Abstract
Antibiotic-associated diarrhea (AAD) is one of the most common complications of most types of antibiotics. Our aim was to determine the efficacy of Clostridium butyricum, Bifidobacterium infantis, and their mixture for AAD treatment in mice. AAD models were administered with single probiotic strain and probiotic mixture for short term and long term to evaluate the changes of the composition and diversity of intestinal microbiota, histopathology of the colon, and the systemic inflammation. Our data indicated that long-term probiotic therapy, but not short-term course, exerted beneficial effects on the restoration of the intestinal microbiota, the recovery of the tissue architecture, and attenuation of systemic inflammation. All predominant fecal bacteria reached normal level after the long-term probiotic mixture treatment, while IL-10, IFN-γ, and TNF-α also returned to normal level. However, the efficacy for AAD was time dependent and probiotic strain specific. Short-term administration of probiotic strains or mixture showed no apparent positive effects for AAD. In addition, the beneficial effects of C. butyricum combined with B. infantis probiotic mixture were superior to their single strain. This research showed that supplementation with C. butyricum combined with B. infantis probiotic mixture may be a simple and effective method for AAD treatment.
1. Introduction
Antibiotic-associated diarrhea (AAD) is the most common adverse effect of antimicrobial therapy, especially those with a relatively broad spectrum such as aminopenicillins, cephalosporins, and clindamycin [1, 2]. Generally, the mechanism by which AAD occurs most likely relates to disturbances of microbiota in the gastrointestinal tract, shifting the gastrointestinal microbiota from eubiosis to severe dysbiosis [3]. Evidence has shown that cytokines disturbance was observed after antibiotic treatment [4–6]. Approximately 1000 species of bacteria inhabit the gastrointestinal tract, and a balance of these microorganisms is crucial to normal gastrointestinal function [7]. Dysbiosis of the gastrointestinal tract may disturb the metabolism of carbohydrates, resulting in malabsorption of osmotically active particles (i.e., diarrhoea) [8]. Probiotics are live nonpathogenic microorganisms which provide a health benefit to the host when administered in adequate amounts. Previous studies have demonstrated that a variety of different types of probiotics such as Saccharomyces boulardii, Lactobacillus rhamnosus GG, and probiotic mixtures were shown as effective therapies for AAD, which can restore the altered intestinal microbiota [9]. As probiotics, Clostridium butyricum produces high levels of butyrate, which can decrease the intestinal permeability and reinforce various components of the colonic defense barrier such as the promotion of epithelial migration and the induction of mucins, intestinal trefoil factor, transglutaminase activity, antimicrobial peptides, and heat shock proteins [10]. Bifidobacterium infantis exhibits a decrease in colonic permeability, an attenuation of colonic inflammation, and a decrease in interferon-gamma secretion [11]. The probiotic mixture of C. butyricum combined with B. infantis has been used to treat the observed dysbiosis in China for several years. However, it is unclear whether similar benefits occur in AAD, as most of clinical observational studies were only focused on the improvement of symptoms and signs. Using an AAD model in male C57BL/6 mice, our present study aimed to evaluate the modulation role of the probiotic mixture on the fecal microbiota and inflammatory cytokines with different courses.
2. Materials and Methods
2.1. Probiotic Strains
Two freeze-dried probiotic strains and its mixture (Changlekang) were used in present study, Clostridium butyricum (CGMCC 0313-1) and Bifidobacterium infantis (CGMCC 0313-2), which were kindly provided by Shandong Kexing Bioproducts Co., Ltd. (China). The freeze-dried C. butyricum powder contained viable bacteria at 5.6 × 109 colony-forming units (CFU)/g and spore number at 4.4 × 109 CFU/g, and B. infantis powder contained viable bacteria at 2.3 × 1011 CFU/g, while the probiotic mixture contained viable C. butyricum at 2.4 × 108 CFU/g and viable B. infantis at 1.8 × 109 CFU/g. Directly before administration, the probiotic products were reconstituted in sterile saline for 15 min at 37°C. The finial concentrations were 2.3 × 109 CFU/mL for C. butyricum, 5 × 1010 CFU/mL for B. infantis, and 1.0 × 108 CFU/mL C. butyricum and 1.0 × 109 CFU/mL B. infantis in the probiotic mixture for further use.
2.2. Animals and Experimental Design
One hundred and twenty specific pathogen-free (SPF) male C57BL/6 mice (20 ± 1.3 g), purchased from the Experimental Animal Center of Zhejiang Province (Zhejiang, China), were included in our present study. AAD mice models were administered with ceftriaxone (8 g/kg body weight, Rocephin, Shanghai Roche Pharmaceuticals Ltd., Shanghai, China) intragastrically through a ball-tipped stainless steel gavage needle once daily for 5 days except the normal group [5, 6]. In order to explore the time-dependent effects of the probiotics, AAD mice models were administered with probiotics for short term (5 days) and long term (15 days). These mice were randomized into six groups of ten mice each. Besides the normal control group and AAD model groups, other four AAD groups were treated with sterile saline, C. butyricum (1.2 × 109 CFU), B. infantis (2 × 1010 CFU), and probiotic mixture (4 × 107 CFU C. butyricum and 4 × 108 CFU B. infantis), respectively. The weight and stool characteristics of the mice were monitored daily. At the end of the experimental period, the mice were anesthetized by an intraperitoneal injection of 400 mg/kg body weight chloral hydrate (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) to collect their colon contents, serum, and colon tissues for further analysis. All animals were allowed to adjust to these conditions for 1 week prior to experiments, which were kept under stable housing conditions with a 12-hour light/dark cycle and free access to water and food throughout the experiment. The study protocol was approved by the Animal Care Committee of Zhejiang University, China.
2.3. Bacterial Genomic DNA Extraction
Frozen colon contents were thawed, and bacterial genomic DNA was extracted using QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, with the additional glass-bead beating steps on a Precellys 24 homogenizer (Bertin Technologies, Montigny, France). Bacterial genomic DNA was eluted in 60 μL of elution buffer and stored at −20°C for further analysis
2.4. PCR-DGGE Analysis
For amplification of bacterial DNA, universal bacterial primers 341F and 534R for the V3 regions of 16S rRNA genes were used and the reaction conditions were set as described by our previous studies [12, 13]. DGGE analysis of the PCR products were performed as described by Muyzer et al. [14, 15] and Ling et al. [13] with 35% to 60% gradient, using a D-Code system (Bio-Rad, Hercules, CA, USA).
2.5. qPCR for Fecal Predominant Bacteria
The qPCR assay was performed with a Power SYBR Green PCR Master Mix (Takara, Dalian, China) on ABI ViiA7 real-time PCR system (Applied Biosystems, Carlsbad, CA) according to the manufacturer's instructions. Bacterial specific primer sets and the reaction conditions used for qPCR were performed according to previous study [16]. The copy number of target DNA was determined by comparison with a 10-log-fold diluting standards plasmid DNA running on the same plate. Data analysis was conducted with ViiA 7 software v1.1. All reactions were carried out in triplicate repeats and a nontemplate control was performed in every analysis. Bacterial quantity was presented as log 10 bacteria per gram of feces (wet weight).
2.6. Histopathology
Samples from colon were fixed, paraffin-embedded, sectioned at 5 μm, processed for H&E staining, and then examined under light microscopy. Tissue slides were examined in an Olympus microscope (Olympus, Tokyo, Japan).
2.7. Serum Cytokines Analysis
Serum interleukin-1β (IL-1β), IL-10, tumor necrosis factor α (TNF-α), and interferon γ were determined by commercially available mouse Raybio sandwich enzyme-linked immunosorbent assay (ELISA) kits (RayBiotech, Norcross GA, USA). Lower limit of detection for each assay was 5 pg/mL. Standard curves were generated for every plate and the average zero standard optical densities were subtracted from the rest of the standards, controls, and samples to obtain a corrected concentration.
2.8. Statistical Analysis
The DGGE analysis and the similarities among different bacterial DGGE profiles by Quantity One 1-D Analysis software were carried out as described previously [13]. A similarity matrix was constructed using Dice's similarity coefficient. A dendrogram was constructed by the unweighted pair group method, using arithmetic averages (UPGMA). The normally distributed continuous data are presented as the mean ± standard deviation (SD) and the differences between groups were evaluated by one-way ANOVA or Student's t-test. The differences between categorical data and the correlations between variables were tested by Fisher's exact test and Spearman rank correlation, respectively. All statistical analyses were performed using SPSS 18.0 (SPSS Inc, Chicago, IL) and were considered statistically significant if P < 0.05.
3. Results
3.1. Structural Modulation of the Fecal Predominant Microbiota by Probiotic Therapy
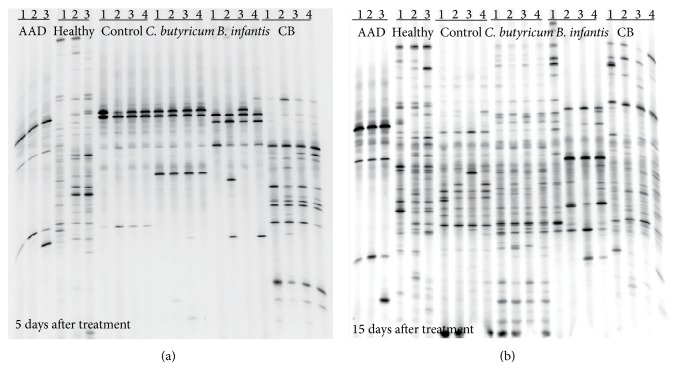
As shown in Figure 1, PCR-DGGE profiles showed the overall structure and diversity of the fecal predominant bacteria after treatment with probiotics. In the AAD mice model, most of the bacteria in the fecal microbiota have been eliminated after antibiotics treatment. After short-term probiotic therapy, the bacterial profiles were still not restored, although the numbers of bands in the probiotic mixture group were more than those in single strain groups (Figure 1(a)). When the probiotic therapy was prolonged to 15 days, there were dramatic changes in the richness and diversity of the fecal predominant bacteria in the C. butyricum and probiotic mixture groups, which indicated the restoration of fecal microbiota after treatment (Figure 1(b)). However, B. infantis could not restore the fecal microbiota successfully even after long-term administration. Cluster analysis of the DGGE profiles, which was based on the similarity indices, also demonstrated that long-term administration of probiotic mixture could restore the fecal microbiota (see Figure S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2015/582048).
Figure 1.
PCR-DGGE analysis of the predominant fecal microbiota in mice. PCR-DGGE fingerprints analyzed the fecal microbiota of samples from AAD mice model treated with different probiotic strains or probiotic mixture for short-term (a) or long-term (b) course. Each lane represented one subject which was selected in its group at random.
3.2. Quantification of Predominant Fecal Bacteria by qPCR
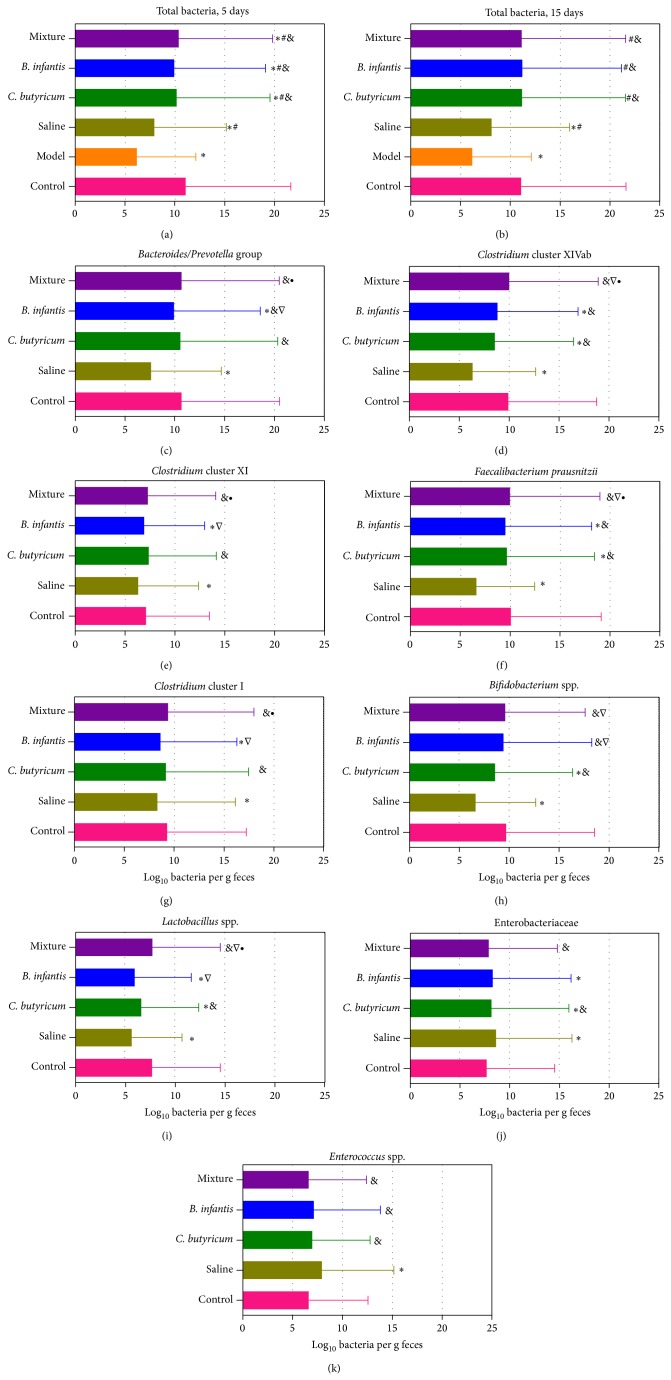
The differences in predominant fecal bacteria after treatment with different probiotic strains and different terms were detected by qPCR (Figure 2). After 5-day treatment, the total bacteria in these treated groups could not reach healthy levels, with approximately one order of magnitude decrease when compared with the healthy mice. However, the total bacteria returned to normal level after 15-day treatment except the saline control group, which indicated that long-term administration of probiotic strains or probiotic mixture demonstrated a positive effect on modulating the intestinal microbiota in mice. In order to explore the specific modulation of the fecal microbiota, other nine predominant bacteria were detected after 15-day treatment. Our data demonstrated that Bacteroides-Prevotella group, Clostridium cluster XI, Clostridium cluster I, and Enterococcus were restored into normal level in the C. butyricum group, while only Bifidobacterium and Enterococcus reversed in the B. infantis group. Intriguingly, all predominant fecal bacteria reached normal level after the probiotic mixture treatment. Clostridium cluster XIVab, F. prausnitzii, Bifidobacterium, and Lactobacillus were significantly increased in the probiotic mixture group when compared with the C. butyricum group, while Bacteroides-Prevotella group, Clostridium cluster XIVab, Clostridium cluster XI, F. prausnitzii, Clostridium cluster I, and Lactobacillus were obviously higher in the probiotic mixture group than that in the B. infantis group. These observations suggested that the combined C. butyricum with B. infantis could restore the fecal microbiota more efficiently than the single probiotic strain.
Figure 2.
Bacterial loads in fecal microbiota as measured by qPCR (log10 copies/g fresh feces). The total bacteria were detected in the short-term and long-term treatment, while other predominant fecal microbiota were only detected in long-term treatment. Graph values are reported as the mean and standard deviation of the mean. Comparisons among the groups were calculated with Student's t-tests. P < 0.05 was labeled; ∗ compared with healthy control; # compared with AAD mice model; & compared with saline control; ∇ compared with C. butyricum treated group; ∙ compared with B. infantis treated group.
3.3. Histopathology of the Colon
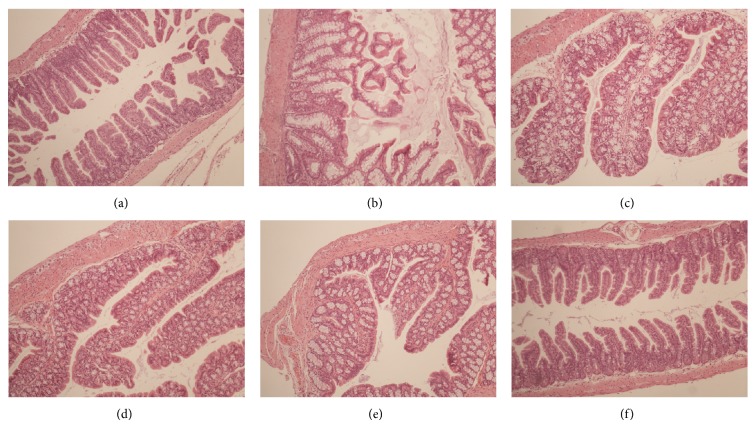
The evident damage of colon architecture after antibiotic treatment was loss of homogenously distributed and integrated villi (Figure 3). Most of the colon epithelial cells showed severe swelling and partially rupturing. After 15-day actively treatment, the tissue architecture of the colon was restored significantly in the C. butyricum combined with B. infantis group, with the villi homogenously distributing as the healthy control. However, the severe villous swelling and extending could still not return to normal level in the C. butyricum group and the B. infantis group. Our data suggested that the C. butyricum combined with B. infantis could help to restore the colon mucosa successfully in a relative long-term course.
Figure 3.
Histopathology of the colon after treatment. Images represent sections of the distal colon (magnification, ×40). (a) Healthy control; (b) AAD mice model; (c) saline control; (d) C. butyricum treated group; (e) B. infantis treated group; (f) probiotic mixture treated group.
3.4. Comparison of Serum Cytokines after Probiotic Treatment
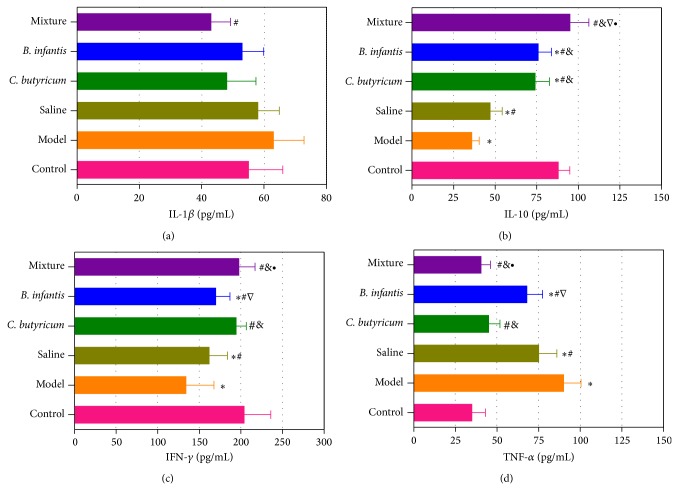
In the AAD mice model, the TNF-α level increased and the IL-10 and IFN-γ levels decreased significantly, which might be involved in the systemic inflammation of the mice (Figure 4). However, IL-1β was not significantly altered in the AAD mice. The serum levels of IL-10 and IFN-γ increased significantly after long-term C. butyricum administration, while the TNF-α decreased obviously, which were also observed in the B. infantis group (P < 0.05). However, those altered serum IL-10, IFN-γ, and TNF-α were still not reaching healthy levels in the B. infantis group, whereas only IL-10 was still lower in the C. butyricum group than that in healthy mice. It was unexpected that the concentration of IL-10, IFN-γ, and TNF-α returned to normal levels in the probiotic mixture group (P > 0.05). Our results indicated that the C. butyricum combined with B. infantis could attenuate systemic inflammation in the AAD mice.
Figure 4.
Comparison of cytokines among the six groups. P < 0.05 was labeled. ∗ compared with healthy control; # compared with AAD mice model; & compared with saline control; ∇ compared with C. butyricum treated group; ∙ compared with B. infantis treated group.
4. Discussion
The emergence of variety of antibiotics has been used extensively in human and veterinary medicine, for the purpose of preventing (prophylaxis) or treating microbial infections since 1928. However, antibiotic therapy has been widely overused and misused during the past decade despite considerable evidence that antibiotic treatment of microbial infections shortens its course or prevents the development of secondary bacterial infections. In fact, widespread use of broad-spectrum antibiotics can affect not only the target pathogen but also commensal inhabitants of the human host. Normal commensal bacteria play vital roles in maintaining host-microbe homeostasis and inhibiting pathogen colonization and overgrowth (i.e., colonization resistance). These processes are attributable to the existence of a stable and diverse population of resident microorganisms that compete with an invading pathogen directly for niches and nutrients or through production of antibacterial substances [17, 18]. As a common complication of most types of antibiotics, AAD affects variety of populations including outpatients, hospitalized patients, and residents of long-term care facilities and results in extended hospital stays, increased medical care costs, and increased diagnostic procedures [1, 19]. Beniwal et al. have considered that AAD pathogenesis may be related to altered short-chain fatty acids in the intestine, functional disturbance of carbohydrate, and bile acid metabolism due to alteration of the microbiota or toxic effects on the intestinal mucosa and pharmacological effect on motility [3, 20]. So far, there are no other current effective preventive measures for AAD, except for discontinuing the inciting antibiotic or switching to an antibiotic with a narrower spectrum of action.
Increasing evidence has shown that probiotics are a promising strategy for the prevention and treatment of AAD, which can help to restore intestinal microbiota and reestablish intestinal homeostasis [9, 21]. Probiotics are living microbes taken to provide a health benefit on the host, which offer promise for a wide diversity of diseases and have an excellent safety-benefit ratio. However, the efficacy is probiotic strain specific. C. butyricum (Clostridium cluster I) is a typical butyric-acid producing gram-positive anaerobe found in the intestines of healthy human, which has been used for modulating gastrointestinal microbiota and treating intestinal disorders [22–24]. C. butyricum can produce endospores, which is a key characteristic related to its ability to survive at lower pH, at relatively higher bile concentrations, and in the presence of coadministered antibiotics [23, 25]. B. infantis is a normal component of the intestinal microbiota in humans and animals and is frequently associated with health-promoting effects. As a lactic acid bacterium, B. infantis has been proved to be useful probiotics that can specifically relieve many of the symptoms of irritable bowel syndrome [26]. Previous studies also demonstrated that C. butyricum promotes the growth of Lactobacillus and Bifidobacterium and inhibits AAD in human and mice [23, 27, 28]. In our present study, two probiotic strains and their mixtures mentioned above were used to treat the AAD. In the AAD mice model, the intestinal microbiota was almost eliminated as only few bands in the DGGE profiles, and the tissue architecture of the colon was damaged dramatically. Regardless of the short-term or long-term follow-up time, the intestinal microbiota and the tissue architecture of the colon could still not recover naturally. Our data demonstrated that the probiotic strains and mixtures exerted beneficial effects on the restoration of the intestinal microbiota and the recovery of the tissue architecture. However, the effects were time dependent and strain specific. Short-term treatment had little influence on the composition and diversity of the intestinal microbiota. In addition, the probiotic C. butyricum was more effective than B. infantis, even though high dose of B. infantis was used. The discrepancy of the therapeutic effects might be associated with their different metabolites, especially short-chain fatty acids [29]. These metabolites might improve the ecosystem of the gastrointestinal tract via promoting the growth of healthy symbionts and/or enhancing the barrier function of epithelial cells [30]. Wong et al. have shown that butyrate, rather than lactic acid, plays a central role in maintaining gut homeostasis [31]. However, it was apparent that the C. butyricum combined with B. infantis probiotic mixture was superior to their single probiotic strain in the treatment of AAD. The predominant bacteria and the tissue architecture that were broken down by antibiotics were almost restored after a relative long-term probiotic mixture treatment. The advantage of using this probiotic mixture containing C. butyricum and B. infantis may derive from the synergistic effects of the two bacteria.
In addition, the development of AAD was also accompanied with systemic inflammation as the proinflammatory cytokines increased and anti-inflammatory cytokines decreased significantly. In present study, the probiotic strains and probiotic mixture showed immunomodulatory effects in the AAD mice. The increase of TNF-α and decreases of IL-10 and IFN-γ in the AAD mice were significantly recovered in all probiotic groups. Previous studies have demonstrated that C. butyricum could suppress intestinal immune disorders by regulating IL-10 production [32], while B. infantis could attenuate inflammation in DSS-induced colitis in rats [33]. Our study also indicated that the immunomodulatory effects of the probiotic mixture were superior to the single strain of C. butyricum and B. infantis. These cytokines seemed to be involved in the beneficial effects of probiotics on AAD; however, the mechanisms by which the probiotic mixture modulated immune function were still unclear. Future researches on the mechanisms of action of the probiotic mixture in vitro are required.
5. Conclusion
In present study, we found that long-term administration of C. butyricum combined with B. infantis probiotic mixture exerted beneficial effects on the restoration of the intestinal microbiota and the recovery of the tissue architecture of the colon, which was superior to their single probiotic strain in the treatment of AAD. Moreover, the possible protective role might be associated with the immunomodulatory effects of the probiotic mixture. According to our present results, supplementation with C. butyricum combined with B. infantis probiotic mixture is a simple and effective method to treat AAD.
Supplementary Material
Figure S1: UPGMA dendrogram of the DGGE profiles for short-term (A) and long-term (B) treatment. Based on the similarity indices, our present cluster analysis of the DGGE profiles demonstrated that long-term administration of probiotic mixture could restore the fecal microbiota.
Acknowledgments
This present work was funded by National Natural Science Foundation of China under Grant no. 81400586, Zhejiang Provincial Natural Science Foundation of China under Grant no. LQ14H030002, and the National Basic Research Program of China (973 Program) under Grant no. 2013CB531404.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Zongxin Ling and Xia Liu contributed equally to this work.
References
- 1.McFarland L. V. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiology. 2008;3(5):563–578. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 2.Ling Z., Liu X., Jia X., et al. Impacts of infection with different toxigenic Clostridium difficile strains on faecal microbiota in children. Scientific Reports. 2014;4, article 7485 doi: 10.1038/srep07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jernberg C., Löfmark S., Edlund C., Jansson J. K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156(11):3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 4.Sekirov I., Tam N. M., Jogova M., et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infection and Immunity. 2008;76(10):4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M., Li W. H., Wen S., Liu Y. H., Tang L. Effects of ceftriaxone-induced intestinal dysbacteriosis on dendritic cells of small intestine in mice. Microbiology and Immunology. 2013;57(8):561–568. doi: 10.1111/1348-0421.12068. [DOI] [PubMed] [Google Scholar]
- 6.Gao F., Li M., Liu Y. H., Gao C., Wen S., Tang L. Intestinal dysbacteriosis induces changes of T lymphocyte subpopulations in Peyer's patches of mice and orients the immune response towards humoral immunity. Gut Pathogens. 2012;4(1, article 19) doi: 10.1186/1757-4749-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson J. K., Holmes E., Wilson I. D. Gut microorganisms, mammalian metabolism and personalized health care. Nature Reviews Microbiology. 2005;3(5):431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 8.Vanderhoof J. A., Whitney D. B., Antonson D. L., Hanner T. L., Lupo J. V., Young R. J. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. Journal of Pediatrics. 1999;135(5):564–568. doi: 10.1016/s0022-3476(99)70053-3. [DOI] [PubMed] [Google Scholar]
- 9.McFarland L. V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. American Journal of Gastroenterology. 2006;101(4):812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamer H. M., Jonkers D., Venema K., Vanhoutvin S., Troost F. J., Brummer R.-J. Review article: the role of butyrate on colonic function. Alimentary Pharmacology & Therapeutics. 2008;27(2):104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 11.Ewaschuk J. B., Diaz H., Meddings L., et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. The American Journal of Physiology—Gastrointestinal and Liver Physiology. 2008;295(5):G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 12.Ling Z., Kong J., Jia P., et al. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microbial Ecology. 2010;60(3):677–690. doi: 10.1007/s00248-010-9712-8. [DOI] [PubMed] [Google Scholar]
- 13.Ling Z., Kong J., Liu F., et al. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics. 2010;11(1, article 488) doi: 10.1186/1471-2164-11-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muyzer G., de Waal E. C., Uitterlinden A. G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Microbiology. 1993;59(3):695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartosch S., Fite A., Macfarlane G. T., McMurdo M. E. T. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Applied and Environmental Microbiology. 2004;70(6):3575–3581. doi: 10.1128/aem.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y., Yang F., Lu H., et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 17.Adamu B. O., Lawley T. D. Bacteriotherapy for the treatment of intestinal dysbiosis caused by Clostridium difficile infection. Current Opinion in Microbiology. 2013;16(5):596–601. doi: 10.1016/j.mib.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawley T. D., Walker A. W. Intestinal colonization resistance. Immunology. 2013;138(1):1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFarland L. V. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Digestive Diseases. 1998;16(5):292–307. doi: 10.1159/000016879. [DOI] [PubMed] [Google Scholar]
- 20.Beniwal R. S., Arena V. C., Thomas L., et al. A randomized trial of yogurt for prevention of antibiotic-associated diarrhea. Digestive Diseases and Sciences. 2003;48(10):2077–2082. doi: 10.1023/a:1026155328638. [DOI] [PubMed] [Google Scholar]
- 21.McFarland L. V. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe. 2009;15(6):274–280. doi: 10.1016/j.anaerobe.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M., Taguchi H., Yamaguchi H., Osaki T., Komatsu A., Kamiya S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunology and Medical Microbiology. 2004;41(3):219–226. doi: 10.1016/j.femsim.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Kong Q., He G.-Q., Jia J.-L., Zhu Q.-L., Ruan H. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Current Microbiology. 2011;62(2):512–517. doi: 10.1007/s00284-010-9737-8. [DOI] [PubMed] [Google Scholar]
- 24.Yang C. M., Cao G. T., Ferket P. R., et al. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poultry Science. 2012;91(9):2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- 25.Courvalin P. Antibiotic resistance: the pros and cons of probiotics. Digestive and Liver Disease. 2006;38(supplement 2):S261–S265. doi: 10.1016/s1590-8658(07)60006-1. [DOI] [PubMed] [Google Scholar]
- 26.Whorwell P. J., Altringer L., Morel J., et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. The American Journal of Gastroenterology. 2006;101(7):1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B., Yang X., Guo Y., Long F. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Archives of Animal Nutrition. 2011;65(4):329–339. doi: 10.1080/1745039x.2011.568274. [DOI] [PubMed] [Google Scholar]
- 28.Imase K., Takahashi M., Tanaka A., et al. Efficacy of Clostridium butyricum preparation concomitantly with Helicobacter pylori eradication therapy in relation to changes in the intestinal microbiota. Microbiology and Immunology. 2008;52(3):156–161. doi: 10.1111/j.1348-0421.2008.00026.x. [DOI] [PubMed] [Google Scholar]
- 29.Macia L., Thorburn A. N., Binge L. C., et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunological Reviews. 2012;245(1):164–176. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 30.Round J. L., Mazmanian S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong J. M. W., De Souza R., Kendall C. W. C., Emam A., Jenkins D. J. A. Colonic health: fermentation and short chain fatty acids. Journal of Clinical Gastroenterology. 2006;40(3):235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi A., Sato T., Kamada N., et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host and Microbe. 2013;13(6):711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Osman N., Adawi D., Molin G., Ahrne S., Berggren A., Jeppsson B. Bifidobacterium infantis strains with and without a combination of Oligofructose and Inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterology. 2006;6, article 31 doi: 10.1186/1471-230x-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: UPGMA dendrogram of the DGGE profiles for short-term (A) and long-term (B) treatment. Based on the similarity indices, our present cluster analysis of the DGGE profiles demonstrated that long-term administration of probiotic mixture could restore the fecal microbiota.