration, and increases TGF-β expression.
Keloid scarring is a benign, recurring, skin tumor with excessive fibroblastic tissue resulting from abnormal wound healing. Scars can be disfiguring and can limit mobility and growth by restricting skin movement and there is currently no treatment for prevention in susceptible individuals. The ECM is implicated in both hypertrophic and keloid scarring.181 Keloid fibroblasts synthesize less decorin with longer GAG chains than normal skin cells.182 The expression of the aggrecan gene (ACAN) increased an average of 175-fold in skin biopsies from the margins of keloid scars from four patients compared with corresponding areas of normal skin.183 This suggests a change of cell phenotype leading to a dysregulation of proteoglycan metabolism in this scar tissue.
Proteoglycans in aging skin
Aging is an important factor to consider when studying the proteoglycan content of skin and its ability to heal. The molecular aspects of skin aging has been recently reviewed.184 Changes in aging skin can occur both internally due to chronological age (intrinsic) and from environmental factors such as climatic, particularly sun, exposure (extrinsic). While HA is decreased in intrinsically aged skin,185 proteoglycans are increased and abnormally distributed in photo-aged skin.186
Intrinsic aging of skin can be studied in body regions usually clothed and hence rarely exposed to the elements. Sections of aged buttock skin biopsies had reduced Alcian blue, HA, HS, decorin, and SDC-1 staining compared with young skin in both genders.187 CS GAGs and perlecan staining were also reduced but only in female aged skin. Versican staining increased only in male aged skin.187 Decreases were observed in the size of the GAG chains attached to decorin with skin aging; the smaller GAG chains would result in changes in collagen fibril packing and skin mechanical properties.188
Skin damaged by the sun has an increased content of GAGs that appears to be mostly CS on the versican core protein more superficially localized in the dermis compared with versican's localization in normal skin.189 Versican increases in skin exposed to UVB radiation when reactive oxygen species are generated in an inflammatory response.190 However, pathological and phenotypical changes in skin following solar elastosis can lead to a loss of the HA-binding ability of versican with attendant effects on the hydration and viscoelastic properties of the dermis.13 Increased UV irradiation increases protein levels for HS-proteoglycans in human skin (perlecan, SDC-1, -4) and CD44 v3.191
Increases in GAGs due to UV irradiation have been studied using the hairless SKH-1 mouse, however, the results have been mixed.192 Increases in CS,193 DS,194 and HS195 in UV-exposed skin have been variously reported, with HA either increasing,193,194 not changing195 or decreasing196,197 after UV exposure. If cultured human dermal fibroblasts are UV-irradiated, gene expression of all the SLRPs, versican and perlecan decrease, with only SDC-1 increasing.198 Expression of type I and III collagen changes with aging and, following UV irradiation (photo-aging), there is a decrease in the normal collagen to decorin ratio, resulting in the appearance of smaller collagen fiber bundles and consequential changes in the biomechanical properties of skin.199 A decrease in skin DCN expression was found following short-term UV exposure.200 The free-radical scavenging properties of HA provide protection against solar radiation that can lead to damaging levels of free radical generation,144 however, chronic UV irradiation of mice downregulated HA synthases, resulting in lower levels of this GAG in mouse dermis.197
Future Directions
Members of the SLRPs have well-known capability to control TGF-β and have been examined as a means of inhibiting excessive TGF-β activity in tissues that lead to fibrosis and impaired organ function.50 Decorin and biglycan in particular would appear prime candidates for further examination of their therapeutic potential in the prevention of skin fibrosis. For example, decorin GAG chains sequester the growth factors, EGF, IGF, and TGFβ in the ECM surrounding cells and may regulate their bioavailability. Decorin can be extensively processed by MMPs, MT1-MMP, and isoforms of BMP-1 to produce a fragment named decorunt.10,57,201 This contains the LRR region of decorin with a TGFβ-binding site and a strong interactive site for collagen. Decorunt is thus capable of sequestering TGFβ and influencing collagen fibril assembly processes but not processes mediated by the other growth factors and cytokines. Most recently, a heptapeptide (LQVVYLH) TGF-β binding segment of the biglycan core protein, Peniel-2000 (P2K) has been used to repair experimental defects in the intervertebral disc.202 Reattainment of disc height was achieved, with P2K significantly upregulating anabolic gene expression (aggrecan, type II collagen). Significantly, the mode of P2K action was by inhibiting Smad-1/5/8 cell signaling with TGF-β, while Smad-2, -3 signaling through ALK1 was still detected. Thus, P2K selectively switched off the anti-anabolic effects of TGF-β but not its beneficial anabolic gene effects. Similar approaches using bio-active peptides, which directly target functional aspects of components in skin such as TGF-β to promote or inhibit a specific functional property, may also be exploitable for potential skin repair therapies. For such approaches to succeed, it is crucial that we obtain a firm understanding of the biology of key functional components and how they must to be manipulated to provide a positive outcome.
A number of synthetic skin substitutes have been developed including Matriderm®, Integra® bilayer artificial dermis, JACE® cultured epidermal autograft, MyDerm® bilayered human skin equivalent, Suprathel® synthetic skin substitute, Renoskin®, and Hyalomatrix®.203,204 These have been seeded with autologous stem cells harvested from debrided human burn skin205 or from adipose tissue206 or human amniotic fluid207 and used in a number of skin repair strategies. Advances in nano-fabrication technology for the preparation of artificial matrices such as those that can be assembled by the Nanospider NS200 using collagen-based electrospinning of nanofibrous textile meshes to produce matrices of controlled 3D morphology are also now possible.208 These matrices are also suitable for seeding with stem cells to effect repair of large skin defects.209 Artificial skin substitutes with a microvascular supply have also been developed and applied to melanoma research.210 Epidermal electronics offers particularly exciting possibilities in the area of advanced materials design and in the remote sensing of skin repair processes.211 Transparent, optical, pressure sensitive artificial skin with large area stretchable electronics have been developed.212 These ultrathin (600 μm) devices are laminated on to the skin in a similar manner to a temporary transfer tattoo and are fully contained with their own power supply and communication components. The intrinsic sensing capability of such advanced materials have already found application in smart dressings with the capability of sensing bacterial infection in wounds213 and in development of Theragauze®, a smart dressing with the ability to regulate antibiotic and moisture delivery across diabetic wounds of the foot,214 to promote skin repair processes, and prevent infection in pediatric burns patients.215 The use of proteoglycans to sequester cell mediators and refine ECM structure will augment this skin repair technology and has the potential for exciting possibilities in the future.
Take Home Messages
• Proteoglycans are a multifunctional diverse group of matrix and cell-associated proteins each with a distinct localization in skin.
• Proteoglycans can interact with other matrix molecules such as collagen to form and stabilize the ECM, thus producing the hydrated integrated structures required for responsive cells in a healthy intact skin.
• Proteoglycans can bind and sequester growth factors in the skin ECM and either inhibit their activity or present them to cognitive receptors to initiate cell signaling, proliferation, and matrix production.
• Manipulation of proteoglycan metabolism and homeostasis in skin may lead to effective therapies to accelerate scarless wound healing.
Abbreviations and Acronyms
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- ALK
activin receptor-like kinase
- BGN
biglycan
- BMP
bone morphogenetic protein
- CD
cytoplasmic domain
- CS
chondroitin sulfate
- CSPG
chondroitin sulfate proteoglycan
- DCN
decorin
- DS
dermatan sulfate
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ED
extracellular domain
- ERK
extracellular regulated kinase
- FGF
fibroblast growth factor
- FMOD
fibromodulin
- GAG
glycosaminoglycan
- GPC
glypican
- GPI
glycosylphosphatidylinositol
- HA
hyaluronan
- HS
heparan sulfate
- HSPG2
heparin sulfate proteoglycan 2; perlecan
- IGD
interglobular domain
- IGF
insulin growth factor
- KERA
keratocan
- KS
keratan sulfate
- LRR
leucine-rich repeat
- LTBP
latent transforming growth factor beta-binding protein
- MMP
matrix metalloproteinase
- MT1-MMP
membrane type-1 matrix metalloproteinase
- NG2
nerve-glial antigen-2
- PDGF
platelet-derived growth factor
- PKCα
protein kinase C alpha
- PRELP
proline/arginine-rich and leucine-rich repeat protein; prolargin
- SDC
syndecan
- SLRP
small leucine-rich repeat proteoglycan
- TβRI/TβRII/TβRIII
transforming growth factor-β receptor I, II, III
- TGF
transforming growth factor
- TMD
transmembrane domain
- TNF
tumor necrosis factor
- UV
ultraviolet
- VEGF
vascular endothelial growth factor
- WARP
von Willebrand factor A domain-related protein
Acknowledgments and Funding Sources
We gratefully acknowledge the contribution of Susan Smith for technical assistance with the immunohistology. The National Health and Medical Research Council of Australia provided funding for a portion of this work.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used in the creation of this article.
About the Authors
Dr. Margaret Mary Smith and Dr. James Melrose are senior research fellows in the Raymond Purves Research Laboratories at the Kolling Institute (University of Sydney) at Royal North Shore Hospital in Sydney. Both authors study the role of proteoglycans in extracellular matrix structure, function and disease pathology.
References
- 1.Cummings RD: The repertoire of glycan determinants in the human glycome. Mol Biosyst 2009; 5:1087. [DOI] [PubMed] [Google Scholar]
- 2.Caterson B, Mahmoodian F, Sorrell JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A, and Muir H: Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci 1990; 97(Pt 3):411. [DOI] [PubMed] [Google Scholar]
- 3.Caterson B. Fell-Muir Lecture: chondroitin sulphate glycosaminoglycans: fun for some and confusion for others. Int J Exp Pathol 2012; 93:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nugent MA. Heparin sequencing brings structure to the function of complex oligosaccharides. Proc Natl Acad Sci USA 2000; 97:10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull JE. Heparan sulfate glycomics: towards systems biology strategies. Biochem Soc Trans 2010; 38:1356. [DOI] [PubMed] [Google Scholar]
- 6.Humphries DE, and Stevens RL: Regulation of the gene that encodes the peptide core of heparin proteoglycan and other proteoglycans that are stored in the secretory granules of hematopoietic cells. Adv Exp Med Biol 1992; 313:59. [DOI] [PubMed] [Google Scholar]
- 7.Malgouries S, Thibaut S, and Bernard BA: Proteoglycan expression patterns in human hair follicle. Br J Dermatol 2008; 158:234. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann DR, and Ruoslahti E: Multiple domains of the large fibroblast proteoglycan, versican. EMBO J 1989; 8:2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dours-Zimmermann MT, and Zimmermann DR: A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem 1994; 269:32992. [PubMed] [Google Scholar]
- 10.Carrino DA, Calabro A, Darr AB, Dours-Zimmermann MT, Sandy JD, Zimmermann DR, Sorrell JM, Hascall VC, and Caplan AI: Age-related differences in human skin proteoglycans. Glycobiology 2011; 21:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, and Apte SS: ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell 2009; 17:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, and Clowes AW: Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem 2001; 276:13372. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa K, Yoneda M, Kuwabara H, Miyaishi O, Itano N, Ohno A, Zako M, and Isogai Z: Versican, a major hyaluronan-binding component in the dermis, loses its hyaluronan-binding ability in solar elastosis. J Invest Dermatol 2007; 127:1657. [DOI] [PubMed] [Google Scholar]
- 14.Aspberg A, Adam S, Kostka G, Timpl R, and Heinegård D: Fibulin-1 is a ligand for the C-type lectin domains of aggrecan and versican. J Biol Chem 1999; 274:20444. [DOI] [PubMed] [Google Scholar]
- 15.Olin AI, Morgelin M, Sasaki T, Timpl R, Heinegard D, and Aspberg A: The proteoglycans aggrecan and Versican form networks with fibulin-2 through their lectin domain binding. J Biol Chem 2001; 276:1253. [DOI] [PubMed] [Google Scholar]
- 16.Du WW, Yang BB, Shatseva TA, Yang BL, Deng Z, Shan SW, Lee DY, Seth A, and Yee AJ: Versican G3 promotes mouse mammary tumor cell growth, migration, and metastasis by influencing EGF receptor signaling. PLoS One 2010; 5:e13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez D, Miquel-Serra L, Docampo MJ, Marco-Ramell A, Cabrera J, Fabra A, and Bassols A: V3 versican isoform alters the behavior of human melanoma cells by interfering with CD44/ErbB-dependent signaling. J Biol Chem 2011; 286:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gambichler T, Kreuter A, Grothe S, Altmeyer P, Brockmeyer NH, and Rotterdam S: Versican overexpression in cutaneous malignant melanoma. Eur J Med Res 2008; 13:500. [PubMed] [Google Scholar]
- 19.Velasco J, Li J, DiPietro L, Stepp MA, Sandy JD, and Plaas A: Adamts5 deletion blocks murine dermal repair through CD44-mediated aggrecan accumulation and modulation of transforming growth factor beta1 (TGFbeta1) signaling. J Biol Chem 2011; 286:26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauder DN, Dekoven J, Champagne P, Croteau D, and Dupont E: Neovastat (AE-941), an inhibitor of angiogenesis: randomized phase I/II clinical trial results in patients with plaque psoriasis. J Am Acad Dermatol 2002; 47:535. [DOI] [PubMed] [Google Scholar]
- 21.Liu N, Lapcevich RK, Underhill CB, Han Z, Gao F, Swartz G, Plum SM, Zhang L, and Green SJ: Metastatin: a hyaluronan-binding complex from cartilage that inhibits tumor growth. Cancer Res 2001; 61:1022. [PubMed] [Google Scholar]
- 22.Xu XM, Chen Y, Chen J, Yang S, Gao F, Underhill CB, Creswell K, and Zhang L: A peptide with three hyaluronan binding motifs inhibits tumor growth and induces apoptosis. Cancer Res 2003; 63:5685. [PubMed] [Google Scholar]
- 23.Knox S, Fosang AJ, Last K, Melrose J, and Whitelock J: Perlecan from human epithelial cells is a hybrid heparan/chondroitin/keratan sulfate proteoglycan. FEBS Lett 2005; 579:5019. [DOI] [PubMed] [Google Scholar]
- 24.Melrose J, Smith SM, Cake M, Read R, and Whitelock JM: Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study. Histochem Cell Biol 2005; 124:225. [DOI] [PubMed] [Google Scholar]
- 25.Melrose J, Smith SM, Cake M, Read R, and Whitelock JM: Perlecan displays variable spatial and temporal immunolocalisation patterns in the articular and growth plate cartilages of the ovine stifle joint. Histochem Cell Biol 2005; 123:561. [DOI] [PubMed] [Google Scholar]
- 26.Melrose J, Hayes AJ, Whitelock JM, and Little CB: Perlecan, the “jack of all trades” proteoglycan of cartilaginous weight-bearing connective tissues. Bioessays 2008; 30:457. [DOI] [PubMed] [Google Scholar]
- 27.Whitelock JM, Melrose J, and Iozzo RV: Diverse cell signaling events modulated by perlecan. Biochemistry 2008; 47:11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes A, Lord MS, Smith SM, Smith MM, Whitelock JM, Weiss AS, and Melrose J: Colocalization in vivo and association in vitro of perlecan and elastin. Histochem Cell Biol 2011; 136:437. [DOI] [PubMed] [Google Scholar]
- 29.Hayes AJ, Smith SM, and Melrose J: Comparative immunolocalisation of fibrillin-1 and perlecan in the human foetal, and HS-deficient hspg2 exon 3 null mutant mouse intervertebral disc. Histochem Cell Biol 2013; 139:1. [DOI] [PubMed] [Google Scholar]
- 30.Chuang CY, Lord MS, Melrose J, Rees MD, Knox SM, Freeman C, Iozzo RV, and Whitelock JM: Heparan sulfate-dependent signaling of fibroblast growth factor 18 by chondrocyte-derived perlecan. Biochemistry 2010; 49:5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes AJ, Smith SM, Gibson MA, and Melrose J: Comparative immunolocalisation of the elastin fibre associated proteins fibrillin-1, LTBP2 and MAGP-1 with components of the collagenous and proteoglycan matrix of the foetal human IVD. Spine 2011; 36:E1365. [DOI] [PubMed] [Google Scholar]
- 32.Gheduzzi D, Guerra D, Bochicchio B, Pepe A, Tamburro AM, Quaglino D, Mithieux S, Weiss AS, and Pasquali Ronchetti I: Heparan sulphate interacts with tropoelastin, with some tropoelastin peptides and is present in human dermis elastic fibers. Matrix Biol 2005; 24:15. [DOI] [PubMed] [Google Scholar]
- 33.Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, and Soininen R: Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. Embo J 2003; 22:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou ZP, Wang J, Cao R, Morita H, Soininen R, Chan KM, Liu BS, Cao Y, and Tryggvason K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res 2004; 64:4699. [DOI] [PubMed] [Google Scholar]
- 35.Jung M, Lord MS, Cheng B, Lyons JG, Alkhouri H, Hughes JM, McCarthy SJ, Iozzo RV, and Whitelock JM: Mast cells produce novel shorter forms of perlecan that contain functional endorepellin: a role in angiogenesis and wound healing. J Biol Chem 2013; 288:3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Hook M, Reed CC, and Iozzo RV: Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through alpha2beta1 integrin. J Cell Biol 2004; 166:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bix G, Iozzo RA, Woodall B, Burrows M, McQuillan A, Campbell S, Fields GB, and Iozzo RV. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the alpha2beta1-integrin receptor. Blood 2007; 109:3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, Greenspan DS, and Iozzo RV: BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J Biol Chem 2005; 280:7080. [DOI] [PubMed] [Google Scholar]
- 39.Whitelock JM, Murdoch AD, Iozzo RV, and Underwood PA: The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem 1996; 271:10079. [DOI] [PubMed] [Google Scholar]
- 40.Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, Bruckner-Tuderman L, Bruckner P, and Hansen U: The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J Biol Chem 2012; 287:18700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sher I, Zisman-Rozen S, Eliahu L, Whitelock JM, Maas-Szabowski N, Yamada Y, Breitkreutz D, Fusenig NE, Arikawa-Hirasawa E, Iozzo RV, Bergman R, and Ron D: Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J Biol Chem 2006; 281:5178. [DOI] [PubMed] [Google Scholar]
- 42.Hocking AM, Shinomura T, and McQuillan DJ: Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol 1998; 17:1. [DOI] [PubMed] [Google Scholar]
- 43.Kalamajski S, and Oldberg A: The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol 2010; 29:248. [DOI] [PubMed] [Google Scholar]
- 44.Iozzo RV, and Schaefer L: Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J 2010; 277:3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang CH, Culshaw GJ, Liu MM, Lu CC, French AT, Clements DN, and Corcoran BM: Canine tissue-specific expression of multiple small leucine rich proteoglycans. Vet J 2012; 193:374. [DOI] [PubMed] [Google Scholar]
- 46.Scott IC, Imamura Y, Pappano WN, Troedel JM, Recklies AD, Roughley PJ, and Greenspan DS: Bone morphogenetic protein-1 processes probiglycan. J Biol Chem 2000; 275:30504. [DOI] [PubMed] [Google Scholar]
- 47.Onnerfjord P, Heathfield TF, and Heinegard D: Identification of tyrosine sulfation in extracellular leucine-rich repeat proteins using mass spectrometry. J Biol Chem 2004; 279:26. [DOI] [PubMed] [Google Scholar]
- 48.Tillgren V, Onnerfjord P, Haglund L, and Heinegard D: The tyrosine sulfate-rich domains of the LRR proteins fibromodulin and osteoadherin bind motifs of basic clusters in a variety of heparin-binding proteins, including bioactive factors. J Biol Chem 2009; 284:28543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roughley PJ: The structure and function of cartilage proteoglycans. Eur Cell Mater 2006; 12:92. [DOI] [PubMed] [Google Scholar]
- 50.Iozzo RV, Goldoni S, Berendsen AD, and Young MF: Chapter 6. Small leucine-rich proteoglycans. In: The Extracellular Matrix: an Overview, Biology of Extracellular Matrix, edited by Mecham RP. Berlin: Heidelberg Springer-Verlag, 2011, pp. 197–266 [Google Scholar]
- 51.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, and Iozzo RV: Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997; 136:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orgel JP, Eid A, Antipova O, Bella J, and Scott JE: Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS One 2009; 4:e7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott JE, and Stockwell RA: Cartilage elasticity resides in shape module decoran and aggrecan sumps of damping fluid: implications in osteoarthrosis. J Physiol 2006; 574:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geng Y, McQuillan D, and Roughley PJ: SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol 2006; 25:484. [DOI] [PubMed] [Google Scholar]
- 55.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, and Okada Y: Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J 1997; 322(Pt 3):809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monfort J, Tardif G, Reboul P, Mineau F, Roughley P, Pelletier JP, and Martel-Pelletier J: Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site. Arthritis Res Ther 2006; 8:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Marschall Z, and Fisher LW: Decorin is processed by three isoforms of bone morphogenetic protein-1 (BMP1). Biochem Biophys Res Commun 2010; 391:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mimura T, Han KY, Onguchi T, Chang JH, Kim TI, Kojima T, Zhou Z, and Azar DT: MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res 2009; 46:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran KT, Lamb P, and Deng J-S: Matrikines and matricryptins: Implications for cutaneous cancers and skin repair. J Dermatol Sci 2005; 40:11. [DOI] [PubMed] [Google Scholar]
- 60.Fleischmajer R, Fisher LW, MacDonald ED, Jacobs L, Perlish JS, and Termine JD: Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol 1991; 106:82. [DOI] [PubMed] [Google Scholar]
- 61.Maccarana M, Kalamajski S, Kongsgaard M, Magnusson SP, Oldberg A, and Malmström A: Dermatan sulfate epimerase 1-deficient mice have reduced content and changed distribution of iduronic acids in dermatan sulfate and an altered collagen structure in skin. Mol Cell Biol 2009; 29:5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antonsson P, Heinegård D, and Oldberg Å: Structure and deduced amino acid sequence of the human fibromodulin gene. Biochim Biophys Acta 1993; 1174:204. [DOI] [PubMed] [Google Scholar]
- 63.Roughley PJ, White RJ, Cs-Szabo G, and Mort JS: Changes with age in the structure of fibromodulin in human articular cartilage. Osteoarthritis Cartilage 1996; 4:153. [DOI] [PubMed] [Google Scholar]
- 64.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, and Birk DE: Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol 2000; 151:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Velez-Delvalle C, Marsch-Moreno M, Castro-Munozledo F, Bolivar-Flores YJ, and Kuri-Harcuch W: Fibromodulin gene is expressed in human epidermal keratinocytes in culture and in human epidermis in vivo. Biochem Biophys Res Commun 2008; 371:420. [DOI] [PubMed] [Google Scholar]
- 66.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, and Carroll H: Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol 1998; 141:1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heathfield TF, Onnerfjord P, Dahlberg L, and Heinegard D: Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J Biol Chem 2004; 279:6286. [DOI] [PubMed] [Google Scholar]
- 68.Chakravarti S, Stallings RL, SundarRaj N, Cornuet PK, and Hassell JR: Primary structure of human lumican (keratan sulfate proteoglycan) and localization of the gene (LUM) to chromosome 12q21.3-q22. Genomics 1995; 27:481. [DOI] [PubMed] [Google Scholar]
- 69.Kalamajski S, and Oldberg A: Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding. J Biol Chem 2009; 284:534. [DOI] [PubMed] [Google Scholar]
- 70.Svensson L, Närlid I, and Oldberg A: Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett 2000; 470:178. [DOI] [PubMed] [Google Scholar]
- 71.Hedbom E, and Heinegård D: Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem 1993; 268:27307. [PubMed] [Google Scholar]
- 72.Neame PJ, Kay CJ, McQuillan DJ, Beales MP, and Hassell JR: Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci 2000; 57:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Aoki T, Mori Y, Ahmad M, Miyamori H, Takino T, and Sato H: Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res 2004; 64:7058. [DOI] [PubMed] [Google Scholar]
- 74.Sifaki M, Assouti M, Nikitovic D, Krasagakis K, Karamanos NK, and Tzanakakis GN: Lumican, a small leucine-rich proteoglycan substituted with keratan sulfate chains is expressed and secreted by human melanoma cells and not normal melanocytes. IUBMB Life 2006; 58:606. [DOI] [PubMed] [Google Scholar]
- 75.Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, and Conrad GW: Molecular cloning and tissue distribution of keratocan. Bovine corneal keratan sulfate proteoglycan 37A. J Biol Chem 1996; 271:9759. [DOI] [PubMed] [Google Scholar]
- 76.Chen J, Wong-Chong J, and SundarRaj N: FGF-2- and TGF-β1-induced downregulation of lumican and keratocan in activated corneal keratocytes by JNK signaling pathway. Invest Ophthalmol Vis Sci 2011; 52:8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bengtsson E, Neame PJ, Heinegard D, and Sommarin Y: The primary structure of a basic leucine-rich repeat protein, PRELP, found in connective tissues. J Biol Chem 1995; 270:25639. [DOI] [PubMed] [Google Scholar]
- 78.Grover J, and Roughley PJ: Characterization of the human proline/arginine-rich end leucine-rich repeat protein (PRELP) gene promoter and identification of a repressor element. Biochem J 1998; 336(Pt 1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grover J, and Roughley PJ: Characterization and expression of murine PRELP. Matrix Biol 2001; 20:555. [DOI] [PubMed] [Google Scholar]
- 80.Bengtsson E, Morgelin M, Sasaki T, Timpl R, Heinegard D, and Aspberg A: The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J Biol Chem 2002; 277:15061. [DOI] [PubMed] [Google Scholar]
- 81.Kadoya K, Fukushi J, Matsumoto Y, Yamaguchi Y, and Stallcup WB: NG2 proteoglycan expression in mouse skin: altered postnatal skin development in the NG2 null mouse. J Histochem Cytochem 2008; 56:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trotter J, Karram K, and Nishiyama A: NG2 cells: properties, progeny and origin. Brain Res Rev 2010; 63:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, and Stallcup WB: The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol 1991; 114:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Price MA, Colvin Wanshura LE, Yang J, Carlson J, Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA, and McCarthy JB: CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res 2011; 24:1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campoli M, Ferrone S, and Wang X: Functional and clinical relevance of chondroitin sulfate proteoglycan 4. Adv Cancer Res 2010; 109:73. [DOI] [PubMed] [Google Scholar]
- 86.Mayayo SL, Prestigio S, Maniscalco L, La Rosa G, Arico A, De Maria R, Cavallo F, Ferrone S, Buracco P, and Iussich S: Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J 2011; 190:e26. [DOI] [PubMed] [Google Scholar]
- 87.Goretzki L, Burg MA, Grako KA, and Stallcup WB: High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem 1999; 274:16831. [DOI] [PubMed] [Google Scholar]
- 88.Burg MA, Tillet E, Timpl R, and Stallcup WB: Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem 1996; 271:26110. [DOI] [PubMed] [Google Scholar]
- 89.Tillet E, Ruggiero F, Nishiyama A, and Stallcup WB: The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J Biol Chem 1997; 272:10769. [DOI] [PubMed] [Google Scholar]
- 90.Fang X, Burg MA, Barritt D, Dahlin-Huppe K, Nishiyama A, and Stallcup WB: Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol Biol Cell 1999; 10:3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stallcup WB: The NG2 proteoglycan: past insights and future prospects. J Neurocytol 2002; 31:423. [DOI] [PubMed] [Google Scholar]
- 92.Alexopoulou AN, Multhaupt HA, and Couchman JR: Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol 2007; 39:505. [DOI] [PubMed] [Google Scholar]
- 93.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, and Lose EJ: Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol 1992; 8:365. [DOI] [PubMed] [Google Scholar]
- 94.Manon-Jensen T, Itoh Y, and Couchman JR: Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J 2010; 277:3876. [DOI] [PubMed] [Google Scholar]
- 95.Lundqvist K, and Schmidtchen A: Immunohistochemical studies on proteoglycan expression in normal skin and chronic ulcers. Br J Dermatol 2001; 144:254. [DOI] [PubMed] [Google Scholar]
- 96.Bachy S, Letourneur F, and Rousselle P: Syndecan-1 interaction with the LG4/5 domain in laminin-332 is essential for keratinocyte migration. J Cell Physiol 2008; 214:238. [DOI] [PubMed] [Google Scholar]
- 97.Stepp MA, Liu Y, Pal-Ghosh S, Jurjus RA, Tadvalkar G, Sekaran A, Losicco K, Jiang L, Larsen M, Li L, and Yuspa SH. Reduced migration, altered matrix and enhanced TGFbeta1 signaling are signatures of mouse keratinocytes lacking Sdc1. J Cell Sci 2007; 120:2851. [DOI] [PubMed] [Google Scholar]
- 98.O'Connell MP, Fiori JL, Kershner EK, Frank BP, Indig FE, Taub DD, Hoek KS, and Weeraratna AT: Heparan sulfate proteoglycan modulation of Wnt5A signal transduction in metastatic melanoma cells. J Biol Chem 2009; 284:28704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stepp MA, Pal-Ghosh S, Tadvalkar G, Rajjoub L, Jurjus RA, Gerdes M, Ryscavage A, Cataisson C, Shukla A, and Yuspa SH: Loss of syndecan-1 is associated with malignant conversion in skin carcinogenesis. Mol Carcinog 2010; 49:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomas D, Vucic M, Situm M, and Kruslin B: The expression of syndecan-1 in psoriatic epidermis. Arch Dermatol Res 2008; 300:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee JH, Park H, Chung H, Choi S, Kim Y, Yoo H, Kim TY, Hann HJ, Seong I, Kim J, Kang KG, Han IO, and Oh ES: Syndecan-2 regulates the migratory potential of melanoma cells. J Biol Chem 2009; 284:27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin F, Ren XD, Doris G, and Clark RA: Three-dimensional migration of human adult dermal fibroblasts from collagen lattices into fibrin/fibronectin gels requires syndecan-4 proteoglycan. J Invest Dermatol 2005; 124:906. [DOI] [PubMed] [Google Scholar]
- 103.Nagy N, Nemeth IB, Szabad G, Szolnoky G, Belso N, Bata-Csorgo Z, Dobozy A, Kemeny L, and Szell M: The altered expression of syndecan 4 in the uninvolved skin of venous leg ulcer patients may predispose to venous leg ulcer. Wound Repair Regen 2008; 16:495. [DOI] [PubMed] [Google Scholar]
- 104.Capurro MI, Xu P, Shi W, Li F, Jia A, and Filmus J: Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell 2008; 14:700. [DOI] [PubMed] [Google Scholar]
- 105.Filmus J, and Selleck SB: Glypicans: proteoglycans with a surprise. J Clin Invest 2001; 108:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iglesias BV, Centeno G, Pascuccelli H, Ward F, Peters MG, Filmus J, Puricelli L, and de Kier Joffe EB: Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol Histopathol 2008; 23:1333. [DOI] [PubMed] [Google Scholar]
- 107.Litwack ED, Ivins JK, Kumbasar A, Paine-Saunders S, Stipp CS, and Lander AD: Expression of the heparan sulfate proteoglycan glypican-1 in the developing rodent. Dev Dyn 1998; 211:72. [DOI] [PubMed] [Google Scholar]
- 108.Ivins JK, Litwack ED, Kumbasar A, Stipp CS, and Lander AD: Cerebroglycan, a developmentally regulated cell-surface heparan sulfate proteoglycan, is expressed on developing axons and growth cones. Dev Biol 1997; 184:320. [DOI] [PubMed] [Google Scholar]
- 109.Watanabe K, Yamada H, and Yamaguchi Y: K-glypican: a novel GPI-anchored heparan sulfate proteoglycan that is highly expressed in developing brain and kidney. J Cell Biol 1995; 130:1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kolset SO, and Tveit H: Serglycin—structure and biology. Cell Mol Life Sci 2008; 65:1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pejler G, Abrink M, and Wernersson S: Serglycin proteoglycan: regulating the storage and activities of hematopoietic proteases. Biofactors 2009; 35:61. [DOI] [PubMed] [Google Scholar]
- 112.Niemann CU, Abrink M, Pejler G, Fischer RL, Christensen EI, Knight SD, and Borregaard N: Neutrophil elastase depends on serglycin proteoglycan for localization in granules. Blood 2007; 109:4478. [DOI] [PubMed] [Google Scholar]
- 113.Grujic M, Braga T, Lukinius A, Eloranta ML, Knight SD, Pejler G, and Abrink M: Serglycin-deficient cytotoxic T lymphocytes display defective secretory granule maturation and granzyme B storage. J Biol Chem 2005; 280:33411. [DOI] [PubMed] [Google Scholar]
- 114.Ronnberg E, Melo FR, and Pejler G: Mast cell proteoglycans. J Histochem Cytochem 2012; 60:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim JS, and Werth VP: Identification of specific chondroitin sulfate species in cutaneous autoimmune disease. J Histochem Cytochem 2011; 59:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dawicki W, and Marshall JS: New and emerging roles for mast cells in host defence. Curr Opin Immunol 2007; 19:31. [DOI] [PubMed] [Google Scholar]
- 117.Malaviya R, Ikeda T, Ross E, and Abraham SN: Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 1996; 381:77. [DOI] [PubMed] [Google Scholar]
- 118.Proudfoot AE: The biological relevance of chemokine-proteoglycan interactions. Biochem Soc Trans 2006; 34:422. [DOI] [PubMed] [Google Scholar]
- 119.Ellyard JI, Simson L, Bezos A, Johnston K, Freeman C, and Parish CR: Eotaxin selectively binds heparin. An interaction that protects eotaxin from proteolysis and potentiates chemotactic activity in vivo. J Biol Chem 2007; 282:15238. [DOI] [PubMed] [Google Scholar]
- 120.Milstone LM, Hough-Monroe L, Kugelman LC, Bender JR, and Haggerty JG: Epican, a heparan/chondroitin sulfate proteoglycan form of CD44, mediates cell-cell adhesion. J Cell Sci 1994; 107(Pt 11):3183. [DOI] [PubMed] [Google Scholar]
- 121.Tuhkanen AL, Tammi M, and Tammi R: CD44 substituted with heparan sulfate and endo-beta-galactosidase-sensitive oligosaccharides: a major proteoglycan in adult human epidermis. J Invest Dermatol 1997; 109:213. [DOI] [PubMed] [Google Scholar]
- 122.Quintanilla M, Ramirez JR, Perez-Gomez E, Romero D, Velasco B, Letarte M, Lopez-Novoa JM, and Bernabeu C: Expression of the TGF-beta coreceptor endoglin in epidermal keratinocytes and its dual role in multistage mouse skin carcinogenesis. Oncogene 2003; 22:5976. [DOI] [PubMed] [Google Scholar]
- 123.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, and Letarte M: Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem 1992; 267:19027. [PubMed] [Google Scholar]
- 124.Gougos A, and Letarte M: Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem 1990; 265:8361. [PubMed] [Google Scholar]
- 125.O'Connell PJ, McKenzie A, Fisicaro N, Rockman SP, Pearse MJ, and d'Apice AJ: Endoglin: a 180-kD endothelial cell and macrophage restricted differentiation molecule. Clin Exp Immunol 1992; 90:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ge AZ, and Butcher EC: Cloning and expression of a cDNA encoding mouse endoglin, an endothelial cell TGF-beta ligand. Gene 1994; 138:201. [DOI] [PubMed] [Google Scholar]
- 127.Yamashita H, Ichijo H, Grimsby S, Moren A, ten Dijke P, and Miyazono K: Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-beta. J Biol Chem 1994; 269:1995. [PubMed] [Google Scholar]
- 128.Bernabeu C, Lopez-Novoa JM, and Quintanilla M: The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim Biophys Acta 2009; 1792:954. [DOI] [PubMed] [Google Scholar]
- 129.Bilandzic M, and Stenvers KL: Betaglycan: a multifunctional accessory. Mol Cell Endocrinol 2011; 339:180. [DOI] [PubMed] [Google Scholar]
- 130.ten Dijke P, Goumans MJ, and Pardali E: Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008; 11:79. [DOI] [PubMed] [Google Scholar]
- 131.Rulo HF, Westphal JR, van de Kerkhof PC, de Waal RM, van Vlijmen IM, and Ruiter DJ: Expression of endoglin in psoriatic involved and uninvolved skin. J Dermatol Sci 1995; 10:103. [DOI] [PubMed] [Google Scholar]
- 132.Dharmapatni AA, Smith MD, Ahern MJ, Simpson A, Li C, Kumar S, and Roberts-Thomson PJ: The TGF beta receptor endoglin in systemic sclerosis. Asian Pac J Allergy Immunol 2001; 19:275. [PubMed] [Google Scholar]
- 133.Dawn G, and MacKie RM: Expression of endoglin in human melanocytic lesions. Clin Exp Dermatol 2002; 27:153. [DOI] [PubMed] [Google Scholar]
- 134.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, and ten Dijke P: Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J 2004; 23:4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schmidt-Weber CB, Letarte M, Kunzmann S, Ruckert B, Bernabeu C, and Blaser K: TGF-b signaling of human T cells is modulated by the ancillary TGF-b receptor endoglin. Int Immunol 2005; 17:921. [DOI] [PubMed] [Google Scholar]
- 136.Morris E, Chrobak I, Bujor A, Hant F, Mummery C, Ten Dijke P, and Trojanowska M: Endoglin promotes TGF-beta/Smad1 signaling in scleroderma fibroblasts. J Cell Physiol 2011; 226:3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maring JA, Trojanowska M, and ten Dijke P: Role of endoglin in fibrosis and scleroderma. Int Rev Cell Mol Biol 2012; 297:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rossi E, Sanz-Rodriguez F, Eleno N, Duwell A, Blanco FJ, Langa C, Botella LM, Cabanas C, Lopez-Novoa JM, and Bernabeu C: Endothelial endoglin is involved in inflammation: role in leukocyte adhesion and transmigration. Blood 2013; 121:403. [DOI] [PubMed] [Google Scholar]
- 139.Lopez-Casillas F, Payne HM, Andres JL, and Massague J: Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J Cell Biol 1994; 124:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, and Vale W: Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 2000; 404:411. [DOI] [PubMed] [Google Scholar]
- 141.Yang L, and Peng R: Unveiling hair follicle sten cells. Stem Cell Rev 2010; 6:658. [DOI] [PubMed] [Google Scholar]
- 142.Brown TA, Bouchard T, St John T, Wayner E, and Carter WG: Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J Cell Biol 1991; 113:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Penneys NS: CD44 expression in normal and inflamed skin. J Cutan Pathol 1993; 20:250. [DOI] [PubMed] [Google Scholar]
- 144.Chen WY, and Abatangelo G: Functions of hyaluronan in wound repair. Wound Repair Regen 1999; 7:79. [DOI] [PubMed] [Google Scholar]
- 145.Kaya G, Rodriguez I, Jorcano JL, Vassalli P, and Stamenkovic I: Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev 1997; 11:996. [DOI] [PubMed] [Google Scholar]
- 146.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, and Tang DG: The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011; 17:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goodison S, Urquidi V, and Tarin D: CD44 cell adhesion molecules. Mol Pathol 1999; 52:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yasasever V, Tas F, Duranyildiz D, Camlica H, Kurul S, and Dalay N: Serum levels of the soluble adhesion molecules in patients with malignant melanoma. Pathol Oncol Res 2000; 6:42. [DOI] [PubMed] [Google Scholar]
- 149.Sy MS, Guo YJ, and Stamenkovic I: Inhibition of tumor growth in vivo with a soluble CD44-immunoglobulin fusion protein. J Exp Med 1992; 176:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Artlett CM: Inflammasomes in wound healing and fibrosis. J Pathol 2013; 229:157. [DOI] [PubMed] [Google Scholar]
- 151.Diegelmann RF, and Evans MC: Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9:283. [DOI] [PubMed] [Google Scholar]
- 152.Gibran NS, Boyce S, and Greenhalgh DG: Cutaneous wound healing. J Burn Care Res 2007; 28:577. [DOI] [PubMed] [Google Scholar]
- 153.Namazi MR, Fallahzadeh MK, and Schwartz RA: Strategies for prevention of scars: what can we learn from fetal skin? Int J Dermatol 2011; 50:85. [DOI] [PubMed] [Google Scholar]
- 154.Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, and Schaefer L: Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J 2013; 280:2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ghatak S, Misra S, and Toole BP: Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem 2002; 277:38013. [DOI] [PubMed] [Google Scholar]
- 156.Gao F, Liu Y, He Y, Yang C, Wang Y, Shi X, and Wei G: Hyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesis. Matrix Biol 2010; 29:107. [DOI] [PubMed] [Google Scholar]
- 157.Tammi R, Ripellino JA, Margolis RU, Maibach HI, and Tammi M: Hyaluronate accumulation in human epidermis treated with retinoic acid in skin organ culture. J Invest Dermatol 1989; 92:326. [DOI] [PubMed] [Google Scholar]
- 158.Tammi RH, and Tammi MI: Hyaluronan accumulation in wounded epidermis: a mediator of keratinocyte activation. J Invest Dermatol 2009; 129:1858. [DOI] [PubMed] [Google Scholar]
- 159.Tammi R, Ripellino JA, Margolis RU, and Tammi M: Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol 1988; 90:412. [DOI] [PubMed] [Google Scholar]
- 160.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, and Simon JC: Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 2002; 195:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, and Simon JC: Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol 2000; 165:1863. [DOI] [PubMed] [Google Scholar]
- 162.Borthwick LA, Wynn TA, and Fisher AJ: Cytokine mediated tissue fibrosis. Biochim Biophys Acta 2013; 1832:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Klingberg F, Hinz B, and White ES: The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 2013; 229:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, and Hynes RO: The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 2012; 11:M111..014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Graham GJ, and Locati M: Regulation of the immune and inflammatory responses by the ‘atypical’ chemokine receptor D6. J Pathol 2013; 229:168. [DOI] [PubMed] [Google Scholar]
- 166.Kapetanaki MG, Mora AL, and Rojas M: Influence of age on wound healing and fibrosis. J Pathol 2013; 229:310. [DOI] [PubMed] [Google Scholar]
- 167.Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, and Adzick NS: Adult skin wounds in the fetal environment heal with scar formation. Ann Surg 1994; 219:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lorenz HP, Lin RY, Longaker MT, Whitby DJ, and Adzick NS: The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg 1995; 96:1251.; discussion 1260–1. [DOI] [PubMed] [Google Scholar]
- 169.Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, and Adzick NS: Scarless wound repair: a human fetal skin model. Development 1992; 114:253. [DOI] [PubMed] [Google Scholar]
- 170.Rolfe KJ, Irvine LM, Grobbelaar AO, and Linge C: Differential gene expression in response to transforming growth factor-beta1 by fetal and postnatal dermal fibroblasts. Wound Repair Regen 2007; 15:897. [DOI] [PubMed] [Google Scholar]
- 171.Eslami A, Gallant-Behm CL, Hart DA, Wiebe C, Honardoust D, Gardner H, Häkkinen L, and Larjava HS: Expression of integrin alphavbeta6 and TGF-beta in scarless vs scar-forming wound healing. J Histochem Cytochem 2009; 57:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, and Stern R: Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg 1991; 213:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gawronska-Kozak B, Bogacki M, Rim J-S, Monroe WT, and Manuel JA: Scarless skin repair in immunodeficient mice. Wound Repair Regen 2006; 14:265. [DOI] [PubMed] [Google Scholar]
- 174.Sayani K, Dodd CM, Nedelec B, Shen YJ, Ghahary A, Tredget EE, and Scott PG: Delayed appearance of decorin in healing burn scars. Histopathology 2000; 36:262. [DOI] [PubMed] [Google Scholar]
- 175.Armour A, Scott PG, and Tredget EE: Cellular and molecular pathology of HTS: basis for treatment. Wound Repair Regen 2007; 15:S6. [DOI] [PubMed] [Google Scholar]
- 176.Honardoust D, Varkey M, Hori K, Ding J, Shankowsky HA, and Tredget EE: Small leucine-rich proteoglycans, decorin and fibromodulin, are reduced in postburn hypertrophic scar. Wound Repair Regen 2011; 19:368. [DOI] [PubMed] [Google Scholar]
- 177.Honardoust D, Varkey M, Marcoux Y, Shankowsky HA, and Tredget EE: Reduced decorin, fibromodulin, and transforming growth factor-beta3 in deep dermis leads to hypertrophic scarring. J Burn Care Res 2012; 33:218. [DOI] [PubMed] [Google Scholar]
- 178.Zheng Z, Nguyen C, Zhang X, Khorasani H, Wang JZ, Zara JN, Chu F, Yin W, Pang S, Le A, Ting K, and Soo C: Delayed wound closure in fibromodulin-deficient mice is associated with increased TGF-beta3 signaling. J Invest Dermatol 2011; 131:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yeh JT, Yeh LK, Jung SM, Chang TJ, Wu HH, Shiu TF, Liu CY, Kao WW, and Chu PH: Impaired skin wound healing in lumican-null mice. Br J Dermatol 2010; 163:1174. [DOI] [PubMed] [Google Scholar]
- 180.Ojeh N, Hiilesvuo K, Warri A, Salmivirta M, Henttinen T, and Maatta A: Ectopic expression of syndecan-1 in basal epidermis affects keratinocyte proliferation and wound re-epithelialization. J Invest Dermatol 2008; 128:26. [DOI] [PubMed] [Google Scholar]
- 181.Sidgwick GP, and Bayat A: Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 2012; 26:141. [DOI] [PubMed] [Google Scholar]
- 182.Koźma EM, Olczyk K, Głowacki A, and Bobiński R: An accumulation of proteoglycans in scarred fascia. Mol Cell Biochem 2000; 203:103. [DOI] [PubMed] [Google Scholar]
- 183.Shih B, McGrouther DA, and Bayat A: Identification of novel keloid biomarkers through profiling of tissue biopsies versus cell cultures in keloid margin specimens compared to adjacent normal skin. Eplasty 2010; 10:e24. [PMC free article] [PubMed] [Google Scholar]
- 184.Naylor EC, Watson REB, and Sherratt MJ: Molecular aspects of skin ageing. Maturitas 2011; 69:249. [DOI] [PubMed] [Google Scholar]
- 185.Ghersetich I, Lotti T, Campanile G, Grappone C, and Dini G: Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol 1994; 33:119. [DOI] [PubMed] [Google Scholar]
- 186.Waller JM, and Maibach HI: Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol 2006; 12:145. [DOI] [PubMed] [Google Scholar]
- 187.Oh JH, Kim YK, Jung JY, Shin JE, and Chung JH: Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Exp Dermatol 2011; 20:454. [DOI] [PubMed] [Google Scholar]
- 188.Nomura Y: Structural change in decorin with skin aging. Connect Tissue Res 2006; 47:249. [DOI] [PubMed] [Google Scholar]
- 189.Bernstein EF, Underhill CB, Hahn PJ, Brown DB, and Uitto J: Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Br J Dermatol 1996; 135:255. [DOI] [PubMed] [Google Scholar]
- 190.Kunisada M, Yogianti F, Sakumi K, Ono R, Nakabeppu Y, and Nishigori C: Increased expression of versican in the inflammatory response to UVB- and reactive oxygen species-induced skin tumorigenesis. Am J Pathol 2011; 179:3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jung JY, Oh JH, Kim YK, Shin MH, Lee D, and Chung JH: Acute UV irradiation increases heparan sulfate proteoglycan levels in human skin. J Korean Med Sci 2012; 27:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Werth BB, Bashir M, Chang LY, and Werth VP: Ultraviolet irradiation induces the accumulation of chondroitin sulfate, but not other glycosaminoglycans, in human skin. PLoS One 2011; 6:e14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schwartz E: Connective tissue alterations in the skin of ultraviolet irradiated hairless mice. J Invest Dermatol 1988; 91:158. [DOI] [PubMed] [Google Scholar]
- 194.Koshiishi I, Horikoshi E, Mitani H, and Imanari T: Quantitative alterations of hyaluronan and dermatan sulfate in the hairless mouse dorsal skin exposed to chronic UV irradiation. Biochim Biophys Acta 1999; 1428:327. [DOI] [PubMed] [Google Scholar]
- 195.Margelin D, Fourtanier A, Thevenin T, Medaisko C, Breton M, and Picard J: Alterations of proteoglycans in ultraviolet-irradiated skin. Photochem Photobiol 1993; 58:211. [DOI] [PubMed] [Google Scholar]
- 196.Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, and Simon JC: Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol 2007; 127:687. [DOI] [PubMed] [Google Scholar]
- 197.Dai G, Freudenberger T, Zipper P, Melchior A, Grether-Beck S, Rabausch B, de Groot J, Twarock S, Hanenberg H, Homey B, Krutmann J, Reifenberger J, and Fischer JW: Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am J Pathol 2007; 171:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shin J-E, Oh J-H, Kim YK, Jung J-Y, and Chung JH: Transcriptional regulation of proteoglycans and glycosaminoglycan chain-synthesizing glycosyltransferases by UV irradiation in cultured human dermal fibroblasts. J Korean Med Sci 2011; 26:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lochner K, Gaemlich A, Südel KM, Venzke K, Moll I, Knott A, Stäb F, Wenck H, Döring O, Böttger M, and Gallinat S: Expression of decorin and collagens I and III in different layers of human skin in vivo: a laser capture microdissection study. Biogerontology 2007; 8:269. [DOI] [PubMed] [Google Scholar]
- 200.Gambichler T, Tomi NS, Skrygan M, Altmeyer P, and Kreuter A: Significant decrease of decorin expression in human skin following short-term ultraviolet exposures. J Dermatol Sci 2007; 45:203. [DOI] [PubMed] [Google Scholar]
- 201.Carrino DA, Onnerfjord P, Sandy JD, Cs-Szabo G, Scott PG, Sorrell JM, Heinegard D, and Caplan AI: Age-related changes in the proteoglycans of human skin. Specific cleavage of decorin to yield a major catabolic fragment in adult skin. J Biol Chem 2003; 278:17566. [DOI] [PubMed] [Google Scholar]
- 202.Kwon Y-J, Lee J-W, Moon E-J, Chung YG, Kim O-S, and Kim H-J: Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration. Spine 2013; 38:E49. [DOI] [PubMed] [Google Scholar]
- 203.Philandrianos C, Andrac-Meyer L, Mordon S, Feuerstein J-M, Sabatier F, Veran J, Magalon G, and Casanova D: Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 2012; 38:820. [DOI] [PubMed] [Google Scholar]
- 204.Hartmann-Fritsch F, Biedermann T, Braziulis E, Meuli M, and Reichmann E: A new model for preclinical testing of dermal substitutes for human skin reconstruction. Pediatr Surg Int 2013; 29:479. [DOI] [PubMed] [Google Scholar]
- 205.Natesan S, Zamora DO, Wrice NL, Baer DG, and Christy RJ: Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J Burn Care Res 2013; 34:18. [DOI] [PubMed] [Google Scholar]
- 206.Chang P, and Tao K: The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast Reconstr Surg 2013; 131:116e. [DOI] [PubMed] [Google Scholar]
- 207.Hartmann-Fritsch F, Hosper N, Luginbuhl J, Biedermann T, Reichmann E, and Meuli M: Human amniotic fluid derived cells can competently substitute dermal fibroblasts in a tissue-engineered dermo-epidermal skin analog. Pediatr Surg Int 2013; 29:61. [DOI] [PubMed] [Google Scholar]
- 208.Alamein MA, Stephens S, Liu Q, Skabo S, and Warnke PH: Mass production of nanofibrous extracellular matrix with controlled 3D morphology for large-scale soft tissue regeneration. Tissue Eng Part C Methods 2013; 19:458. [DOI] [PubMed] [Google Scholar]
- 209.Leonardi D, Oberdoerfer D, Fernandes MC, Meurer RT, Pereira-Filho GA, Cruz P, Vargas M, Chem RC, Camassola M, and Nardi NB: Mesenchymal stem cells combined with an artificial dermal substitute improve repair in full-thickness skin wounds. Burns 2012; 38:1143. [DOI] [PubMed] [Google Scholar]
- 210.Gibot L, Galbraith T, Huot J, and Auger FA: Development of a tridimensional microvascularized human skin substitute to study melanoma biology. Clin Exp Metastasis 2013; 30:83. [DOI] [PubMed] [Google Scholar]
- 211.Kim DH, Lu N, Ma R, Kim YS, Kim RH, Wang S, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim TI, Chowdhury R, Ying M, Xu L, Li M, Chung HJ, Keum H, McCormick M, Liu P, Zhang YW, Omenetto FG, Huang Y, Coleman T, and Rogers JA: Epidermal electronics. Science 2011; 333:838. [DOI] [PubMed] [Google Scholar]
- 212.Ramuz M, Tee BC, Tok JB, and Bao Z: Transparent, optical, pressure-sensitive artificial skin for large-area stretchable electronics. Adv Mater 2012; 24:3223. [DOI] [PubMed] [Google Scholar]
- 213.Brocklesby KL, Johns SC, Jones AE, Sharp D, and Smith RB: Smart bandages—A colourful approach to early stage infection detection & control in wound care. Med Hypotheses 2013; 80:237. [DOI] [PubMed] [Google Scholar]
- 214.Landsman A, Agnew P, Parish L, Joseph R, and Galiano RD: Diabetic foot ulcers treated with becaplermin and TheraGauze, a moisture-controlling smart dressing: a randomized, multicenter, prospective analysis. J Am Podiatr Med Assoc 2010; 100:155. [DOI] [PubMed] [Google Scholar]
- 215.Jenkins AT, and Young A: Smart dressings for the prevention of infection in pediatric burns patients. Expert Rev Anti Infect Ther 2010; 8:1063. [DOI] [PubMed] [Google Scholar]
- 216.Smith SM, Whitelock JM, Iozzo RV, Little CB, and Melrose J: Topographical variation in the distributions of versican, aggrecan and perlecan in the foetal human spine reflects their diverse functional roles in spinal development. Histochem Cell Biol 2009; 132:491. [DOI] [PubMed] [Google Scholar]
- 217.Smith SM, Shu C, and Melrose J: Comparative immunolocalisation of perlecan with collagen II and aggrecan in human foetal, newborn and adult ovine joint tissues demonstrates perlecan as an early developmental chondrogenic marker. Histochem Cell Biol 2010; 134:251. [DOI] [PubMed] [Google Scholar]
- 218.Ochoa PC, Smith OD, and Swerdlow M: The dermal-epidermal junction: a preliminary study with periodic acid Schiff stain. AMA Arch Derm 1957; 75:70. [DOI] [PubMed] [Google Scholar]
- 219.Wislocki GB, Fawcett DW, and Dempsey EW: Staining of stratified squamous epithelium of mucous membranes and skin of man and monkey by the periodic-Schiff method. Anat Rec 1951; 110:359. [DOI] [PubMed] [Google Scholar]
- 220.Zugibe FT: Positive periodic acid—Schiff staining of acid mucopolysaccharides. Histochem J 1970; 2:191. [DOI] [PubMed] [Google Scholar]
- 221.Horii-Hayashi N, Okuda H, Tatsumi K, Ishizaka S, Yoshikawa M, and Wanaka A: Localization of chondroitin sulfate proteoglycan versican in adult brain with special reference to large projection neurons. Cell Tissue Res 2008; 334:163. [DOI] [PubMed] [Google Scholar]
- 222.O'Connell MP, and Weeraratna AT: A spoonful of sugar makes the melanoma go: the role of heparan sulfate proteoglycans in melanoma metastasis. Pigment Cell Melanoma Res 2011; 24:1133. [DOI] [PubMed] [Google Scholar]
- 223.Breitkreutz D, Koxholt I, Thiemann K, and Nischt R: Skin basement membrane: the foundation of epidermal integrity—BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res Int 2013: 179784, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yasaka N, Furue M, and Tamaki K: CD44 expression in normal human skin and skin tumors. J Dermatol 1995; 22:88. [DOI] [PubMed] [Google Scholar]
- 225.Ahn JY, Park S, Yun YS, and Song JY: Inhibition of type III TGF-b receptor aggravates lung fibrotic process. Biomed Pharmacother 2010; 64:472. [DOI] [PubMed] [Google Scholar]
- 226.Hasebe Y, Hasegawa S, Hashimoto N, Toyoda M, Matsumoto K, Umezawa A, Yagami A, Matsunaga K, Mizutani H, Nakata S, and Akamatsu H: Analysis of cell characterization using cell surface markers in the dermis. J Dermatol Sci 2011; 62:98. [DOI] [PubMed] [Google Scholar]