Abstract
Barrett’s esophagus (BE) is defined as any metaplastic columnar epithelium in the distal esophagus which replaces normal squamous epithelium and which predisposes to cancer development. It is this second requirement, the predisposition to cancer, which makes this condition both clinically highly relevant and an important area for ongoing research. While BE has been defined pathologically since the 1950’s (Allison and Johnstone, Thorax 1955), and identified as a risk factor for esophageal adenocarcinoma since the 1970’s (Naef A.P., et.al. J Thorac Cardiovasc Surg. 1975), our understanding of the molecular events giving rise to this condition remains limited. Herein we will examine what is known about the intestinal features of BE and how well it recapitulates the intestinal epithelium, including stem identity and function. Finally, we will explore laboratory models of this condition presently in use and under development, to identify new insights they may provide into this important clinical condition.
Keywords: Gastroesophageal reflux disease (GERD), Barrett’s esophagus (BE), esophageal adenocarcinoma (EAC), tissue engineering, human 3D organotypic model systems (OTC), Multi-layered epithelium (MLE), BMP4, Hedgehog, Cdx2, Sox2
Introduction
Barrett’s esophagus (BE) occurs in the in the distal esophagus in the setting of chronic gastroesophageal reflux disease (GERD) and is histopathologically defined as the replacement of the normal squamous epithelium with an intestinalized columnar epithelium (1). Clinically, the importance of Barrett’s esophagus lies in the observation that it is an important risk factor for esophageal adenocarcinoma (EAC) (2). This is not unique, as metaplasia has been linked to malignant transformation in other tissues including the stomach, where gastric intestinal metaplasia precedes gastric cancer, and in the cervix and lung, where squamous metaplasia in the columnar epithelium precedes the onset of cancer (3).
Efforts directed at understanding the pathogenesis of BE and its progression to EAC have been increasing over the last 15 years. Driving this increased focus has been the well-established observation that the rates of EAC have been increasing within the U.S. and western European populations over the last 3 decades (2). While this imperative to better understand BE onset and progression to dysplasia and cancer has become clearer, much about the pathogenesis of this disease remains poorly understood. Herein we review the intestinal differentiation of BE and explore approaches currently used to study BE in the clinics and laboratories.
Molecular features of BE overlap with but do not completely recapitulate normal intestinal differentiation in the esophagus
Histologically BE is characterized as the replacement of the normal multilayered squamous epithelium with a specialized columnar-lined epithelium containing intestinal-type goblet cells. In fact, there are three types of columnar epithelium observed in the distal esophagus including (1) a junctional (cardia-type) epithelium, (2) a gastric fundic-type epithelium with parietal and chief cells, and (3) a specialized, intestinal-type metaplasia with prominent goblet cells (4). However, in a widely circulated position statement, the AGA defined Barrett’s esophagus as “the condition in which any extent of metaplastic columnar epithelium that predisposes to cancer development replaces the stratified squamous epithelium ….” (5). Therefore, it is this risk of progression to EAC that has led to the clinical focus on intestinalized metaplasia, although there are many questioning whether for clinical surveillance the focus should be broadened to include all columnar epithelium in the distal esophagus.
BE is referred to as an “intestinal” metaplasia because all four of the main intestinal epithelial cell lineages have been detected in BE tissues, including enterocytes, Paneth cells, enteroendocrine cells, and of course goblet cells. However, these cell lineages are not typically fully mature. At the ultrastructural level, enterocytes are frequently described as “pseudoabsorptive” displaying apical microvilli, but mature cells with a well defined brush border are rare (6). Nevertheless, these “pseudoabsorptive” BE cells express many genes associated with absorptive enterocytes.
BE and associated tissues have been characterized using squamous-cell specific and glandular epithelial-cell specific cytokeratins (CK) and differentiation markers. CK7 and CK20 staining patterns may specifically distinguish BE from other histologically-related conditions including intestinal metaplasia of the gastric antrum and cardia (7, 8). Goblet cells are much more abundant in BE than in normal small intestine, and they are marked by the presence of acidic mucins detected by Alcian Blue staining, with Mucin 2 (MUC2) being perhaps the most important (9). Like the cytokeratins, mucin expression patterns have been used to distinguish BE from cardia and antrum metaplasia, with MUC1 and MUC6 expression in BE distinct from mucin expression in the cardia and antrum (10).
Gene expression profiling revealed not only a similarity between normal upper GI (i.e. gastric and duodenal) mucosa and BE but stark differences between the squamous normal esophageal tissues and BE. Amongst genes relatively specific to BE are CK8, CK20, MUC2, MUC5AC, MUC6 and mucin-associated trefoil factors TFF1, TFF2 and TFF3 (11, 12). Analyzing multiple datasets from six independent studies several transcription factors (CDX1, CDX2, HNF1, and HNF4) and the TGF-β/BMP pathway have been identified being significantly enriched in BE (13). Homeobox transcription factors CDX1 and CDX2 are required for normal intestinal development, although their role in BE intestinal metaplasia and cancer is presently unclear. Both are induced in esophageal keratinocytes exposed to bile and acids in vitro and in vivo (14-16). In addition, our own gene array analysis of BE identified CDX1 and the c-myc pathway as possible candidate transcription factors cooperating to induce mucin production and changes in keratin expression in the BE epithelium (17).
Cell of origin of Barrett’s esophagus
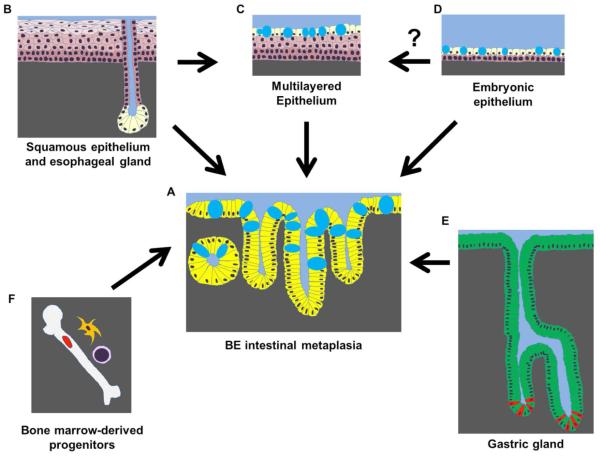
There are several accepted hypotheses concerning which cells give rise to BE in adults with GERD (Figure 1). They include 1) “transdifferentiation” of squamous epithelial cells into columnar BE cells; 2) migration upward of subesophageal gland cells; 3) migration of an embryonic population residing at the squamo-columnar junction; 4) migration of columnar epithelia cells from the gastric cardia; and finally, 5) migration in of bone marrow progenitors. There are published studies in support of all these possibilities, and none have yet been formally excluded. In support of the first premise, scanning electron microscopy has revealed a unique multilayered epithelium (MLE) at the squamo-columnar junction and within columnar mucosa (Figure 2). MLE comprises 4-8 layers of distinctive stratified squamous-like cells defined by intercellular ridges topped with superficial mucinous epithelial cells expressing microvilli (18, 19). MLE has been postulated as an early or intermediate stage of columnar metaplasia (19, 20). MLE expresses both squamous-cell and glandular differentiation markers (20, 21) in line with the “transdifferentiation” hypothesis. In addition, MLE develops in a rat model of gastroesophageal reflux-induced BE (22), as well as our study targeting the intestine-specific transcription factor Cdx2 into the murine esophagus (14).
Figure 1.
Multiple tissue and cell types have been hypothesized to serve as the cell of origin for BE comprising intestinal-type columnar epithelial cells with goblet cells (deposition of mucins are depicted as blue oval shapes) in (A). Esophageal squamous epithelial cells (keratinocytes) or submucosal esophageal gland ductal epithelial cells (B) may give rise to BE directly. MLE is suspected as a BE precursor lesion (C). MLE has been linked to esophageal glands in human. BE may be accounted for by migration of residual embryonic esophageal cells or reactivation of the developmental pathways (e.g. BMP4, Hh)(D). Migration of gastric cells (e.g. Lgr5-positive stem cells in red at the grand base)(E) and circulating bone marrow progenitor (F) remain the possibilities.
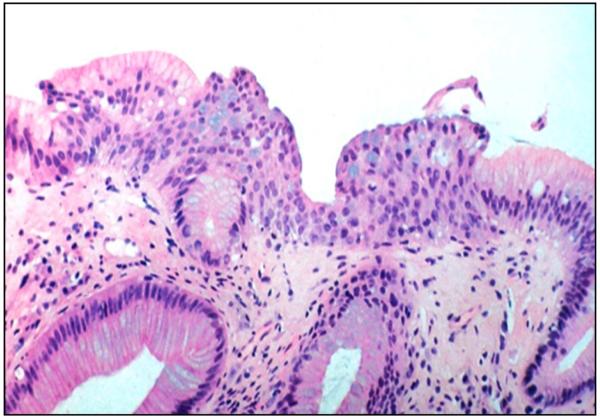
Figure 2.
Multilayered epithelium features distinct stratified squamous epithelium-like cell layers topped by a layer of columnar cells containing goblet cells. Photomicrograph, a courtesy of Dr. Nirag Jhala, MD, University of Pennsylvania Perelman School of Medicine.
In other studies, comparing gland morphology and immunohistochemical staining patterns has led several groups to conclude that BE and MLE may arise from the esophageal gland duct epithelial cells (Table 1) (20, 23). Corroborating this premise, Braxton et al. have recently reported that esophageal submucosal glands display unique reflux-induced metaplastic changes (24). However, the lack of esophageal submucosal glands in rodents limits experimental modeling and testing of this hypothesis
Table 1.
Molecular markers defining BE and of potential cell origin of BE
| BE and potential cell of origin | Histologic Markers |
|---|---|
| BE intestinal metaplasia | CK7, CK8, CK18, CK20 MUC1, MUC2, MUC5AC, MUC6, CDX1, CDX2, BMP4 |
| Gastric glands | MUC1, MUC5AC, MUC6 |
| Esophageal squamous epithelium | p63, SOX2, CK14, CK13 MUC1, MUC4 |
| Esophageal gland | p63, CK13 CK7, CK8, CK18, CK20, MUC5B |
| Multilayered epithelium | p63, SOX2, CK4, CK13, CK7, CK8, CK18, CK20, MUC2, MUC5AC, MUC6, CDX2 |
| Embryonic esophageal cells | < ~embryonic day 15.5-17.5: CK7, CK8, CK18, MUC4, BMP4 > ~ embryonic day: p63, SOX2, CK14, CK13 |
| Bone marrow progenitors | Donor cell Y-chromosome |
CK, cytokeratin. CK7, CK8, CK18 and CK20 are columnar cell-specific; CK14, CK4, CK13 are squamous cell-specific. MUC, mucin. MUC2, MUC5AC, MUC5B and MUC6 are secretory mucins. MUC1 and MUC4 are membrane-anchored mucins.
Another potential cell of origin of human BE is a unique cell population described by Wang et al. (25). Rodent stomach consists of the forestomach and distal stomach, lined by the squamous and glandular epithelium, respectively. The transcription factor p63 is required for normal development of the squamous epithelium of the forestomach and esophagus in mice (26). In p63−/− mice, the squamous epithelium fails to form, and there is a compensatory expansion of cells from the glandular compartment. In particular there is expansion of a population normally observed on the border of the squamous and glandular epithelium (25). These cells are thought to be embryonic remnants since similar cells are observed during esophageal development. However, these embryonic mouse cells fail to express the intestine-specific transcription factor Cdx2, which is very frequently observed in human BE (25). This suggests this model may not be truly representative of the human disease.
Developmental signaling pathways regulate cell fates and differentiation in BE
During embryogenesis, the primitive foregut endoderm develops into the esophageal tube consisting of simple epithelium topped with a superficial layer of ciliated epithelial cells and supported by the surrounding mesoderm. The ciliated epithelium is replaced by a stratified squamous epithelium beginning at the fourth month of gestation (27). In addition, we know from the mouse that in the developing esophagus, the foregut epithelial cell fate is subjected to regulation by transcription factors NKX2.1, p63 and Sox2. While NKX2.1 facilitates columnar cell differentiation in the trachea, p63 and Sox2 cooperate to promote squamous-cell differentiation in the esophagus (28). p63 and Sox2 define the development of the normal esophageal epithelium; loss of either results in the failure of squamous epithelial formation and the persistence of mucus-producing ciliated columnar epithelial cells in embryonic and newborn mice (29, 30).
Esophageal development is also regulated by other embryonic signaling pathways including Hedgehog (Hh), Wingless (Wnt), and bone morphogenic protein (BMP) (31). Hh signaling is required for a proper separation of the single anterior foregut tube into the esophagus and trachea (32, 33). Canonical Wnt signaling functions to promote columnar cell differentiation in the trachea by inducing NKX2.1 and downregulating SOX2 (34). Lastly, excess BMP signaling can lead to failure of the transition from ciliated columnar epithelium to the differentiated, multilayered squamous epithelium characteristic of a mature esophagus and forestomach (35). Together these observations suggest a complex, interconnected web of developmental signaling pathways and transcription factors serve to coordinated the emergence of a mature, multilayered squamous epithelium in the developing esophagus. It is interesting to speculate whether BE represents a failure of this network.
To explore this possibility, the roles of these and other transcription factors and developmental signaling pathways have been examined in human BE tissues (Table 2). p63, which is not expressed in BE or esophageal adenocarcinoma but is frequently present in BE- associated MLE (20), can be downregulated in primary esophageal epithelial cells when they are treated with bile salts and/or acid, suggesting that p63-mediated squamous-cell commitment may be impaired upon gastroduodenal reflux conditions (36). The role of BMP4 in BE pathogenesis is more complex. BMP4 is overexpressed in GERD esophagitis and BE epithelium, as well as a rat model of reflux esophagitis. In in vitro culture studies, BMP4 treatment of squamous epithelial cells induced a significant shift in the gene expression toward that of cultured BE cells (37), and BMP4 overexpression alone leads to non-specialized columnar metaplasia in mice (38).
Table 2.
Developmental factors implicated in BE
| Factors | Biological functions | ||
|---|---|---|---|
| General function | Normal esophagus | BE | |
| BMP4 | Ligand (BMP pathway) |
Epithelial stratification and differentiation during the esophageal development |
Upregulated to promote intestinalization in concert with CDX2 |
| Hh | Ligand (Hh pathway) |
Tracheoesophageal separation | intestinalization by inducing MUC2 |
| SOX2 | Transcription factor |
Squamous epithelial homeostasis/stem cell homeostasis Cooperates with p63 Inhibits CDX2 |
Downregulated |
| P63 | Transcription factor |
Squamous epithelial homeostasis/stem cell homeostasis |
Downregulated |
| CDX2 | Transcription factor |
Not expressed | Upregulated to promote Intestinalization |
BMP4 was also found to be a key mediator of Hh signaling. Hh is induced in esophageal epithelial cells exposed to bile salts and acids. Hh is targeted at stromal fibroblasts, where it induces the secretion of BMP4, which feeds back to the epithelium where it enhances the epithelial expression of SOX9 and the induction of columnar cell differentiation markers (39). SOX9, but not CDX2, appeared to be sufficient to drive columnar differentiation of squamous epithelium and expression of intestinal differentiation markers including CK8 (40). In summary, multiple developmental signaling pathways and downstream transcription factors are likely to have functional roles driving the formation of Barrett’s esophagus in the setting of GERD.
Identification of BE stem cells
There has not yet been any definitive identification of a stem cell functionally or based on broadly cell markers. Several groups have identified populations of cells expressing intestinal stem cell markers Lgr5 (41), doublecortin and CaM kinase-like-1 (DCAMKL-1) (42) and SOX9(43). However, expression of these markers does not confer stem cell status alone. Although controversial, label retaining cell (LRC) populations have been implicated as stem cells in some tissues. In a very novel in vivo study in humans, a rare slow-cycling LRC population, representing <0.1% of epithelial cells, has been detected in the crypt base in BE. Moreover, >99% of these LRC do not express lineage-specific markers such as defensin-5, MUC2 and chromogranin A (44). BE stem cells have also been investigated in a mouse model with genetic fate mapping, identifying a Lgr5+ gastric cardia stem cell which may serve as the stem cell in this BE-like metaplasia (45). Clearly, much more work needs to be carried out to identify stem cell populations giving rise to BE.
Studying Barrett’s Esophagus in the laboratory
It is certainly true that animal models, particularly mouse models, have become a critical component of biomedical research, as they allow for the evaluation of genetic alterations and/or environmental variables in a controlled manner. However, animal models for BE, in particular mouse models, have been difficult to develop. Despite some notable successes (14, 38, 45, 46), mouse models have several important limitations. One problem is that mice lack submucosal glands which are, as we have observed, a potential cell population from which BE cells emerge (23). Moreover, mice have a squamous epithelium-lined forestomach, which means the squamo-columnar junction in mice occurs in the stomach rather than at the distal esophagus as it does in humans. Lastly, the squamous epithelium in the mouse esophagus and forestomach is keratinized, while in humans the esophagus is lined by non-keratinized squamous epithelium. This argues that developmental and gene expression pathways are likely different between mice and humans, thereby giving rise to different epithelial structures. Together, these observations do questions about the utility of the mouse as a model animal for human BE, and suggest that novel human cell-based tissue-engineered culture systems may be a better way to accurately model the in vivo events giving rise to Barrett’s esophagus.
Immortalized Human Cell Lines in Barrett’s Research
One great limitation to basic laboratory research into the pathogenesis of BE and EAC has been the dearth of reliable cell lines and cell culture model systems. Adding to this difficulty, in 2010 it was revealed that four highly utilized cell lines thought to be human EAC (TE-7, SEG-1, BIC-1, and SK-GT-5) were in fact cancers from other tissues entirely (47, 48). Therefore, when reviewing the literature prior to 2010, care must be taken to confirm which cell lines were used. At present there are 10 confirmed human EAC cell lines (Table 3 and Figure 3) (48, 49). A PubMed search for papers using these cell lines found only 191 published reports, establishing the limited research that has been carried out thus far on this cancer. Clearly, the biology and treatment of esophageal adenocarcinomas is significantly understudied and therefore less well understood compared to other human cancers.
Table 3.
Validated human cell lines used in Barrett’s esophagus and esophageal adenocarcinoma research
| Cell Line | Type | Background | Ref |
|---|---|---|---|
| EPC2-hTert | INE | Squamous esophageal epithelium from male non-BE patient–immortalized by hTERT |
(50, 51). |
| HET1A | INE | Squamous esophageal epithelium–immortalized by SV40 virus Large T antigen |
(50, 60, 61) |
| NES-B3T | INE | Squamous esophageal epithelium from BE patient– immortalized by hTERT |
(50, 51). |
| NES-G2T | INE | Squamous esophageal epithelium from non-BE patient–immortalized by hTERT |
(50, 51). |
| BAR-T | IBE | hTERT immortalized non dysplastic Barrett’s esophagus from a 69 year-old male |
(52) |
| CP-A | IBE | hTERT immortalized non dysplastic Barrett’s esophagus |
(53) |
| CP-B | IBE | hTERT immortalized dysplastic Barrett’s esophagus | (53) |
| CP-C | IBE | hTERT immortalized dysplastic Barrett’s esophagus | (53) |
| CP-D | IBE | hTERT immortalized dysplastic Barrett’s esophagus | (53) |
| ESO26 | EAC | Established from a 51 year-old Caucasian male with a primary tumor located at the gastroesophageal junction and distal esophagus with known lymph node and distant metastases. |
(48, 49) |
| ESO51 | EAC | Established from a 74 year-old Caucasian male with a primary adenocarcinoma of the distal esophagus and Barrett’s esophagus. |
(48, 49) |
| FLO-1 | EAC | Established from a primary distal esophageal adenocarcinoma in a 68 year-old Caucasian male. |
(48, 49) |
| JH-EsoAd1 | EAC | Established from a distal esophageal adenocarcinoma (moderately to poorly differentiated) within Barrett’s esophagus. |
(48, 49) |
| KYAE-1 | EAC | Established from the pleural effusion from a from a 60 year old Asian male with a distal esophageal adenocarcinoma. |
(48, 49) |
| OACM5.1 | EAC | Established from a lymph node metastasis from a 47 year-old Caucasian female with primary adenocarcinoma of distal esophagus and Barrett’s esophagus. The patient also exhibited pleural metastases. |
(74) |
| OACP4C | EAC | Established from a primary gastric cardia tumor in a 55 year-old Caucasian male. The patient also exhibited lymph node and pleural metastases. |
(48, 49) |
| OE19 | EAC | Also known as JROECL19, was established from a well-differentiated adenocarcinoma of gastric cardia/esophageal gastric junction of a 72 year old male patient. |
(75) |
| OE33 | EAC | Also known as JROECL33, was established from a poorly differentiated adenocarcinoma of the lower esophagus with Barrett’s metaplasia from a 73 year old female patient. |
(75) |
| SK-GT-4 | EAC | Established from a primary well-differentiated adenocarcinoma arising in the distal esophagus within Barrett esophagus of an 89 year-old Caucasian male. |
(76) |
INE: immortalized normal esophagus; IBE: immortalized Barrett’s esophagus; EAC: Esophageal adenocarcinoma; hTERT: human telomerase gene;
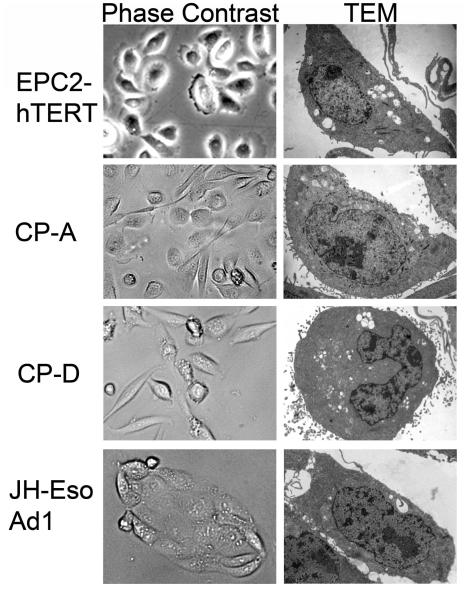
Figure 3.
Cell lines commonly used in Barrett’s esophagus and esophageal adenocarcinoma research cultured on a 2-dimensional support. Images are of cells using phase-contrast light microscopy or tranmission electron microscopy (TEM). Images provided by Jianping Kong MD PhD and Ali Soroush, University of Pennsylvania Perelman School of Medicine.
There has also been an emphasis and some success in establishing immortalized cell lines from BE tissues (Figure 3). Five immortalized Barrett’s epithelial cell lines have been established using retroviral transduction of human telomerase reverse transcriptase (hTERT) (50, 51). Two of these cell lines, CP-A and BAR-T cells, originated from non-dysplastic BE biopsies, while CP-B, CP-C, and CP-D were all derived from Barrett’s with HGD. Moreover, compared to the other cell lines, the BAR-T cells are the most “normal” in that they maintain a diploid status and respond normally to contact inhibition (52). In contrast the CP-A, B, C, and D cell lines all have documented chromosomal abnormalities (53), including 17p loss of heterozygosity or p53 mutations documented in CP-B, C, and D lines. Many of the studies using these cell lines in traditional 2-dimensional (2D) culture environments have explored the effects of Barrett’s etiologic agents, acid and bile acid exposure, on cellular responses at the molecular level, including production of reactive oxygen species, anti-oxidant responses, DNA damage, and nuclear factor kappaB activation (54-57). Perhaps most revealing has been the induction of a malignant transformed phenotype in non-dysplastic BAR-T cells either by chronic acid exposure or the introduction of defined genetic changes (58, 59).
Lastly, there are four published immortalized human squamous esophageal cell lines, EPC2-hTert, NES-B3T, NES-G2T, and HET1A (Figure 3) (50, 60, 61). The first three are immortalized by ectopic expression of human telomerase, and the fourth, HET1A cells, are immortalized by ectopic expression of the SV40 T antigen, a viral oncoprotein which inactivates p53 and the retinoblastoma protein (pRb), among other targets (62). Many of the published studies using these immortalized human esophageal cell lines have explored the responses of these cells to a variety of Barrett’s etiologic agents, including chronic acid and bile acid treatment (16), BMP-4 (37), retinoic acid (63, 64), and the intestine-specific transcription factors Cdx1 and Cdx2 (17, 61, 65).
Treatment of esophageal keratinocytes with acid or bile acid pulses can induce low levels of intestinal transcription factors like Cdx2, but has not induced a true intestinal metaplasia (16, 66). Moreover, esophageal keratinocytes treated with BMP-4 begin expressing BE-associated cytokeratins and a more intestinalized pattern of gene expression (37). In another study using HET1A cells, Cdx2 expression was associated with increased cell proliferation and an intestinal pattern of gene expression (67). In summary, while these treatments did yield increased expression of intestine-specific genes and changes in cell morphology, none has yet succeeded at inducing a complete BE phenotype from human keratinocytes.
Engineered Human Tissue Systems to Model Barrett’s Esophagus
Physiologically relevant research models must strive to best mimic human in vivo microenvironments in an in vitro setting. As we discussed, animal models have many benefits as well as significant limitations. Moreover, traditional 2-dimensional cell culture approaches using immortalized human cells fail to capture the significant contributions of the multicellular microenvironment inherent in vivo. The development of the organotypic culture (OTC) systems that allows for the co-culture of immortalized human epithelial cell lines together with primary fibroblasts in 3-dimensional (3D) tissue reconstructions represents a novel means by which to perform in vitro experiments that are still physiologically relevant (68).
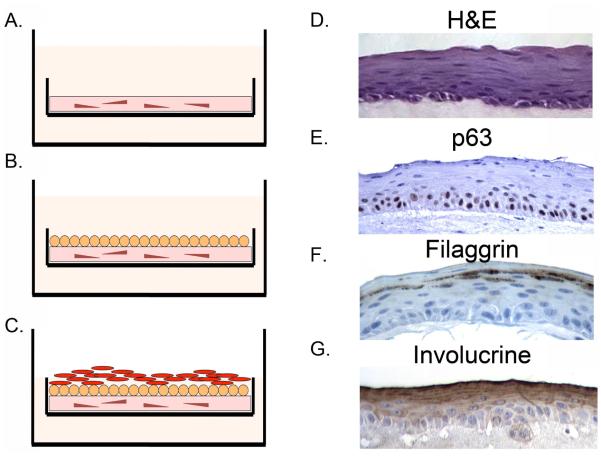
Organotypic cultures are more complex to initiate but yield a well differentiated multilayered squamous epithelium that is physiologically similar to that observed in the human esophagus. A collagen matrix is poured that includes human embryonic fibroblast cells (Figure 4A) (68). This culture is allowed to mature for 4 days, then human primary esophageal keratinocytes or an immortalized cell line like EPC2-hTert are layered on top and maintained in culture for an additional four days (Figure 4B). For the final maturation of the culture, the media level is reduced until the epithelium is at the air-liquid interface which provokes additional proliferation and differentiation in the epithelial cells (Figure 4C). The final epithelium that emerges is a fully differentiated, multilayered epithelium which closely mimics the normal squamous epithelium seen in esophageal biopsies including localization of stem cell marker p63 in the basal cell layer and differentiation markers filaggrin and involucrin in the differentiated suprabasal compartment (Figure 4D-G). This physiologically relevant engineered tissue thus provides a novel platform for the study of many human esophageal diseases.
Figure 4.
Organotypic culture (OTC) technique to model human esophageal epithelium and Barrett’s esophagus in an in vitro system. A. Culture is initiated by plating human embryonic fibroblasts in a collagen matrix for 4 days. B. Human esophageal keratinocytes or Barrett’s epithelial cells are then plated on top of the collagen matrix. C. Once eithelial cells are confluent, the level of the cell culture media is reduced to place the epithelium at the air-liquid interface. D. Example of normal appearing multi-layered human esophageal epithelium that forms after EPC2-hTERT cells are grown under OTC conditions. H&E: stained by hematoxylin and eosin. E. p63 immunohistochemistry staining of an OTC culture. Immunohistochemistry for differentiation markers F. Filaggrin and G. Involucrin. Images provided by Jianping Kong MD PhD University of Pennsylvania Perelman School of Medicine.
Barrett’s immortalized cell lines can also be cultured under OTC conditions, and this revealed several unrecognized properties. Most strikingly, CP-A cells yielded mucin-producing goblet cells when cultured under OTC conditions, though they were also were unexpectedly highly invasive into the collagen matrix despite their origin from a non-dysplastic BE sample (69). These phenotypes are not apparent when these cells are cultured under traditional culture conditions, emphasizing the importance of migrating future research into these more physiologically relevant culture systems.
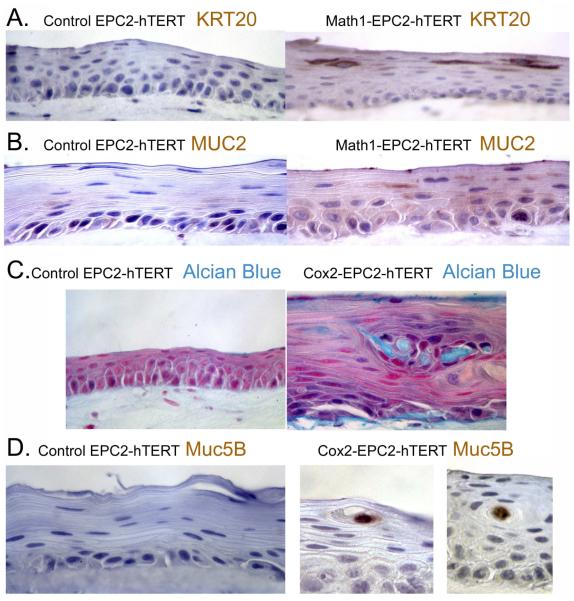
Our laboratory has utilized this culture system for a number of years, and have on several occasions observed new cellular phenotypes emerge when cells were cultured under OTC conditions, including new gene expression. In a series of studies to determine if human esophageal keratinocytes can be induced to adopt an intestinalized, BE like morphology, we introduced expression of several genes associated with BE, including transcription factors (Cdx1, Cdx2, Atoh1/Math1), Wnt signaling (β-catenin), and pro-inflammatory Cyclooxygenase-2 (Cox2-the rate limiting enzyme in eicosanoid synthesis) in human EPC2-hTert cells (17, 65, 70, 71). Typically, these genes had little effect on cell morphology or gene expression when cells were maintained under more classic 2-dimensional culture conditions. However, we observed significant induction of small intestine and BE-associated genes when these cells were cultured under OTC conditions. For example, expression of the goblet cell transcription factor Atoh1/Math1 in EPC2-hTERT cells induced expression of the BE markers Keratin 20 (K20) and Mucin 2 (MUC2) (Figure 5A, B), but only when grown under OTC conditions (65, 70, 71). Most significant was the response of the cells to the inflammation-associated enzyme Cox-2, where we induced cells capable of polarized secretion of Alcian blue positive intestinal mucins including MUC5B (Figure 5C,D) (70). In addition, using this same OTC system, we determined through a genetic approach that the onset of a true BE from keratinocytes is a multistep process that requires increased proliferation, senescence inhibition, and epigenetic alterations (65).
Figure 5.
Introduction of Math1 or Cox2 expression in EPC2-hTERT cells leads to new intestinal and BE associated gene expression in OTC cultured cells. Immunohistochemistry for A. KT20 and B. MUC2 in EPC2-hTERT cells expressing Math1. C. Alcian blue histochemical identification of intestinal mucins expressed by EPC2-hTERT cells expressing the pro- inflammatory enzyme cyclooxygenase-2 (Cox2). D. Immunohistochemistry for intestinal MUC5B in EPC2-hTERT cells expressing Cox2. Images provided by Jianping Kong MD PhD University of Pennsylvania Perelman School of Medicine.
We and others are presently working on developing approaches to increase the complexity OTC cultures in order to better model the microenvironment of BE. One approach is to include other relevant cell types including immune, endothelial, smooth muscle, and neural cells (72, 73). Coincident with this, we will need a much better understanding of the cells present within the BE microenvironment. It is anticipated that this approach may yield new insights into what drives disease progression. Together, this novel multicellular OTC culture system and new primary human squamous keratinocyte and Barrett’s cell culture methods will lead to innovative engineered human esophageal and Barrett’s tissues that can better model physiologic responses and therefore serve as a far superior research platform than any that are presently available.
Summary
Barrett’s esophagus is the replacement of the normal stratified squamous epithelium with a columnar epithelium containing intestinal mucin-producing goblet cells. BE has been strongly associated with chronic GERD and is a well recognized risk factor for EAC. Of concern, the rates of EAC have been increasing within the U.S. and western European populations, which is why there is now greater urgency for research into this disease. We have made progress in characterizing BE at the ultrastructural and molecular levels. BE is referred to as an “intestinal” metaplasia because all four of the main intestinal epithelial cell lineages have been detected in BE, however these cell lineages are typically not fully mature. We also have evidence that a number of important developmental signaling pathways (BMP4, Hh, Wnt, and Notch) and transcription factors (Cdx2, Myc, Atoh1/Math1, Sox2, and Sox9) are critically important for regulating both normal developmental events at the distal esophagus as well as the induction of Barrett’s epithelial phenotype. However, much remains unknown yet regarding BE, in particular, which cell population the BE cell emerges from, and what are the molecular events or steps by which BE progresses to EAC. These are critical questions for investigators that, once answered, will significantly impact disease prevention and management. Despite the presently limited set of experimental model systems in which to study BE and EAC, innovations in tissue engineering and human cell based organotypic culture systems hold great promise as platforms for future investigations into disease pathogenesis and progression.
Practice Points.
Barrett’s esophagus occurs in the in the distal esophagus and is defined as the replacement of the normal stratified squamous epithelium with an intestinalized, columnar epithelium.
Barrett’s Esophagus has been strongly associated with chronic gastroesophageal reflux disease (GERD), and is an important risk factor for esophageal adenocarcinoma.
Esophageal adenocarcinoma incidence is increasing in the US and other Western countries, therefore identification and treatment of patients with Barrett’s esophagus is a clinical imperative.
Research agenda.
The cell of origin for the Barrett’s Esophagus remains unknown. Definitive studies identifying the molecular pathogenesis and cell of origin are needed.
There are a limited number of model systems and cell lines by which Barrett’s esophagus can be studied in the laboratory; innovative, more physiologically relevant models are urgently needed.
The stem cell for Barrett’s has not yet been identified and should be a focus for future investigation.
An improved understanding of the role of the network of developmental and signaling pathways in the pathogenesis of Barrett’s esophagus is needed
Advances in tissue engineering of innovative, multicellular organotypic culture systems that recapitulate in vivo structure and function and permit hypothesis testing for BE and EAC pathogenesis.
Acknowledgements
This work is supported as part of the Integrated Microphysiological Systems program and the Intestinal Stem Cell Consortium with funding to JPL (TR 000536 and DK 085551). This work was also supported by an NCI Program Project PO1 CA098101 and the Morphology, Cell Culture, and Molecular Biology Core Facilities of the Center for Molecular Studies in Digestive and Liver Disease at the University of Pennsylvania (P30-DK050306 and PO1 CA098101). These sponsors had no role in the collection, analysis and interpretation of data and in the writing of this manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflict of interest.
Contributor Information
Hiroshi Nakagawa, Research Associate Professor of Medicine, Division of Gastroenterology, 421 Curie Boulevard, 956 Biomedical Research Building, Philadelphia, PA 19104, Office: 215-573-1867, Fax: 215-573-2024.
Kelly Whelan, Post Doctoral Fellow, Division of Gastroenterology, 421 Curie Boulevard, 956 Biomedical Research Building, Philadelphia, PA 19104, Office: 215-573-1867, Fax: 215-573-2024.
John P. Lynch, Associate Professor of Medicine, Division of Gastroenterology, University of Pennsylvania Perelman School of Medicine, 421 Curie Blvd. /912 BRB, Philadelphia, PA 19104, Tel (215) 898-0155, Fax (215) 573-2024
REFERENCES
- 1.Falk GW, Jacobson BC, Riddell RH, Rubenstein JH, El-Zimaity H, Drewes AM, et al. Barrett's esophagus: prevalence-incidence and etiology-origins. Ann N Y Acad Sci. 2011 Sep;1232:1–17. doi: 10.1111/j.1749-6632.2011.06042.x. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen NJ. What is behind the remarkable increase in esophageal adenocarcinoma? Am J Gastroenterol. 2014 Mar;109(3):345–7. doi: 10.1038/ajg.2014.35. [DOI] [PubMed] [Google Scholar]
- 3.Stairs DB, Kong J, Lynch JP. Cdx genes, inflammation, and the pathogenesis of intestinal metaplasia. Progress in Molecular Biology and Translational Science. 2010;96:231–70. doi: 10.1016/B978-0-12-381280-3.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The histologic spectrum of Barrett's esophagus. N Engl J Med. 1976 Aug 26;295(9):476–80. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011 Mar;140(3):1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Levine DS, Reid BJ, Haggitt RC, Rubin CE, Rabinovitch PS. Correlation of ultrastructural aberrations with dysplasia and flow cytometric abnormalities in Barrett's epithelium. Gastroenterology. 1989 Feb;96(2):355–67. doi: 10.1016/s0016-5085(89)91559-x. Pt 1. [DOI] [PubMed] [Google Scholar]
- 7.Couvelard A, Cauvin JM, Goldfain D, Rotenberg A, Robaszkiewicz M, Flejou JF. Cytokeratin immunoreactivity of intestinal metaplasia at normal oesophagogastric junction indicates its aetiology. Gut. 2001 Dec;49(6):761–6. doi: 10.1136/gut.49.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odze R. Cytokeratin 7/20 immunostaining: Barrett's oesophagus or gastric intestinal metaplasia? Lancet. 2002 May 18;359(9319):1711–3. doi: 10.1016/S0140-6736(02)08661-0. [DOI] [PubMed] [Google Scholar]
- 9.McIntire MG, Soucy G, Vaughan TL, Shahsafaei A, Odze RD. MUC2 is a highly specific marker of goblet cell metaplasia in the distal esophagus and gastroesophageal junction. Am J Surg Pathol. 2011 Jul;35(7):1007–13. doi: 10.1097/PAS.0b013e318218940d. [DOI] [PubMed] [Google Scholar]
- 10.Glickman JN, Shahsafaei A, Odze RD. Mucin core peptide expression can help differentiate Barrett's esophagus from intestinal metaplasia of the stomach. Am J Surg Pathol. 2003 Oct;27(10):1357–65. doi: 10.1097/00000478-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Gomes LI, Esteves GH, Carvalho AF, Cristo EB, Hirata R, Jr., Martins WK, et al. Expression profile of malignant and nonmalignant lesions of esophagus and stomach: differential activity of functional modules related to inflammation and lipid metabolism. Cancer Research. 2005 Aug 15;65(16):7127–36. doi: 10.1158/0008-5472.CAN-05-1035. [DOI] [PubMed] [Google Scholar]
- 12.Hao Y, Triadafilopoulos G, Sahbaie P, Young HS, Omary MB, Lowe AW. Gene expression profiling reveals stromal genes expressed in common between Barrett's esophagus and adenocarcinoma. Gastroenterology. 2006 Sep;131(3):925–33. doi: 10.1053/j.gastro.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Qin R, Ma Y, Wu H, Peters H, Tyska M, et al. Differential gene expression in normal esophagus and Barrett's esophagus. J Gastroenterol. 2009;44(9):897–911. doi: 10.1007/s00535-009-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong J, Crissey MA, Funakoshi S, Kreindler JL, Lynch JP. Ectopic Cdx2 Expression in Murine Esophagus Models an Intermediate Stage in the Emergence of Barrett's Esophagus. PLoS ONE. 2011;6(4):e18280. doi: 10.1371/journal.pone.0018280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazumori H, Ishihara S, Kinoshita Y. Roles of caudal-related homeobox gene Cdx1 in oesophageal epithelial cells in Barrett's epithelium development. Gut. 2009 May;58(5):620–8. doi: 10.1136/gut.2008.152975. [DOI] [PubMed] [Google Scholar]
- 16.Kazumori H, Ishihara S, Rumi MA, Kadowaki Y, Kinoshita Y. Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett's epithelium. Gut. 2006 Jan;55(1):16–25. doi: 10.1136/gut.2005.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stairs DB, Nakagawa H, Klein-Szanto A, Mitchell SD, Silberg DG, Tobias JW, et al. Cdx1 and c-Myc foster the initiation of transdifferentiation of the normal esophageal squamous epithelium toward Barrett's esophagus. PLoS ONE. 2008;3(10):e3534. doi: 10.1371/journal.pone.0003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawhney RA, Shields HM, Allan CH, Boch JA, Trier JS, Antonioli DA. Morphological characterization of the squamocolumnar junction of the esophagus in patients with and without Barrett's epithelium. Dig Dis Sci. 1996 Jun;41(6):1088–98. doi: 10.1007/BF02088224. [DOI] [PubMed] [Google Scholar]
- 19.Shields HM, Zwas F, Antonioli DA, Doos WG, Kim S, Spechler SJ. Detection by scanning electron microscopy of a distinctive esophageal surface cell at the junction of squamous and Barrett's epithelium. Dig Dis Sci. 1993 Jan;38(1):97–108. doi: 10.1007/BF01296780. [DOI] [PubMed] [Google Scholar]
- 20.Glickman JN, Chen YY, Wang HH, Antonioli DA, Odze RD. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus. Am J Surg Pathol. 2001 May;25(5):569–78. doi: 10.1097/00000478-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Boch JA, Shields HM, Antonioli DA, Zwas F, Sawhney RA, Trier JS. Distribution of cytokeratin markers in Barrett's specialized columnar epithelium. Gastroenterology. 1997 Mar;112(3):760–5. doi: 10.1053/gast.1997.v112.pm9041237. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Qin R, Liu B, Ma Y, Su Y, Yang CS, et al. Multilayered epithelium in a rat model and human Barrett's esophagus: similar expression patterns of transcription factors and differentiation markers. BMC Gastroenterol. 2008;8:1. doi: 10.1186/1471-230X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson AM, Graham TA, Simpson A, Humphries A, Burch N, Rodriguez-Justo M, et al. Barrett's metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut. 2011 Dec 26; doi: 10.1136/gutjnl-2011-301174. [DOI] [PubMed] [Google Scholar]
- 24.Braxton DR, Nickleach DC, Liu Y, Farris AB., 3rd Necrotizing sialometaplasia-like change of the esophageal submucosal glands is associated with Barrett's esophagus. Virchows Arch. 2014 Aug;465(2):135–43. doi: 10.1007/s00428-014-1590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, et al. Residual Embryonic Cells as Precursors of a Barrett's-like Metaplasia. Cell. 2011 Jun 24;145(7):1023–35. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007 May 4;129(3):523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 27.Schoenwolf GC. Larsen's Human Embryology. 4TH Churchill, Livingston, Elsevier; Philadelphia: 2009. [Google Scholar]
- 28.Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. The Journal of clinical investigation. 2014 Apr 1;124(4):1636–45. doi: 10.1172/JCI71545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009 Jun;136(11):1899–907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004 Jul;287(1):C171–81. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 31.Pavlov K, Meijer C, van den Berg A, Peters FT, Kruyt FA, Kleibeuker JH. Embryological signaling pathways in Barrett's metaplasia development and malignant transformation; mechanisms and therapeutic opportunities. Crit Rev Oncol Hematol. 2014 May 12; doi: 10.1016/j.critrevonc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007 Jul;134(13):2521–31. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs IJ, Ku WY, Que J. Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Developmental biology. 2012 Sep 1;369(1):54–64. doi: 10.1016/j.ydbio.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proceedings of the National Academy of Sciences of the United States of America. 2009 Sep 22;106(38):16287–92. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez P, Da Silva S, Oxburgh L, Wang F, Hogan BL, Que J. BMP signaling in the development of the mouse esophagus and forestomach. Development. 2010 Dec;137(24):4171–6. doi: 10.1242/dev.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roman S, Petre A, Thepot A, Hautefeuille A, Scoazec JY, Mion F, et al. Downregulation of p63 upon exposure to bile salts and acid in normal and cancer esophageal cells in culture. American journal of physiology Gastrointestinal and liver physiology. 2007 Jul;293(1):G45–53. doi: 10.1152/ajpgi.00583.2006. [DOI] [PubMed] [Google Scholar]
- 37.Milano F, van Baal JW, Buttar NS, Rygiel AM, de Kort F, DeMars CJ, et al. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology. 2007 Jun;132(7):2412–21. doi: 10.1053/j.gastro.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Mari L, Milano F, Parikh K, Straub D, Everts V, Hoeben KK, et al. A pSMAD/CDX2 complex is essential for the intestinalization of epithelial metaplasia. Cell Rep. 2014 May 22;7(4):1197–210. doi: 10.1016/j.celrep.2014.03.074. [DOI] [PubMed] [Google Scholar]
- 39.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, et al. Aberrant epithelial- mesenchymal Hedgehog signaling characterizes Barrett's metaplasia. Gastroenterology. 2010 May;138(5):1810–22. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clemons NJ, Wang DH, Croagh D, Tikoo A, Fennell CM, Murone C, et al. Sox9 drives columnar differentiation of esophageal squamous epithelium: a possible role in the pathogenesis of Barrett's esophagus. American journal of physiology Gastrointestinal and liver physiology. 2012 Dec 15;303(12):G1335–46. doi: 10.1152/ajpgi.00291.2012. [DOI] [PubMed] [Google Scholar]
- 41.Becker L, Huang Q, Mashimo H. Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett's esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010 Feb;23(2):168–74. doi: 10.1111/j.1442-2050.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- 42.Vega KJ, May R, Sureban SM, Lightfoot SA, Qu D, Reed A, et al. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett's esophagus and esophageal adenocarcinoma. J Gastroenterol Hepatol. 2012 Apr;27(4):773–80. doi: 10.1111/j.1440-1746.2011.06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darlavoix T, Seelentag W, Yan P, Bachmann A, Bosman FT. Altered expression of CD44 and DKK1 in the progression of Barrett's esophagus to esophageal adenocarcinoma. Virchows Arch. 2009 Jun;454(6):629–37. doi: 10.1007/s00428-009-0769-z. [DOI] [PubMed] [Google Scholar]
- 44.Pan Q, Nicholson AM, Barr H, Harrison LA, Wilson GD, Burkert J, et al. Identification of lineage- uncommitted, long-lived, label-retaining cells in healthy human esophagus and stomach, and in metaplastic esophagus. Gastroenterology. 2013 Apr;144(4):761–70. doi: 10.1053/j.gastro.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, et al. Bile Acid and inflammation activate gastric cardia stem cells in a mouse model of barrett-like metaplasia. Cancer Cell. 2012;21(1):36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang DH, Tiwari A, Kim ME, Clemons NJ, Regmi NL, Hodges WA, et al. Hedgehog signaling regulates FOXA2 in esophageal embryogenesis and Barrett's metaplasia. J Clin Invest. 2014 Aug 1; doi: 10.1172/JCI66603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boonstra JJ, van der Velden AW, Beerens EC, van Marion R, Morita-Fujimura Y, Matsui Y, et al. Mistaken identity of widely used esophageal adenocarcinoma cell line TE-7. Cancer Res. 2007 Sep 1;67(17):7996–8001. doi: 10.1158/0008-5472.CAN-07-2064. [DOI] [PubMed] [Google Scholar]
- 48.Boonstra JJ, van Marion R, Beer DG, Lin L, Chaves P, Ribeiro C, et al. Verification and unmasking of widely used human esophageal adenocarcinoma cell lines. J Natl Cancer Inst. 2010 Feb 24;102(4):271–4. doi: 10.1093/jnci/djp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez H, Koorstra JB, Hong SM, Boonstra JJ, Dinjens WN, Foratiere AA, et al. Establishment and characterization of a bona fide Barrett esophagus-associated adenocarcinoma cell line. Cancer Biol Ther. 2008 Nov;7(11):1753–5. doi: 10.4161/cbt.7.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B, et al. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res. 2003 Aug;1(10):729–38. [PubMed] [Google Scholar]
- 51.Morales CP, Gandia KG, Ramirez RD, Wright WE, Shay JW, Spechler SJ. Characterisation of telomerase immortalised normal human oesophageal squamous cells. Gut. 2003 Mar;52(3):327–33. doi: 10.1136/gut.52.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaiswal KR, Morales CP, Feagins LA, Gandia KG, Zhang X, Zhang HY, et al. Characterization of telomerase-immortalized, non-neoplastic, human Barrett's cell line (BAR-T) Dis Esophagus. 2007;20(3):256–64. doi: 10.1111/j.1442-2050.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 53.Palanca-Wessels MC, Klingelhutz A, Reid BJ, Norwood TH, Opheim KE, Paulson TG, et al. Extended lifespan of Barrett's esophagus epithelium transduced with the human telomerase catalytic subunit: a useful in vitro model. Carcinogenesis. 2003 Jul;24(7):1183–90. doi: 10.1093/carcin/bgg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang HY, Hormi-Carver K, Zhang X, Spechler SJ, Souza RF. In benign Barrett's epithelial cells, acid exposure generates reactive oxygen species that cause DNA double-strand breaks. Cancer Res. 2009 Dec 1;69(23):9083–9. doi: 10.1158/0008-5472.CAN-09-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huo X, Juergens S, Zhang X, Rezaei D, Yu C, Strauch ED, et al. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-kappaB activation in benign Barrett's epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G278–86. doi: 10.1152/ajpgi.00092.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang HY, Zhang X, Hormi-Carver K, Feagins LA, Spechler SJ, Souza RF. In non-neoplastic Barrett's epithelial cells, acid exerts early antiproliferative effects through activation of the Chk2 pathway. Cancer Res. 2007 Sep 15;67(18):8580–7. doi: 10.1158/0008-5472.CAN-07-2023. [DOI] [PubMed] [Google Scholar]
- 57.Hormi-Carver K, Zhang X, Zhang HY, Whitehead RH, Terada LS, Spechler SJ, et al. Unlike esophageal squamous cells, Barrett's epithelial cells resist apoptosis by activating the nuclear factor- kappaB pathway. Cancer Res. 2009 Jan 15;69(2):672–7. doi: 10.1158/0008-5472.CAN-08-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das KM, Kong Y, Bajpai M, Kulkarni D, Geng X, Mishra P, et al. Transformation of benign Barrett's epithelium by repeated acid and bile exposure over 65 weeks: a novel in vitro model. Int J Cancer. 2011 Jan 15;128(2):274–82. doi: 10.1002/ijc.25343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Yu C, Wilson K, Zhang HY, Melton SD, Huo X, et al. Malignant transformation of non-neoplastic Barrett's epithelial cells through well-defined genetic manipulations. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0013093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feagins LA, Zhang HY, Zhang X, Hormi-Carver K, Thomas T, Terada LS, et al. Mechanisms of oxidant production in esophageal squamous cell and Barrett's cell lines. Am J Physiol Gastrointest Liver Physiol. 2008 Feb;294(2):G411–7. doi: 10.1152/ajpgi.00373.2007. [DOI] [PubMed] [Google Scholar]
- 61.Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, et al. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007 Sep 21;28(2):488–96. doi: 10.1093/carcin/bgl176. [DOI] [PubMed] [Google Scholar]
- 62.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005 Nov 21;24(52):7729–45. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 63.Cooke G, Blanco-Fernandez A, Seery JP. The effect of retinoic acid and deoxycholic acid on the differentiation of primary human esophageal keratinocytes. Dig Dis Sci. 2008 Nov;53(11):2851–7. doi: 10.1007/s10620-008-0240-z. [DOI] [PubMed] [Google Scholar]
- 64.Chang CL, Lao-Sirieix P, Save V, De La Cueva Mendez G, Laskey R, Fitzgerald RC. Retinoic acid-induced glandular differentiation of the oesophagus. Gut. 2007 Jul;56(7):906–17. doi: 10.1136/gut.2006.097915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kong J, Nakagawa H, Isariyawongse B, Funakoshi S, Silberg D, Rustgi AK, et al. Induction of Intestinalization in Human Esophageal Keratinocytes is a Multi-step Process. Carcinogenesis. 2009;30(1):122–30. doi: 10.1093/carcin/bgn227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchetti M, Caliot E, Pringault E. Chronic acid exposure leads to activation of the cdx2 intestinal homeobox gene in a long-term culture of mouse esophageal keratinocytes. J Cell Sci. 2003 Apr 15;116:1429–36. doi: 10.1242/jcs.00338. Pt 8. [DOI] [PubMed] [Google Scholar]
- 67.Bellin C, de Wiza DH, Wiernsperger NF, Rosen P. Generation of reactive oxygen species by endothelial and smooth muscle cells: influence of hyperglycemia and metformin. Horm Metab Res. 2006 Nov;38(11):732–9. doi: 10.1055/s-2006-955084. [DOI] [PubMed] [Google Scholar]
- 68.Kalabis J, Wong GS, Vega ME, Natsuizaka M, Robertson ES, Herlyn M, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc. 2012 Feb;7(2):235–46. doi: 10.1038/nprot.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kosoff RE, Gardiner KL, Merlo LM, Pavlov K, Rustgi AK, Maley CC. Development and characterization of an organotypic model of Barrett's esophagus. J Cell Physiol. 2011 Aug 31; doi: 10.1002/jcp.23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kong J, Crissey MA, Stairs DB, Sepulveda AR, Lynch JP. Cox2 and β-catenin/TCF signaling intestinalize human esophageal keratinocytes when cultured under organotypic conditions. Neoplasia. 2011;13(9):792–805. doi: 10.1593/neo.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong J, Crissey MA, Sepulveda AR, Lynch JP. Math1/Atoh1 contributes to intestinalization of esophageal keratinocytes by inducing the expression of Muc2 and Keratin-20. Dig Dis Sci. 2012 Apr;57(4):845–57. doi: 10.1007/s10620-011-1998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartman KG, Bortner JD, Jr., Falk GW, Ginsberg GG, Jhala N, Yu J, et al. Modeling human gastrointestinal inflammatory diseases using microphysiological culture systems. Exp Biol Med (Maywood) 2014 Apr 29; doi: 10.1177/1535370214529388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartman KG, Bortner JD, Falk GW, Yu J, Martin MG, Rustgi AK, et al. Modeling inflammation and oxidative stress in gastrointestinal disease development using novel organotypic culture systems. Stem Cell Res Ther. 2013;4(Suppl 1):S5. doi: 10.1186/scrt366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Both NJ, Wijnhoven BP, Sleddens HF, Tilanus HW, Dinjens WN. Establishment of cell lines from adenocarcinomas of the esophagus and gastric cardia growing in vivo and in vitro. Virchows Arch. 2001 May;438(5):451–6. doi: 10.1007/s004280000358. [DOI] [PubMed] [Google Scholar]
- 75.Rockett JC, Larkin K, Darnton SJ, Morris AG, Matthews HR. Five newly established oesophageal carcinoma cell lines: phenotypic and immunological characterization. Br J Cancer. 1997;75(2):258–63. doi: 10.1038/bjc.1997.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Altorki N, Schwartz GK, Blundell M, Davis BM, Kelsen DP, Albino AP. Characterization of cell lines established from human gastric-esophageal adenocarcinomas. Biologic phenotype and invasion potential. Cancer. 1993 Aug 1;72(3):649–57. doi: 10.1002/1097-0142(19930801)72:3<649::aid-cncr2820720305>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]