Abstract
More than twenty years ago, hydroxyapatite (HA), calcium phosphate ceramics, was introduced as a coating for cementless hip prostheses. The choice of this ceramic is due to its composition being similar to organic apatite bone crystals. This ceramic is biocompatible, bioactive, and osteoconductive. These qualities facilitate the primary stability and osseointegration of implants. Our surgical experience includes the implantation of more than 4,000 cementless hydroxyapatite coated hip prostheses since 1990. The models implanted are coated with HA in the acetabulum and in the metaphyseal area of the stem. The results corresponding to survival and stability of implants were very satisfactory in the long-term. From our experience, HA-coated hip implants are a reliable alternative which can achieve long term survival, provided that certain requirements are met: good design selection, sound choice of bearing surfaces based on patient life expectancy, meticulous surgical technique, and indications based on adequate bone quality.
1. Introduction
Since Charnley's first design of low friction arthroplasty which emerged in the 1960s [1] a number of improvements have gradually arisen, affecting design, materials, primary and secondary implant fixation systems, and biomechanical and biological adaptations which occur in the bone after joint replacement. The main objective in all instances has been to improve the survival of the implant in the long term [2], a goal shared by all surgeons. Implant to bone fixation was initially achieved by means of acrylic cement, which was also introduced by Charnley. Although long-term outcomes of cemented hip arthroplasty have been good and modern cementing techniques can improve implant survival even further, cemented hip implants have always been a concern in young and more active patients. Cementless hip arthroplasty emerged at the end of the 1970s, as an alternative to cemented systems. Primary fixation of cementless designs is based on a tight press-fit of the implant into the bone, and secondary or definitive fixation depends on a biological anchoring in bone, needed to ensure long-term survival of the implant [3]. In early cementless designs biological fixation was poor, and loosening was common. Primary bone-implant stability is critical because although 50 to 150 μm wide micromovements still allow new bone formation [4–7], the greater the magnitude of micromovements, the lower the amount of bone formation. Thus, bone-implant anchorage could turn into a fibrous tissue which leads to loosening.
In the late 1970s, porous coatings were added to implant surfaces in order to improve osseointegration [8–12]; however, a high incidence of thigh pain, bead shedding, and loosening was found. Implant osseointegration of these designs took a long time, and surgical technique was demanding. Further research on porous coatings [13] brought improvements in manufacturing which immediately improved clinical outcomes.
The search for a type of coating capable of enhancing implant osseointegration led to growing interest in calcium phosphate ceramics, which were first used for coating dental implants and then were brought into the orthopaedics field. Hydroxyapatite (HA) was chosen from calcium phosphate ceramics for its chemical characteristics and for being a major component of bone mineral content. The Leiden Biomaterials Research Group, Gloot and Geesing [14, 15], Furlong [16], Manley [17], and Epinette [18] pioneered the orthopaedic use of HA, as an osteoconductive material which promotes osseointegration of implants improving their long-term survival. Currently, HA-coated implants have been in use for nearly 30 years with excellent results.
Hydroxyapatite: Structure and Properties. Synthetic calcium phosphate ceramics have similar chemical and crystalline properties to biological apatite crystals. Among them, the HA (Ca10(PO4)6(OH)2) is the most similar to biological apatite crystals, but its atomic ratio is 1.67 times greater than that of bone or dentine, and it is the least soluble of all calcium phosphate ceramics [19]. HA is biocompatible (it does not cause toxic or inflammatory responses in vivo) [20], bioactive, and osteoconductive, but HA is not osteoinductive [19]. Its mechanical characteristics include high compressive strength (700 MPa) but low tensile (250 MPa) and fatigue strength [21]. HA is used for creating a coating on prosthetic cup or stem surfaces, which are usually made of a titanium alloy (TiAlV). HA deposition is often achieved through the plasmaspray technique, which is performed at high temperature (15000°) and under vacuum, by projecting HA particles onto the metallic material at a speed of 300 m/s. The metallic substrate has a rough surface to promote adhesion. The other manufacturing method achieves HA deposition by electrochemical means, although it appears that the plasma spray technique is associated with improved bone ongrowth [22]. Advisable HA coating thickness is 50 μm because coats 15–20 μm thick are quickly dissolved, and coats 100–150 μm thick may suffer delamination as a result of the tensile forces produced in uploading [21], even though good results have been reported with thicker (200 microns) coatings [16]. HA coating should have pores of about 100–200 microns and an acceptable porosity index to promote osteoconductivity, although coating strength decreases with porosity [19]. Another key characteristic of HA coating is crystallinity, which is associated with increased bioactivity and bone growth and with decreased bone resorption [23].
Other important matters are the HA coating resorption after implantation, or the coating delamination process, most likely in thicker coatings (150–200 microns) and unstable implants [24].
No strong evidence can be found in literature about the HA resorption process and loss of HA coating. However, two phases in HA loss have been suggested [25]. The first one, in the immediate postoperative period, is when micromovements in the HA-bone interface lead to the formation of a fibrous membrane with high metabolic activity, containing fibroblasts and macrophages which are able to remove the HA coating. This inflammatory response, described as transient by Geesink et al. [21], along with an increased fluid content and a low pH, helps to dissolve the less crystalline HA, releasing calcium ions which may have biological activity [19]. These calcium ions can be incorporated into the remaining HA coating, increasing its crystallinity and thus reducing the subsequent coating loss, which takes years to disappear [26]. The bone around the implant is also undergoing a remodeling process, adaptive remodeling, regulated by biomechanical changes, among other factors. Osteoclastic activity linked to adaptative remodeling contributes to HA resorption [19, 26–31], which is related to the thickness of the coating layer [28]. Later the lost HA coating is replaced by new bone [26–31] leading to implant osseointegration. New bone apposition on the HA coating surface begins at third postoperative week [21, 32–34], and initially it has a lamellar structure which is gradually replaced by a Haversian structure as remodeling progresses [21].
2. Materials and Methods
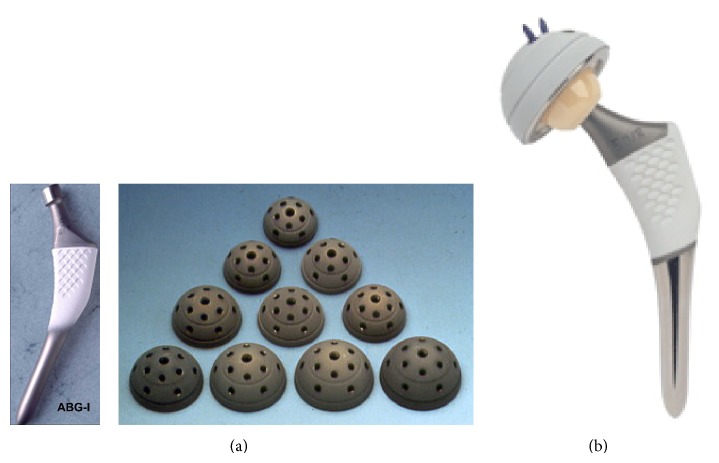
In 1990 our department started using cementless HA-coated hip implants, in particular the ABG I prosthesis (Stryker). It consists of an anatomical HA-coated stem with press-fit metaphyseal fixation and an HA-coated hemispherical cup. The ABG I implant is made of a titanium alloy (Ti6Al4V) with Young's modulus of 110 GPa (Figure 1(a)). The HA coating, applied through a plasma spraying process, was 50 μm thick and of about 80% crystallinity after the manufacturing process. Until September 1999, this design was implanted in 1637 patients (bilateral in 277 of them), with a total of 1914 hip arthroplasties. The bearing surfaces were conventional polyethylene with metal or zirconia heads in all cases. In 1999, the ABG I system was replaced by a new design, the ABG II model (Figure 1(b)). The new cup has only five holes through which spikes or screws can be inserted for proper primary fixation, and hole plugs are supplied for sealing unused holes. The “shoulder” of the stem is higher and its metaphyseal region has a decreased volume. The diaphyseal portion of the stem also has a smaller diameter and length and is highly polished. Titanium alloy was improved with the addition of molybdenum, zirconium, and ferrous (iron), reducing Young's modulus to 74–85 GPa. HA coating, manufactured by a Stryker patented process, keeps the same coating thickness but crystallinity has been improved to 98%. Highly cross-linked polyethylene (Duration) liners with metallic or zirconium heads and ceramic on ceramic heads were used as bearing couplings. From September 1999 to December 2013, 1694 patients were operated on; 428 of them were bilateral, bringing the number to 2122 total hip arthroplasties.
Figure 1.
(a) ABG-I stem and acetabular cups; (b) ABG-II stem and acetabular cup.
A similar surgical technique was used in both ABG models, and only the surgical instruments for acetabular and femoral preparation varied somewhat between them. The same posterolateral approach, intravenous antibiotic prophylaxis (2nd generation cephalosporins), and antithrombotic prophylaxis protocol (low molecular weight heparin) were used in all cases. Over the years, the only significant changes in postoperative management have been a shorter postoperative immobilization and a reduced length in hospital stay.
Regardless of our participation in an international multicenter follow-up study on ABG I outcomes [35], several long-term follow-up studies have been carried out in our department.
A ten-year follow-up study, on 630 ABG I prostheses implanted in 579 patients, was reported [36]. Clinical outcomes were assessed with the Merle D'Aubigne-Postel score [37], and bone was quality scored, on plain preoperative radiographs, according to the modified Singh scale [38]. Different radiographic items were evaluated at the first, fifth, and tenth postoperative years. Description of radiological findings was done according to the Gruen zones [39] in proximal femur and De Lee and Charnley zones [40] in periacetabular bone. Broker scale for heterotopic ossifications was used [41]. Polyethylene wear was assessed with the Livermore method [42], and granulomatous and osteolytic lesions, secondary to wear debris particles, were also examined. Position of the cup in relation to the anatomic hip rotation centre, cup inclination (opening) angle, and size of the stem in relation to the diameter of the femur were studied too.
On the other hand, 196 ABG II arthroplasties, implanted in 168 patients, were followed up for a mean of 11.3 years [43]. In this case, clinical outcomes were assessed with Harris hip score [44] and subjective outcomes with the EuroQolGroup EQ-5D questionnaire [45]. The Livermore method [42] was used again to evaluate polyethylene wear, even though evaluation was done by means of a computer program since digital radiology had become available. Granulomatous and osteolytic lesions were measured, in this case, according to the scale proposed by Goetz et al. [46].
Both in the ABG I and in the ABG II studies, a statistical χ 2 analysis for categorical data and percentages comparison and a Student's t-test for means comparison of isolated data or between pairs of related data with Pearson correlation were used. The level of significance was set at P < .05.
To assess the femoral remodeling changes which occur after stem implantation, a group of patients with ABG I implants had DXA exams which were recorded in the preoperative and throughout the follow-up period (15th day, 3rd, 6th, and 12th month, and annually until the 10th postoperative year) [47]. Similarly, another group of patients with ABG II implants had DXA exams throughout a five-year follow-up period [48].
Finally, simulations were made with the ABG I and ABG II stems, by means of the finite element method, to assess the biomechanical changes which occur in the femur after stem implantation. Simulation results were compared with their respective DXA studies in each model [49–51].
3. Results
The gender distribution in the ABG I group was 55.39% male, with a mean age of 58.10 years, and 44.61% female, with a mean age of 61.32 years. In the ABG II group, 70.83% were men and 29.17% were women, and the mean age was 11.26 ± 58.84 years (SD) with a range of 23–77 years.
Clinical outcomes in each implant group are specified below: in the ABG I group the mean preoperative Merle D'Aubigne-Postel score was 7.91, and it increased to 16.21 (range 9–18) at the 10th year; subjective assessment was excellent or good in 82.1% of cases. In the ABG II group, the mean preoperative Harris hip score was 32.55, and the average postoperative score rose to 85.80 (range 26.05–95.82); subjective assessment was excellent or good in 90.32% of cases.
ABG I implants survival at 10-year follow-up was 97.1%. Although all acetabular components were stable, 1.35% of these patients needed revision surgery because of an excessive polyethylene wear. In these cases, the liner was replaced by a highly cross-linked polyethylene, and femoral and/or periacetabular osteolytic lesions were cleaned and grafted. But the prosthetic cup remained stable in all cases and was not replaced.
At 17-year follow-up with ABG I, 18 patients needed revision surgery for major acetabular and/or femoral osteolytic lesions. In such cases both the stem and the cup remained stable; therefore only polyethylene liner was replaced and osteolytic lesions were curetted and grafted. At 20-year follow-up, 21 patients needed revision surgery for major acetabular and/or femoral osteolytic lesions. Only in three patients a replacement of the acetabular cup and femoral stem was performed, implanting a cemented prosthesis in all of them. In the remaining 18 the implants were stable despite osteolytic lesions; therefore only polyethylene liner was replaced and osteolytic lesions were curetted and grafted (Figures 2 to 5).
Figure 2.
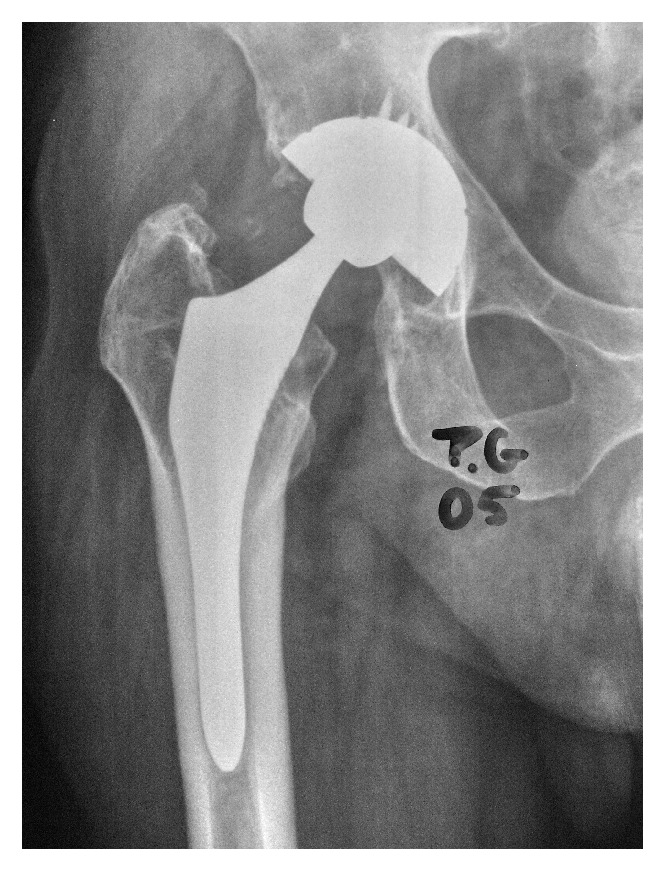
X-ray image of patient with follow-up at 12 y. Osteolysis in acetabulum produced by excessive polyethylene wear.
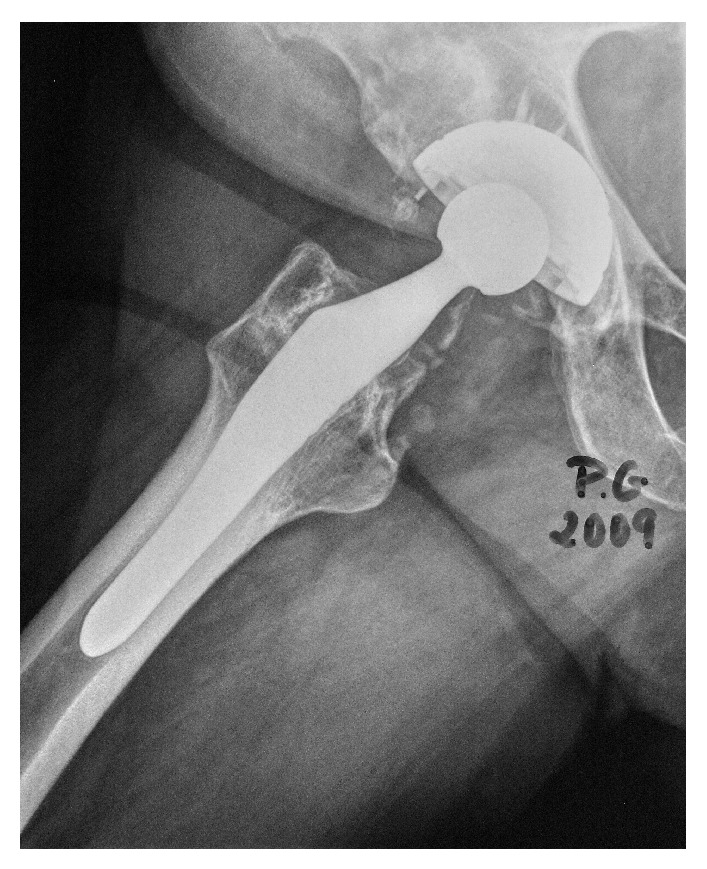
Figure 5.
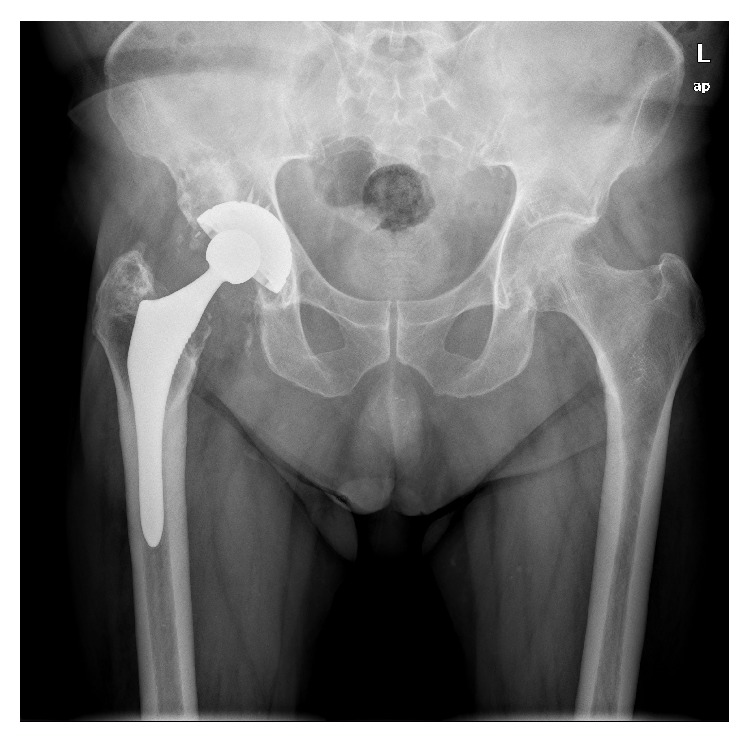
Same case as in Figures 2, 3, and 4. X-ray control image in 2013, after 8 y. follow-up of second surgery with original implant since 1993.
ABG II prosthesis survival at a mean of 11.3 years of follow-up was 98.30%, with all acetabular components being stable and with no signs of migration.
The key difference between the two model outcomes is polyethylene wear. Duration polyethylene, used in the ABG II model, has shown a 54.55% less wear rate than conventional polyethylene used in the ABG I model. The ABG I polyethylene wear has been greatest in acetabular cups placed in a low position (P = .038), with opening angles greater than 46°, and in patients under the age of 65. At ten years of follow-up, the incidence of periacetabular granulomatous lesions in the ABG I group was 44.23% in zone I, 37.11% in zone II, and 15% in zone III; and it was 78% in Gruen zone I and 91.73% in Gruen zone 7. Despite this, all the acetabular and femoral components were stable, even though some stems showed some subsidence. In the ABG II group, granulomatous lesions were very small and only occurred in metal on polyethylene or zirconium on polyethylene couplings. In this group, 9.52% of cases showed osteolytic lesions in acetabular zone I and 12.7% in Gruen zones 1 and 7. Decreased incidence of osteolytic lesions in the ABG II model is due to the lesser wear of the new polyethylene but, in our opinion, the sealing of unused cup holes may also have played a role.
Stem subsidence has been evaluated in both models and significant differences have been found. In the ABG I group, of the cases in which the size of the stem was deemed appropriate, mean subsidence was 1.51 mm at the first year and increased to 3 mm at the 10th year. However, when the size of the stem was deemed large, mean subsidence was 2.29 mm at the first year and reaches 4.17 mm at the 10th year. In the ABG II group, lesser subsidence has been found among the cases in which the size of the stem was deemed appropriate; mean subsidence at the first year was 0.514 mm and rose to 0.638 ± 0.180 (SD) at the end of follow-up, but oversized stems showed 2.435 mm and 2.830 mm, respectively. In both studies oversized stems were associated with a significantly greater subsidence (P = .0001).
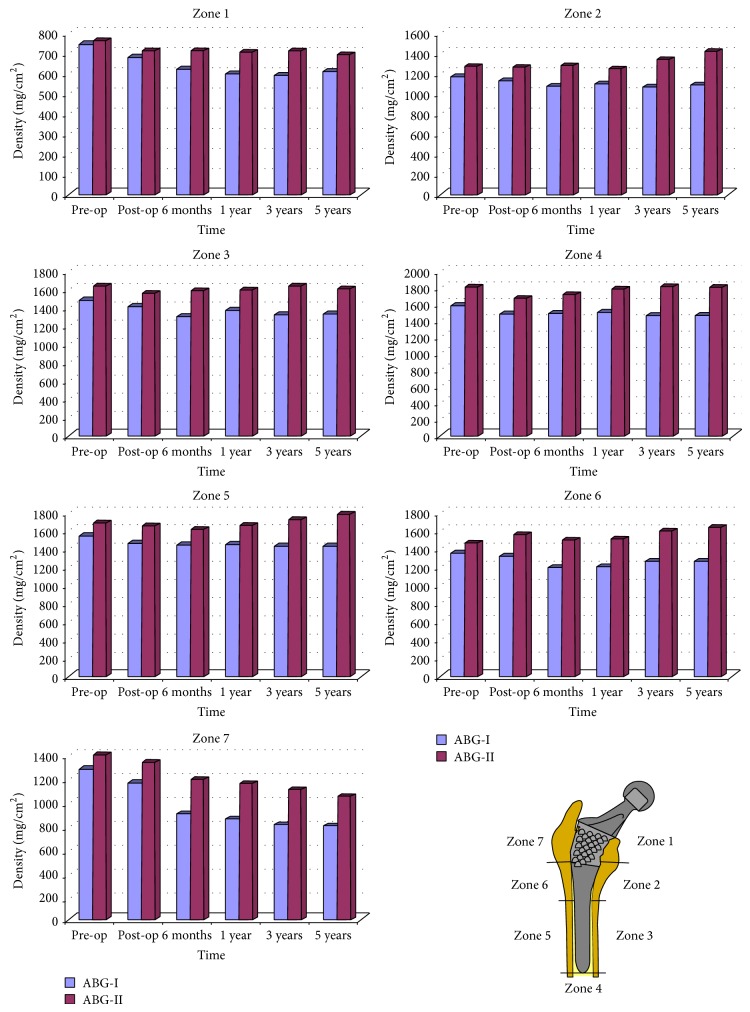
Femoral remodeling has also shown to be significantly different between the two groups. Up to 90% of cases in the ABG I group showed evident bone devitalization in Gruen zones 1 and 7, while bone loss was less marked in the ABG II group in which it was only detected in 42.07% of cases. Cancellous bone densification in zones 2 and 6 of Gruen was present in 89.42% and 83.26% of cases in the ABG I group, respectively, while in the ABG II group this finding was detected in zone 2 in 34.43% of cases and in zone 6 in 29% of cases. Cancellous bone densification is associated with larger stems (P = .002). The high rates of devitalized bone in zones 1 and 7 are caused by the stress-shielding effect which occurs after insertion of a femoral stem. Stress-shielding in femoral zones 1 and 7 is strongly associated with females (P = .001), older age (P = .001), and low preoperative Singh index (P = .001) in both stem models. Comparisons of DXA studies at five-year follow-up show a 13.07% bone loss in zone 1 and 37.5% in zone 7 in the ABG I group, while in the ABG II group the results are 9.07% in zone 1 and 23.88% in zone 7 (Figure 6). These data may mean that design changes in the ABG II stem have achieved a better load transmission.
Figure 6.
Evolution of bone mass density for ABG I (blue) and ABG II (red), corresponding to five-year follow-up, in the Gruen zones.
4. Discussion
Our 23 years of experience in routine use of HA-coated hip prostheses is quite satisfactory as regards the long-term stability of implants, in agreement with Geesink [52]. Primary implant stability is favored by HA coating, which provides improved contact between bone and implant [26, 52–56], and osteointegration of HA-coated implants has been sufficiently demonstrated in many studies [26–31, 33, 34]. Through the years, as resorption of the HA coating is caused by chemical dissolution or osteoclastic action, new bone formation replaces it in a percentage which could rise to 78%, according to a number of publications [26, 27, 30, 31, 33, 57]. It is clear, however, that HA-coated implants achieve a stable fixation despite osseointegration not being complete. Moreover, it is well documented that HA debris particles cause no osteolytic reaction [21, 28, 30, 57]. In our personal experience no osteolytic reaction was detected along more than 23 years.
The most important problem we have experienced with the ABG I model is excessive wear of conventional polyethylene and subsequent periprosthetic osteolysis (Figures 7 and 8), although fortunately implants remained stable at 20 years of follow-up. Concerning the ABG II model, with Duration highly crosslinked polyethylene, it has shown much lower wear rates and osteolityc lesions have been significantly less frequent. We believe that sealing unused cup holes has limited the migration of wear debris to acetabular bone, helping to reduce the incidence of osteolytic acetabular lesions. Good peripheral osseointegration of the cup could also have acted as a barrier to wear debris migration [58]. The lower incidence of osteolytic lesions that we have also found in the femur can be explained by the reduced rate of wear debris particles in the ABG II model. But in addition, changes in design of the stem and improved HA crystallinity could have played a role in enhanced osseointegration, which would prevent debris migration into the femoral implant-bone interface [59].
Figure 7.
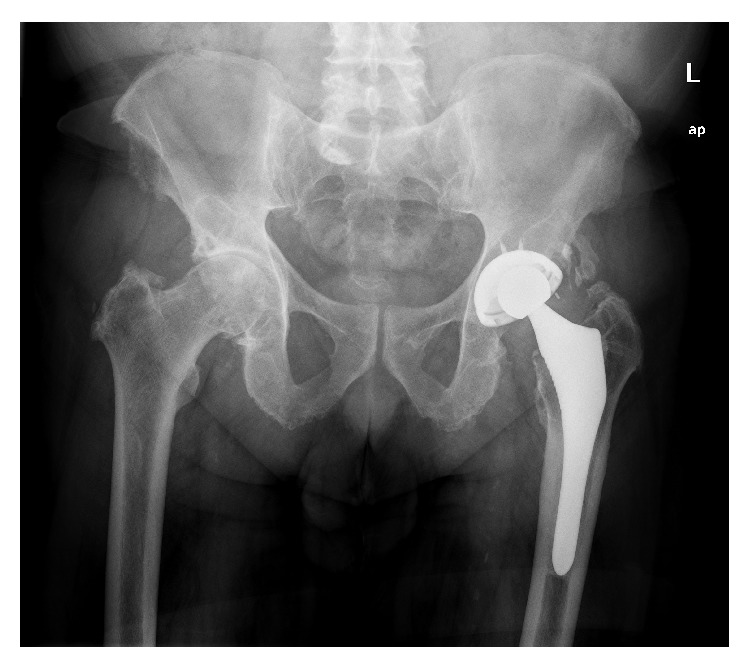
X-ray image of patient with follow-up at 12 y. Excessive polyethylene wear. Osteolysis in metaphyseal of femur.
Figure 8.
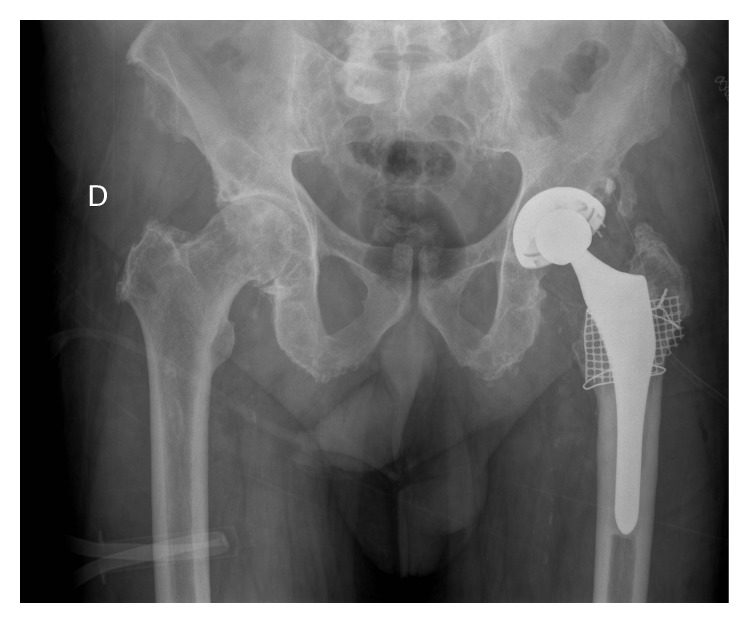
Same case as in Figure 7, after changing polyethylene and fulfilling with bone graft and femoral mesh. No change of original implant. Control at 20 y. of primary surgery.
Concerning loads acting on the hip, there are previous works [60, 61] that include a comparative analysis for different combinations of muscle loads, concluding that the more appropriate cases are those that consider the load comprising gluteus medius, iliotibial tract, and psoas iliacus, or only the action from abductor muscle, which produce compression in the femur. For the simulations carried out by our group, the last option was chosen in accordance with the majority of authors [62–65]. Orthoload's database values were used to apply hip reaction forces at the head of the stem and abductor, respectively [66].
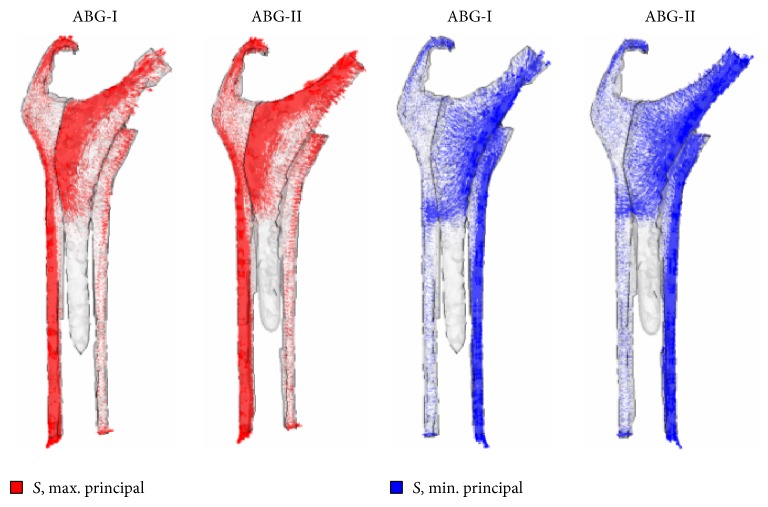
Hip arthroplasty modifies the initial tensional state of the hip joint. In the healthy femur, loads are transferred from the femoral head to the lesser trochanter which distributes the compressive forces to the femoral diaphysis [30]. Load distribution can explain the anatomical structure of the primary trabecular bundles of the healthy femur: the arch shape, formed by traction forces, and the principal compression group of Delbet, formed by compression forces [31]. Despite this load pattern is inverted after hip replacement, so that the stresses are transferred fundamentally from the prosthetic head to the stem, which transmits mechanical loads to the zone of the femur corresponding with the end of stem HA coating. Thus, a bottleneck effect is produced, as was demonstrated in the simulation (Figure 9), which leads to stress-shielding. Due to these changes in the transmission of forces all implants cause remodeling changes in the proximal femur, though cemented stems do it to a lesser extent [67]. Adaptative remodeling is due to an alteration in loads transmission produced by the femoral stem. It is regulated by Wolf's Law [67, 68] and is a multifactorial process influenced by the bone quality and stiffness, implant design and stiffness, type of bone fixation, and forces acting on the femur [64, 69–75]. As Huiskes et al. [68] pointed out, preoperative bone mass of the proximal femur is a very important factor in adaptative remodeling. ABG stems theoretically have a metaphyseal anchorage and, like other similar designs, were intended to transmit loads from proximal to distal femur and avoid stress-shielding. But so far, this goal has not been achieved as McAuley et al. [76] demonstrated. Loads are mostly transmitted through the distal end of the metaphyseal bone, right where stem coating ends. Lack of loading on the proximal femur is a common problem to all anatomical stem designs [77–79]. The biomechanical finite element (FE) studies we have conducted on both ABG stems [49–51] support this assertion (Figure 9). The highest incidence of cancellous bone densification and cortical bone sclerosis detected in zones 2 and 6 of Gruen, in oversized stems, is explained by the tight fit of the implant into the medullary canal, which causes higher stresses in these zones (Figure 10).
Figure 9.
Maximum and minimum principal stress flow in the models with prosthesis (from a FE simulation).
Figure 10.
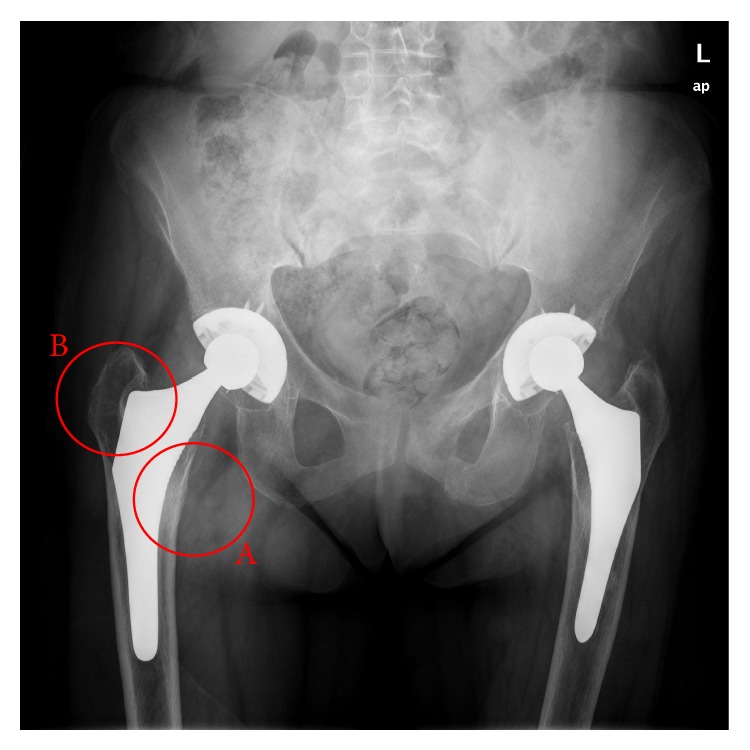
X-ray image of oversized stem in right femur with cancellous bone densification in support area (zone A) and bone resorption (zone B).
Correlation between the data obtained from finite element simulations and those obtained from DXA with ABG I at 10 years [47] and ABG II at 5 years [48] shows that the ABG-II stem is more effective than the ABG-I model, because the former generates higher tensional values on femoral bone, resulting in lesser bone loss (Figure 6). Thus, improved loads transmission matches biological findings obtained with DXA. We believe that the design and the alloy of stem have major importance in the transmission of loads in the femur. Changes in the lateral metaphyseal area and shoulder of the ABG II with a more trapezoidal (tapered) design have possibly contributed to improving the transmission, which is in accordance with the experience of Leali and Fetto with a lateral flare stem [80].
On the other hand, the lower length and volume of the ABG II stem allow us to better preserve the cancellous bone in the proximal femur which is an important factor for adaptive remodeling after implanting a femoral stem [67]. The decrease in volume of the metaphyseal area of stem ABG II has not affected the primary and secondary stability thereof, as shown by the values of mean subsidence in ABG II rods, which are 78.73% lower than the corresponding to the ABG I stem, confirming the effectiveness of design changes.
Adaptative remodeling and loads transmission influence the replacement of HA coating by new bone. Osseointegration rate is higher in heaviest loaded areas according to Wolf's law [26], which is supported by our findings: increased osseointegration in the metaphyseal-diaphyseal transition area and persistence of HA coating in the proximal metaphyseal area for more than 8 years after implantation of the stem [31].
A good preoperative planning [79] and meticulous surgical technique is needed in cementless hip arthroplasty to achieve a perfect press-fit of implants which provides an adequate primary fixation. Acetabular reaming must progress carefully down to bleeding subchondral bone, which is essential for secondary fixation, that is, osseointegration [81]. A similar technique should be used in femoral preparation. HA-coated implants require the same careful technique because HA coating does not solve technical errors. Critical analysis of our experience makes us understand that we have made incorrect indications in older patients with poor bone quality, who required oversized stems resulting in significant subsidence. Therefore, we reaffirm that appropriate indication and preoperative planning are essential requirements for good outcomes. Although design improvements in the ABG II stem have led to a decreased incidence of stress-shielding and subsidence, we have to bear in mind that bone mass index is critical to minimize stress-shielding, as Huiskes et al. [68] noted. The new Duration polyethylene has decreased wear by 54.55% compared to the conventional one, but alternative bearing surfaces should be considered in young patients with more demanding physical activity (ceramic-ceramic, in which we observed no measurable wear over an 11-year follow-up period).
Our HA-coated hip implants series has high long-term survival, in line with other author series [34, 59, 77, 82–96]. The excellent stability of HA-coated implants has been demonstrated even in elderly patients [97]. Other studies find no advantage in HA-coated implants over metallic-coated designs, mainly in the acetabular cups [98–104]. Some even believe that HA coating is a risk factor which contributes to loosening and is associated with poor long-term results in acetabular cups [105] in the HA coating stems, finding no advantage over metallic-coated implants [106]. We think it is risky attributing the mobilization of acetabular cups only to the possible delamination of the HA, and the consequent release of particles, when it coexists with excessive polyethylene wear in these cases, which we consider primarily responsible for osteolytic lesions. In our experience with the ABG I prosthesis, which presents an excessive polyethylene wear, 21 patients with 20-year follow-up needed major revision surgery for acetabular and/or femoral osteolytic lesions. The replacement of the acetabular cup and femoral stem was performed only in three patients; the rest had perfect stability of their implants, demonstrating the advantages of HA coating for reaching an excellent osseointegration. The data of Danish Hip Arthroplasty Registry show excellent medium-term survival of HA-coated and non-HA-coated implants [107] and the data of the Finnish Arthroplasty Register showed a better survival of HA-coated implants in young patients [108]; the Norwegian Arthroplasty Register showed that one brand of HA-coated stem had better survival than some non-HA-coated components [109]. Our wide and long-term experience in HA-coated hip implants and our outcome with excellent survival go against these assertions, in agreement with many authors [34, 59, 77, 82–96], but HA is not a magic powder [89] and the indication and surgical technique must be careful and correct in the HA-coated prostheses.
The survival of HA coated acetabular cups is better than of cemented cups especially in younger people which have a high percentage of long-term loosening, while the long-term survival of cemented stems reaches 85–95% according to papers published, depending on follow-up time, cementing techniques, and patient age [107–118]. Our personal experience with cemented hip prosthesis has similar survival rates. Survival of cementless HA-coated prostheses is superior in the acetabular components to the published results of cemented prostheses [59, 77, 83–91] and it is comparable in the femoral stems. Moreover if in the long term it is necessary to perform a replacement this will be technically easier in cementless prostheses because we will have more bone stock for future revision surgery, considering that today hip arthroplasties are implanted at very young patients with high functional demands [119–121] that possibly will need in the long term this type of surgery.
In conclusion, HA-coated hip implants are a reliable alternative, mainly in young people, which can achieve long-term survival provided that certain requirements are met: good design selection, sound choice of bearing surfaces based on patient life expectancy, meticulous surgical technique, and indications based on adequate bone quality.
Figure 3.
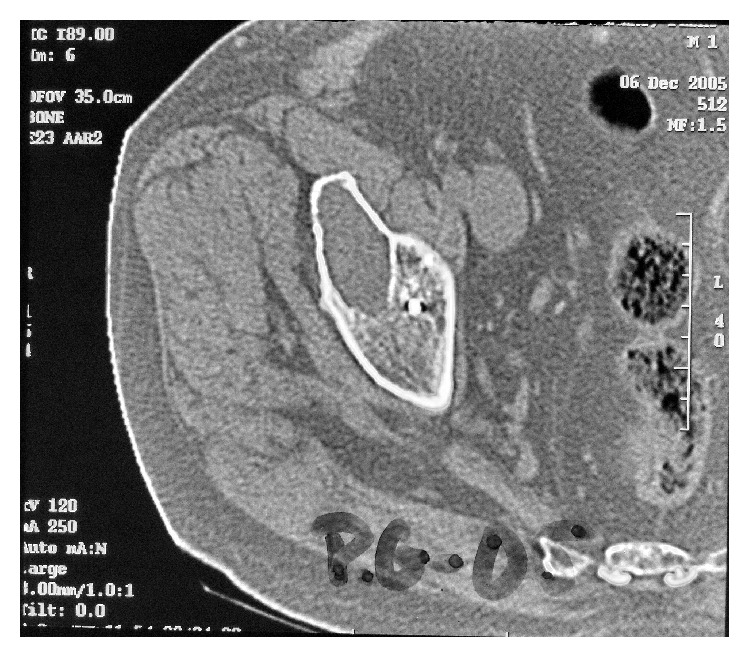
Computed tomography of the same case in Figure 2. Osteolysis in acetabulum.
Figure 4.
X-ray control of patient in Figures 2 and 3, after 4 y follow-up, after changing polyethylene and fulfilling with bone graft the acetabulum osteolysis. No change of original implant.
Abbreviations
- HA:
Hydroxyapatite
- ABG:
Anatomique benoist giraud
- DXA:
Dual-emission X-ray absorptiometry
- FE:
Finite elements.
Conflict of Interests
The authors declare that they have no conflict of interests.
Authors' Contribution
Antonio Herrera and Luis Gracia conceived the design of the study and coordinated the work between surgeons and engineers. Jesús Mateo, Jorge Gil-Albarova, and Antonio Lobo-Escolar performed the surgery and supervised the postoperative follow-up. Elena Ibarz, Sergio Gabarre, and Yolanda Más conceived and developed the finite element models and carried out all the simulations and results processing. All authors participated in the drawing up of the paper and read and approved the final paper.
References
- 1.Charnley J. Arthroplasty of the hip. A new operation. The Lancet. 1961;277(7187):1129–1132. doi: 10.1016/s0140-6736(61)92063-3. [DOI] [PubMed] [Google Scholar]
- 2.Harris W. H. The first 50 years of total hip arthroplasty: lessons learned. Clinical Orthopaedics and Related Research. 2009;467(1):28–31. doi: 10.1007/s11999-008-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engh C. A., Bobyn J. D., Glassman A. H. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. Journal of Bone and Joint Surgery—Series B. 1987;69(1):45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 4.Pilliar R. M., Lee J. M., Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clinical Orthopaedics and Related Research. 1986;(208):108–113. [PubMed] [Google Scholar]
- 5.Kienapfel H., Sprey C., Wilke A., Griss P. Implant fixation by bone ingrowth. The Journal of Arthroplasty. 1999;14(3):355–368. doi: 10.1016/s0883-5403(99)90063-3. [DOI] [PubMed] [Google Scholar]
- 6.Jasty M., Bragdon C., Burke D., O'Connor D., Lowenstein J., Harris W. H. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. The Journal of Bone and Joint Surgery—Series A. 1997;79(5):707–714. doi: 10.2106/00004623-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Udofia I. J., Liu F., Jin Z., Roberts P., Grigoris P. The initial stability and contact mechanics of a press-fit resurfacing arthroplasty of the hip. The Journal of Bone and Joint Surgery—Series B. 2007;89(4):549–556. doi: 10.1302/0301-620x.89b4.18055. [DOI] [PubMed] [Google Scholar]
- 8.Cameron H. U., Macnab I., Pilliar R. M. A porous metal system for joint replacement surgery. The International Journal of Artificial Organs. 1978;1(2):104–109. [PubMed] [Google Scholar]
- 9.Judet R., Siguier M., Brumpt B., Judet T. Prothese totale de hanche en poro-metal sans ciment. Revue de Chirurgie Orthopédique et Réparatrice de l'Appareil Moteur. 1978;64(supplement 2):14–21. [PubMed] [Google Scholar]
- 10.Lord G., Marotte J. H., Blanchard J. P., Guillamon J. L., Gory M. The fixation of madreporic total hip prosthesis; an experimental study (author's transl) Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur. 1978;64(6):459–470. [PubMed] [Google Scholar]
- 11.Pilliar R. M., Cameron H. U., Welsh R. P., Binnington A. G. Radiographic and morphologic studies of load-bearing porous-surfaced structured implants. Clinical Orthopaedics and Related Research. 1981;156:249–257. [PubMed] [Google Scholar]
- 12.Engh C. A. Hip arthroplasty with a Moore prosthesis with porous coating. A five-year study. Clinical Orthopaedics and Related Research. 1983;(176):52–66. [PubMed] [Google Scholar]
- 13.Pilliar R. M. Porous-surfaced metallic implants for orthopedic applications. Journal of Biomedical Materials Research. 1987;21(supplement A1):1–33. [PubMed] [Google Scholar]
- 14.de Groot K., Geesink R., Klein C. P. A. T., Serekian P. Plasma sprayed coatings of hydroxylapatite. Journal of Biomedical Materials Research. 1987;21(12):1375–1381. doi: 10.1002/jbm.820211203. [DOI] [PubMed] [Google Scholar]
- 15.Geesink R. G. T., de Groot K., Klein C. P. A. T. Chemical implant fixation using hydroxyl-apatite coatings. The development of a human total hip prosthesis for chemical fixation to bone using hydroxyl-apatite coatings on titanium substrates. Clinical Orthopaedics and Related Research. 1987;(225):147–170. [PubMed] [Google Scholar]
- 16.Furlong R. J., Osborn J. F. Fixation of hip prostheses by hydroxyapatite ceramic coatings. The Journal of Bone and Joint Surgery—Series B. 1991;73(5):741–745. doi: 10.1302/0301-620X.73B5.1654336. [DOI] [PubMed] [Google Scholar]
- 17.Manley M. T., Koch R. Clinical results with the hydroxyapatite-coated Omnifit hip stem. Dental Clinics of North America. 1992;36(1):257–262. [PubMed] [Google Scholar]
- 18.Epinette J.-A., Manley M. T., D'Antonio J. A., Edidin A. A., Capello W. N. A 10-year minimum follow-up of hydroxyapatite-coated threaded cups: clinical, radiographic and survivorship analyses with comparison to the literature. The Journal of Arthroplasty. 2003;18(2):140–148. doi: 10.1054/arth.2003.50039. [DOI] [PubMed] [Google Scholar]
- 19.Passuti N. New concepts in bone substitution. In: Fulford P., Horan F., Jakob R. P., editors. European Instructinal Course Lectures. Vol. 4. London, UK: The British Editorial Society of Bobe and Joint Surgery; 1999. [Google Scholar]
- 20.Hoogendoorn H. A., Renooij W., Akkermans L. M. A. Long-term study of large ceramic implants (porous hydroxyapatite) in dog femora. Clinical Orthopaedics and Related Research. 1984;187:281–288. [PubMed] [Google Scholar]
- 21.Geesink R. G. T., De Groot K., Klein C. P. A. T. Bonding of bone to apatite-coated implants. The Journal of Bone and Joint Surgery—Series B. 1988;70(1):17–22. doi: 10.1302/0301-620X.70B1.2828374. [DOI] [PubMed] [Google Scholar]
- 22.Daugaard H., Elmengaard B., Bechtold J. E., Jensen T., Soballe K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. Journal of Biomedical Materials Research Part A. 2010;92(3):913–921. doi: 10.1002/jbm.a.32303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overgaard S., Bromose U., Lind M., Bünger C., Søballe K. The influence of crystallinity of the hydroxyapatite coating on the fixation of implants. Mechanical and histomorphometric results. The Journal of Bone and Joint Surgery Series B. 1999;81(4):725–731. doi: 10.1302/0301-620x.81b4.9282. [DOI] [PubMed] [Google Scholar]
- 24.Røkkum M., Reigstad A., Johansson C. B. HA particles can be released from well-fixed HA-coated stems: histopathology of biopsies from 20 hips 2–8 years after implantation. Acta Orthopaedica Scandinavica. 2002;73(3):298–306. doi: 10.1080/000164702320155293. [DOI] [PubMed] [Google Scholar]
- 25.Rahbet O. O. S., Soballe K. Fifteen Years of Clinical Experience with Hydroxyapatite Coating in Joint Arthroplasty. Paris, France: Springer; 2004. Calcium phosphate coating for implants fixation; pp. 35–52. [Google Scholar]
- 26.Bauer T. W., Geesink R. C. T., Zimmerman R., McMahon J. T. Hydroxyapatite-coated femoral stems. Histological analysis of components retrieved at autopsy. The Journal of Bone and Joint Surgery—Series A. 1991;73(10):1439–1452. [PubMed] [Google Scholar]
- 27.Overgaard S., Søballe K., Josephsen K., Hansen E. S., Bunger C. Role of different loading conditions on resorption of hydroxyapatite coating evaluated by histomorphometric and stereological methods. Journal of Orthopaedic Research. 1996;14(6):888–894. doi: 10.1002/jor.1100140607. [DOI] [PubMed] [Google Scholar]
- 28.Overgaard S., Lind M., Rahbek O., Bünger C., Søballe K. Improved fixation of porous-coated versus grit-blasted surface texture of hydroxyapatite-coated implants in dogs. Acta Orthopaedica Scandinavica. 1997;68(4):337–343. doi: 10.3109/17453679708996173. [DOI] [PubMed] [Google Scholar]
- 29.Hardy D. C. R., Frayssinet P., Krallis P., et al. Histopathology of a well-functioning hydroxyapatite-coated femoral prosthesis after 52 months. Acta Orthopaedica Belgica. 1999;65(1):72–82. [PubMed] [Google Scholar]
- 30.Tonino A. J., Therin M., Doyle C. Hydroxyapatite-coated femoral stems. Histology and histomorphometry around five components retrieved at post mortem. Journal of Bone and Joint Surgery. 1999;81(1):148–154. doi: 10.1302/0301-620x.81b1.8948. [DOI] [PubMed] [Google Scholar]
- 31.Tonino A. J., van der Wal B. C. H., Heyligers I. C., Grimm B. Bone remodeling and hydroxyapatite resorption in coated primary hip prostheses. Clinical Orthopaedics and Related Research. 2009;467(2):478–484. doi: 10.1007/s11999-008-0559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tisdel C. L., Goldberg V. M., Parr J. A., Bensusan J. S., Staikoff L. S., Stevenson S. The influence of a hydroxyapatite and tricalcium-phosphate coating on bone growth into titanium fiber-metal implants. Journal of Bone and Joint Surgery—Series A. 1994;76(2):159–171. doi: 10.2106/00004623-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Tonino A., Oosterbos C., Rahmy A., Thèrin M., Doyle C. Hydroxyapatite-coated acetabular components. Histological and histomorphometric analysis of six cups retrieved at autopsy between three and seven years after successful implantation. The Journal of Bone and Joint Surgery—Series A. 2001;83(6):817–825. [PubMed] [Google Scholar]
- 34.Chambers B., St. Clair S. F., Froimson M. I. Hydroxyapatite-coated tapered cementless femoral components in total hip arthroplasty. Journal of Arthroplasty. 2007;22(4, supplement 1):71–74. doi: 10.1016/j.arth.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Herrera A., Canales V., Anderson J., García-Araujo C., Murcia-Mazón A., Tonino A. J. Seven to 10 years followup of an anatomic hip prosthesis: an international study. Clinical Orthopaedics and Related Research. 2004;(423):129–137. doi: 10.1097/01.blo.0000128973.73132.0b. [DOI] [PubMed] [Google Scholar]
- 36.Canales V., Panisello J. J., Herrera A., Sola A., Mateo J. J., Caballero M. J. Extensive osteolysis caused by polyethylene particle migration in an anatomical hydroxyapatite-coated hip prosthesis: 10 years' follow-up. Journal of Arthroplasty. 2010;25(7):1115.e1–1124.e1. doi: 10.1016/j.arth.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 37.D'Aubigne R. M., Postel M. Function al results of hip arthroplasty with acrylic prosthesis. The Journal of Bone and Joint Surgery. American volume. 1954;36(3):451–475. [PubMed] [Google Scholar]
- 38.Singh M., Nagrath A. R., Maini P. S. Changes in trabecular pattern of the upper end of the femur as an index of osteoporosis. The Journal of Bone and Joint Surgery Series A. 1970;52(3):457–467. [PubMed] [Google Scholar]
- 39.Gruen T. A., McNeice G. M., Amstutz H. C. 'Modes of failure' of cemented stem-type femoral components. A radiographic analysis of loosening. Clinical Orthopaedics and Related Research. 1979;141:17–27. [PubMed] [Google Scholar]
- 40.DeLee J. G., Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clinical Orthopaedics and Related Research. 1976;121:20–32. [PubMed] [Google Scholar]
- 41.Brooker A. F., Bowerman J. W., Robinson R. A., Riley L. H., Jr. Ectopic ossification following total hip replacement. Incidence and a method of classification. The Journal of Bone and Joint Surgery. American Volume. 1973;55(8):1629–1632. [PubMed] [Google Scholar]
- 42.Livermore J., Ilstrup D., Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. The Journal of Bone and Joint Surgery—Series A. 1990;72(4):518–528. [PubMed] [Google Scholar]
- 43.Herrera A., Mateo J., Lobo-Escolar A., Panisello J. J., Ibarz E., Gracia L. Long-term outcomes of a new model of anatomical hydroxyapatite-coated hip prosthesis. The Journal of Arthroplasty. 2013;28(7):1160–1166. doi: 10.1016/j.arth.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 44.Harris W. H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. The Journal of Bone and Joint Surgery American Volume. 1969;51(4):737–755. [PubMed] [Google Scholar]
- 45.Szende A., Williams A., editors. Measuring Self-Reported Population Health: An International Perspective based on EQ-5D. The EuroQol Group's International Task Force on Self-Reported Health; 2004. [Google Scholar]
- 46.Goetz D. D., Smith E. J., Harris W. H. The prevalence of femoral osteolysis associated with components inserted with or without cement in total hip replacements. A retrospective matched- pair series. The Journal of Bone and Joint Surgery—Series A. 1994;76(8):1121–1129. doi: 10.2106/00004623-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Panisello J. J., Herrero L., Canales V., Herrera A., Martínez A. A., Mateo J. Long-term remodeling in proximal femur around a hydroxyapatite-coated anatomic stem: ten years densitometric follow-up. The Journal of Arthroplasty. 2009;24(1):56–64. doi: 10.1016/j.arth.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Panisello J. J., Canales V., Herrero L., Herrera A., Mateo J., Caballero M. J. Changes in periprosthetic bone remodelling after redesigning an anatomic cementless stem. International Orthopaedics. 2009;33(2):373–379. doi: 10.1007/s00264-007-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrera A., Panisello J. J., Ibarz E., Cegoñino J., Puértolas J. A., Gracia L. Long-term study of bone remodelling after femoral stem: a comparison between dexa and finite element simulation. Journal of Biomechanics. 2007;40(16):3615–3625. doi: 10.1016/j.jbiomech.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Herrera A., Panisello J. J., Ibarz E., Cegoñino J., Puértolas J. A., Gracia L. Comparison between DEXA and finite element studies in the long-term bone remodeling of an anatomical femoral stem. Journal of Biomechanical Engineering. 2009;131(4) doi: 10.1115/1.3072888.041013 [DOI] [PubMed] [Google Scholar]
- 51.Gracia L., Ibarz E., Puértolas S., et al. Study of bone remodeling of two models of femoral cementless stems by means of DEXA and finite elements. BioMedical Engineering Online. 2010;9, article 22 doi: 10.1186/1475-925x-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geesink R. D. Fixation strategies in total hip arthroplasty. Surgical Technology International. 2011;21:240–247. [PubMed] [Google Scholar]
- 53.Soballe K. Hydroxyapatite ceramic coating for bone implant fixation: mechanical anf histological studies in dogs. Acta Orthopaedica Scandinavica, Supplementum. 1993;64(255):1–58. doi: 10.3109/17453679309155636. [DOI] [PubMed] [Google Scholar]
- 54.Karrholm J., Malchau H., Snorrason F., Herberts P. Micromotion of femoral stems in total hip arthroplasty. A randomized study of cemented, hydroxyapatite-coated and porous-coated stems with roentgen stereophotogrammetric analysis. The Journal of Bone and Joint Surgery—Series A. 1994;76(11):1692–1705. doi: 10.2106/00004623-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Coathup M. J., Blunn G. W., Flynn N., Williams C., Thomas N. P. A comparison of bone remodelling around hydroxyapatite-coated, porous-coated and grit-blasted hip replacements retrieved at post-mortem. The Journal of Bone and Joint Surgery Series B. 2001;83(1):118–123. doi: 10.1302/0301-620x.83b1.10062. [DOI] [PubMed] [Google Scholar]
- 56.Geesink R. G. T. Osteoconductive coatings for total joint arthroplasty. Clinical Orthopaedics and Related Research. 2002;(395):53–65. doi: 10.1097/00003086-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Aebli N., Krebs J., Schwenke D., Stich H., Schwalder P., Theis J.-C. Degradation of hydroxyapatite coating on a well-functioning femoral component. The Journal of Bone and Joint Surgery British Volume. 2003;85(4):499–503. doi: 10.1302/0301-620x.85b4.13605. [DOI] [PubMed] [Google Scholar]
- 58.Coathup M. J., Blackburn J., Goodship A. E., Cunningham J. L., Smith T., Blunn G. W. Role of hydroxyapatite coating in resisting wear particle migration and osteolysis around acetabular components. Biomaterials. 2005;26(19):4161–4169. doi: 10.1016/j.biomaterials.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Emans P. J., Broeke R. H., van Mulken J. M., Kuijer R., van Rhijn L. W., Geesink R. G. Results of total hip arthroplasties in the young patient; further evidence for a barrier against articular wear debris by hydroxyapatite coatings. Hip International. 2009;19(4):343–351. doi: 10.1177/112070000901900408. [DOI] [PubMed] [Google Scholar]
- 60.Rybicki E. F., Simonen F. A., Weis E. B., Jr. On the mathematical analysis of stress in the human femur. Journal of Biomechanics. 1972;5(2):203–215. doi: 10.1016/0021-9290(72)90056-5. [DOI] [PubMed] [Google Scholar]
- 61.Domínguez-Hernández V. M., Carbajal M. F., Urriolagoitia G., et al. Biomecánica de un fémur sometido a carga. Desarrollo de un modelo tridimensional por medio del método del elemento finito. Revista Mexicana de Ortopaedica Traumatologie. 1999;13(6):633–638. [Google Scholar]
- 62.Weinans H., Huiskes R., Grootenboer H. J. Effects of fit and bonding characteristics of femoral stems on adaptive bone remodeling. Journal of Biomechanical Engineering. 1994;116(4):393–400. doi: 10.1115/1.2895789. [DOI] [PubMed] [Google Scholar]
- 63.van Rietbergen B., Huiskes R. Load transfer and stress shielding of the hydroxyapatite-ABG hip: a study of stem length and proximal fixation. Journal of Arthroplasty. 2001;16(8) supplement 1:55–63. doi: 10.1054/arth.2001.28369. [DOI] [PubMed] [Google Scholar]
- 64.Kerner J., Huiskes R., van Lenthe G. H., et al. Correlation between pre-operative periprosthetic bone density and post-operative bone loss in THA can be explained by strain-adaptive remodelling. Journal of Biomechanics. 1999;32(7):695–703. doi: 10.1016/s0021-9290(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 65.Domínguez-Hernández V. M., Ramos V. H., Feria C. V., Urriolagoitia G., Hernández L. H. Efecto del espesor de la capa de cemento en el componente femoral de una prótesis de Charnley. Anaílisis biomecánico mediante el método del elemento finito. Revista Mexicana de Ortopaedica Traumatologie. 2000;14(6):443–448. [Google Scholar]
- 66.Bergmann G. E. A. Orthoload data base. Loading of Orthopaedic Implants, 2013, http://www.orthoload.com/
- 67.Herrera A., Rebollo S., Ibarz E., Mateo J., Gabarre S., Gracia L. Mid-Term study of bone remodeling after femoral cemented stem implantation: comparison between DXA and finite element simulation. The Journal of Arthroplasty. 2014;29(1):90–100. doi: 10.1016/j.arth.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 68.Huiskes R., Weinans H., Dalstra M. Adaptive bone remodeling and biomechanical design considerations for noncemented total hip arthroplasty. Orthopedics. 1989;12(9):1255–1267. doi: 10.3928/0147-7447-19890901-15. [DOI] [PubMed] [Google Scholar]
- 69.Bobyn J. D., Mortimer E. S., Glassman A. H., Engh C. A., Miller J. E., Brooks C. E. Producing and avoiding stress shielding: laboratory and clinical observations of noncemented total hip arthroplasty. Clinical Orthopaedics and Related Research. 1992;(274):79–96. [PubMed] [Google Scholar]
- 70.Huiskes R., van Rietbergen B. Preclinical testing of total hip stems. The effects of coating placement. Clinical Orthopaedics and Related Research. 1995;(319):64–76. [PubMed] [Google Scholar]
- 71.D'Antonio J. A., Capello W. N., Manley M. T. Remodeling of bone around hydroxyapatite-coated femoral stems. Journal of Bone and Joint Surgery—Series A. 1996;78(8):1226–1234. doi: 10.2106/00004623-199608000-00013. [DOI] [PubMed] [Google Scholar]
- 72.Rosenthall L., Bobyn J. D., Tanzer M. Bone densitometry: influence of prosthetic design and hydroxyapatite coating on regional adaptive bone remodelling. International Orthopaedics. 1999;23(6):325–329. doi: 10.1007/s002640050383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sychterz C. J., Claus A. M., Engh C. A., Wolf R. What we have learned about long-term cementless fixation from autopsy retrievals. Clinical Orthopaedics and Related Research. 2002;(405):79–91. doi: 10.1097/00003086-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Rahmy A. I. A., Gosens T., Blake G. M., Tonino A., Fogelman I. Periprosthetic bone remodelling of two types of uncemented femoral implant with proximal hydroxyapatite coating: a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss. Osteoporosis International. 2004;15(4):281–289. doi: 10.1007/s00198-003-1546-5. [DOI] [PubMed] [Google Scholar]
- 75.Chandran P., Azzabi M., Andrews M., Bradley J. G. Periprosthetic bone remodeling after 12 years differs in cemented and uncemented hip arthroplasties. Clinical Orthopaedics and Related Research. 2012;470(5):1431–1435. doi: 10.1007/s11999-011-2134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McAuley J. P., Sychterz C. J., Engh C. A., Sr. Influence of porous coating level on proximal femoral remodeling: a postmortem analysis. Clinical Orthopaedics and Related Research. 2000;(371):146–153. doi: 10.1097/00003086-200002000-00018. [DOI] [PubMed] [Google Scholar]
- 77.Oosterbos C. J. M., Rahmy A. I. A., Tonino A. J., Witpeerd W. High survival rate of hydroxyapatite-coated hip prostheses: 100 consecutive hips followed for 10 years. Acta Orthopaedica Scandinavica. 2004;75(2):127–133. doi: 10.1080/00016470412331294365. [DOI] [PubMed] [Google Scholar]
- 78.Capello W. N., D'Antonio J. A., Geesink R. G., Feinberg J. R., Naughton M. Late remodeling around a proximally ha-coated tapered titanium femoral component. Clinical Orthopaedics and Related Research. 2009;467(1):155–165. doi: 10.1007/s11999-008-0550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Della Valle A. G., Padgett D. E., Salvati E. A. Preoperative planning for primary total hip arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2005;13(7):455–462. doi: 10.5435/00124635-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Leali A., Fetto J. F. Preservation of femoral bone mass after total hip replacements with a lateral flare stem. International Orthopaedics. 2004;28(3):151–154. doi: 10.1007/s00264-004-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morscher E. W. Zementfrei pfannenverankerung nach dem ‘Press-Fit-Konzept’ bei der Totalprothesen-Arthroplastik der Hufte. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca. 2002;69(1):8–15. [PubMed] [Google Scholar]
- 82.Reikerås O., Gunderson R. B. Excellent results of HA coating on a grit-blasted stem: 245 patients followed for 8–12 years. Acta Orthopaedica Scandinavica. 2003;74(2):140–145. doi: 10.1080/00016470310013851. [DOI] [PubMed] [Google Scholar]
- 83.Capello W. N., D'Antonio J. A., Jaffe W. L., Geesink R. G., Manley M. T., Feinberg J. R. Hydroxyapatite-coated femoral components: 15-year minimum followup. Clinical Orthopaedics and Related Research. 2006;(453):75–80. doi: 10.1097/01.blo.0000246534.44629.b2. [DOI] [PubMed] [Google Scholar]
- 84.Rajaratnam S. S., Jack C., Tavakkolizadeh A., et al. Long-term results of a hydroxyapatite-coated femoral component in total hip replacement: a 15- to 21-year follow-up study. The Journal of Bone and Joint Surgery— Series B. 2008;90(1):27–30. doi: 10.1302/0301-620x.90b1.19731. [DOI] [PubMed] [Google Scholar]
- 85.Epinette J.-A., Manley M. T. Uncemented stems in hip replacement—hydroxyapatite or plain porous: does it matter? Based on a prospective study of HA Omnifit stems at 15-years minimum follow-up. HIP International. 2008;18(2):69–74. doi: 10.1177/112070000801800201. [DOI] [PubMed] [Google Scholar]
- 86.Camazzola D., Hammond T., Gandhi R., Davey J. R. A randomized trial of hydroxyapatite-coated femoral stems in total hip arthroplasty: a 13-year follow-up. Journal of Arthroplasty. 2009;24(1):33–37. doi: 10.1016/j.arth.2008.01.129. [DOI] [PubMed] [Google Scholar]
- 87.Bidar R., Kouyoumdjian P., Munini E., Asencio G. Long-term results of the ABG-1 hydroxyapatite coated total hip arthroplasty: analysis of 111 cases with a minimum follow-up of 10 years. Orthopaedics and Traumatology: Surgery and Research. 2009;95(8):579–587. doi: 10.1016/j.otsr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Baker P. N., McMurtry I. A., Chuter G., Port A., Anderson J. Tha with the abg i prosthesis at 15 years: excellent survival with minimal osteolysis. Clinical Orthopaedics and Related Research. 2010;468(7):1855–1861. doi: 10.1007/s11999-009-1066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vidalain J.-P. Twenty-year results of the cementless Corail stem. International Orthopaedics. 2011;35(2):189–194. doi: 10.1007/s00264-010-1117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sariali E., Mouttet A., Mordasini P., Catonné Y. High 10-year survival rate with an anatomic cementless stem (SPS) Clinical Orthopaedics and Related Research. 2012;470(7):1941–1949. doi: 10.1007/s11999-012-2300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Epinette J. A., Asencio G., Essig J., Llagonne B., Nourissat C. Clinical results, radiological findings and survival of a proximally hydroxyapatite-coated hip ABG II stem at a minimum of ten years' follow-up: results of a consecutive multicentre study of 1148 hips in 1053 patients. The Bone & Joint Journal B. 2013;95(12):1610–1616. doi: 10.1302/0301-620x.95b12.31167. [DOI] [PubMed] [Google Scholar]
- 92.Gabbar O. A., Rajan R. A., Londhe S., Hyde I. D. Ten- to twelve-year follow-up of the furlong hydroxyapatite-coated femoral stem and threaded acetabular cup in patients younger than 65 years. The Journal of Arthroplasty. 2008;23(3):413–417. doi: 10.1016/j.arth.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 93.Wangen H., Lereim P., Holm I., Gunderson R., Reikerås O. Hip arthroplasty in patients younger than 30 years: excellent ten to 16-year follow-up results with a HA-coated stem. International Orthopaedics. 2008;32(2):203–208. doi: 10.1007/s00264-006-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flecher X., Pearce O., Parratte S., Aubaniac J. M., Argenson J. N. Custom cementless stem improves hip function in young patients at 15-year followup. Clinical Orthopaedics and Related Research. 2010;468(3):747–755. doi: 10.1007/s11999-009-1045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gottliebsen M., Rahbek O., Ottosen P. F., Søballe K., Stilling M. Superior 11-year survival but higher polyethylene wear of hydroxyapatite-coated Mallory-Head cups. HIP International. 2012;22(1):35–40. doi: 10.5301/hip.2012.9075. [DOI] [PubMed] [Google Scholar]
- 96.Nakashima Y., Sato T., Yamamoto T., et al. Results at a minimum of 10 years of follow-up for AMS and PerFix HA-coated cementless total hip arthroplasty: impact of cross-linked polyethylene on implant longevity. Journal of Orthopaedic Science. 2013;18(6):962–968. doi: 10.1007/s00776-013-0456-4. [DOI] [PubMed] [Google Scholar]
- 97.Ulivi M., Meroni V., Orlandini L. C., Berjano P., Sansone V. C. Minimum 10 year survivorship analysis of a partially coated hydroxyapatite tapered femoral stem in elderly patients with an average age over 75. The Journal of Arthroplasty. 2013;28(8):1372–1377. doi: 10.1016/j.arth.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 98.Park Y.-S., Lee J.-Y., Yun S.-H., Jung M.-W., Oh I. Comparison of hydroxyapatite- and porous-coated stems in total hip replacement. Acta Orthopaedica Scandinavica. 2003;74(3):259–263. doi: 10.1080/00016470310014166. [DOI] [PubMed] [Google Scholar]
- 99.Lombardi A. V., Jr., Berend K. R., Mallory T. H. Hydroxyapatite-coated titanium porous plasma spray tapered stem: experience at 15 to 18 years. Clinical Orthopaedics and Related Research. 2006;(453):81–85. doi: 10.1097/01.blo.0000238872.01767.09. [DOI] [PubMed] [Google Scholar]
- 100.Yoon K. S., Kim H. J., Lee J. H., Kang S. B., Seong N. H., Koo K.-H. A randomized clinical trial of cementless femoral stems with and without hydroxyapatite/tricalcium-phosphate coating: an 8- to 12-year follow-up study. The Journal of Arthroplasty. 2007;22(4):504–508. doi: 10.1016/j.arth.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 101.Lee J.-M., Lee C.-W. Comparison of hydroxyapatite-coated and non-hydroxyapatite-coated noncemented total hip arthroplasty in same patients. The Journal of Arthroplasty. 2007;22(7):1019–1023. doi: 10.1016/j.arth.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 102.Valancius K., Soballe K., Nielsen P. T., Laursen M. B. No superior performance of hydroxyapatite-coated acetabular cups over porous-coated cups. Acta Orthopaedica. 2013;84(6):544–548. doi: 10.3109/17453674.2013.854665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gandhi R., Davey J. R., Mahomed N. N. Hydroxyapatite coated femoral stems in primary total hip arthroplasty: a meta-analysis. The Journal of Arthroplasty. 2009;24(1):38–42. doi: 10.1016/j.arth.2008.01.299. [DOI] [PubMed] [Google Scholar]
- 104.Li S., Huang B., Chen Y., et al. Hydroxyapatite-coated femoral stems in primary total hip arthroplasty: a meta-analysis of randomized controlled trials. International Journal of Surgery. 2013;11(6):477–482. doi: 10.1016/j.ijsu.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 105.Lazarinis S., Krärholm J., Hailer N. P. Increased risk of revision of acetabular cups coated with hydroxyapatite: a Swedish Hip Arthroplasty Register study involving 8,043 total hip replacements. Acta Orthopaedica. 2010;81(1):53–59. doi: 10.3109/17453670903413178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lazarinis S., Krrholm J., Hailer N. P. Effects of hydroxyapatite coating on survival of an uncemented femoral stem. A Swedish Hip Arthroplasty Register study on 4,772 hips. Acta Orthopaedica. 2011;82(4):399–404. doi: 10.3109/17453674.2011.597699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paulsen A., Pedersen A. B., Johnsen S. P., Riis A., Lucht U., Overgaard S. Effect of hydroxyapatite coating on risk of revision after primary total hip arthroplasty in younger patients: findings from the Danish Hip Arthroplasty Registry. Acta Orthopaedica. 2007;78(5):622–628. doi: 10.1080/17453670710014310. [DOI] [PubMed] [Google Scholar]
- 108.Eskelinen A., Remes V., Helenius I., Pulkkinen P., Nevalainen J., Paavolainen P. Uncemented total hip arthroplasty for primary osteoarthritis in young patients: a mid- to long-term follow-up study from the Finnish Arthroplasty Register. Acta Orthopaedica. 2006;77(1):57–70. doi: 10.1080/17453670610045704. [DOI] [PubMed] [Google Scholar]
- 109.Havelin L. I., Engesæter L. B., Espehaug B., Furnes O., Lie S. A., Vollset S. E. The Norwegian arthroplasty register: 11 years and 73,000 arthroplasties. Acta Orthopaedica Scandinavica. 2000;71(4):337–353. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- 110.Madey S. M., Callaghan J. J., Olejniczak J. P., Goetz D. D., Johnston R. C. Charnley total hip arthroplasty with use of improved techniques of cementing. The results after a minimum of fifteen years of follow-up. Journal of Bone and Joint Surgery. American. 1997;79(1):53–64. doi: 10.1302/0301-620x.79b1.6699. [DOI] [PubMed] [Google Scholar]
- 111.Callaghan J. J., Albright J. C., Goetz D. D., Olejniczak J. P., Johnston R. C. Charnley total hip arthroplasty with cement: minimum twenty-five-year follow-up. The Journal of Bone & Joint Surgery A. 2000;82(4):487–497. doi: 10.2106/00004623-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Berry D. J., Harmsen W. S., Cabanela M. E., Morrey B. F. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: Factors affecting survivorship of acetabular and femoral components. Journal of Bone and Joint Surgery - Series A. 2002;84(2):171–177. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 113.Skutek M., Bourne R. B., Rorabeck C. H., Burns A., Kearns S., Krishna G. The twenty to twenty-five-year outcomes of the Harris design-2 matte-finished cemented total hip replacement: a concise follow-up of a previous report. Journal of Bone and Joint Surgery—Series A. 2007;89(4):814–818. doi: 10.2106/jbjs.f.00837. [DOI] [PubMed] [Google Scholar]
- 114.Callaghan J. J., Bracha P., Liu S. S., Piyaworakhun S., Goetz D. D., Johnston R. C. Survivorship of a Charnley total hip arthroplasty: a concise follow-up, at a minimum of thirty-five years, of previous reports. Journal of Bone and Joint Surgery—Series A. 2009;91(11):2617–2621. doi: 10.2106/jbjs.h.01201. [DOI] [PubMed] [Google Scholar]
- 115.de Kam D. C. J., Gardeniers J. W. M., Veth R. P. H., Schreurs B. W. Good results with cemented total hip arthroplasty in patients between 40 and 50 years of age. Acta Orthopaedica. 2010;81(2):165–170. doi: 10.3109/17453671003717831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caton J., Prudhon J. L. Over 25 years survival after Charnley's total hip arthroplasty. International Orthopaedics. 2011;35(2):185–188. doi: 10.1007/s00264-010-1197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pakvis D., van Hellemondt G., de Visser E., Jacobs W., Spruit M. Is there evidence for a superior method of socket fixation in hip arthroplasty? A systematic review. International Orthopaedics. 2011;35(8):1109–1118. doi: 10.1007/s00264-011-1234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmitz M. W. J. L., Busch V. J., Gardeniers J. W., Hendriks J. C., Veth R. P., Schreurs B. W. Long-term results of cemented total hip arthroplasty in patients younger than 30 years and the outcome of subsequent revisions. BMC Musculoskeletal Disorders. 2013;14, article 37 doi: 10.1186/1471-2474-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lieberman J. R. Two alternative bearings for total hip arthroplasty: more data are needed. Journal of the American Academy of Orthopaedic Surgeons. 2009;17(2):61–62. doi: 10.5435/00124635-200902000-00001. [DOI] [PubMed] [Google Scholar]
- 120.Huch K., Müller K. A. C., Stürmer T., Brenner H., Puhl W., Günther K.-P. Sports activities 5 years after total knee or hip arthroplasty: the Ulm osteoarthritis study. Annals of the Rheumatic Diseases. 2005;64(12):1715–1720. doi: 10.1136/ard.2004.033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chatterji U., Ashworth M. J., Lewis P. L., Dobson P. J. Effect of total hip arthroplasty on recreational and sporting activity. ANZ Journal of Surgery. 2004;74(6):446–449. doi: 10.1111/j.1445-1433.2004.03028.x. [DOI] [PubMed] [Google Scholar]