Significance
The frontopolar cortex (FPC) is a large region occupying the anterior portion of the brain’s frontal lobe, and has been suggested to play a role in complex, higher order behavior. However, the specific contributions of this area toward this type of behavior are still unclear. Using an animal model, we show that localized lesions to the FPC of nonhuman primates selectively impair the ability of the animal to learn rapidly about novel objects and rules, although sparing its ability to draw upon previously established knowledge about objects and rules. These findings suggest that the FPC makes a crucial contribution to the exploration and rapid acquisition of novel behavioral options, which is, in turn, an essential aspect of complex, higher order behavior.
Keywords: frontopolar cortex, prefrontal cortex, primate, learning, neuropsychology
Abstract
Brodmann’s area 10 is one of the largest cytoarchitecturally defined regions in the human cerebral cortex, occupying the most anterior part of the prefrontal cortex [frontopolar cortex (FPC)], and is believed to sit atop a prefrontal hierarchy. The crucial contributions that the FPC makes to cognition are unknown. Rodents do not possess such a FPC, but primates do, and we report here the behavioral effects of circumscribed FPC lesions in nonhuman primates. FPC lesions selectively impaired rapid one-trial learning about unfamiliar objects and unfamiliar objects-in-scenes, and also impaired rapid learning about novel abstract rules. Object recognition memory, shifting between established abstract behavioral rules, and the simultaneous application of two distinct rules were unaffected by the FPC lesion. The distinctive pattern of impaired and spared performance across these seven behavioral tasks reveals that the FPC mediates exploration and rapid learning about the relative value of novel behavioral options, and shows that the crucial contributions made by the FPC to cognition differ markedly from the contributions of other primate prefrontal regions.
Granular prefrontal cortex (gPFC) is unique to anthropoid primates (1), and is believed to underlie the ability to construct novel, complex, structured sequences of intelligent, goal-directed behavior (2). Although the frontopolar cortex (FPC), the most rostral gPFC region, is particularly well developed in hominoids and in humans (3), it is also a substantial cortical structure in monkeys. In both macaques and humans, the lateral, medial, and ventral aspects of the FPC are typically occupied by “area 10” (4–9). FPC connections are also broadly similar across primate species (6, 10–13). The similarity in cytoarchitecture and connections is highly suggestive of some conservation of FPC function across primate species.
Anatomical connections suggest that the FPC sits atop a gPFC hierarchy, yet we do not know what crucial contribution(s) the FPC makes to cognition or how this contribution(s) differs from other gPFC regions. FPC blood oxygen level-dependent activity has been correlated with a bafflingly diverse range of cognitive processes, including implementing task sets (14), multitasking (15), future thinking and prospective memory (16–18), deferring goals and cognitive “branching” (19), exploratory decision making (20), evaluating counterfactual choice (21), complex relational and abstract reasoning (22), integrating outcomes of multiple cognitive operations (23), coordinating internal and external influences on cognition (24), evaluating self-generated information (25), episodic memory retrieval and detailed recollection (26–28), and facing uncertainty or conflict (29–31), for example. Patients with FPC lesions behave inappropriately, particularly in uncertain contexts, and show deficiencies in prospective memory and planning (32–34), but their lesions are large and unselective, and their premorbid performance is unknown. Hence, no consensus has emerged from human neuroimaging and neuropsychology as to what the common underlying contribution(s) of the FPC to cognition might be (23, 35). Targeted electrophysiological recording and circumscribed lesion studies in animal models have had major influences on understanding the contributions to cognition of a broad range of other gPFC areas (2), but such is not the case for the FPC. To date, there are only two primary reports of targeted FPC recordings, (36, 37) and, despite imaging evidence showing FPC activation across a wide range of complex cognitive tasks, many of the patterns of neuronal activity associated with the flexible and complex goal-directed behavior that are usually observed in other gPFC areas (2) were not observed in the FPC (36, 37), suggesting the FPC’s role in these tasks might be different from the role of neighboring areas. Previous lesion studies confirm gPFC areas adjacent to the FPC are necessary for supporting efficient exploitation of current complex tasks/goals (38–44), but no study has yet investigated the effects of circumscribed FPC lesions to identify the FPC’s necessary contribution to cognition. Hence, we aimed to accomplish the following: (i) determine what basic elements of complex, rapidly flexible, goal-directed behavior were crucially dependent upon the FPC; (ii) ascertain whether and how the contribution of the FPC to cognition differs from the rest of gPFC; and (iii) provide an animal model of FPC function to help constrain and inspire hypotheses about the role of the FPC in humans, especially in view of its large volume.
Results
Rapid Learning About the Value of Novel Alternative Stimuli.
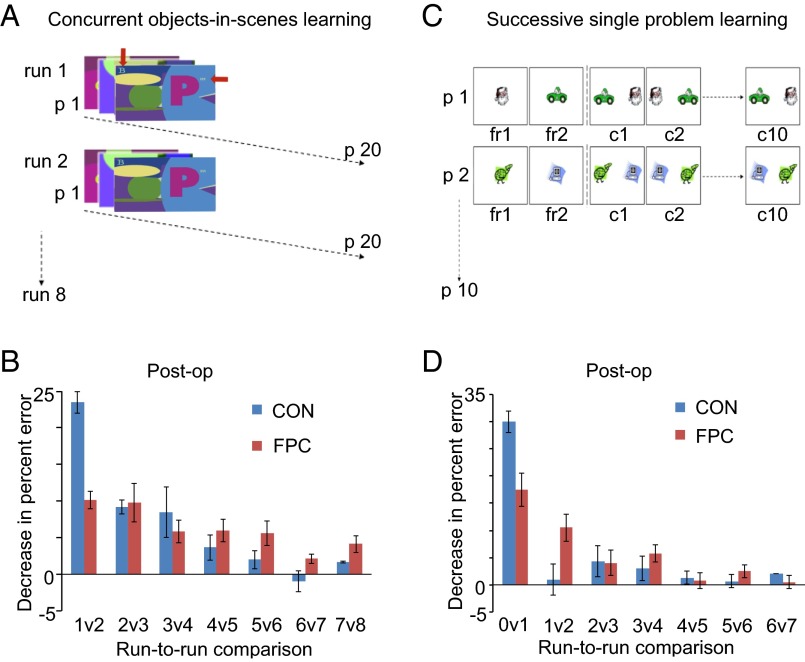
The fundamental role of primate gPFC is said to be the generation of goals that are appropriate to the current context and current needs on the basis of single events, which implies rapid behavioral control (2). Developing and extending this idea about gPFC, we hypothesize that the FPC is important for rapid learning, specifically about the relative values among alternatives, which depends upon self-initiated exploration (35). Therefore, we hypothesized that FPC lesions (Fig.1) might impair rapid learning about novel discriminants but spare gradual learning by repeated reinforcement. To test this hypothesis, we trained seven macaque monkeys on a standard within-session concurrent objects-in-scenes learning task (45), in which the scene unique to each problem and fixed positions of objects on the scene help quick concurrent learning of rewarded (S+) vs. unrewarded (S−) discriminations (experiment 1, Fig. 2A). Once performance was stable, 15 subsequent days provided preoperative data confirming that all animals exhibited rapid within-session learning of 20 novel discriminations each day (Fig. S2). The greatest improvement in performance from one run to the next occurred between the first run [in which animals could only guess between S+ and S−, hence performed at chance (i.e., 50% correct)] and the second run, thereby demonstrating substantial one-trial learning. Subsequent run-to-run increases in accuracy mediated by repeated reinforcement were increasingly modest (Fig. S2). Four animals received bilateral FPC lesions (Fig. S1) and were retested about 2 wk postoperatively across 15 more consecutive days. The remaining three monkeys rested for an equivalent period before retesting [control (CON) group]. The FPC group was markedly and significantly impaired at one-trial learning (i.e., run 2) [t5(group [preoperative minus postoperative]) = 4.48, P = 0.004 (one-tailed test)] but remained unimpaired across runs 3–8 (F1,5[preoperative/postoperative × group] = 2.75, P = 0.158; F[preoperative/postoperative × group × run] < 1). Analyses of run-to-run differences in performance data (e.g., second run percent correct minus first run percent correct) confirmed that the improvement from the first run to the second run (1v2, Fig. 2B) was significantly less in the FPC group than in the CON group [t5(group[preoperative minus postoperative]) = 5.12, P = 0.002 (one-tailed test)], whereas the groups did not significantly differ on other run-to-run comparisons (F1,5[preoperative/postoperative × group] = 2.78, P = 0.157; F[preoperative/postoperative × group × run] < 1). The FPC is necessary for rapid one-trial learning about unfamiliar stimuli but not for gradual learning by repeated reinforcement. The FPC deficit magnitude in run 2 did not depend on whether problems were guessed correctly (1C) or incorrectly (1W) on the first run (F1,5[preoperative/postoperative × group] = 20.92, P = 0.006; F[preoperative/postoperative × group × 1C_vs._1W] < 1). FPC function therefore dissociates from three adjacent gPFC regions because a dorsolateral PFC lesion did not impair this task, orbitofrontal cortex lesions impaired performance across all runs, and ventrolateral PFC lesions impaired performance on all repetitions of 1W trials (46–48).
Fig. 1.
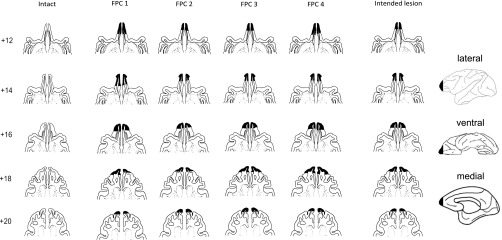
Drawings of intended and actual extents of the FPC lesion and histological verification. The intended lesion extent is shown on the far right in drawings of lateral, ventral, and medial surfaces, as well as in a series of drawings of horizontal sections. The sections on the left (one column per FPC-lesioned animal and one intact animal on the far left for comparison) show the actual extent of the lesions, as evident from the horizontal stained sections presented in Fig. S1. Numbers represent the approximate distance in millimeters above the interaural plane.
Fig. 2.
Concurrent objects-in-scenes learning and successive single problem learning. (A) Schematic of concurrent objects-in-scenes learning task depicting the relationship between the 20 problems per run (p1 to p20) and the eight runs that make up a daily session (run 1 to run 8). The superimposed red arrows in the figure, not present in the stimuli, indicate the objects’ locations in this example scene. The locations of S+ and S− objects in the scene in each problem were fixed. (B) Mean postoperative (Post-op) data plotted separately for the FPC and CON groups, in terms of the run-to-run change in performance [positive values on the y axis indicate improvement between runs (e.g., 1v2 depicts the percentage of erroneous responses on the second run subtracted from the percentage of erroneous responses on the first run)]. (C) Schematic of successive single problem learning task depicting the relationship between the two initial forced response (fr1 and fr2) phases and the 10 subsequent successive choices to this problem (c1 to c10) within each problem, and with 10 problems (p1 to p10) constituting a single session. (D) Postoperative performance for the FPC and CON groups plotted in the same way as in B, depicting improvement across successive attempts at the same problem for the successive single problem learning task (curtailed at 6v7 for comparison with B). All error bars depict ±SEM.
To rule out task-specific effects, we also assessed one-trial learning in a successive single problem learning task (experiment 2, Fig. 2C) in which animals had to make 10 successive discriminations between the same S+ vs. S− problem (presented on a blank background with left/right randomization of stimuli). Importantly, before the first choice, animals had to touch the S+ and the S− once while presented alone (S+/S− order randomized), from which they acquired some knowledge about their relative values by means of the outcome (reward or no reward) experienced after touching the stimulus (Fig. 2C). The FPC group was impaired relative to the CON group on the first choice trial akin to one-trial learning [t5(group [preoperative − postoperative]) = 1.998, P = 0.048 (one-tailed test)] but were unimpaired across the following nine trials (F[preoperative/postoperative × group] < 1; F[preoperative/postoperative × group × run] < 1). Analyses of run-to-run differences in performance data (Fig. 2D) confirmed that the improvement from chance performance before learning (i.e., 50%) to the first choice trial (0v1, 4f) was significantly less in the FPC group than in the CON group [t5(group [preoperative − postoperative]) = 2.036, P = 0.048 (one-tailed test)], whereas the groups did not differ across all other run-to-run comparisons (F[preoperative/postoperative × group] < 1; F[preoperative/postoperative × group × run-to-run improvement] < 1). Preoperative and postoperative run-to-run improvement associated with the initial one-trial learning and the next run-to-run improvement for both the objects-in-scenes and successive single problem learning tasks are entered into one repeated measures ANOVA. The selectivity of the postoperative deficit for one-trial learning across both tasks in the FPC group was robust (F1,5[preoperative/postoperative × group × run-to-run improvement] = 45.442, P = 0.001; whereas F[preoperative/postoperative × group × run-to-run improvement × task] < 1 and F[preoperative/postoperative × group × task] < 1), providing strong support to our hypothesis.
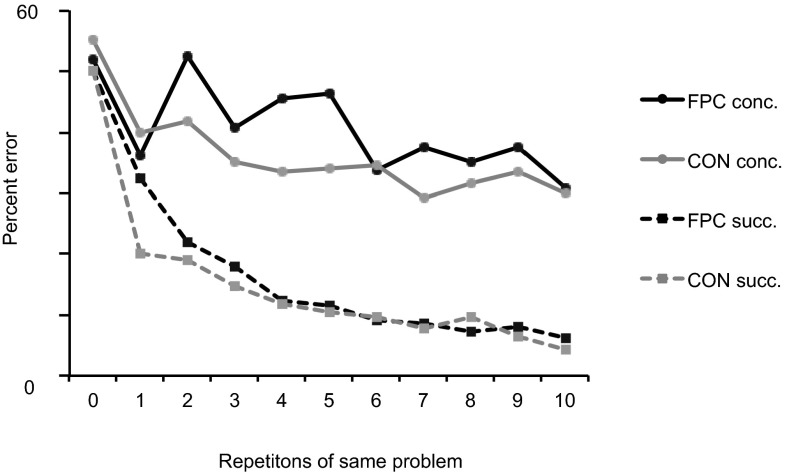
As expected, the FPC group was also unimpaired on a standard test of concurrent object discrimination learning for 10 problem sets learned gradually across days (experiment 3). Across the two separate, 10-problem, postoperative discrimination sets, the mean total errors to criterion per set were 193 and 192 for the CON and FPC groups, respectively, so there is no difference between groups in learning to criterion. Fig. 3 shows that this task is acquired gradually (the data are averaged across both sets of 10 problems). The performance on the second run through the sets of problems is still not significantly better than chance performance (50%) in either set 1 [t2 = 0.667] or set 2 [t2 = 0.199], or in an analysis of data from both sets combined [t2 = 0.184]. We conclude that there is no significant one-trial learning effect in concurrent object discrimination learning.
Fig. 3.
Comparison of learning rate for concurrent vs. successive discrimination learning tasks. Mean postoperative error rates for the CON group and FPC-lesioned group averaged across two 10-problem sets of concurrent discriminations and across many instances of 10-trial repetitions of successively presented single problems are depicted. In both cases, the error rate is plotted for the first 10 choices made to individual problems, choices that were in successive trials (succ.) in the single problem learning task but were separated by the intervening problems in the concurrent learning task (conc.). In the concurrent discrimination learning task, the repetition corresponding to zero is the first presentation of a problem to which the animals necessarily have to guess (hence, averaging around 50%). In the successive task, the repetition corresponding to zero is the forced response phase to S+ and S−, where no choice is actually made between S+ and S−; hence, we plot the data at precisely 50% (chance) to facilitate visual comparison between the data from both tasks.
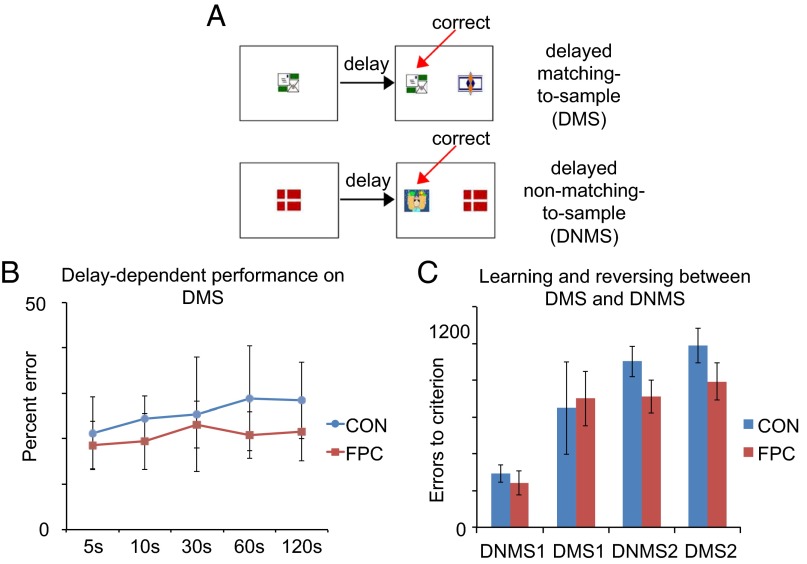
To determine if the aforementioned deficits in one-trial learning in experiments 1 and 2 reflected deficits in one-trial learning about the relative value of chosen and unchosen alternatives as opposed to learning to choose stimuli on the basis of a single experience per se, we next introduced the animals to two tasks in which they had to respond on the basis of a single experience, albeit one in which there were no unchosen alternatives, namely, delayed matching-to-sample (DMS) and delayed nonmatching-to-sample (DNMS) (Fig. 4A). DMS and DNMS (experiments 4 and 5) also had a secondary purpose, given that gPFC is known to be crucial for performance on DMS and DNSM (49, 50); the use of these tasks allowed us to determine whether the FPC could be further dissociated in function from neighboring gPFC if FPC lesions did not impair DMS/DNMS. We did not expect an FPC lesion to impair DMS and DNMS because there are no alternatives to value in the sample phases of DMS/DNMS and because familiarity judgments, which can be used to distinguish the sample stimulus at test, are not associated with the FPC (28). When we commenced the standard series of performance tests to plot mean percent correct across different delay lengths between sample and choice, the FPC group was clearly not impaired (Fig. 4B). As expected, FPC lesions also failed to impair either acquisition of DNMS or subsequent relearning of these two rules across repeated reversals of these familiar rules (Fig. 4C and SI Experimental Procedures).
Fig. 4.
No effect of FPC lesions on recognition memory. (A) Schematic of DMS and DNMS tasks respectively. (B) Mean postoperative percent error rates for the CON group and FPC-lesioned group on five different delays (in seconds) during performance tests for DMS. (C) Mean errors to criterion for the CON and FPC group on DNMS and the repeated reversals between DMS and DNMS tasks. All error bars depict ±SEM.
Rapid Learning About the Value of a Novel Alternative Rule.
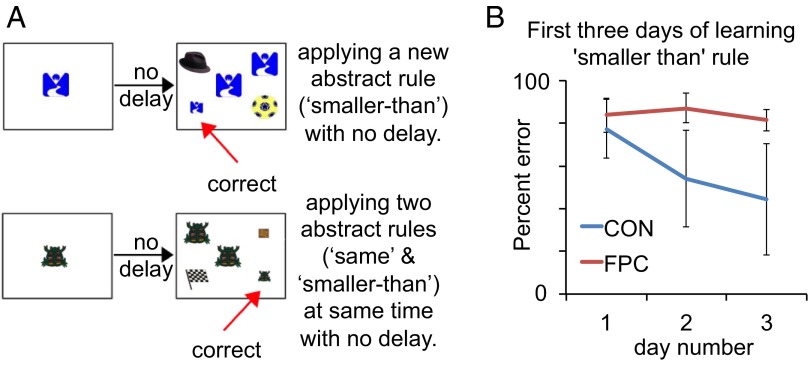
The anatomical connectivity and activation pattern (20, 21, 51) of the FPC leads us to hypothesize a more general role in exploring the relative value of unfamiliar alternatives beyond rapid learning about the relative value of unfamiliar alternative stimuli, as shown above. Therefore, we trained the seven monkeys on a new task postoperatively, which required animals to learn about the relative value of novel alternative rules (experiment 6). As described above, the animals had experience of delayed response tasks. In experiment 6, across a series of stages (more details are provided in SI Experimental Procedures), we proceeded to remove the delay from DNMS (to avoid any possible memory confound) and add in the additional test items [because we also wanted to evaluate the animal’s ability to apply two rules concurrently (experiment 7), which requires two match items of differing sizes and two nonmatch items of differing sizes] to expose the animals to a new four-choice, simultaneous discrimination task (experiment 6). The FPC-lesioned monkeys showed prominent deficits when a novel rule, “smaller than,” was introduced into this task for the very first time (i.e., three S− test items were the same size as the sample, one matching in identity and two not, whereas the S+ test item was smaller than the sample but matched in identity) (Fig. 5A, Upper). On the first day, animals were reluctant to touch the S+ (Fig. 5B). Improvement in mean percent correct from the first day to the second day (Fig. 5B) was significantly different between groups (F1,5 = 10.16, P = 0.024), with a significant reduction in mean percent error across the first 3 d only observed in the CON group (F2,10[day × group] = 5.22, P = 0.028). We presume that both FPC-lesioned and CON group animals failed initially to appreciate the potential value of the novel small S+, but once the S+ had been selected a few times, the CON group monkeys could start to establish the new rule as an alternative, whereas FPC-lesioned animals could not. Furthermore, once the novel rule began to emerge, animals with an intact FPC may have been able, on both correct and error trials, to infer the relative value of the novel emerging rule such that the emerging novel rule could consistently accrue value in this way. Such incremental effects associated with exploring and valuing chosen and unchosen alternatives may have underscored the subsequent dramatic change in choice behavior on the second day onward, when only the CON group chose the S+ increasingly often (Fig. 5B).
Fig. 5.
Performance of CON and FPC groups in learning new abstract rules. (A) Schematic of the new abstract rule “smaller than” task and also the following task in which two rules were required to be applied at the same time. (B) Mean postoperative percent error in the first 3 d of the four-choice simultaneous discrimination task after the “smaller than” rule was introduced. Error bars depict ±SEM.
Correction trials were implemented from the fourth day onward, and animals in both groups quickly attained ≥90% correct performance at the same rate (SI Experimental Procedures). This finding suggests that the deficit is specific to rule acquisition that is dependent upon self-initiated learning and exploration. We also investigated (experiment 7) whether the FPC was necessary for applying two rules at the same time, given neuroimaging studies have indicated that FPC is more active when considering simultaneous multiple relations (52). Therefore, on the next day, we replaced one of the large nonmatch S− items in each trial with a small nonmatch S− item so that animals now had to apply two rules, “same as” and “smaller than”, at the same time to attain a reward (Fig. 5A, Lower). FPC-lesioned animals were unimpaired compared with CON animals in learning to criterion (≥90% correct).
Discussion
Circumscribed bilateral lesions to the macaque FPC produced a unique and distinctive pattern of spared and impaired performance across seven different behavioral tasks (a concise summary is provided in Table S1). The entire pattern may be accounted for and understood in terms of our hypothesis that the FPC is important for rapid learning about the relative value of alternatives. The FPC is confirmed to be crucial for valuing not only alternative stimuli (one-trial learning deficits in experiments 1 and 2) but also alternative rules (new rule learning deficits in experiment 6).
In the concurrent objects-in-scenes learning task (experiment 1), normal animals exhibited robust one-trial learning. However, they did not necessarily learn all there was about the relative value of the S+ and S− in the first run (Fig. S2), despite the fact that unambiguous information about the value of the S+ and S− was provided by the feedback after choice. Likewise, in the successive single problem learning task (experiment 2), although there was a one-trial learning effect after the initial forced response phase (fr1 and fr2 in Fig. 2C), normal animals failed to learn all there was about the relative value of the S+ and S− solely from the experience in the forced response phase (Fig. 3), despite unambiguous information about the value of the S+ and S− provided by the feedback in the second forced response phase (fr2). However, in these contexts (i.e., rapid learning about unfamiliar alternatives), there is a clear benefit to gain from learning more about the relative value of the S+ and S−. Specifically, if an animal could update the relative value of both chosen and unchosen stimuli, its maximum rate of rapid learning would be faster compared with animals that could only update the value of the chosen stimulus. This advantage is maximally effective in the one-trial learning phase because as problems become more familiar, across many repeats, progressively smaller “refinements” to value of S+ and S− can be accumulated. Indeed, the concurrent object discrimination learning task, which was acquired very gradually (over days) and did not exhibit a robust one-trial learning phase (Fig. 3 and SI Experimental Procedures), did not benefit significantly from the aforementioned FPC contribution to learning, thereby remaining unaffected by the lesion. Hence, the results from these memory tasks are consistent with our hypothesis that the FPC is crucial for rapid learning about the relative value of unfamiliar stimuli.
The only published studies to date that targeted FPC neurons (36, 37) showed task-related activity around the time of delayed feedback (reward/no reward) following a self-generated decision, but not if the delayed feedback followed a cued response in a control task. In both the experimental and control tasks, the animals chose between two directions, but in the former, they had to integrate information about their memory for their response on the previous trial with the feedback to find the meaning of the feedback (stay or switch). This result is consistent with our finding that the acquisition of novel rules is impaired only when learning is dependent upon a self-initiated choice of alternative options, and not once correction trials are introduced (experiment 6). However, because the task paradigm in the study by Tsujimoto et al. (36, 37) did not include novel stimuli, actions, or rules, it is difficult to relate their findings to the behavioral changes that we observed in the present study after FPC lesions (SI Experimental Procedures).
No other theory of FPC function derived from human FPC neuroimaging and/or neuropsychology can account for the overall pattern of spared, impaired, and enhanced performance across our battery of tasks. For example, the gateway hypothesis (53), which postulates FPC involvement in the coordination of stimulus-independent and stimulus-oriented cognition in cognitively demanding situations, does not predict the deficit we found in a very simple, successive, single problem learning task. The same difficulty is faced by the cognitive branching (52) and prospective memory underlying multitasking (34) hypotheses of FPC function. Likewise, the integration of the several cognitive operations hypothesis (23) fails to account for why application of two rules remains unimpaired yet rapid one-trial learning of simple object discriminations is impaired. The latter result is also not easily accounted for by theories of a key role for the FPC in episodic recollection (26–28). Christoff et al. (25) have proposed that FPC activity during complex cognitive tasks could be interpreted in terms of explicit evaluation of internally generated information that is not directly provided by the environment. Although these authors relate their hypothesis to novel information of a complex and abstract nature, including inferences, hypotheses, relations, and plans, an extended interpretation of their hypothesis may be developed on the basis of our findings that the FPC may also be important for learning about the relative value of simple options in cases where internal inferences are made about the value of alternatives without having to rely on direct experience with the outcome of selecting each alternative. Unlike the other hypotheses reviewed above, this hypothesis can account for the deficit we found after FPC lesions in one-trial learning of our simple object discriminations. Nevertheless, to account fully for all of our current results, it is important, as in our own hypothesis, to also emphasize rapid learning about novel alternatives, or else the reason why macaques with FPC lesions are not impaired in other contexts wherein internal inferences about the relative value of unchosen alternatives may also be highly relevant (e.g., unimpaired relearning of DMS/DNMS in our study) remains unexplained. Hence, to date, it appears that many of the diverse theories of human FPC function have arguably failed to capture underlying core functions of the FPC that may be common to all primates. This hypothesis is an important way in which a new animal model can help constrain and inspire theorizing about human FPC function.
Our finding that the macaque FPC is necessary for supporting rapid learning about the relative value of a broad range of alternatives is consistent with its anatomical connectivity. The FPC has substantial bidirectional corticocortical connections with gPFC, as well as with more posterior cortical areas, including predominantly cingulate and retrosplenial midline areas, the superior temporal sulcus (dorsal bank) and gyrus and parahippocampal and temporal poles in the temporal lobe, and the rostral insula cortex (4, 12, 54–56). In contrast to other gPFC areas, its direct connections to parietal, inferior temporal, and occipital lobes are typically of lower density (55). These robust supramodal connections implicate the FPC in processing and influencing the brain’s most abstract conceptual representations. Also, FPC interactions with the superior temporal sulcus and gyrus regions, which are associated in both macaques and humans with interpretation of socially relevant signals, such as the intentions and emotions of others (57, 58), as well as with more advanced forms of mentalizing in humans (59), may implicate the FPC in valuing alternatives in the social realm.
The broadly similar connectivity profile of the macaque and human FPC (13) strongly suggests some basic conservation of function across species. In humans, the FPC is occupied by Brodmann’s area 10 (BA10), and because humans have larger brains than other primates, their BA10 is correspondingly larger. In addition, and although estimates vary, the proportional volume of BA10 relative to the rest of the brain may be greatest in humans (3, 60). BA10 is also one of the largest cytoarchitecturally defined areas of the human cerebral cortex. Hence, one may theorize that the human FPC is imbued with greater processing power than the monkey FPC, enabling humans to explore the relative value of a wider range of novel alternatives that support our more advanced goal-directed behaviors. Nonetheless, precursors of these complex human functions, such as highly developed flexibility in learning and shifting between potential goals in complex changing environments, may exist in macaques (12, 13). Although cross-species comparison of functional connectivity fingerprints matched a medial subdivision of the human FPC (FPm) to macaque area 10, a lateral subregion of the FPC (FPl) in humans could not be similarly matched to any macaque prefrontal region, leading to the suggestion that FPl is a uniquely human FPC subregion that could support distinctively human cognitive processes (11). However, Neubert et al. (11) confounded species difference with difference in cognitive state (anesthetized animals vs. resting human participants), and although it is true that somewhat similar patterns of resting-state coactivations have previously been reported in anesthetized vs. awake humans (61) and macaques (62, 63), anesthesia certainly influences cerebral blood flow and is considered to underlie some of the observed changes in correlation strengths, localizations, and inclusion of areas within networks in some studies (64). Given that our study confirmed an essential role of the FPC in exploring the value of alternatives, and given the fact that MRI participants at rest engage in uncontrolled thought processes, including mentalizing about alternatives and attending to distractors, more robust claims about functionally unique FPC areas require functional connectivity comparisons to be made while both species are actively exploring the value of alternatives in similar tasks. Indeed, Boorman et al. (21) showed that changes in human FPC functional connectivity occur when participants finally decide to switch to an alternative. These authors also reported in the same study that a region of the human ventral and medial FPC exhibited activity that correlated with the difference between the chosen and unchosen subjective expected values during the time of the decision (i.e., relative chosen value) (21). This finding indicates correspondence between our monkey lesion data and human imaging, because the area concerned was within area FPm, which Neubert et al. (11) matched to macaque area 10m.
In conclusion, although the FPC has been linked to exploratory decision making between stimuli (20, 21), our data and the pattern of anatomical connections indicate that the crucial function of the FPC in exploration of value is broader in scope. The FPC may be important for facilitating initial assessment of the relative values of wide-ranging novel alternatives, ranging from simple individual stimuli to more complex abstract rules, and particularly so when this process relies on self-initiated exploration and evaluation. Furthermore, even in the absence of novel information or stimuli, FPC cells encode information about choices at the time of feedback (36, 37), which is crucial for the evaluation of recent choices to determine whether a behavioral adjustment should be implemented. Therefore, the FPC may both play a key role in acquiring evidence in favor of behavioral adjustment and influence posterior cortical areas to implement adjustment (65) when sufficient evidence accrues.
Experimental Procedures
Animals.
Seven female macaque monkeys (Macaca mulatta) without previous training experience with any experimental task were used in this study; the seven animals were divided into two subgroups (four with FPC lesions and three intact control animals), and, where possible, analyses were conducted on group differences on sensitive within-subject differences (e.g., preoperative vs. postoperative performance).
All animal training, surgery, and experimental procedures were done in accordance with the guidelines of the UK Animals (Scientific Procedures) Act of 1986, licensed by the UK Home Office, and approved by Oxford’s Committee on Animal Care and Ethical Review. Further details about husbandry and housing and statistical power are provided in SI Experimental Procedures.
Surgery.
As in the human, macaque area 10 occupies the dorsal, medial, and ventral aspects of the macaque polar region (4, 66, 67). The caudal limit of the lesion on the dorsal surface was 2 mm posterior to the rostral tip of the principal sulcus; the caudal limits on the orbital and medial surfaces were also limited to an imaginary vertical line drawn 2 mm posterior to the rostral end of the principal sulcus. Further details of the aseptic surgical method are provided in SI Experimental Procedures.
Histology.
A series of horizontal drawings of the sections through the lesions is shown in Fig. 1. Further details of the method for preparing histology are provided in SI Experimental Procedures and Fig. S1.
Apparatus.
The tasks were provided in automated test apparatuses as described in detail in our previous studies (38). Summary details of the apparatuses are provided in SI Experimental Procedures.
Behavioral Tasks.
A summary of the key elements of each of the seven behavioral tasks used is provided below. More detailed descriptions of all tasks are provided in SI Experimental Procedures.
Experiment 1 (concurrent objects-in-scenes learning).
The task and stimulus material were as described previously (45, 68), and a schematic is shown in Fig. 2A. Briefly, each trial consists of a discrimination problem between a pair of small typographic “objects” superimposed on a computer-constructed scene according to an algorithm that can generate a very large number of unique scenes (one unique complex scene for each pair of foreground objects). The background scene occupies the whole area of the display of the touch-screen. The foreground objects are randomly selected, small, colored typographic characters each placed in a constant location within its individual scene. In each scene, one of the two foreground objects was always correct (S+, rewarded) and the other incorrect (S−, nonrewarded). All correct choices delivered visual feedback (the object flashed for 2 s); a reward pellet was delivered; and the screen then went blank, with an intertrial interval (ITI) of 10 s before the next trial. All incorrect choices triggered an immediate screen blanking and an ITI. In the first run only, the ITI after an error was also followed by a forced-response trial comprising the same scene to which the error was made, but with the S− omitted, and animals were just required to touch the S+ (i.e., no choice was possible) to progress to another ITI preceding the next problem. Touches anywhere else in the scene caused the screen to go blank, and the trial was repeated. Hence, monkeys learned which object in each scene was correct by trial and error, and expressed rapid within-session learning for 20 new problems each day.
Experiment 2 (successive single problem learning).
In a daily session, monkeys worked through 10 problems, with each problem consisting of 10 repetitions of a single discrimination problem between an S+ and an S− (Fig. 2C). Each problem began with the presentation of either the S+ or S− for that problem alone, chosen randomly, in the center of the screen. A touch to this stimulus resulted in the delivery of a reward if that stimulus was the S+ or no reward if that stimulus was the S−. The other stimulus was then shown in the center of the screen, and a touch to this stimulus resulted in the delivery of a reward if that stimulus was the S+ or no reward if that stimulus was the S−. Following these forced-response sample phases (fr1 and fr2 in Fig. 2C), and after an ITI of 5 s, both the S+ and the S− were now presented as a discrimination problem for a further 10 trials, separated by 5-s ITIs, after which the stimuli used for that problem were discarded and not used for the remainder of the experiment. As in the forced-response phases, a touch to the S+ resulted in a reward and no reward was delivered if the stimulus touched was the S−. Following these 10 trials of the same problem, a new pair of objects was chosen from the pool and another forced-response sample phase began. A total of 10 problems were given per session in this way. Analyses were conducted, as in the objects-in-scenes task, on the arcsin-transformed preoperative vs. postoperative percent error data.
Experiment 3 (concurrent discrimination learning for objects).
Before training began, a set of 20 stimuli was randomly arranged into 10 pairs; one of each pair of stimuli was then randomly assigned to be the correct choice (S+) and was subsequently always rewarded, whereas the other was assigned to be the incorrect choice (S−) and was always unrewarded. Once these stimulus–reward associations were assigned, they were maintained throughout the experiments. Two such sets were created. The positions in which the two stimuli in each trial appeared on the screen were randomized (left vs. right) from trial to trial.
Before each trial began, there was an ITI of 10 s. Any touch to the screen during the ITI restarted the 10-s interval. After the ITI, the pair of stimuli was presented on the touch screen. At any point, if the subject touched anywhere on the screen other than to an S+ or S−, any stimuli present remained until a correct or incorrect choice was subsequently made. If the subject touched the S+, a reward pellet was delivered with an audible click from the automatic pellet dispenser. The screen then went blank, apart from the S+ itself, which remained on the screen for a further 1 s before the 10-s ITI before the next trial commenced. Alternatively, if the subject touched the S−, the screen immediately went blank, no reward pellet was dispensed, and the next trial commenced after a time-out interval of 20 s. The subjects first learned set 1 to criterion and then the other (criterion was 90% correct within a single daily session; each subject performed around 10 repeats of the set per session).
Experiment 4 (DMS) and experiment 5 (DNMS) task acquisition, performance tests, and subsequent alternations.
We trained the initial set of seven animals, postoperatively, to respond to trial unique object stimuli on the basis of an abstract rule (DMS) that could be applied to any novel stimulus. The training occurred in a series of stages. In every trial, the animals were first shown a central sample item, which was required to be touched; after a delay, the identical sample (S+) and a nonmatch item (S−) were then presented together, one on the left of the screen and one on the right of the screen (left/right positions selected at random). A reward pellet was delivered in response to a touch to the S+ (which remained on the screen for a further 1 s), whereas no reward and immediate screen blanking were delivered in response to a touch to the S−. Either outcome was followed by commencement of an ITI of 10 s. Once the task rule was acquired to >90% correct within a single daily session, we commenced a standard series of performance tests to plot mean percent correct across different delay lengths between sample and choice. The performance tests consisted of testing many repetitions of five different trial types that differed according to having one of the following delays: 5 s, 10 s, 30 s, 60 s, or 120 s (results are discussed in SI Experimental Procedures).
Next, we switched the reinforced rule in every trial to DNMS (i.e., the nonmatch is rewarded rather than the match in the choice phase) and required the animals to learn this new abstract rule not previously encountered to a criterion of ≥90% correct within a single daily session. This phase took several days, after which we reversed the rule back to DMS. We repeated reversing the rule until the animals had experienced the following sequence of rule reversals: DNMS, DMS, DNMS, and DMS.
Experiment 6 [learning new abstract rule (“smaller than”)] and experiment 7 [combining two rules (“smaller than” and “same as”)].
The animals were trained on a series of seven different training stages. Stages 1–5 trained the animal to perform a variation of the simple DMS task already acquired. Stages 6 and 7 involved the acquisition of a new abstract rule, “smaller than”. Finally, stage 8 involved the combination of the rules “same as” and “smaller than”. The details of each stage are provided in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank G. Daubney for histological support. The work was funded by a UK Medical Research Council (MRC) Programme Grant (to Matthew Rushworth, M.J.B., and coapplicants) and a UK MRC Programme Grant (to David Gaffan).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419649112/-/DCSupplemental.
References
- 1.Preuss TM. Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J Cogn Neurosci. 1995;7(1):1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Passingham D, Wise SP. The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight. Oxford Univ Press; Oxford: 2012. [Google Scholar]
- 3.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. Am J Phys Anthropol. 2001;114(3):224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286(3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346(3):366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 6.Petrides M, Pandya DN. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Elsevier; Amsterdam: 1994. pp. 17–58. [Google Scholar]
- 7.Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73(1):59–86. [Google Scholar]
- 8.Brodmann K. Vergleichende lokalisationslehre der grosshirnrinde in ihren prinzi pen dargestellt auf grund des zellenbaus. Barth; Leipzig, Germany: 1909. German. [Google Scholar]
- 9.Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 10.Burman KJ, Reser DH, Yu HH, Rosa MGP. Cortical input to the frontal pole of the marmoset monkey. Cereb Cortex. 2011;21(8):1712–1737. doi: 10.1093/cercor/bhq239. [DOI] [PubMed] [Google Scholar]
- 11.Neubert FX, Mars RB, Thomas AG, Sallet J, Rushworth MF. Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron. 2014;81(3):700–713. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27(43):11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallet J, et al. The organization of dorsal frontal cortex in humans and macaques. J Neurosci. 2013;33(30):12255–12274. doi: 10.1523/JNEUROSCI.5108-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci. 2006;26(4):1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess PW. Strategy application disorder: The role of the frontal lobes in human multitasking. Psychol Res. 2000;63(3-4):279–288. doi: 10.1007/s004269900006. [DOI] [PubMed] [Google Scholar]
- 16.Burgess PW, Gonen-Yaacovi G, Volle E. Functional neuroimaging studies of prospective memory: What have we learnt so far? Neuropsychologia. 2011;49(8):2246–2257. doi: 10.1016/j.neuropsychologia.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Okuda J, et al. Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19(4):1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- 18.Okuda J, et al. Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time- and event-based prospective memory. Int J Psychophysiol. 2007;64(3):233–246. doi: 10.1016/j.ijpsycho.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399(6732):148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 20.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441(7095):876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62(5):733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Bunge SA, Helskog EH, Wendelken C. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. Neuroimage. 2009;46(1):338–342. doi: 10.1016/j.neuroimage.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 24.Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117(6):1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- 26.Ranganath C, Johnson MK, D’Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;20(22):RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds JR, McDermott KB, Braver TS. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cereb Cortex. 2006;16(4):519–528. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler MA, Stuss DT. Remembering and knowing in patients with frontal lobe injuries. Cortex. 2003;39(4-5):827–846. doi: 10.1016/s0010-9452(08)70866-9. [DOI] [PubMed] [Google Scholar]
- 29.Badre D, Wagner AD. Selection, integration, and conflict monitoring; Assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41(3):473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- 30.Koechlin E, Corrado G, Pietrini P, Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci USA. 2000;97(13):7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida W, Ishii S. Resolution of uncertainty in prefrontal cortex. Neuron. 2006;50(5):781–789. doi: 10.1016/j.neuron.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38(6):848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 33.Dreher JC, Koechlin E, Tierney M, Grafman J. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS ONE. 2008;3(9):e3227. doi: 10.1371/journal.pone.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volle E, Gonen-Yaacovi G, Costello AdeL, Gilbert SJ, Burgess PW. The role of rostral prefrontal cortex in prospective memory: a voxel-based lesion study. Neuropsychologia. 2011;49(8):2185–2198. doi: 10.1016/j.neuropsychologia.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsujimoto S, Genovesio A, Wise SP. Frontal pole cortex: Encoding ends at the end of the endbrain. Trends Cogn Sci. 2011;15(4):169–176. doi: 10.1016/j.tics.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Tsujimoto S, Genovesio A, Wise SP. Evaluating self-generated decisions in frontal pole cortex of monkeys. Nat Neurosci. 2010;13(1):120–126. doi: 10.1038/nn.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsujimoto S, Genovesio A, Wise SP. Neuronal activity during a cued strategy task: Comparison of dorsolateral, orbital, and polar prefrontal cortex. J Neurosci. 2012;32(32):11017–11031. doi: 10.1523/JNEUROSCI.1230-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley MJ, et al. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325(5936):52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- 39.Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318(5852):987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- 40.Wilson CRE, Gaffan D, Mitchell AS, Baxter MG. Neurotoxic lesions of ventrolateral prefrontal cortex impair object-in-place scene memory. Eur J Neurosci. 2007;25(8):2514–2522. doi: 10.1111/j.1460-9568.2007.05468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka K, Matsumoto K, Mansouri FA, Buckley MJ. Functional division among monkey prefrontal areas in goal-directed behaviour. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford Univ Press; Oxford: 2013. [Google Scholar]
- 42.Rudebeck PH, et al. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28(51):13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noonan MP, et al. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci USA. 2010;107(47):20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16(8):1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaffan D. Scene-specific memory for objects: A model of episodic memory impairment in monkeys with fornix transection. J Cogn Neurosci. 1994;6(4):305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- 46.Baxter MG, Browning PG, Mitchell AS. Perseverative interference with object-in-place scene learning in rhesus monkeys with bilateral ablation of ventrolateral prefrontal cortex. Learn Mem. 2008;15(3):126–132. doi: 10.1101/lm.804508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Orbital prefrontal cortex is required for object-in-place scene memory but not performance of a strategy implementation task. J Neurosci. 2007;27(42):11327–11333. doi: 10.1523/JNEUROSCI.3369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Dorsolateral prefrontal lesions do not impair tests of scene learning and decision-making that require frontal-temporal interaction. Eur J Neurosci. 2008;28(3):491–499. doi: 10.1111/j.1460-9568.2008.06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35(7):999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 50.Voytko ML. Cooling orbital frontal-cortex disrupts matching-to-sample and visual-discrimination learning in monkeys. Physiological Psychology. 1985;13(4):219–229. [Google Scholar]
- 51.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 52.Christoff K, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14(5):1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- 53.Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007s;11(7):290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10(3):220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- 55.Markov NT, et al. A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb Cortex. 2014;24(1):17–36. doi: 10.1093/cercor/bhs270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228(1):105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 57.Jellema T, Baker CI, Wicker B, Perrett DI. Neural representation for the perception of the intentionality of actions. Brain Cogn. 2000;44(2):280–302. doi: 10.1006/brcg.2000.1231. [DOI] [PubMed] [Google Scholar]
- 58.Wicker B, Perrett DI, Baron-Cohen S, Decety J. Being the target of another’s emotion: A PET study. Neuropsychologia. 2003;41(2):139–146. doi: 10.1016/s0028-3932(02)00144-6. [DOI] [PubMed] [Google Scholar]
- 59.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holloway RL. Brief communication: How much larger is the relative volume of area 10 of the prefrontal cortex in humans? Am J Phys Anthropol. 2002;118(4):399–401. doi: 10.1002/ajpa.10090. [DOI] [PubMed] [Google Scholar]
- 61.Greicius MD, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29(7):839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantini D, et al. Default mode of brain function in monkeys. J Neurosci. 2011;31(36):12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 64.Hutchison RM, Everling S. Monkey in the middle: Why non-human primates are needed to bridge the gap in resting-state investigations. Front Neuroanatomy. 2012;6:29. doi: 10.3389/fnana.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat Neurosci. 2003;6(1):75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- 66.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 67.Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991;310(4):429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- 68.Browning PGF, Easton A, Buckley MJ, Gaffan D. The role of prefrontal cortex in object-in-place learning in monkeys. Eur J Neurosci. 2005;22(12):3281–3291. doi: 10.1111/j.1460-9568.2005.04477.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.