Significance
Insects are among the most robust organisms on the planet, surviving in virtually all environments and capable of surmounting a range of environmental stresses including desiccation and cold. Although desiccation and cold tolerance share many common traits, potential mechanisms for such linked responses remain unclear. Here we show that an insect neuropeptide gene is associated with tolerance of both desiccation and cold in Drosophila melanogaster, suggesting a novel mechanism in renal tubule epithelia that enhances survival of both desiccation and cold. Also, we can reverse RNAi-induced stress tolerance phenotypes in intact flies using rationally designed peptide mimetic analogs. We thus demonstrate the power of intervention in physiological processes controlled by neuropeptides, with potential for insect pest control.
Keywords: environmental stress, insects, neuropeptides, capa, desiccation and cold tolerance
Abstract
The success of insects is linked to their impressive tolerance to environmental stress, but little is known about how such responses are mediated by the neuroendocrine system. Here we show that the capability (capa) neuropeptide gene is a desiccation- and cold stress-responsive gene in diverse dipteran species. Using targeted in vivo gene silencing, physiological manipulations, stress-tolerance assays, and rationally designed neuropeptide analogs, we demonstrate that the Drosophila melanogaster capa neuropeptide gene and its encoded peptides alter desiccation and cold tolerance. Knockdown of the capa gene increases desiccation tolerance but lengthens chill coma recovery time, and injection of capa peptide analogs can reverse both phenotypes. Immunohistochemical staining suggests that capa accumulates in the capa-expressing Va neurons during desiccation and nonlethal cold stress but is not released until recovery from each stress. Our results also suggest that regulation of cellular ion and water homeostasis mediated by capa peptide signaling in the insect Malpighian (renal) tubules is a key physiological mechanism during recovery from desiccation and cold stress. This work augments our understanding of how stress tolerance is mediated by neuroendocrine signaling and illustrates the use of rationally designed peptide analogs as agents for disrupting protective stress tolerance.
All organisms live in variable environments, and the ability to adapt to change, via either evolution or phenotypic plasticity, is critical for survival. Insects are ectotherms with high surface area to volume ratios; maintaining water balance and tolerating temperature fluctuations thus are essential adaptations. In desiccating environments, a key mechanism used by insects to maintain water balance is to reduce the rate of water loss (1, 2). In low-temperature environments insects face both chilling and low availability of water, thus requiring that they be both cold and desiccation tolerant (3). Both cold and desiccation stress result in decreased hemolymph volume and increased hemolymph osmolarity (4), so it is reasonable to expect mechanistic overlap between these stresses. Indeed, similar molecular mechanisms, including calcium signaling pathways, appear to modulate cold and desiccation responses (5, 6). Several studies have shown that freeze-tolerant insects can improve their cold tolerance in response to a mild desiccation stress (7–9), and artificial selection for desiccation tolerance in Drosophila melanogaster impacts the ability to recover from chill coma (10).
Recent work has demonstrated that disrupted ion and water gradients between the insect gut and hemocoel contribute to low-temperature injury and that osmotic balance must be restored following exposure to cold (11–13). Ion and water balance in insects is regulated by the balance between excretion by the Malpighian tubules and absorption by the midgut and hindgut/rectum (14). Insect renal (Malpighian) tubules move fluid at the highest rates observed in biology and play key roles in transport and excretion of ions and water via transporters and water channels (15). Given the role of Malpighian tubules in osmoregulation, it is possible that tubule epithelia play additional, still undefined, roles in cold tolerance, in addition to those described for gut (16).
In arid environments, cuticular and respiratory water losses are the main routes of water loss in Drosophila species (17). Desiccation in drosophilids also is accompanied by changes in the expression of genes associated with environmental sensing and cuticular structure (18), and one study has shown that selection for desiccation-tolerance is linked to polymorphisms in Malpighian tubule ion transport genes (19). The latter observation thus implicates tubule function in abiotic stress tolerance, providing a physiological link between desiccation and cold tolerance via ion- and water-transport mechanisms.
Furthermore, although a potential role for the CNS in cold tolerance has been suggested (20), little is known about the control mechanisms that govern physiological responses to cold tolerance in insects. Such control mechanisms could occur via neuroendocrine signaling, in which Malpighian tubule function may act as an integrating physiological process for desiccation and cold tolerance, especially because insect osmoregulation is subject to highly sophisticated endocrine control, and several families of neuropeptides regulate diuresis (21). Among these are the capa peptides encoded by the capability (capa) neuropeptide gene (22). Capa peptides are distributed throughout the Insecta (23), including crop pests and disease vectors. In dipteran insects, capa is diuretic, acting on the Malpighian tubules to modulate cell-signaling and ion-transport pathways (24). CapaR, the G protein-coupled receptor for the capa peptides, is localized exclusively in tubule principal cells, and we have shown previously that targeted knockdown of capaR increases whole-fly survival under desiccation stress caused by reduced capa-stimulated diuresis (25). Capa/capaR signaling is functionally conserved in the tubules of dipteran disease vector species including mosquitoes and tsetse flies (24) and is of increasing interest as a target for insect control through the design of peptide mimetic analogs (26). Such agents overcome the inherent limitations of peptide physicochemical characteristics and increase their therapeutic potential, because blocking or overstimulating insect neuropeptide receptors may lead to reduction of pest fitness and/or death (27).
Here, using a combination of molecular genetics, physiology, and synthetic peptide mimetic analogs, we show that tolerance to desiccation and cold in D. melanogaster are dramatically impacted by capa peptide signaling. Furthermore, we provide data suggesting a key novel physiological role for Malpighian tubules in cold stress survival.
Results
Capa mRNA Levels Are Induced by Desiccation and Cold.
To determine capa gene sensitivity to previously published abiotic stressors in D. melanogaster, capa gene expression was assessed in wild-type flies subjected to cold, desiccation, starvation, heat, and osmotic, oxidative, and immune stressors. Capa mRNA levels were unchanged following immune challenge (Gram-negative or -positive), oxidative stress (H2O2 in the diet), osmotic stress (sorbitol or high salt in the diet) (Fig. 1A), or heat shock, but desiccation elicited significant increases in capa mRNA levels (more than twofold after 24 h compared with untreated controls), with expression returning to control levels within 6 h of recovery (Fig. S1A). Starvation stress in the presence of water failed to increase capa mRNA levels (Fig. 1A and Fig. S1B). Nonlethal cold stress (0 °C for 24 h) elicited a significant increase in capa mRNA levels, higher than the increases observed in response to desiccation (Fig. 1A). Capa expression increased steadily with the duration of cold stress (Fig. S1C) and returned to basal levels within 4 h of recovery. To determine if cold- and desiccation-induced capa up-regulation also occurred in Drosophila species originating from different environments, as well as in other dipteran species, cold- and/or desiccation-induced capa mRNA changes were assessed in Drosophila montana, a cold-tolerant species (28), Drosophila mojavensis, a desert-dwelling species adapted to harsh conditions including desiccation and high temperature (18, 29), and two warm-acclimatized mosquito species, Aedes aegypti and Anopheles gambiae (Fig. 1B). Capa mRNA was increased significantly by cold and desiccation in D. montana and in D. melanogaster but not in D. mojavensis and by desiccation in both mosquito species. Although only a small sampling of Diptera has been tested in this study, these data suggest that capa is a desiccation- and cold stress-responsive gene in diverse dipteran species.
Fig. 1.
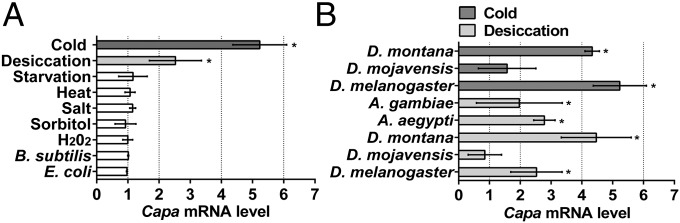
Desiccation- and cold stress-specific up-regulation of capa mRNA levels. (A) capa mRNA expression in D. melanogaster adults subjected to 24 h cold, desiccation, starvation, or heat stress, or fed for 24 h with NaCl, sorbitol, or H2O2, or injected with Gram-positive Bacillus subtilis bacteria or Gram-negative Escherichia coli bacteria. (B) capa mRNA expression in D. montana, D. mojavensis, and D. melanogaster during cold stress and in desiccated A. gambiae, A. aegypti, and Drosophila species. Data are expressed as fold change compared with nonstressed controls ± SEM (n = 3). Asterisks indicate a significant increase (P < 0.05, Student’s t test) compared with nonstressed control.
Capa Neuropeptides Are Released not During Desiccation and Cold Stress but During Recovery.
The D. melanogaster neuropeptide gene capa encodes two capa peptides (Drm-capa-1 and Drm-capa-2), homologs of the first identified capa peptide, Manduca sexta (Manse)-CAP2b (30), together with a third peptide, Drm-PK-1, which is a member of the pyrokinin peptide family (22). Both Drm-capa-1 and Drm-capa-2 perform a diuretic role in D. melanogaster Malpighian tubules (22), but the function of Drm-PK-1 is largely unknown. Drm-capa-1, Drm-capa-2, and Drm-PK-1 are produced from three pairs of ventral neuroendocrine cells in the abdominal neuromeres (Va neurons) (Fig. 2A) (22). The neurite network of the two anterior Va neurons pairs is confined to the dorsal ganglion and forms a neurohemal area, suggesting release of capa peptides into the hemolymph, whereupon they activate capaR, the G protein-coupled receptor for Drm-capa-1 and Drm-capa-2 (31), which is localized exclusively in Malpighian tubule principal cells (25).
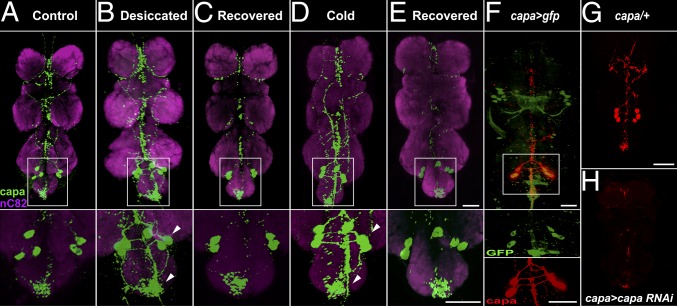
Fig. 2.
Capa precursor levels in capa-producing Va neurons under desiccation and cold stress and in targeted capa RNAi flies as shown by the intensity of capa precursor immunofluorescence (green) in the thoracoabdominal ganglion of wild-type D. melanogaster adults, in which neuropil were counterstained with anti-nC82 Mab (magenta). (A–E, Upper) Flies were fed normally (A) or desiccated for 24 h (B) and left to recover for 24 h on normal food (C) or were cold stressed for 24 h (D) and left to recover for 4 h on normal food (E). (A–E, Insets) Z-stack merge reveals capa precursor immunoreactivity in three pairs of Va neurons in the abdominal ganglion and the meshwork of varicosities in the ventral ganglion. (A–E, Lower) Higher magnification of abdominal ganglion shows increased intensity of immunoreactivity of Va neuron cell bodies and their projections (arrowheads) in response to desiccation and cold exposure compared with control; the capa precursor immunoreactivity was reduced under recovery conditions. (Scale bars, 50 μm.) (F) Capa-Gal4 drives expression in Va neurons. (Top) Localization of capa neurons using anti-capa precursor antibody (22) (red in Inset and Bottom showing a Va neuron) in capa-Gal4 > gfp progeny (green in Inset and Middle). The top image shows colocalization (yellow, merge). (Scale bar, 100 µm.) (G and H) Capa precursor immunoreactivity in control (capa-Gal4/+) (G) and progeny of targeted capa knockdown, capa-Gal4 > capa RNAi (capa > capa RNAi) (H).
To determine if capa mRNA levels are associated with capa precursor levels and are correlated with capa peptide release, we evaluated the quantity of capa peptides in the Va neurons under desiccation, cold, and recovery conditions. We used an antiserum specific to capa precursor peptide (22) to analyze the intensity of capa precursor immunofluorescence in the Va neurons of wild-type D. melanogaster flies that had been normally fed, desiccated, or chilled at 0 °C for 24 h and then left to recover on normal food (Fig. 2). Desiccated and cold-stressed flies displayed higher levels of capa precursor than controls (Fig. 2 B and D) as visualized by the intensity of immunoreactivity in the cell body of the Va neurons and their projections to the median and posterior ventral ganglion. In flies that recovered from both stresses, capa precursor levels decreased considerably and returned to levels similar to those in control flies (Fig. 2 C and E). A complete absence of immunoreactivity for some capa neuroendocrine cells following recovery likely indicates that these neurons discharge their content at high rates (Fig. 2E). Increased capa precursor immunofluorescence also was seen in capa neuroendocrine cells in desiccated larvae (Fig. S2 A and B), which also showed increased capa mRNA levels (Fig. S2C). Thus, it appears that capa peptides are released from neurons and neurites not during desiccation and cold stress (Fig. 2 B and D) but rather during recovery (Fig. 2 C and E). The high capa mRNA levels induced by desiccation and cold thus may be a priming response in preparation for the release of capa peptides upon recovery from desiccation and cold.
Silencing capa Gene Expression Results in Desiccation Tolerance.
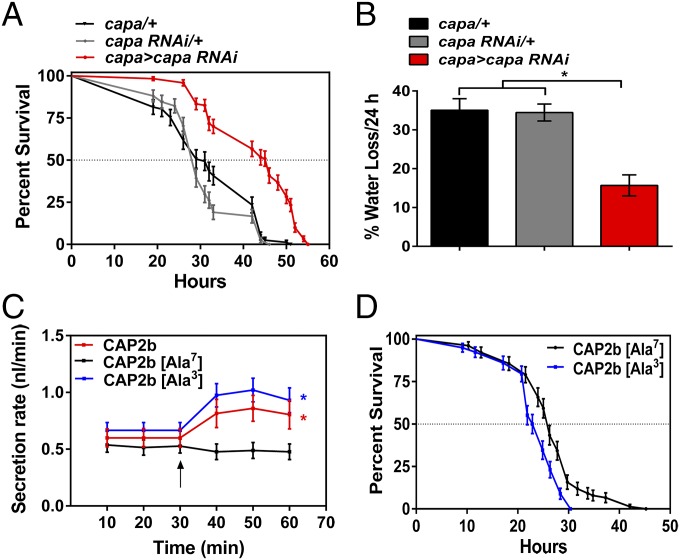
Given the induction of capa mRNA levels by desiccation and cold stress, we asked if capa gene expression in the Va neurons plays a functional role in organismal stress tolerance by targeted knockdown of capa, achieved by generation of a specific capa-Gal4 driver line for binary gene expression of capa RNAi. Capa-Gal4 clearly drives gene expression (gfp, marker gene) in the Va neurons (Fig. 2F), as demonstrated by colocalization of capa precursor antibody staining and GFP fluorescence. This capa-GAL4 line also shows higher specificity than other, previously available, capa-Gal4 lines (Fig. S3). Knockdown of capa gene expression using RNAi in only the capa-expressing neurons of adult flies was achieved using the capa-Gal4 driver line. This capa knockdown line (capa > capa RNAi) showed an ∼90% decrease in capa mRNA levels compared with parental control lines (Fig. S4) and greatly reduced capa precursor levels compared with controls (compare Fig. 2 G and H). Next, we investigated survival following desiccation and starvation stress in flies with reduced capa levels. During desiccation stress, capa > capa RNAi flies survived longer than controls (median life span of ∼46 h, as compared with the median control life span of ∼30 h) (Fig. 3A and Fig. S5A). Under starvation stress, no survival phenotype was observed in capa-knockdown flies (Fig. S5 B and C).
Fig. 3.
Consequence of targeted capa RNAi and injection of Manse-CAP2b [Ala3] capa peptide analogs on desiccation stress. (A) Reduced capa levels in Va neurons (capa > capa RNAi, red trace) alter survival of desiccated D. melanogaster flies. Stress tolerance was significantly higher in capa > capa RNAi flies than in controls (P < 0.001 against both controls; log-rank test; n = 100–130 male flies for the different genotypes). (B) Water loss decreases in desiccated capa-knockdown flies. capa > capa RNAi flies were exposed to desiccation, and water loss over 24 h was calculated for each genotype by subtracting the water content at 24 h from that at 0 h. A significant decrease in water loss was seen in the capa > capa RNAi flies. *P < 0.001, one-way ANOVA; n = 90–110 flies for the three genotypes). (C) Manse-CAP2b, Manse-CAP2b [Ala3], and Manse-CAP2b [Ala7] peptide- stimulated fluid transport rates in isolated tubules from wild-type adult D. melanogaster. Data are expressed as mean fluid transport rate (in nanoliters per minute) ± SEM, n = 6–10. *P < 0.05, basal rate compared with stimulated rates, Student’s t test. (D) Individual capa-knockdown capa > capa RNAi male flies were injected with Manse-CAP2b [Ala3] or Manse-CAP2b [Ala7] peptide analogs and subjected to desiccation. Stress tolerance was significantly lower after Manse-CAP2b [Ala3] peptide injection than after control peptide injection (P < 0.001; log-rank test; n > 80 male flies).
In D. melanogaster, desiccation resistance is improved by decreasing the rate of water loss or by increasing the water content of the body (1, 17, 32), and under desiccation conditions desiccated flies with reduced capa levels retained markedly larger abdomens, indicating a potentially higher hemolymph volume, compared with parental controls (Fig. S6A). To confirm that the abdominal phenotype was caused by increased body water content, we compared water loss in flies desiccated for 24 h with normally fed flies. Capa-knockdown male adults were significantly heavier than control flies (Fig. S6B) and lost only half as much of their body water following 24 h of desiccation (Fig. 3B). Main routes for water loss are thought to be via the cuticle and respiration (17). Thus, the reduced rate of water loss in capa-knockdown flies may be caused by combined reduction in respiratory, cuticular, and excretory water loss. However, the direct action of capa peptides on capaR exclusively expressed in the Malpighian tubules (25) suggests that excretory water loss may contribute more to desiccation tolerance than previously suspected.
Capa Peptide Analogs Reverse Desiccation Tolerance.
To determine a direct role for capa peptides in desiccation tolerance, we injected peptide analogs of capa, based on Manse-CAP2b (33, 34). As members of the same family, Manse-CAP2b shares an identical C-terminal 7-mer with Drm-capa-1 (LYAFPRVamide) with an additional pGlu cap, i.e., pGlu-LYAFPRVamide, which renders the sequence resistant to amino peptidases; thus it would be expected to have a longer residence time in the hemolymph. Also, the activity of Manse-CAP2b and Tyr3 Manse-CAP2b [Ala3] is substantial when applied to dipteran tubules (34). Indeed, application of these peptides to wild-type D. melanogaster tubules (Fig. 3C) showed stimulation of fluid secretion by Tyr3 Manse-CAP2b [Ala3] (EC50 = 33 nM) at rates similar to those of Manse-CAP2b (EC50 = 53 nM) (33) and the endogenous Drm-capa-1 (22), suggesting that Manse-CAP2b and the Tyr3 Manse-CAP2b [Ala3] analog act via capaR in D. melanogaster tubules. Replacement of the critical Arg7 Manse-CAP2b (Manse-CAP2b [Ala7]) (34) led to an analog with no significant activity (Fig. 3C). Manse-CAP2b [Ala3] and Manse-CAP2b [Ala7] then were injected into capa-knockdown flies to determine if circulating capa family peptides can modulate desiccation tolerance. Flies injected with the active Manse-CAP2b [Ala3] analog exhibited significantly reduced desiccation tolerance compared with flies injected with the control peptide Manse-CAP2b [Ala7] (median life spans of ∼23 h and 26 h, respectively) (Fig. 3D). These data thus suggest that capa peptides directly influence desiccation tolerance.
Capa-Knockdown Flies Display Slower Chill Coma Recovery.
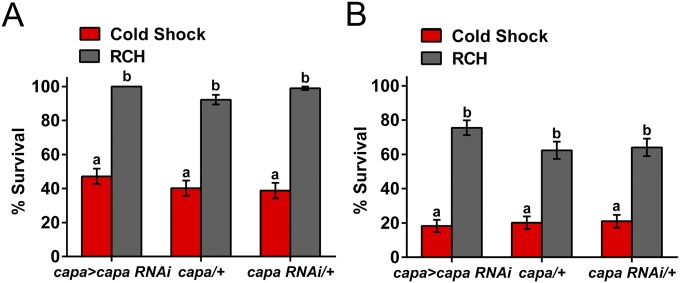
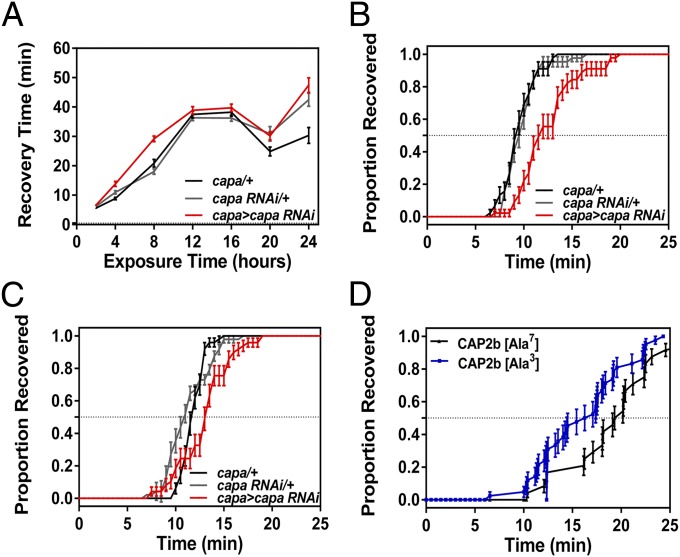
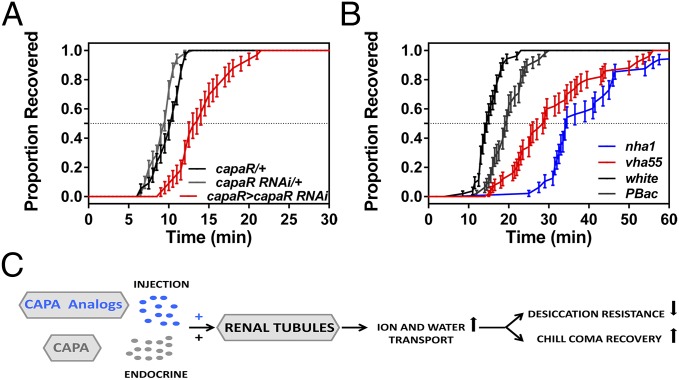
In addition to desiccation stress, cold stress induced a significant increase in capa mRNA levels. Therefore we assessed whether capa is involved in rapid responses to low temperature. Specifically, we measured whether capa knockdown impacts acute cold shock tolerance, the capacity for rapid cold hardening (RCH), and chill coma recovery time (CCR). RCH is an extreme case of rapid acclimation whereby brief (minutes to hours) nonlethal chilling significantly enhances cold-shock tolerance (35). CCR is a commonly used assay to assess the time required to regain coordinated movement after nonlethal chilling injury and frequently is used as an indicator of cold tolerance, especially in Drosophila species (16, 36). Our data show that sex, treatment, and their interaction impacted cold shock or RCH survival, but line did not (Table S1). None of the lines (male or female) differed in their intrinsic cold-shock tolerance (Fig. 4), and all lines showed a substantial increase in survival following RCH. For CCR experiments, capa-knockdown flies and parental lines were subject to different exposure times (4, 8, 12, 16, 20, and 24 h) and were assessed for recovery (Fig. 5A). Both exposure time and line had significant effects on CCR (Table S2) with capa-knockdown flies having longer recovery times than either control line across the entire range of exposure times (Table S3). In all lines, CCR increased linearly with exposure time but reached a plateau following 12 h of exposure, and differences in recovery time between capa-knockdown and control lines were more pronounced with shorter exposure times. Therefore a 4-h exposure was used for subsequent experiments with capa peptide analogs and genes downstream of capa, because capa has a clear impact on recovery time at 4-h exposure. Chill coma exposure experiments (0 °C, 4 h) were conducted with a larger sample size of the capa lines, and we found that capa strongly impacted CCR; mean recovery time of capa-knockdown male flies was >2 min, 25%, longer than in the parental control lines (capa > capa RNAi vs. capa/+, P < 1E-7; capa > capa RNAi vs. capa RNAi/+, P < 1E-4) (Fig. 5B). In contrast, the parental control lines did not differ in recovery times (P > 0.99). As seen in Fig. 5C, females showed the same patterns as males in the chill coma experiment.
Fig. 4.
Capa does not affect the ability to survive cold shock or undergo RCH. Cold shock and RCH in male (A) and female (B) flies. Bars represent proportion surviving, including SE. Different letters represent significant differences in survival between groups [generalized linear model (GLM), post hoc tests, P < 0.05]. (Table S1).
Fig. 5.
Capa improves chill coma recovery. (A) CCR for capa-knockdown males as a function of exposure time (in hours) at 0 °C. Data are expressed as mean time ± SEM; n = 19–22 flies per group. (B and C) Chill coma recovery curves for capa-knockdown male (B) and female (C) flies which were exposed to 0 °C for 4 h. In B and C, points represent the proportion recovered for each line at 30-s intervals, with SEs (Table S3). (D) Capa-knockdown male flies were exposed to 0 °C for 4 h and individually injected with Manse-CAP2b [Ala3] and Manse-CAP2b [Ala7] peptide analogs. Recovery time was significantly faster after Manse-CAP2b [Ala3] peptide injection than after control peptide injection (Mantel–Cox; P = 0.0067; n > 70 male flies).
Finally, we asked if capa peptides modulate cold-stress survival by testing the ability of capa peptide analogs to influence CCR. Capa-knockdown flies were preexposed to nonlethal chilling (0 °C, 4 h), were injected individually with the active [Manse-CAP2b (Ala3)] and inactive [Manse-CAP2b (Ala7)] analogs as in previous experiments, and were assessed for recovery. CCR was ∼20% shorter after Manse-CAP2b [Ala3] peptide injection than after control peptide injection (Fig. 5D). Because treatment of cold-stressed capa-knockdown flies with an active capa analog during the recovery period improves CCR, this result suggests that release of endogenous capa peptides during recovery from cold stress helps restore ion and water homeostasis. These data thus provide evidence that capa peptides directly modulate cold-stress tolerance.
CCR Is Associated with Capa-Modulated Ion Homeostasis.
Increased desiccation tolerance caused by reduced water loss in capa-knockdown flies was associated with increased CCR. Both these phenotypes were capa-dependent and could be reversed with active capa peptide analogs. CapaR is localized exclusively in Malpighian tubule principal cells, and we have shown previously that targeted knockdown of capaR mediates whole-fly desiccation tolerance (25). We also show that knockdown of capaR significantly increases CCR (Fig. 6A), suggesting that functional capa/capaR signaling in Malpighian tubules is necessary for CCR. Recent work indicates that CCR is influenced primarily by the magnitude of ion disruption in the gut during cold exposure and the time taken to restore ion and water balance to regain motor control, potentially via ion-motive epithelial ATPases (12). Vacuolar H+-ATPase (V-ATPase) is most highly expressed in Malpighian tubule principal cells (37), which also uniquely express capaR (25). Capa increases V-ATPase activity by elevating mitochondrial calcium and ATP levels (38), increasing tubule diuresis. V-ATPase sets up a proton gradient, which then drives Na+ and K+ excretion through one or more exchangers such as sodium/proton exchanger (NHA) 1 and 2 (39). Thus, we propose that activation of capa signaling mediates recovery from nonlethal chill coma either by minimizing ion and water dysregulation during cold or by stimulating the redistribution of ions and water via the diuretic action of capa peptides on the tubule during recovery, or by both mechanisms (12). Therefore, we tested the possibility that capa-induced ion transport via V-ATPase and NHA1 mediates recovery from chilling injury by assessing CCR in a V-ATPase subunit mutant, vha55 (40), and an nha1 mutant. Accordingly, we observed significantly delayed CCR for both mutants compared with controls, with the nha1 mutant displaying the longest CCR (Fig. 6B). This finding indicates that CCR is associated with ion transport in the tubule by V-ATPase and NHA1.
Fig. 6.
Knockdown of capaR and ion transport genes alter chill coma recovery. (A) Reduced capaR levels in the Malpighian tubule (capaR > capaR RNAi, red trace) lengthened CCR. (B) Recovery curves for vha55 (red trace) and nha1 (blue trace) ion transport mutants compared with two control strains, white (w1118) and Exelixis PBac flies. In A and B, points represent the proportion recovered for each line at 30-s intervals, with SEs (n > 80 male flies). (C) Model for a role for capa in cold and desiccation tolerance. When capa signaling is elevated, fluid excretion is increased and impedes desiccation tolerance, but the altered ion and osmolyte levels protect against chilling and allow rapid recovery. Accordingly, artificial stimulation of capa signaling reduces survival after desiccation and speeds recovery from cold.
Discussion
Low-temperature and desiccation tolerance are closely linked at the molecular level (5, 6), and our data provide evidence that neuropeptide signaling may provide potential unifying pathway(s) between cold and desiccation tolerance, because capa mRNA levels are elevated in response to both stresses. We also provide functional data from manipulation of capa gene expression specifically in capa-expressing neurons, which show a significant impact on desiccation tolerance. Capa-knockdown flies display improved desiccation tolerance that can be reversed by capa peptide analogs. Is this desiccation phenotype specific only to the capa neuropeptides? The capa gene encodes three peptides, Drm-capa-1 and Drm-capa-2, which are diuretic, and also Drm-PK-1, which activates the Drm-PK-1 receptor (41) but not capaR (25). The Drm-PK-1 receptor gene is highly expressed in salivary gland, carcass, and the tracheal system (37), implying a potential function of Drm-PK-1 in feeding and/or cuticular and respiratory transpiration, which are associated with desiccation stress responses. Finally, we cannot exclude the possibility that capa neurons may release capa/PK-1 peptides in a paracrine fashion within the CNS, as do the insulin-producing cells, which are known to be involved in modulating responses to temperature and desiccation stress (42, 43).
Several studies suggest that cold tolerance is mediated by ion and water movements in insect gut (12, 16) and/or muscle (13, 44). At low temperatures, ion and water homeostasis is disrupted by reduced active transport by ion-motive ATPases. This disruption leads to the loss of Na+ and water from hemolymph into the gut and to increased K+ levels in hemolymph (12), resulting in reduced nerve or muscle excitation.
We show here that capa has a significant effect on cold tolerance by regulating Drosophila Malpighian tubule osmoregulation. By manipulating capa peptide expression precisely only in capa-producing neurons, we show that CCR is lengthened significantly in capa-knockdown flies. CCR is thought to be determined by the time required to restore cellular ion gradients during recovery (12, 16); this model suggests that flies with faster recovery times have lower levels of ion disequilibrium in response to cold, restore ion gradients more quickly, or a combination of the two. In our experiments, CCR increased with length of cold exposure, reaching a plateau at 12 h of exposure. However, capa knockdown lengthened CCR only during the linear increase in CCR observed during the first 8 h of cold exposure. This observation may be explained by one or more of the following scenarios: (i) All lines eventually reach the same level of ion disequilibrium after 12 h of exposure, which is why recovery times reach a plateau, but capa-knockdown flies reach this new equilibrium more quickly. This scenario is consistent with current models of the nature of the recovery plateau observed in chill coma experiments (16). (ii) Recovery time during the plateau and/or postplateau (16) is dependent on osmoregulation by an epithelium other than the tubules, such as the midgut or hindgut, that is not regulated by capa. (iii) Recovery time during the plateau is determined by some other form of chilling injury (e.g., membrane or protein damage), and repair of this injury is capa independent. Although teasing apart these mechanisms is beyond the scope of the current study, our data suggest that capa influences CCR, at least in part, by regulating tubule osmoregulation and ion/water transport during recovery. Specifically, capa’s role during recovery is supported by the following data: immunohistochemical staining shows that that capa may be released only during recovery from cold stress (Fig. 2); capa-knockdown flies have the same unstimulated tubule activity as control flies (Fig. S7); and application of capa analogs during recovery restores CCR in capa-knockdown flies. Moreover, based on a standard temperature coefficient (Q10) estimate of 2.5 for Drosophila metabolism (45), the metabolic rate of flies at 0 °C is only ∼10% of that of flies at 25 °C, so the ability to maintain energetically expensive ion gradients while at 0 °C is likely minimal, and it is unlikely that action potentials are driving the release of neuropeptides.
Recent work also has demonstrated a role for the Na+/K+ ATPase in cold tolerance in Drosophila; cold-acclimatized Drosophila species have reduced Na+/K+ ATPase activity (46). In D. melanogaster, Na+/K+ ATPase gene expression is highest in Malpighian tubules (37), and the transporter is localized at the basolateral membrane of the tubule principal cells (47). These observations further suggest that the tubule, in addition to gut, may be a key epithelium in cold tolerance. We confirm this idea by demonstrating that capa-induced tubule ion transport via other ATPases and transporters (V-ATPase and NHA1) mediates recovery to chilling injury. By increasing V-ATPase activity and therefore the apical membrane transport of Na+ and K+ from the cell into the lumen, capa also may speed CCR by potentially stimulating excretion of excess hemolymph K+ (44) and redistribution of water caused by the diuretic action of capa peptides. Taken together, our results suggest a key role for capa/capaR-associated CCR, which occurs via ion and water homeostasis in Malpighian tubules. Interestingly, expression levels of candidate genes for cold tolerance (smp-30, several hsps, and frost) (48–50) are all enriched in tubules (37), further suggesting a key physiological role for Malpighian tubules in cold stress survival.
Finally, although we provide definitive data for a previously unidentified role of capa peptides in cold and desiccation tolerance, it is possible that other neuropeptides that modulate tubule water and ion homeostasis, e.g., DH 31, DH44, and insect kinin (51), also may affect cold and desiccation responses. However, unlike the capa peptides, the targets of these other peptides may not be tubule specific.
In conclusion, we have shown that neuroendocrine responses to desiccation and cold are altered by the action of capa neuropeptides on Malpighian tubule ion transport and also can be modulated by synthetic peptide analogs (Fig. 6C). Understanding the molecular basis for organismal survival tolerance to specific environmental stresses may augment the development of stratagems to control insect pest populations.
Materials and Methods
Drosophila Stocks and Generation of Transformants.
All lines were maintained on a standard Drosophila diet at 22 °C, 55% humidity with a 12:12 h light:dark photoperiod. Stocks and generation of the capa-Gal41 driver are described in SI Materials and Methods.
Quantitative RT-PCR.
capa mRNA levels in Canton-S flies were assessed as described in SI Materials and Methods.
Malpighian Tubule Fluid Transport Assays.
Assays were carried out on intact, live tubules as described in SI Materials and Methods.
Immunofluorescence.
Immunofluorescence in larval and adult nervous systems was assayed using anti-GFP, -nc82, and -capa antibodies as described in SI Materials and Methods.
Gravimetric Estimates of Body Water.
Gravimetric estimates of body water were made by measuring wet and dry body weight after desiccation in parental and capa > capa RNAi flies; see SI Materials and Methods for further details.
Assays of Gene Expression and/or Survival in Response to Different Stresses.
Flies and/or mosquitoes were subject to different stressors as described in SI Materials and Methods, and capa gene expression (Table S4) in either tubules and/or organismal survival was scored in response to each stress.
Peptide Analog Injections.
Peptide analog nanoinjections were performed on capa > capa RNAi flies as described in SI Materials and Methods.
Data Analysis.
Data analysis was performed for each experimental condition using relevant methods as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank K. Lukowiak, W. Bendena, and A. Dornan for discussions and comments on the manuscript; G. Overend and L. Ranford-Cartwright for mosquitoes; W. Etges for D. mojavensis; and D. Parker for D. montana bioinformatics. This work was funded by grants from the UK Biotechnology and Biological Sciences Research Council (BB/G020620 and BB/L002647/1) (to S.-A.D., J.A.T.D., and S.T.); US Department of Agriculture/Department of Defense Deployed War Fighters Protection Grant Initiative (#6202-22000-029-00D) and US–Israel Binational Agricultural Research and Development Fund (BARD) (IS-4205-09C) (R.J.N.); and the National Science Foundation (IOS-0840772) (D.L.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501518112/-/DCSupplemental.
References
- 1.Gibbs AG, Matzkin LM. Evolution of water balance in the genus Drosophila. J Exp Biol. 2001;204(Pt 13):2331–2338. doi: 10.1242/jeb.204.13.2331. [DOI] [PubMed] [Google Scholar]
- 2.Chown SL, Sørensen JG, Terblanche JS. Water loss in insects: An environmental change perspective. J Insect Physiol. 2011;57(8):1070–1084. doi: 10.1016/j.jinsphys.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Denlinger DL, Lee RE., Jr . Low Temperature Biology of Insects. Cambridge Univ Press; Cambridge, UK: 2010. [Google Scholar]
- 4.Zachariassen KE, Einarson S. Regulation of body fluid compartments during dehydration of the tenebrionid beetle Rhytinota praelonga. J Exp Biol. 1993;182:283–289. [Google Scholar]
- 5.Teets NM, Denlinger DL. Surviving in a frozen desert: Environmental stress physiology of terrestrial Antarctic arthropods. J Exp Biol. 2014;217(Pt 1):84–93. doi: 10.1242/jeb.089490. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair BJ, Ferguson LV, Salehipour-shirazi G, MacMillan HA. Cross-tolerance and cross-talk in the cold: Relating low temperatures to desiccation and immune stress in insects. Integr Comp Biol. 2013;53(4):545–556. doi: 10.1093/icb/ict004. [DOI] [PubMed] [Google Scholar]
- 7.Sinclair BJ, Chown SL. Rapid responses to high temperature and desiccation but not to low temperature in the freeze tolerant sub-Antarctic caterpillar Pringleophaga marioni (Lepidoptera, Tineidae) J Insect Physiol. 2003;49(1):45–52. doi: 10.1016/s0022-1910(02)00225-1. [DOI] [PubMed] [Google Scholar]
- 8.Hayward SA, Rinehart JP, Sandro LH, Lee RE, Jr, Denlinger DL. Slow dehydration promotes desiccation and freeze tolerance in the Antarctic midge Belgica antarctica. J Exp Biol. 2007;210(Pt 5):836–844. doi: 10.1242/jeb.02714. [DOI] [PubMed] [Google Scholar]
- 9.Levis NA, Yi SX, Lee RE., Jr Mild desiccation rapidly increases freeze tolerance of the goldenrod gall fly, Eurosta solidaginis: Evidence for drought-induced rapid cold-hardening. J Exp Biol. 2012;215(Pt 21):3768–3773. doi: 10.1242/jeb.076885. [DOI] [PubMed] [Google Scholar]
- 10.Sinclair BJ, Nelson S, Nilson TL, Roberts SP, Gibbs AG. The effect of selection for desiccation resistance on cold tolerance of Drosophila melanogaster. Physiol Entomol. 2007;32:322–327. [Google Scholar]
- 11.Kostál V, Vambera J, Bastl J. On the nature of pre-freeze mortality in insects: Water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. J Exp Biol. 2004;207(Pt 9):1509–1521. doi: 10.1242/jeb.00923. [DOI] [PubMed] [Google Scholar]
- 12.MacMillan HA, Williams CM, Staples JF, Sinclair BJ. Reestablishment of ion homeostasis during chill-coma recovery in the cricket Gryllus pennsylvanicus. Proc Natl Acad Sci USA. 2012;109(50):20750–20755. doi: 10.1073/pnas.1212788109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Findsen A, Andersen JL, Calderon S, Overgaard J. Rapid cold hardening improves recovery of ion homeostasis and chill coma recovery time in the migratory locust, Locusta migratoria. J Exp Biol. 2013;216(Pt 9):1630–1637. doi: 10.1242/jeb.081141. [DOI] [PubMed] [Google Scholar]
- 14.Dow JAT. Excretion and salt and water regulation. In: Chapman RF, Simpson SJ, Douglas AE, editors. The Insects, Structure and Function. 5th Ed Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 15.Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–374. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 16.Macmillan HA, Sinclair BJ. Mechanisms underlying insect chill-coma. J Insect Physiol. 2011;57(1):12–20. doi: 10.1016/j.jinsphys.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs AG, Fukuzato F, Matzkin LM. Evolution of water conservation mechanisms in Drosophila. J Exp Biol. 2003;206(Pt 7):1183–1192. doi: 10.1242/jeb.00233. [DOI] [PubMed] [Google Scholar]
- 18.Rajpurohit S, Oliveira CC, Etges WJ, Gibbs AG. Functional genomic and phenotypic responses to desiccation in natural populations of a desert drosophilid. Mol Ecol. 2013;22(10):2698–2715. doi: 10.1111/mec.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telonis-Scott M, Gane M, DeGaris S, Sgrò CM, Hoffmann AA. High resolution mapping of candidate alleles for desiccation resistance in Drosophila melanogaster under selection. Mol Biol Evol. 2012;29(5):1335–1351. doi: 10.1093/molbev/msr294. [DOI] [PubMed] [Google Scholar]
- 20.Yoder JA, Benoit JB, Denlinger DL, Rivers DB. Stress-induced accumulation of glycerol in the flesh fly, Sarcophaga bullata: Evidence indicating anti-desiccant and cryoprotectant functions of this polyol and a role for the brain in coordinating the response. J Insect Physiol. 2006;52(2):202–214. doi: 10.1016/j.jinsphys.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Schooley DA, Horodyski FM, Coast GM. Hormones controlling homeostasis in insects. In: Gilbert LI, editor. Insect Endocrinology. Elsevier; Oxford: 2012. pp. 366–429. [Google Scholar]
- 22.Kean L, et al. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1297–R1307. doi: 10.1152/ajpregu.00584.2001. [DOI] [PubMed] [Google Scholar]
- 23.Predel R, Wegener C. Biology of the CAPA peptides in insects. Cell Mol Life Sci. 2006;63(21):2477–2490. doi: 10.1007/s00018-006-6187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies SA, et al. Signaling by Drosophila capa neuropeptides. Gen Comp Endocrinol. 2013;188:60–66. doi: 10.1016/j.ygcen.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Terhzaz S, et al. Mechanism and function of Drosophila capa GPCR: A desiccation stress-responsive receptor with functional homology to human neuromedinU receptor. PLoS ONE. 2012;7(1):e29897. doi: 10.1371/journal.pone.0029897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachman RJ, et al. Evaluation of insect CAP2b analogs with either an (E)-alkene, trans- or a (Z)-alkene, cis-Pro isostere identifies the Pro orientation for antidiuretic activity in the stink bug. Peptides. 2013;41:101–106. doi: 10.1016/j.peptides.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Nachman RJ, Pietrantonio PV, Coast GM. Toward the development of novel pest management agents based upon insect kinin neuropeptide analogues. Ann N Y Acad Sci. 2009;1163:251–261. doi: 10.1111/j.1749-6632.2008.03633.x. [DOI] [PubMed] [Google Scholar]
- 28.Vesala L, Salminen TS, Laiho A, Hoikkala A, Kankare M. Cold tolerance and cold-induced modulation of gene expression in two Drosophila virilis group species with different distributions. Insect Mol Biol. 2012;21(1):107–118. doi: 10.1111/j.1365-2583.2011.01119.x. [DOI] [PubMed] [Google Scholar]
- 29.Matzkin LM. Ecological genomics of host shifts in Drosophila mojavensis. Adv Exp Med Biol. 2014;781:233–247. doi: 10.1007/978-94-007-7347-9_12. [DOI] [PubMed] [Google Scholar]
- 30.Huesmann GR, et al. Amino acid sequence of CAP2b, an insect cardioacceleratory peptide from the tobacco hawkmoth Manduca sexta. FEBS Lett. 1995;371(3):311–314. doi: 10.1016/0014-5793(95)00929-4. [DOI] [PubMed] [Google Scholar]
- 31.Park Y, Kim YJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA. 2002;99(17):11423–11428. doi: 10.1073/pnas.162276199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folk DG, Bradley TJ. Evolved patterns and rates of water loss and ion regulation in laboratory-selected populations of Drosophila melanogaster. J Exp Biol. 2003;206(Pt 16):2779–2786. doi: 10.1242/jeb.00498. [DOI] [PubMed] [Google Scholar]
- 33.Davies SA, et al. CAP2b, a cardioacceleratory peptide, is present in Drosophila and stimulates tubule fluid secretion via cGMP. Am J Physiol. 1995;269(6 Pt 2):R1321–R1326. doi: 10.1152/ajpregu.1995.269.6.R1321. [DOI] [PubMed] [Google Scholar]
- 34.Nachman RJ, Coast GM. Structure-activity relationships for in vitro diuretic activity of CAP2b in the housefly. Peptides. 2007;28(1):57–61. doi: 10.1016/j.peptides.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Lee RE, Jr, Chen CP, Denlinger DL. A rapid cold-hardening process in insects. Science. 1987;238(4832):1415–1417. doi: 10.1126/science.238.4832.1415. [DOI] [PubMed] [Google Scholar]
- 36.Ransberry VE, MacMillan HA, Sinclair BJ. The relationship between chill-coma onset and recovery at the extremes of the thermal window of Drosophila melanogaster. Physiol Biochem Zool. 2011;84(6):553–559. doi: 10.1086/662642. [DOI] [PubMed] [Google Scholar]
- 37.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39(6):715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 38.Terhzaz S, et al. Differential gel electrophoresis and transgenic mitochondrial calcium reporters demonstrate spatiotemporal filtering in calcium control of mitochondria. J Biol Chem. 2006;281(27):18849–18858. doi: 10.1074/jbc.M603002200. [DOI] [PubMed] [Google Scholar]
- 39.Day JP, et al. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci. 2008;121(Pt 15):2612–2619. doi: 10.1242/jcs.033084. [DOI] [PubMed] [Google Scholar]
- 40.Allan AK, Du J, Davies SA, Dow JAT. Genome-wide survey of V-ATPase genes in Drosophila reveals a conserved renal phenotype for lethal alleles. Physiol Genomics. 2005;22(2):128–138. doi: 10.1152/physiolgenomics.00233.2004. [DOI] [PubMed] [Google Scholar]
- 41.Cazzamali G, Torp M, Hauser F, Williamson M, Grimmelikhuijzen CJ. The Drosophila gene CG9918 codes for a pyrokinin-1 receptor. Biochem Biophys Res Commun. 2005;335(1):14–19. doi: 10.1016/j.bbrc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 42.Broughton SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102(8):3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J, Becnel J, Nichols CD, Nässel DR. Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT1A receptor. Cell Mol Life Sci. 2012;69(3):471–484. doi: 10.1007/s00018-011-0789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Findsen A, Pedersen TH, Petersen AG, Nielsen OB, Overgaard J. Why do insects enter and recover from chill coma? Low temperature and high extracellular potassium compromise muscle function in Locusta migratoria. J Exp Biol. 2014;217(Pt 8):1297–1306. doi: 10.1242/jeb.098442. [DOI] [PubMed] [Google Scholar]
- 45.Berrigan D, Partridge L. Influence of temperature and activity on the metabolic rate of adult Drosophila melanogaster. Comp Biochem Physiol A Physiol. 1997;118(4):1301–1307. doi: 10.1016/s0300-9629(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 46.MacMillan HA, et al. Parallel ionoregulatory adjustments underlie phenotypic plasticity and evolution of Drosophila cold tolerance. J Exp Biol. 2015;218:423–432. doi: 10.1242/jeb.115790. [DOI] [PubMed] [Google Scholar]
- 47.Torrie LS, et al. Resolution of the insect ouabain paradox. Proc Natl Acad Sci USA. 2004;101(37):13689–13693. doi: 10.1073/pnas.0403087101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Mol Biol. 2007;16(4):435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 49.Colinet H, Lee SF, Hoffmann A. Knocking down expression of Hsp22 and Hsp23 by RNA interference affects recovery from chill coma in Drosophila melanogaster. J Exp Biol. 2010;213(Pt 24):4146–4150. doi: 10.1242/jeb.051003. [DOI] [PubMed] [Google Scholar]
- 50.Colinet H, Lee SF, Hoffmann A. Functional characterization of the Frost gene in Drosophila melanogaster: Importance for recovery from chill coma. PLoS ONE. 2010;5(6):e10925. doi: 10.1371/journal.pone.0010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coast GM, Garside CS. Neuropeptide control of fluid balance in insects. Ann N Y Acad Sci. 2005;1040:1–8. doi: 10.1196/annals.1327.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.