Significance
Marine organisms produce a wide diversity of natural products with specific bioactivities, representing a valuable source for drug discovery. These secondary metabolites are the result of adaptation and natural selection during the evolution of the producing organisms. However, little is known about the evolutionary persistence and distribution of these secondary metabolites through deep time, because original secondary metabolites of ancient organisms usually are not preserved. Here the unique example of a group of bioactive quinone pigments is shown to have persisted almost unchanged and with a wide distribution from the Early Mesozoic (about 240 MyBP) until today within the echinoderm class Crinoidea, whereas the class underwent a major adaptive radiation and diversification after the end-Permian mass extinction.
Keywords: crinoids, molecular preservation, marine natural products, polyketides, liquid chromatography–mass spectrometry
Abstract
Secondary metabolites often play an important role in the adaptation of organisms to their environment. However, little is known about the secondary metabolites of ancient organisms and their evolutionary history. Chemical analysis of exceptionally well-preserved colored fossil crinoids and modern crinoids from the deep sea suggests that bioactive polycyclic quinones related to hypericin were, and still are, globally widespread in post-Paleozoic crinoids. The discovery of hypericinoid pigments both in fossil and in present-day representatives of the order Isocrinida indicates that the pigments remained almost unchanged since the Mesozoic, also suggesting that the original color of hypericinoid-containing ancient crinoids may have been analogous to that of their modern relatives. The persistent and widespread occurrence, spatially as well as taxonomically, of hypericinoid pigments in various orders during the adaptive radiation of post-Paleozoic crinoids suggests a general functional importance of the pigments, contributing to the evolutionary success of the Crinoidea.
Crinoids are the most ancient group of extant echinoderms with a fossil record extending to the Ordovician (1). Following the end-Permian mass extinction, which almost led to the disappearance of the Crinoidea, the post-Paleozoic crinoids, all grouped in the subclass Articulata (2), underwent a major evolutionary radiation and diversification that led to the development of free-living crinoids and to the offshore displacement of stalked crinoids (2, 3).
Quinone and pyrone pigments are well-known secondary metabolites from present-day free-living comatulids (order Comatulida) (4), the dominant group of modern crinoids. By contrast, the constituents of extant stalked crinoids are still almost unknown due to their occurrence in the deep sea. Although early attempts to characterize the pigments of stalked crinoids were performed in the 19th century (5), until now, only those of the isocrinid Proisocrinus ruberrimus and of the cyrtocrinids Neogymnocrinus richeri and Holopus rangii have been elucidated. A group of brominated anthraquinones (proisocrinins) was isolated from the former (6), whereas a group of brominated phenanthroperylene quinones (gymnochromes) was found in the latter (7, 8).
From the fossil record only a group of polycyclic quinone pigments (fringelites) is known, which was discovered in violet-colored specimens of the Jurassic stalked crinoid Liliocrinus (extinct order Millericrinida) from northern Switzerland (9). These fossil pigments have been described as a series of phenanthroperylene quinones differing in the number of their hydroxy groups (10–12) and were originally interpreted as diagenetic condensation products of primary naphthoquinones (9, 10). Based on comparison with present-day comatulid pigments, the fossil pigments were later thought to be geochemical transformation products of primary anthraquinones or bianthrones (4, 13). However, using more advanced spectroscopic techniques, the pigments have been characterized as a homologous series of hypericinoid pigments (14, 15), closely related to the gymnochromes, suggesting that the fossil pigments may be only slightly changed during diagenesis. Although violet coloration has been occasionally observed in fossil crinoids (e.g., refs. 16 and 17), until now, only very few chemical proofs of quinone pigments have been found for crinoids other than Liliocrinus (14, 15, 18, 19). Recent measurements suggested the presence of quinone-like compounds in Paleozoic (Mississippian) crinoids (20), but the identity of these compounds has been questioned on methodological grounds (21).
To investigate the general occurrence of polycyclic quinone pigments in the Crinoidea, the coloration of numerous fossil crinoids from collection material was evaluated, and diverse samples with colorations ranging from distinct violet to almost gray were chemically analyzed by HPLC–diode array detection–mass spectrometry (HPLC-DAD-MS). For comparison, a set of distinctly colored extant stalked crinoids was analyzed for their pigment contents.
Results and Discussion
Evaluation of fossil crinoids revealed that specimens with a distinct violet coloration occur not only at several localities in the Swiss Jura in the vicinity of the original “Fringeli” locality, but also at many other localities in Europe and even in East Africa (Table S1). Analysis using HPLC-DAD-MS unambiguously showed that the spatial distribution of hypericinoid pigments among fossil crinoids is almost worldwide, the stratigraphic distribution ranges at least back to the Middle Triassic, and the taxonomic distribution comprises representatives from at least four (Encrinida, Isocrinida, Comatulida, and Millericrinida) of the eight post-Paleozoic crinoid orders (Figs. 1, 2A, and 3 and Table 1).
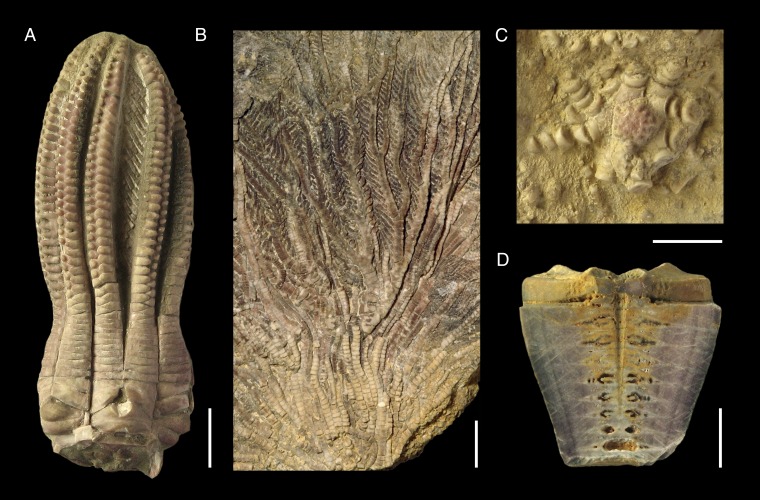
Fig. 1.
Examples of fossil post-Paleozoic crinoids with distinct purple to violet coloration due to preservation of polycyclic quinone pigments (hypericinoids). (A) Encrinid Encrinus aculeatus (GPIT/CR/373), Middle Triassic, Anisian, Górażdże, Poland. (B) Isocrinid Pentacrinites dargniesi (PIMUZ 24616), Middle Jurassic, Bajocian, Develier, Switzerland. (C) Comatulid Solanocrinites beltremieuxi (SMNS 67702), Upper Jurassic, Kimmeridgian, Île de Ré, France. (D) Millericrinid Apiocrinites roissyanus (longitudinal section of cup, MJSN QLP005-884), Upper Jurassic, Oxfordian, Boncourt, Switzerland. (Scale bar, 1 cm.)
Fig. 2.
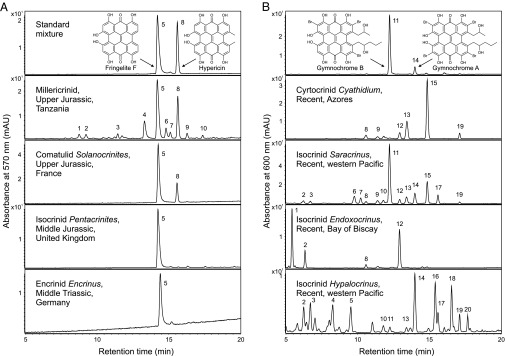
HPLC chromatograms of crinoid pigment analysis. (A) Extracts of fossil crinoids in comparison with reference compounds. From Top to Bottom, the chromatograms correspond to fringelite F and hypericin; millericrinid, Upper Jurassic, Tanzania; comatulid Solanocrinites beltremieuxi, Upper Jurassic, France; isocrinid Pentacrinites dargniesi, Middle Jurassic, United Kingdom; and encrinid Encrinus cf. brahli, Middle Triassic, Germany. (B) Extracts of extant crinoids in comparison with reference compounds. From Top to Bottom, the chromatograms correspond to gymnochrome B and gymnochrome A isolated from Neogymnocrinus richeri, New Caledonia (note that according to ref. 7 the relative positions of the two side chains in gymnochrome B remain to be established); cyrtocrinid Cyathidium foresti, Azores; isocrinid Saracrinus nobilis, western Pacific; isocrinid Endoxocrinus (Diplocrinus) wyvillethomsoni, Bay of Biscay; and isocrinid Hypalocrinus naresianus, western Pacific. Peaks were identified by comparison of the retention times, UV-visible spectra, accurate mass data, and isotopic patterns with those of the standard compounds (Figs. S2 and S3). Molecular formulas of indicated compounds are listed in Tables S3 and S4.
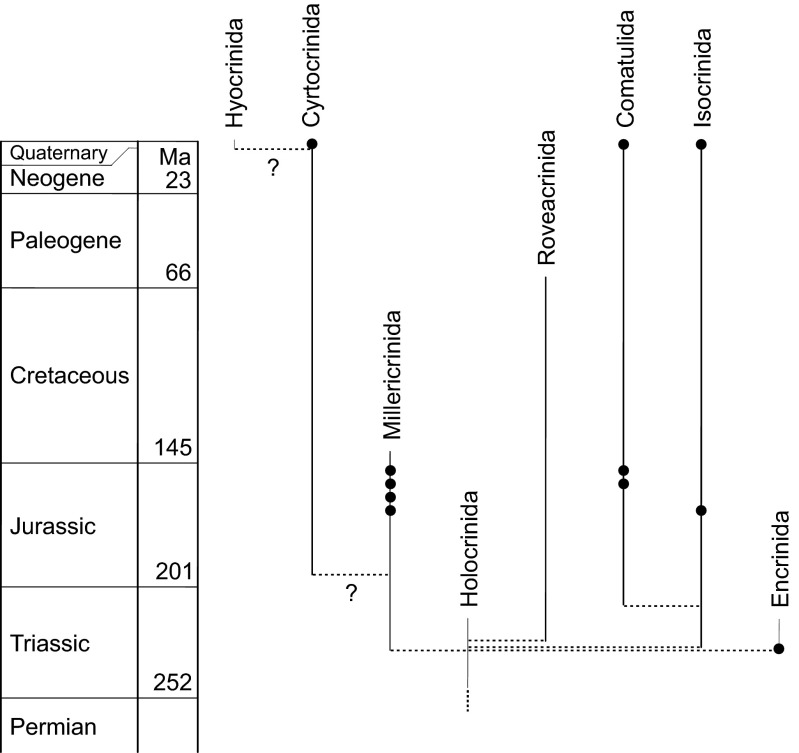
Fig. 3.
Diversification of the post-Paleozoic crinoids (subclass Articulata) with occurrences of hypericinoid pigments (solid circles). Phylogenetic relationships are based on morphological data from ref. 2. Recent investigations based on molecular data support the hypothesis that all living crinoids radiated from a small clade that passed through the end-Permian mass extinction, but suggest an earlier divergence of the order Comatulida from other clades (22, 23). In present-day comatulids the most abundant pigments are anthraquinones, pyrones, and bianthrones, whereas hypericinoid pigments have been detected only as minor pigments (4).
Table 1.
Occurrences of hypericinoid pigments in fossil crinoids
| Species | Stratigraphy | Locality | Sediment | Hypericinoids* (ref.) |
| Millericrinida | ||||
| Millericrinid | U. Jurassic, Ti | Solnhofen, DE | Hardground | 8, 10 |
| Millericrinid | U. Jurassic, Ki/Ti | Tendaguru, TZ | Sandstone | 1–10 |
| Millericrinus lusitanicus | U. Jurassic, Ki | Vestiaria, PT | Marl | 5, 8–10 |
| Liliocrinus polydactylus | U. Jurassic, Ki | Angoulins, FR | Reef flank | 5, 7–10 |
| ?Pomatocrinus mespiliformis | U. Jurassic, Ki | Małogoszcz, PL | Oolite | 5, 7†, 8 (18) |
| Apiocrinites roissyanus | U. Jurassic, Ki | Tonnerre, FR | Chalky limestone | 1, 3–10 |
| Millericrinid | U. Jurassic, Ox/Ki | Hannover, DE | Bioclastic limestone | 1, 3–10 |
| Liliocrinus munsterianus | U. Jurassic, Ox | L’Isle-sur-le-Doubs, FR | Oolite | 1, 3–10 |
| L. munsterianus | U. Jurassic, Ox | Bärschwil, CH | Marl | 5, 7†, 8 (15) |
| A. roissyanus | U. Jurassic, Ox | Boncourt, CH | Reef flank | 1, 3–10 |
| Angulocrinus echinatus | U. Jurassic, Ox | Malton, GB | Oolitic limestone | 1, 3–10 |
| Millericrinid | U. Jurassic, Ox | Villers-sur-Mer, FR | Limestone | 3, 5, 6, 8, 10 |
| Apiocrinites negevensis | M. Jurassic, Ca | Hamakhtesh Hagadol, IL | Marl | 5, 8–10 |
| Apiocrinites parkinsoni | M. Jurassic, Bt | Bradford-on-Avon, GB | Hardground | 1, 3–10 |
| Ailsacrinus abbreviatus | M. Jurassic, Bt | Eastington, GB | Sandy bio-oosparite | 5, 7–10 |
| Comatulida | ||||
| Solanocrinites sp. | U. Jurassic, Ki/Ti | Tendaguru, TZ | Sandstone | 5, 7–10 |
| Solanocrinites beltremieuxi | U. Jurassic, Ki | Île de Ré, FR | Limestone | 5, 8–10 |
| Isocrinida | ||||
| Pentacrinites dargniesi | M. Jurassic, Bt | Malmesbury, GB | Not documented | 5, 7–10 |
| Hispidocrinus leuthardti | M. Jurassic, Bt | Liestal, CH | Marl | 5, 7–10 |
| P. dargniesi | M. Jurassic, Bj | Develier, CH | Oolite | 5, 7–9 |
| Encrinida | ||||
| Chelocrinus schlotheimi | M. Triassic, An | Willebadessen, DE | Limestone | 5, 8–10 |
| Carnallicrinus carnalli | M. Triassic, An | Freyburg/Unstrut, DE | Oolite | 5, 7†, 8 (15) |
| Encrinus aculeatus | M. Triassic, An | Raciborowice, PL | Limestone | 5, 7†, 8 (19) |
| Encrinus cf. aculeatus | M. Triassic, An | Górażdże, PL | Limestone | 5, 7†, 8 (14) |
| Encrinus cf. brahli | M. Triassic, An | Weißenborn, DE | Hardground | 5, 7 |
A more comprehensive dataset is given in Table S1. Geological stages indicated: An, Anisian; Bj, Bajocian; Bt, Bathonian; Ca, Callovian; Ki, Kimmeridgian; Ox, Oxfordian; Ti, Tithonian. M., Middle; U., Upper. Countries indicated: CH, Switzerland; DE, Germany; FR, France; GB, United Kingdom; IL, Israel; PL, Poland; PT, Portugal; TZ, Tanzania.
Molecular formulas of indicated compounds are listed in Table S3.
Indicated compound and/or isomer.
Chemical proofs of fossil hypericinoid pigments were obtained from a large variety of representatives of the order Millericrinida from the Jurassic of Europe, the Middle East, and East Africa (Fig. 2A and Table 1). Moreover, the example of the diverse and exceptionally well-preserved Upper Jurassic millericrinid fauna of the La Rochelle area in France shows that, in addition to the hypericinoid-containing Liliocrinus polydactylus (Table 1), almost all species from this assemblage exhibit a distinct violet coloration (Fig. S1). The combined data suggest that coloration by hypericinoid pigments was ubiquitous in Jurassic millericrinids and, regarding the stratigraphic occurrence of the order (Middle Triassic to Lower Cretaceous) (Fig. 3), likely beyond. Fossil hypericinoids were also detected in representatives of the Triassic order Encrinida, including the oldest polycyclic quinones known (Fig. 2A and Table 1). However, most interesting are chemical proofs of these pigments in Jurassic representatives of two orders that still exist today, Isocrinida and Comatulida (Figs. 2A and 3 and Table 1), allowing direct comparison of ancient and modern crinoid pigments within the same clade.
Indeed, chemical analysis of ethanolic extracts shows that hypericinoid pigments are still widespread in extant isocrinids (Fig. 2B and Table S2) with Hypalocrinus belonging to the same subfamily (Isocrininae) as the fossil Hispidocrinus (Table 1). In addition to the known occurrences in extant cyrtocrinids (7, 8) and comatulids (4), hypericinoid pigments were found in the cyrtocrinid Cyathidium foresti (Fig. 2B and Table S2). Considering the rarity of fossil crinoids with preservation of organic pigments and the poor fossil record of intact Cenozoic crinoids (2, 3), the analytical data of fossil and extant crinoids strongly suggest that hypericinoids were common crinoidal pigments throughout the Mesozoic and Cenozoic, presumably with a monophyletic origin within the ancestral Articulata (Fig. 3).
Fossil as well as extant crinoid pigments show the typical UV-visible spectra of phenanthroperylene quinones (Fig. S2) (24), differing mainly in the position of the long wavelength maxima. Among the extant crinoids, several series of brominated hypericinoid pigments and their isomers were identified by comparison with authentic standards of gymnochrome B and A from the cyrtocrinid Neogymnocrinus (Fig. 2B). Accurate mass data and isotopic patterns indicate that the individual pigments differ in the number of their bromine atoms, the length of the side chains (with characteristic C2H4 intervals due to the biosynthesis of the polyketide compounds via the acetate–malonate pathway), and the presence or lack of sulfate moieties (Fig. S3 and Table S4). Apart from obvious differences in relative amounts, the crinoids show only minor variations in the composition of pigments (Fig. 2B). Based on these results and the observations made by Moseley in 1877 (5), it is very likely that the “purple pentacrinin” pigments extracted from different stalked crinoids were also brominated hypericinoids.
Among the fossil crinoids, several series of nonbrominated hypericinoid pigments and their isomers were identified by comparison with the standard compounds fringelite F and hypericin (Fig. 2A, Fig. S3, and Table S3). Almost the same pigments were found in all samples independent of occurrence, stratigraphy, or taxon of the crinoids (Table 1). Despite minor diagenetic changes, as indicated by the presence of homologs [due to a stepwise demethylation of the side chains during diagenesis (15)] and the predominance of the demethylated fringelite F (Table S1), the pigments preserved in the fossil crinoids show astonishing similarities to those of their extant relatives. In particular, the common occurrence of hypericinoids in fossil and living representatives of the order Isocrinida suggests that the fossil pigments are almost unchanged natural products and not diagenetic condensation products as previously supposed.
Moreover, because halogenated organic compounds generally are not documented in the fossil record (25) (probably as a result of the bromine–carbon bonds being less stable than the carbon–carbon bonds), there is no reasonable explanation why hypericinoid pigments of extant isocrinids are brominated but those of Mesozoic isocrinids were not. Therefore, it is likely that the fossil pigments originally were also brominated and lost the bromine during diagenesis. This is also supported by the corresponding presence of brominated hypericinoids both in extant cyrtocrinids and in isocrinids, with a supposed last common ancestor in the Early Mesozoic (Fig. 3). However, although the fossil hypericinoids represent almost unchanged colorful secondary metabolites, it should be noted that the purple to violet color currently visible in the Mesozoic fossils does not necessarily represent the original color of the crinoids. Because deprotonation and protonation lead to dramatic changes in the absorption behavior of hypericinoid pigments (24) (Fig. S4), and hypericinoid-containing living crinoids (e.g., Cyathidium, Saracrinus, and Hypalocrinus) commonly exhibit a green color (Table S2), hypericinoid-containing ancient crinoids may have exhibited an analogous greenish color.
Structural conservatism in natural products over geological time indicates that the compounds had functions that were important for the organisms that produced them. In the case of the crinoid pigments, these compounds were widespread over a period of about 240 million years while the crinoids adapted to diverse habitats after the end-Permian mass extinction (26). Despite significant morphological changes among crinoids during this diversification, their pigments remained almost unchanged. Hypericinoid pigments occurred in benthic encrinids, millericrinids, and isocrinids as well as in free-living comatulids. Moreover, the pigments can be found both in shallow-water Mesozoic crinoids and in present-day stalked crinoids from the deep sea, suggesting a general functional importance of the pigments.
Although a potential role of the pigments in visual predator–prey interactions cannot be excluded, this would be mainly relevant for shallow-water crinoids. Considering the greenish color of present-day hypericinoid-containing crinoids, a role in camouflage may be suggested rather than a role in aposematism. However, secondary metabolites such as quinones may also play a role in chemical defense. Hypericin and gymnochromes are well known for their biological activity (8, 24), and antifeedant activities against fish have been reported for structurally related anthraquinone pigments from comatulids (27, 28). Previous observations on two extant isocrinids, Endoxocrinus parrae and Neocrinus decorus, suggested that stalked crinoids lack chemical defense against fish predation, supporting the hypothesis that restriction of stalked crinoids to deep-water habitats may have resulted from the Mesozoic radiation of durophagous fishes in shallow seas (29). Chemical analyses also indicated that these crinoid species are virtually devoid of quinone pigments and aromatic polyketides (30). However, the present study shows that many other species of stalked crinoids do contain quinone pigments, indicating that potentially deterrent quinones are common both in comatulids and in stalked crinoids. Therefore, currently there is no evidence for fundamental differences in the chemical defense of comatulids and stalked crinoids that might explain the offshore displacement of stalked crinoids. In contrast to a possible deterrent function of the pigments against fishes, such a function against benthic predation by echinoids is not supported, because indications of predation by echinoids have been observed for (likely hypericinoid-containing) crinoids of the order Encrinida (31). A further defensive function of the pigments might be antifouling, because most echinoderms are free from fouling organisms, and antifouling properties have been reported for ethanolic extracts of echinoderms (32).
It is striking that fossil crinoids with preserved hypericinoid pigments generally are found associated with hardgrounds, oolitic limestones, or reefal carbonates (Table 1), which were deposited in well-oxygenated environments where exceptional preservation of organic substances usually would be least expected. In the case of the Tendaguru locality, which is mainly known for the discovery of dinosaur remains (33), the pigments are preserved even in fossils from a sandstone-dominated coastal lithofacies. The embedding sediments are predominantly light colored and show low contents of organic matter. By contrast, no colored crinoids are known from bituminous sediments such as the Posidonia Black Shale, which is famous for the occurrence of well-preserved isocrinids and fossils with preserved organic tissue material (34). Therefore, it may be supposed that a shallow-water carbonate depositional environment is crucial for the preservation of hypericinoid compounds. Because the pigments occur within the carbonate cement that fills the former pores of the crinoid endoskeleton (15), and early precipitation of carbonate cements is typical for the high-energy depositional environments where the crinoids were buried (35), it is likely that early marine cementation is a key factor for the preservation of hypericinoid pigments in fossil crinoids. Furthermore, the carbonate depositional environment may be critical for the preservation of the pigments, because of the strong acidity of the pigment bay region hydroxy groups that favors the formation of highly insoluble salts with bivalent ions such as Ca2+ (36). It is also striking that the pigments generally are preserved in articulated crinoid remains, suggesting rapid burial of the animals, and in more massive structures such as the roots of millericrinids. The observed occurrence of hypericinoid pigments in fossil crinoids thus very likely is the result of differences among depositional environments of crinoid habitats, preservation potential, and diagenesis and does not reflect the original occurrence of the pigments, which was certainly much more widespread.
In contrast to the Mesozoic crinoids, no traces of hypericinoid pigments could be detected in Paleozoic crinoids (Table S1). This also applies for specimens with a slight violet hue such as Strimplecrinus from the exceptionally well-preserved Mississippian crinoid fauna of LeGrand (Iowa), which is known for species-specific coloration (37, 38). One explanation for the apparent lack of polycyclic quinones in Paleozoic crinoids would be that those pigments have not survived diagenesis. However, although the occurrence of hypericinoids and related pigments in Paleozoic crinoids cannot be excluded, it is also possible that these pigments are an invention of the Articulata, the only crinoid group that survived the end-Permian mass extinction.
Materials and Methods
Samples and Reference Compounds.
Fossil crinoid specimens showing a coloration similar to the characteristic purple to violet of the Liliocrinus specimens from the original Fringeli locality near Bärschwil in Switzerland (9, 15) and additional specimens with a more gray to black coloration were selected from collection material (Table S1). Ethanolic solutions containing dissolved pigments of colored extant crinoids were obtained either by sampling of the ethanolic solutions used for preservation of the specimens or by extraction of dried specimens (C. foresti, Metacrinus levii, and Porphyrocrinus cf. verrucosus) with ethanol (Table S2). For reference, authentic samples of gymnochrome B (containing small amounts of gymnochrome A) from N. richeri (7) and hypericin from St. John’s wort (Hypericum perforatum) as well as a synthetic sample of fringelite F prepared according to ref. 39 were used. Specimens cited in this study are housed in the following institutions: Bayerische Staatssammlung für Paläontologie & Geologie (BSPG), Field Museum of Natural History (FMNH), Institut für Geowissenschaften der Universität Tübingen (GPIT), Geowissenschaftliches Zentrum der Universität Göttingen (GZG), National Natural History Collections at The Hebrew University of Jerusalem (HUJ), Ludwig Maximilians Universität München (LMU), Museum für Naturkunde Berlin (MB), Musée Jurassien des Sciences Naturelles Porrentruy (MJSN), Muséum National d’Histoire Naturelle Paris (MNHN), Natural History Museum London (NHMUK), Naturhistorisches Museum Wien (NHMW), Naturhistorisches Museum Basel (NMB), Nagoya University Museum (NUM), Paläontologisches Institut und Museum der Universität Zürich (PIMUZ), Staatliches Museum für Naturkunde Stuttgart (SMNS), University Museum of the University of Tokyo (UMUT), and Systematische Zoologie am Museum für Naturkunde Berlin (ZMB).
Sample Preparation and Extraction of Fossil Crinoids.
Samples of fossil crinoids were cleaned with acetone. After dissolution of the carbonate with 10 M HCl, the residues were separated by centrifugation, washed thoroughly with distilled water, and dried overnight at room temperature under vacuum (about 10 Torr). Residues were then sequentially extracted by sonication (10 min at 40 °C) and centrifugation in toluene (3×) and dimethyl sulfoxide (DMSO) (1×). The pigment-containing DMSO extracts were cleaned up by solid-phase extraction. The sorbent (Bondesil C18, 40 µm) was conditioned by washing with acetonitrile. The extracts then were loaded onto the column, and the pigments were eluted with acetonitrile.
HPLC-DAD-MS Analysis.
Aliquots of extracts obtained from fossil crinoids and extracts of extant crinoids were analyzed using the same method. HPLC-DAD-MS measurements were carried out on an Agilent 1100 Series HPLC system with a diode array detector coupled to an Agilent 6520 Q-TOF LC/MS mass spectrometer equipped with an electrospray ionization (ESI) source. Separation was performed at 50 °C on a Phenomenex Gemini C18 column (250 × 4.6 mm i.d., 5 µm). The HPLC program consisted of a linear gradient of acetonitrile/20 mM aqueous ammonium acetate [60:40 (vol/vol)] to 100% acetonitrile in 20 min, followed by isocratic elution at 100% acetonitrile at a flow rate of 1 mL⋅min–1. The diode array detector wavelengths were 570 nm, 590 nm, and 600 nm and UV-visible spectra of each peak were recorded in the 200- to 800-nm wavelength range. Extracts were filtered before injection, using 0.2 µm polytetrafluoroethylene filters (ReZist; Schleicher & Schuell). Mass spectra were acquired in the negative-ion mode (nebulizer gas pressure 60 psi, drying gas flow 12 L⋅min–1, drying gas temperature 350 °C, capillary voltage 4.0 kV) over the 100- to 1,300-m/z range. Mass calibration and continuous reference correction were obtained using purine and the HP-0921 acetate adduct (C20H21O8N3P3F24) introduced via an internal reference mass kit. Extracts from two additional fossil samples (Apiocrinites negevensis and millericrinid from Solnhofen) were analyzed only by HPLC-DAD, using an Agilent 1200 Series HPLC system, by applying the same chromatographic conditions as described above.
Absorption Spectroscopy of Extant Crinoid Pigments.
UV-visible spectra were recorded on a Jasco V-630 spectrophotometer, using 1-cm quartz cuvettes. The same amounts of crude Saracrinus pigments were dissolved in ethanol and ethanol containing 1% 1 M HCl. Solutions were filtered before measurements, using 0.2-µm polytetrafluoroethylene filters (ReZist; Schleicher & Schuell).
Supplementary Material
Acknowledgments
I thank J. Ayer (Musée Jurassien des Sciences Naturelles Porrentruy), U. Bielert, S. Charbonnier [Muséum National d’Histoire Naturelle Paris (MNHN)], T. Ewin (Natural History Museum London), L. Kaecke, A. Kroh (Naturhistorisches Museum Wien), S. Lidgard (Field Museum of Natural History), J. Mainguy, C. Neumann (Museum für Naturkunde Berlin), S. Schneider (Bayerische Staatssammlung für Paläontologie & Geologie), G. Schweigert (Staatliches Museum für Naturkunde Stuttgart), S. Seppelt, O. Schmidt [Naturhistorisches Museum Basel (NMB)], A. Weissmüller, and M. A. Wilson (College of Wooster) for fossil crinoid material; N. Améziane and M. Eléaume (MNHN), T. Oji (Nagoya University Museum), M. Wisshak (Senckenberg am Meer), and G. Wörheide [Ludwig Maximilians Universität München (LMU)] for recent crinoid material; M. Iorizzi (University of Molise) and W. Steglich (LMU) for references of gymnochrome B and fringelite F, respectively; H. Furrer (Paläontologisches Institut und Museum der Universität Zürich) for providing the image for Fig. 1B; W. Buchberger (Johannes Kepler Universität Linz) for access to laboratory facilities; J. H. Gross (Universität Heidelberg) for initial ESI-MS measurements of the pigments of Hypalocrinus; T. Oji and M. Roux for information on the natural color of extant crinoid species; and H. Hess (NMB) and H. Falk (Johannes Kepler Universität Linz) for helpful discussions. This work was supported by the Deutsche Forschungsgemeinschaft (WO 1491/1-1 and WO 1491/4-1).
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission. T.K.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417262112/-/DCSupplemental.
References
- 1.Hess H, Ausich WI, Brett CE, Simms MJ, editors. Fossil Crinoids. Cambridge Univ Press; Cambridge, UK: 1999. [Google Scholar]
- 2.Hess H, Messing CG. 2011. Treatise on Invertebrate Paleontology, Part T, Echinodermata 2, Revised, Crinoidea, Vol 3, ed Selden PA (University of Kansas Paleontological Institute, Lawrence, KS)
- 3.Meyer DL, Macurda DB., Jr Adaptive radiation of the comatulid crinoids. Paleobiology. 1977;3(1):74–82. [Google Scholar]
- 4.Rideout JA, Sutherland MD. Pigments of marine animals. XV Bianthrones and related polyketides from Lamprometra palmata gyges and other species of crinoids. Aust J Chem. 1985;38(5):793–808. [Google Scholar]
- 5.Moseley HN. On the colouring matters of various animals, and especially of deep-sea forms, dredged by H.M.S. Challenger. Q J Microsc Sci. 1877;17(65):1–23. [Google Scholar]
- 6.Wolkenstein K, Schoefberger W, Müller N, Oji T. Proisocrinins A-F, brominated anthraquinone pigments from the stalked crinoid Proisocrinus ruberrimus. J Nat Prod. 2009;72(11):2036–2039. doi: 10.1021/np900171h. [DOI] [PubMed] [Google Scholar]
- 7.De Riccardis F, et al. The gymnochromes: Novel marine brominated phenanthroperylenequinone pigments from the stalked crinoid Gymnocrinus richeri. J Org Chem. 1991;56(24):6781–6787. [Google Scholar]
- 8.Kemami Wangun HV, et al. Gymnochromes E and F, cytotoxic phenanthroperylenequinones from a deep-water crinoid, Holopus rangii. J Nat Prod. 2010;73(4):712–715. doi: 10.1021/np900526y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumer M. [Fossil hydrocarbons and dyes in calcareous rocks] Mikrochemie. 1951;36/37(2):1048–1055. German. [Google Scholar]
- 10.Blumer M. Pigments of a fossil echinoderm. Nature. 1960;188(4756):1100–1101. [Google Scholar]
- 11.Blumer M. The organic chemistry of a fossil—I. The structure of the fringelite-pigments. Geochim Cosmochim Acta. 1962;26(2):225–227. [Google Scholar]
- 12.Blumer M. Organic pigments: Their long-term fate. Science. 1965;149(3685):722–726. doi: 10.1126/science.149.3685.722. [DOI] [PubMed] [Google Scholar]
- 13.Needham AE. The Significance of Zoochromes. Springer; Berlin: 1974. [Google Scholar]
- 14.Wolkenstein K. 2005. [Phenanthroperylene quinone pigments in fossil crinoids: Characterization, occurrence and diagenesis]. PhD thesis (University of Heidelberg, Heidelberg). German.
- 15.Wolkenstein K, Gross JH, Falk H, Schöler HF. Preservation of hypericin and related polycyclic quinone pigments in fossil crinoids. Proc R Soc B. 2006;273(1585):451–456. doi: 10.1098/rspb.2005.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.d’Orbigny A. 1840–1841. [General and particular natural history of the living and fossil crinoids including zoological and geological description of the animals]. Histoire Naturelle Generale et Particuliere des Crinoides Vivants et Fossiles Comprenant la Description Zoologique et Geologique de Ces Animaux (d'Orbigny A, Paris). French.
- 17.Taylor PD. Ailsacrinus gen. nov., an aberrant millericrinid from the Middle Jurassic of Britain. Bull Br Mus Nat Hist Geol Ser. 1983;37(2):37–77. [Google Scholar]
- 18.Wolkenstein K, Głuchowski E, Gross JH, Marynowski L. Hypericinoid pigments in millericrinids from the Lower Kimmeridgian of the Holy Cross Mountains (Poland) Palaios. 2008;23(11):773–777. [Google Scholar]
- 19.Niedźwiedzki R, Salamon MA, Wolkenstein K. Encrinus aculeatus (Crinoidea: Encrinida) with exceptional preservation of organic pigments from the Middle Triassic of Lower Silesia (SW Poland) N Jb Geol Paläont Abh. 2011;262(2):163–170. [Google Scholar]
- 20.O’Malley CE, Ausich WI, Chin Y-P. Isolation and characterization of the earliest taxon-specific organic molecules (Mississippian, Crinoidea) Geology. 2013;41(3):347–350. [Google Scholar]
- 21.Wolkenstein K. Isolation and characterization of the earliest taxon-specific organic molecules (Mississippian, Crinoidea): Comment. Geology. 2014;42(3):e320–321, and author reply e322. [Google Scholar]
- 22.Rouse GW, et al. Fixed, free, and fixed: The fickle phylogeny of extant Crinoidea (Echinodermata) and their Permian-Triassic origin. Mol Phylogenet Evol. 2013;66(1):161–181. doi: 10.1016/j.ympev.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Hemery LG, Roux M, Améziane N, Eléaume M. High-resolution crinoid phyletic inter-relationships derived from molecular data. Cah Biol Mar. 2013;54(4):511–523. [Google Scholar]
- 24.Falk H. From the photosensitizer hypericin to the photoreceptor stentorin – the chemistry of phenanthroperylene quinones. Angew Chem Int Ed. 1999;38(21):3116–3136. [PubMed] [Google Scholar]
- 25.Peters KE, Walters CC, Moldowan JM. The Biomarker Guide. 2nd Ed Cambridge Univ Press; Cambridge, UK: 2005. [Google Scholar]
- 26.Hagdorn H. Triassic: The crucial period of post-Palaeozoic crinoid diversification. Swiss J Palaeontol. 2011;130(1):91–112. [Google Scholar]
- 27.Rideout JA, Smith NB, Sutherland MD. Chemical defense of crinoids by polyketide sulphates. Experientia. 1979;35(10):1273–1274. doi: 10.1007/BF01963951. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi D, et al. New quinone sulfates from the crinoids Tropiometra afra macrodiscus and Oxycomanthus japonicus. Chem Pharm Bull. 2002;50(12):1609–1612. doi: 10.1248/cpb.50.1609. [DOI] [PubMed] [Google Scholar]
- 29.McClintock JB, Baker BJ, Baumiller TK, Messing CG. Lack of chemical defense in two species of stalked crinoids: Support for the predation hypothesis for Mesozoic bathymetric restriction. J Exp Mar Biol Ecol. 1999;232(1):1–7. [Google Scholar]
- 30.Kent RA, Smith IR, Sutherland MD. Pigments of marine animals. X. Substituted naphthopyrones from the crinoid Comantheria perplexa. Aust J Chem. 1970;23(11):2325–2335. [Google Scholar]
- 31.Baumiller TK, et al. Post-Paleozoic crinoid radiation in response to benthic predation preceded the Mesozoic marine revolution. Proc Natl Acad Sci USA. 2010;107(13):5893–5896. doi: 10.1073/pnas.0914199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryan PJ, Rittschof D, McClintock JB. Bioactivity of echinoderm ethanolic body-wall extracts: An assessment of marine bacterial attachment and macroinvertebrate larval settlement. J Exp Mar Biol Ecol. 1996;196(1–2):79–96. [Google Scholar]
- 33.Bussert R, Heinrich W-D, Aberhan M. The Tendaguru Formation (Late Jurassic to Early Cretaceous, southern Tanzania): Definition, palaeoenvironments, and sequence stratigraphy. Fossil Record. 2009;12(2):141–174. [Google Scholar]
- 34.Hess H. Lower Jurassic Posidonia Shale of Southern Germany. In: Hess H, Ausich WI, Brett CE, Simms MJ, editors. Fossil Crinoids. Cambridge Univ Press; Cambridge, UK: 1999. pp. 183–196. [Google Scholar]
- 35.Tucker ME, Wright VP. Carbonate Sedimentology. Blackwell Science; Oxford: 1990. [Google Scholar]
- 36.Falk H, Mayr E. Concerning bay salt and peri chelate formation of hydroxyphenanthroperylene quinones (fringelites) Monatsh Chem. 1997;128(4):353–360. [Google Scholar]
- 37.Beane BH. Crinoids varied in color at Le Grand, Iowa. Pan-Am Geol. 1941;76(2):155. [Google Scholar]
- 38.Ausich WI. Lower Mississippian Hampton Formation at LeGrand, Iowa, USA. In: Hess H, Ausich WI, Brett CE, Simms MJ, editors. Fossil Crinoids. Cambridge Univ Press; Cambridge, UK: 1999. pp. 135–138. [Google Scholar]
- 39.Rodewald G, Arnold R, Griesler J, Steglich W. Synthesis of hypericin and related meso-naphthodianthrones by alkaline dimerization of hydroxyanthraquinones. Angew Chem Int Ed Engl. 1977;16(1):46–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.