Significance
Formation of neutrophil extracellular traps (NETs) is a recently described process by which neutrophils combat microbial pathogens. Recent studies demonstrate causative relationships between NETs and debilitating disorders such as rheumatoid arthritis, vasculitis, thrombosis, cystic fibrosis, and acute respiratory distress syndrome. However, the understanding of signaling pathways governing the process termed “NETosis” remains elusive. Two major types of NETosis have been reported; however, the mechanistic differences between these two types are not clearly established. Here we describe that NETosis induced by calcium ionophores is fast, NADPH-oxidase independent, and is mediated by mitochondrial reactive oxygen species (ROS) and a calcium-activated small conductance potassium channel. Thus, drugs that target mitochondrial ROS production or the potassium channels may provide previously unidentified therapeutic approaches for combating disorders with unregulated NETosis.
Keywords: neutrophils, neutrophil extracellular traps, NETosis, NADPH oxidase, SK channels
Abstract
Neutrophils cast neutrophil extracellular traps (NETs) to defend the host against invading pathogens. Although effective against microbial pathogens, a growing body of literature now suggests that NETs have negative impacts on many inflammatory and autoimmune diseases. Identifying mechanisms that regulate the process termed “NETosis” is important for treating these diseases. Although two major types of NETosis have been described to date, mechanisms regulating these forms of cell death are not clearly established. NADPH oxidase 2 (NOX2) generates large amounts of reactive oxygen species (ROS), which is essential for NOX-dependent NETosis. However, major regulators of NOX-independent NETosis are largely unknown. Here we show that calcium activated NOX-independent NETosis is fast and mediated by a calcium-activated small conductance potassium (SK) channel member SK3 and mitochondrial ROS. Although mitochondrial ROS is needed for NOX-independent NETosis, it is not important for NOX-dependent NETosis. We further demonstrate that the activation of the calcium-activated potassium channel is sufficient to induce NOX-independent NETosis. Unlike NOX-dependent NETosis, NOX-independent NETosis is accompanied by a substantially lower level of activation of ERK and moderate level of activation of Akt, whereas the activation of p38 is similar in both pathways. ERK activation is essential for the NOX-dependent pathway, whereas its activation is not essential for the NOX-independent pathway. Despite the differential activation, both NOX-dependent and -independent NETosis require Akt activity. Collectively, this study highlights key differences in these two major NETosis pathways and provides an insight into previously unknown mechanisms for NOX-independent NETosis.
Neutrophils are the first responders to invading pathogens and play a pivotal role in innate immune defense. They are present in large numbers in circulation and transmigrate to the sites of infection in response to chemotactic signals. Once at the site of infection, the activated neutrophils fight pathogens by various means, including oxidative burst, phagocytosis, and the formation and release of neutrophil extracellular traps (NETs) in a process termed “NETosis” (1). NETs are made of DNA and are decorated with antimicrobial proteins and peptides (2, 3).
To date, two distinct forms of NETosis have been described based on their requirement for NADPH oxidases 2 (NOX2). In the NOX-dependent pathway, pharmacological inhibition of NOX2 results in a inhibition of reactive oxygen species (ROS) and subsequent NET formation (1, 4). Furthermore, neutrophils isolated from patients with chronic granulomatous disease (CGD) fail to form NETs for certain stimuli because these cells have deficiencies in NOX-mediated ROS production (5). Therefore, the generation of ROS by NOX enzyme complex is considered to be crucial for NETosis. In contrast, several reports have shown the existence of NOX-independent NETosis that can be induced by certain other stimuli including calcium ionophore (6, 7). Although we are beginning to understand the molecular pathways governing NOX-dependent NETosis, the mechanisms for the NOX-independent pathway of NETosis are not well understood.
It has been shown that the calcium-activated potassium channel of small conductance (SK channel), which is the major calcium-activated potassium channel known to be present on neutrophils (8–11), mediates NOX-independent neutrophil “apoptosis” (11). In this pathway, SK channels activate mitochondrial ROS production. Interestingly, neutrophils exhibit potassium current activated by calcium influx, and potassium has been shown to be important for neutrophil-mediated killing of several microbial pathogens (8, 9, 12, 13). However, the involvement of potassium channels and bacterial killing in the context of NETs has not been examined.
In this study, we set out to investigate the role of mitochondrial ROS in the calcium ionophore-mediated NETosis. We show that calcium ionophore-activated NETosis is fast, NOX-independent, and shows greatly reduced activation of ERK and a moderate level of activation of Akt, whereas the activation of p38 is similar to that of the NOX-dependent pathway. The investigation of the mechanism of the NOX-independent pathway revealed that this pathway requires mitochondrial ROS production. Furthermore, we also show that activation of the SK channel is both necessary and sufficient to induce NETosis. Therefore, we reveal a previously unidentified calcium-induced mitochondrial ROS-dependent but NOX-independent NETosis pathway.
Results
The Calcium Ionophore-Induced NOX-Independent NETosis Is Distinct from NOX-Dependent NETosis.
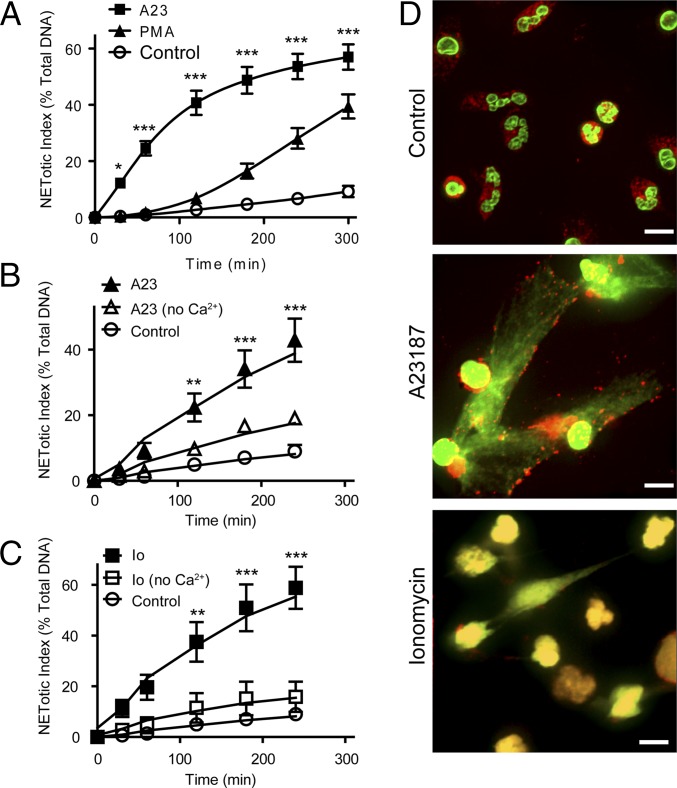
Much of our current understanding of the molecular mechanisms that govern the NOX-dependent pathway results from studies using phorbol 12-myristate 13-acetate (PMA) (14–16). The use of pharmacological agonists such as PMA as the NOX-dependent NET inducer is attractive due to its ability to uniformly activate the cells in culture. To elucidate mechanisms of NOX-independent NETosis, we used a calcium ionophore, A23187. Furthermore, we used PMA to make a side-by-side comparison of the two pathways. To observe the kinetics of NET release induced by the two agonists, a plate reader assay was used. The plate reader assay is routinely used for monitoring NET DNA release (4, 6, 17), and it detects extracellular NETs with a cell impermeable, extracellular DNA dye Sytox Green. The assays show that A23187 activates human neutrophils to release NETs (Fig. 1A). The time course analysis of NET release also confirms that the NETosis induced by PMA and A23187 follows distinct kinetics (Fig. 1A).
Fig. 1.
Calcium ionophores induce rapid NETosis. (A) NET release in response to A23187 or PMA was measured using a plate reader assay (n = 5). A23187- and PMA-mediated NETosis in human neutrophils follow different kinetics of NET release. Ionophore-induced NETosis is faster than that of PMA-induced NETosis. (B and C) Neutrophils were activated with A23187 (B) or ionomycin (C) in the presence or absence of calcium. NETosis was measured using a plate reader assay. NET release is expressed as percentage of total DNA (n = 3). Calcium ionophore-induced NETosis requires extracellular calcium. (D) Neutrophils were incubated in the presence or absence of A23187 (4 µM) or ionomycin (5 µM) for 300 min. Cells were stained for DNA (green) and MPO (red). Immunofluorescence imaging shows that the calcium ionophore A23187 and ionomycin induce NETosis. (Scale bar, 10 µm.) Images are representative of three independent experiments. A23, A23187; Ca2+, calcium chloride; Io, ionomycin. *P < 0.05; **P < 0.01; ***P < 0.001 (A–C, two-way ANOVA).
NETosis induced by certain strains of Staphylococcus aureus has been shown to be both NOX dependent and independent (6, 7). Nevertheless, NETosis induced by the RN4220 strain of S. aureus was inhibited by diphenyleneiodonium (DPI), and thus, NOX dependent (Fig. S1). Therefore, we have tested another NETosis-inducing agonist, ionomycin, a natural calcium ionophore produced by a different Gram-positive bacteria Streptomyces conglobatus. The rapid NETosis induced by A23187 was also observed in NETosis induced by ionomycin (Fig. S2A). Therefore, we used ionomycin as the natural agonist of NOX-independent NETosis for the remainder of the study.
Next, the requirement for the presence of extracellular calcium was assessed in NETosis induced by PMA, A23187, and ionomycin. The plate reader assays show that the activation of neutrophils with A23187 or ionomycin in the absence of extracellular calcium results in a significant reduction of NETosis (P < 0.001; Fig. 1 B and C and Fig. S2B). In contrast, the activation of cells with PMA in the absence of extracellular calcium does not result in a significant reduction in NETosis (Fig. S2B). To verify that NETs are being formed by A23187- and ionomycin-activated cells, an immunofluorescence assay was performed. The immunofluorescence images confirm that the cells activated with the calcium ionophores release NETs as evidenced by the colocalization of extracellular DNA with myeloperoxidase (MPO) (Fig. 1D and Fig. S2C). Taken together, these data show that NETosis induced by the calcium ionophores A23187 and ionomycin is distinct from that induced by PMA.
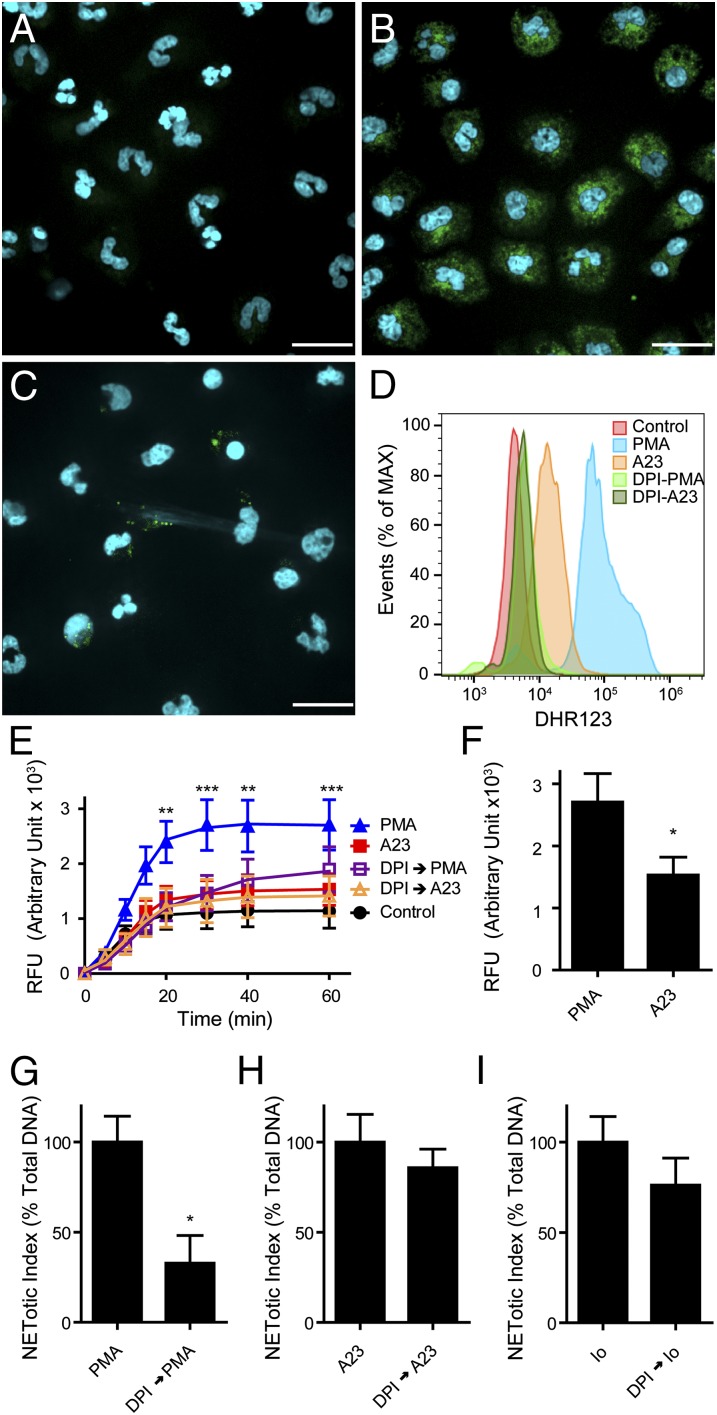
We next asked whether NETosis induced by the calcium ionophores required ROS production by the NOX2 enzyme. To assess the importance of ROS, we first determined whether ROS is produced during PMA- and A23187-induced NETosis. To detect cytosolic ROS produced by NOX2, we used dihydrorhodamine (DHR)123, a fluorescent indicator of cytosolic ROS. Confocal imaging of the cells loaded with DHR123 and activated with PMA or A23187 shows that the activation of cells with PMA leads to an abundant production of cytosolic ROS, whereas A23187-activated neutrophils produce very little cytosolic ROS (Fig. 2 A–C). Quantitative analysis using flow cytometry confirms the above observation (Fig. 2D). Similar results were obtained with a plate reader assay using DHR123 (Fig. 2 E and F). As expected, ROS production in PMA-activated neutrophils is abolished when these cells were preincubated with DPI, a NOX inhibitor, and then activated with PMA (Fig. 2 D and E).
Fig. 2.
Calcium ionophore-mediated NETosis is NOX2 independent. (A–D) Human neutrophils were loaded with cytosolic ROS indicator DHR123 and activated with PMA or A23187. Live cell fluorescence image analysis of DHR123 loaded cells treated with control buffer (A), PMA (B), or A23187 (C). The presence of cytosolic ROS is indicated by the green fluorescence, and the cells were counterstained with Hoechst 33342 live cell DNA stain (blue) (n = 4). (Scale bar, 20 µm.) (D) Representative flow cytometric analysis of cytosolic ROS production in neutrophils loaded with DHR123 and activated with either PMA or A23187, in the presence or absence of DPI (n = 3). (E and F) DHR123-based ROS detection plate reader assays show that PMA induces a significantly greater cytosolic ROS production compared with A23187. (G–I) Neutrophils were activated by PMA, A23187, or ionomycin in the presence or absence of DPI (20 μM) and NET release was measured using a plate reader assay. The results are expressed as percentage of reduction in the presence of DPI compared with the activating agonist alone. DPI significantly reduces PMA-mediated NETosis (G), whereas it fails to inhibit A23187 (H)- and ionomycin (I)-mediated NETosis (n = 3). DPI → PMA, preincubation with DPI and activated with PMA; A23, A23187; DPI → A23, preincubation with DPI and activated with A23187; Io, ionomycin; DPI → Io, preincubation with DPI and activated with ionomycin. *P < 0.05 (E, two-way ANOVA; F–I, Student’s t test).
To directly assess the requirement for NOX2 in PMA- or calcium ionophore-induced NETosis, plate reader assays were performed in the presence or absence of DPI. These assays show that NETosis induced by PMA is NOX dependent, as previously suggested (1, 4), and that the inhibition of ROS production by DPI results in a significant suppression of NETosis induced by PMA (P < 0.001; Fig. 2G). In contrast, A23187- or ionomycin-induced NETosis is less sensitive to suppression by DPI and does not result in a significant reduction in NET release (Fig. 2 H and I, respectively). Therefore, calcium ionophore-induced NOX-independent NETosis is distinct from PMA-mediated NOX-dependent NETosis in terms of its lack of sensitivity to DPI.
Differential Activation of Kinases in NOX-Dependent and -Independent NETosis.
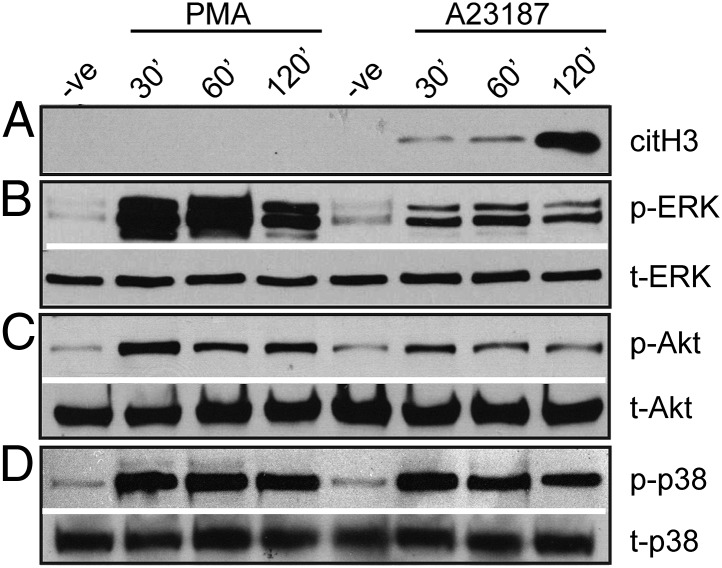
We next sought to determine differences in the signaling pathways that are activated in NOX-dependent and -independent NETosis. Number of key kinases such as ERK (14), Akt (15), and p38 (16) have been shown to be activated in PMA-induced NETosis. Thus, we next assessed differences in the activation of these kinases during PMA- and A23187-induced NETosis over a 120-min period following the activation of neutrophils (Fig. 3). Because the presence of citrullinated histone has previously been shown to be specific to A23187-induced NETosis (18, 19), we first assessed the citrullination of histone H3 in both conditions as a quality control (Fig. 3A and Fig. S3 A and B). Consistent with previous reports (18, 19), hypercitrullination of histone H3 was only observed in the calcium ionophore-mediated NETosis.
Fig. 3.
Immunoblot analysis. (A–D) Human neutrophils were harvested after an activation with PMA, A23187, or negative control (-ve) for the indicated times. The negative (-ve) control samples were incubated at 120 min in the absence of any activator. (A) Immunoblots show that A23187, but not PMA, induces citrullination of histone H3 (citH3) (n = 4). (B–D) The activation of kinases was assessed by immunoblotting for phospho (p)-ERK (B, n = 3), p-Akt (C, n = 3), and p-p38 (D, n = 2). Total kinases t-Akt, t-ERK, and t-p38 were used as loading controls. ERK is highly activated during PMA-induced NETosis. Akt is activated in both forms of NETosis, albeit a moderate level of activation in A23187-induced NETosis. p38 is equally activated in both forms of NETosis.
Immunoblot analyses of the kinases involved in the PMA-induced NETosis show that ERK (Fig. 3B and Fig. S3C) and Akt (Fig. 3C and Fig. S3D) are strongly activated during the NOX-dependent pathway as expected. In contrast, A23187-induced NETosis is accompanied by drastically low activation of ERK (Fig. 3B and Fig. S3C) and moderate reduction in activation of Akt (Fig. 3C and Fig. S3D) compared with the NOX-dependent NETosis. The activation of p38, however, was similar in both pathways (Fig. 3D and Fig. S3E). Together, these results highlight that, while similar kinases are activated in both of these pathways, differences do exist in the extent of activation of the signaling kinases involved in NOX-dependent and NOX-independent pathways of NETosis.
Pathway-Dependent Requirement for Kinases During NETosis.
We next asked whether ERK, Akt, and p38 are required for the NOX-independent NETosis. To do so, we used ERK inhibitor FR180204, Akt inhibitor XI, or p38 inhibitor SB202190, and performed plate reader assays using differentiated HL-60 human neutrophil-like (dHL-60) cells. The results show that PMA-induced NETosis is significantly reduced in the presence of 10 or 20 μM ERK inhibitor FR180204 (IC50 = 300–510 nM; P < 0.01; Fig. S3F) and 5 or 10 μM Akt inhibitor XI (IC50 = 140–310 nM; P < 0.001; Fig. S3H), whereas no change in the NET release is observed in the presence of 1 or 2 μM p38 inhibitor SB202190 (IC50 = 50 and 100 nM; Fig. S3J). On the other hand, A23187-induced NETosis is significantly reduced only in the presence of Akt inhibitor XI (P < 0.01; Fig. S3I), but not ERK or p38 inhibitor (Fig. S3 G and K, respectively). These results suggest that both NOX-dependent and NOX-independent pathways of NETosis are mediated by the activation of Akt, and the NOX-dependent pathway also requires the activation of ERK.
Mitochondrial ROS Is Required for NOX-Independent NETosis.
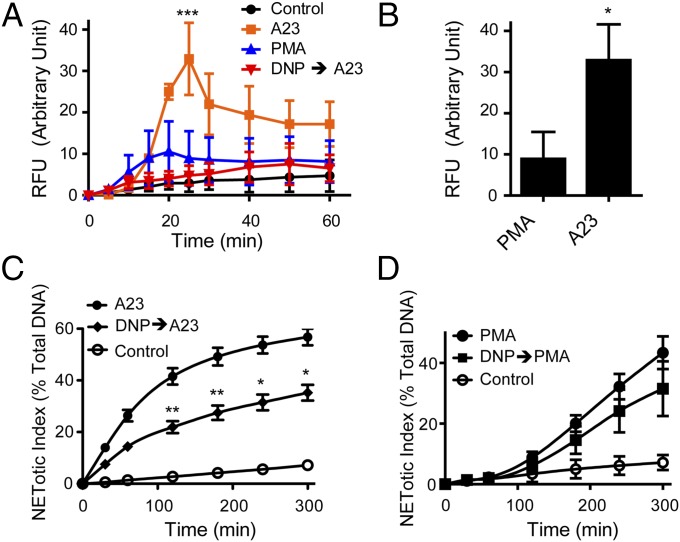
DPI is an inhibitor of NOX, and the above results show that A23187-induced NETosis is NOX independent. It is also known that DPI can partially inhibit mitochondrial ROS production (20, 21). Furthermore, the inhibition of NOX by DPI does not rule out the involvement of ROS from other sources. It is well established that mitochondrial respiration produces ROS (22). Thus, we next asked whether the mitochondrial ROS was produced when cells were activated with PMA or A23187. We used MitoSOX, a mitochondrial ROS-specific fluorescent dye to determine the mitochondrial ROS production. The plate reader assays show that although there is no substantial mitochondrial ROS production in PMA-activated neutrophils, a significantly higher mitochondrial ROS production occurred in A23187-activated cells (Fig. 4 A and B). The involvement of mitochondria in ROS production was confirmed using dinitrophenol (DNP), a mitochondrial uncoupler; the preincubation of cells with DNP completely abolished mitochondrial ROS production (Fig. 4A). In addition to DNP, we also tested another mitochondrial uncoupler, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), and monitored A23187-induced NETosis in dHL-60 cells (Fig. S4). The inhibition of mitochondrial ROS production by DNP leads to a significant (P < 0.001; Fig. S4A) and dose-dependent (P < 0.05; Fig. S4B) reduction in A23187-induced NET release. Preincubation of dHL-60 cells with FCCP also yields a significant (P < 0.001; Fig. S4C) and dose-dependent (P < 0.01; Fig. S4D) reduction in A23187-induced NETosis. Together, these results suggest that mitochondrial ROS generation is required for calcium ionophore-induced NOX-independent NETosis, but not for PMA-induced NOX-dependent NETosis. Therefore, we next asked whether mitochondrial ROS production was required for NETosis in human neutrophils. The plate reader assays show that A23187-induced NETosis is significantly attenuated by the presence of DNP in a dose-dependent manner (P < 0.05; Fig. 4C and Fig. S4E). In contrast, PMA-induced NETosis in the presence of DNP is not reduced significantly even at the highest dose tested (Fig. 4D and Fig. S4F). Collectively, these data show that mitochondrial ROS is important for NOX-independent, but not for NOX-dependent NETosis.
Fig. 4.
A23187-mediated NETosis requires mitochondrial respiration. (A and B) Human neutrophils were loaded with a mitochondrial ROS indicator, MitoSox, and activated with PMA or A23187. A time-course fluorescence plate reader assay for MitoSOX fluorescence shows a larger mitochondrial ROS production in cells activated by A23187 as opposed to PMA-activated cells. A mitochondrial uncoupler, DNP, abolishes mitochondrial ROS production induced by A23187 (n = 3). (B) MitoSOX assays show that A23187-activated cells produce a significantly higher levels of mitochondrial ROS at the peak production at the 25-min time point, compared with the cells activated by PMA (n = 3). (C) A23187-mediated NETosis is reduced in the presence of DNP (750 µM) as shown by time course analysis (n = 7). (D) PMA-mediated NETosis is not significantly reduced in the presence of DNP (750 µM) as shown by time course analysis (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001 compared with the agonist alone (A, C, and D, two-way ANOVA; B, Student’s t test).
Activation of the SK Channel Is Required for NOX-Independent NETosis.
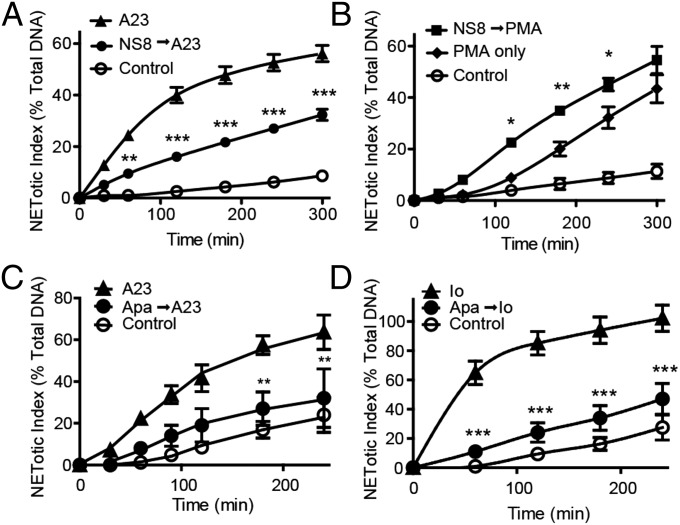
One report has shown that the activation of neutrophils with ionomycin induces cell death mediated by the SK channel activity (11). We therefore asked whether NETosis accounted for the SK channel-mediated cell death reported by Fay et al. (11). We first tested whether the inhibition of SK channels would inhibit NETosis induced by the calcium ionophores. The plate reader assays show that NET release in the NOX-independent pathway is significantly reduced in the presence of a SK channel inhibitor NS8593 in a time- and concentration-dependent manner (P < 0.001; Fig. 5A and Fig. S5A). In contrast, inhibition of the SK channel by NS8593 had no inhibitory effect on the PMA-induced, NOX-dependent NETosis; if any, NS8593 increased the NETosis (Fig. 5B and Fig. S5B). We next tested the requirement of the SK channel in the NOX-independent pathway using another SK channel inhibitor, apamin. The results show that the NETosis induced by both A23187 (P < 0.01; Fig. 5C and Fig. S5C) and ionomycin (P < 0.001; Fig. 5D and Fig. S5D) is significantly inhibited by apamin.
Fig. 5.
SK channel is required for ionophore-mediated NETosis. (A) Human neutrophils were activated with A23187 in the presence of SK channel inhibitor NS8593 (100 µM). The plate reader assay shows that NETosis is significantly reduced in the presence of NS8593 (n = 3), suggesting that A23187-induced NET DNA release in human neutrophils requires the activation of the SK channel. (B) Neutrophils were activated with PMA in the presence of NS8593. The plate reader assays show that NETosis is not inhibited in the presence of NS8593 (100 µM; n = 3), indicating that PMA-mediated NETosis does not require the activation of the SK channel. (C and D) Human neutrophils were activated with A23187 (C) or ionomycin (D) in the presence of SK channel inhibitor apamin (200 nM; n = 3). The plate reader assays show that NETosis is reduced significantly in the presence of apamin; hence, A23187- and ionomycin-induced NETosis requires the activation of the SK channel. A23, A23187; Io, ionomycin. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the agonist alone (two-way ANOVA).
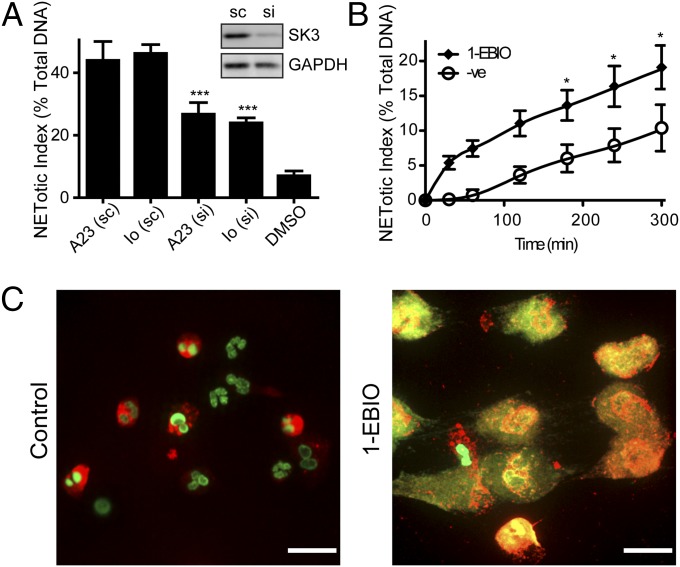
We next sought to identify the specific family member responsible for mediating the NOX-independent NETosis. Of the apamin-sensitive SK channels (SK1–SK3), the expression of SK1 is limited to the neuronal tissue (23). The use of scorpion toxin, scyllatoxin, which has the strongest affinity to SK2, suggests that SK2 is not involved (Fig. S5 E and F). To further test whether the transient receptor potential melastatin (TRPM)7 channel could be involved in NETosis, we used a TRPM7 inhibitor, MK886, which did not inhibit NETosis (Fig. S5 G and H). Therefore, we considered SK3 channel as effectors of NOX-independent NETosis. Furthermore, previous studies show that neutrophils predominantly express SK3 (11). Hence, we sought to directly assess whether SK3 is required for NOX-independent NETosis. To do so, we used siRNA against KCNN3 (gene encoding SK3 channel) transcript in dHL-60 cells. After optimizing and confirming a high transfection efficiency of siRNA in our system (Fig. S6A), we validated the successful knockdown of the SK3 channel protein expression with immunoblot assays (Fig. 6A). We then performed plate reader assays, and the results show that the knockdown of SK3 channels significantly reduces NET release in both A23187- and ionomycin-activated cells (Fig. 6A and Fig. S6 B and C). Together, these results show that NOX-independent NETosis requires the activation of the SK3 channel.
Fig. 6.
SK3 is required for the NOX-independent NETosis. (A) The plate reader assay shows that A23187- or ionomycin-mediated NETosis is significantly reduced in siRNA (si)-transfected dHL-60 cells, compared with scrambled RNA (sc)-transfected control cells at 240 min postactivation (n = 3). Shown above the graph is the result of an immunoblot analysis illustrating a successful knockdown of SK3 protein expression after siRNA transfection (n = 3). Therefore, knockdown of the SK3 channel by siRNA significantly reduces calcium ionophore-induced NETosis. (B and C) SK channel activator 1-EBIO induces NETosis. (B) The plate reader assays were performed to monitor NET release over time in response to the activation with 1-EBIO (n = 5). (C) Immunofluorescence staining confirms that compared with DMSO control, the activation of potassium channels by 1-EBIO induces NET release as shown by the colocalization of DNA (green) and MPO (red) after 300 min. sc, scrambled RNA-transfected cells; si, SK3 siRNA-transfected cells. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the agonist alone (A, one-way ANOVA; B, two-way ANOVA).
We next asked whether the activation of the SK channel could drive NETosis. To do so, we used a SK channel-specific activator, 1-Ethyl-2-benzimidazolinone (EBIO). The plate reader assays show that the potassium channel activator 1-EBIO induces NETosis in human neutrophils (Fig. 6B). In 1-EBIO–mediated NETosis, the release of NETs occurs immediately after the addition of this SK channel agonist (1-EBIO), and increases over time (P < 0.05; Fig. 6B). The formation of NETs was then validated with immunofluorescence assay showing colocalization of extracellular DNA and MPO (Fig. 6C). Together, these results demonstrate that the activation of potassium channels is necessary and sufficient for the induction of NETosis.
Discussion
NETosis can occur via NOX-dependent and NOX-independent pathways (6, 7, 24). However, the mechanism by which the NOX-independent NETosis occurs is not well understood. In this study, we show that the calcium ionophore-induced NOX-independent NETosis occurs more rapidly compared with the NOX-dependent pathway induced by PMA. Activation of ERK and Akt was low in NOX-independent NETosis compared with NOX-dependent NETosis; however, the activation of p38 was similar in both pathways. ERK activation is required for NOX-dependent NETosis, whereas Akt activation is essential for both types of NETosis. We also show that the NOX-independent NETosis requires mitochondrial ROS. Furthermore, the NOX-independent NETosis requires activation of SK3 channel, and direct activation of the SK channel is sufficient to induce NETosis.
The induction of NETosis by the calcium ionophores was first suggested by Neeli and Radic in a study that investigated the role of various agonists in activating peptidyl arginine deiminase 4 (PAD4) (19). Because PAD4 is necessary for the induction of NETosis (25, 26), citrullinated histone has been used as a marker for NET-associated histones in vivo (17, 18, 27). However, the requirement for PAD4 is not universal to all NETotic pathways, as evidenced by the fact that there is a lack of PAD4 activity and histone hypercitrullination in PMA-mediated NETosis (18). Our work shows that the kinetics of calcium ionophore-mediated NETosis is in stark contrast to the PMA-induced NETosis (Fig. 1), which is completely dependent on NOX-mediated ROS production (Fig. 2). We also provide proof that the calcium ionophore does not require NOX-activity to induce NETosis (Fig. 2).
It has recently been shown that BK channel activity is absent in neutrophils and is not required for antimicrobial defense (10, 28). 1-EBIO is an activator of both calcium-activated potassium channel of intermediate conductance (IK) and SK channels (29); however, 1-EBIO does not activate IK channels in human neutrophils (11). Our data show that activation of neutrophils by 1-EBIO induces NETosis (Fig. 6). The use of specific SK channel inhibitors such as NS8593 and apamin confirms that NETosis is mediated through the SK channels (Fig. 5 and Fig. S5). Knockdown experiments using siRNA further show that SK3 channel mediates NOX-independent NETosis (Fig. 6). Thus, it is possible that the SK channel-dependent NOX-independent NETosis is a contributor to the previously described potassium channel-mediated antimicrobial activity of neutrophils (8, 12).
Previous studies suggest the existence of NOX-independent NETosis, as demonstrated by the inability of DPI to suppress NET release (30, 31). Our current work shows that the NOX-independent pathway uses mitochondrial ROS (Fig. 4). Large amount of mitochondrial ROS is being produced during NOX-independent NETosis, but not during NOX-dependent NETosis (Figs. 2 and 4). During NOX-dependent NETosis, production of NOX-dependent ROS is required for the activation of ERK, Akt, and p38 (15, 16). ERK has been implicated as the major kinase involved in NOX-dependent NETosis (14, 16). Our data are consistent with this notion (Fig. 3 and Fig. S3). In contrast, ERK is not substantially activated in NOX-independent NETosis, and its inhibition does not inhibit this form of NETosis. Although p38 was activated in both types of NETosis, p38 inhibitor SB202190 did not inhibit NETosis. Consistent with our previous report (15), we show that Akt is required for NOX-dependent NETosis (Fig. 3 and Fig. S3). In this report, we show that Akt is also required for the NOX-independent pathway. The exact roles of kinases and ROS in NETosis, however, are not clearly understood. Previous studies show that protein tyrosine phosphatases are regulatory targets of ROS (32), which is a second messenger necessary to inactivate protein tyrosine phosphatase or MAP kinase phosphatase to induce the activation of ERK in ML-1 cells (33). Thus, it is possible that a similar mechanism is at play for the induction of ROS-mediated NETosis, whether it is NOX dependent or independent.
There have been numerous reports describing the cross-talk between mitochondrial superoxide and NOX activity (34–36). In endothelial cells, mitochondrial superoxide can stimulate cytoplasmic NOX (36). Our results show that although mitochondrial superoxide is generated by the calcium ionophore A23187, it does not lead to the generation of cytoplasmic ROS by NOX, as suggested by the DHR123 staining (Fig. 2). Conversely, there is very little, if any, mitochondrial ROS being generated in the PMA-induced NOX-dependent pathway (Fig. 4). These data suggest very little interplay between mitochondrial superoxide and NOX activity during NETosis.
Overall, mitochondrial respiration and potassium current at the mitochondrial level are tightly linked (37). Recent reports demonstrate the presence of the mitochondrial SK channel in neuronal cell types and show that potassium influx into mitochondria causes changes in the membrane potential (38, 39). Although such mitochondrial potassium channels have not been identified in neutrophils, our results suggest that activation of SK channels leads to the production of mitochondrial ROS in neutrophils (Fig. 4). Moreover, although apamin is a cell-impermeable inhibitor peptide, it exerts an inhibitory effect on NETosis that suggests the engagement of cell surface SK3 in this process. It has been shown that changes in cytosolic potassium levels can change potassium flux and mitochondrial respiration (39). Furthermore, a unique feature of the SK channel complex is that it contains a calmodulin-binding domain. This calcium sensor is responsible for the conformational change and the channel gate opening (40, 41). The entire protein complex of the SK channel includes two other regulators—protein kinase CK2 and protein phosphatase 2A (42). These two proteins regulate the calcium sensitivity of the calmodulin subunit, whereby the phosphorylation of calmodulin at tyrosine 80 enhances its sensitivity to calcium (42). Therefore, NETosis regulated by calcium-induced potassium channels could occur via different regulatory events.
In this study, the depletion of extracellular calcium led to a significant, but partial decrease in the A23187- and ionomycin-induced NOX-independent NETosis (Fig. 1 and Fig. S2). However, the calcium ionophores also induce the release of stored calcium (43, 44). Thus, it is likely that release of stored calcium leads to a partial activation of the NOX-independent pathway. Similarly, the effect of the mitochondrial uncoupler, DNP, also resulted in partial effects and 1-EBIO had a modest effect on the activation of the NOX-independent NETosis compared with the calcium ionophore (Fig. 6). The robust effect of the calcium ionophore on the induction of NOX-independent NETosis also suggests that the calcium signaling is an upstream initiator of this pathway. The partial effects of the chemical inhibitors and inducers likely arise from the fact that the inhibitors and activators used were chemical compounds that did not achieve full inhibition. Furthermore, it is also possible that other redundant parallel pathways exist. Collectively, our study demonstrates that the calcium ionophore-induced NETosis does not require NOX2 activity and is distinct from PMA-induced, NOX-dependent NETosis. We further show that this NOX-independent pathway requires the activation of the calcium-activated SK3 channel, providing insight into mechanisms by which NOX-independent NETosis occurs.
Materials and Methods
Human Peripheral Neutrophils.
The protocol was approved by the Hospital for Sick Children ethics committee and signed informed consent was obtained from each subject enrolled. Peripheral blood from healthy donors was collected in K2 EDTA blood collection tubes (Becton, Dickinson and Co.), and neutrophils were isolated from the whole blood using PolymorphPrep (Axis-Shield) according to the manufacturer’s instructions with minor modifications (SI Materials and Methods). Some assays were conducted in dHL-60 neutrophil-like cells [e.g., kinase inhibitor assays and siRNA SK3 channel knockdown studies (SI Materials and Methods has additional details)].
NETosis Analysis.
NETosis was monitored by a plate reader assay in the presence of Sytox Green cell-impermeable nucleic acid stain (5 µM). NETosis was confirmed by imaging the colocalization of myeloperoxidase, and DNA was stained with Sytox Green after the fixation and permeablization. For some studies, Western blots were used for determining citH3, the activation states of kinases (ERK, Akt, and p38), and the protein levels of the SK3 channel. Inhibitors such as DPI, NS8593, scyllatoxin, apamin, MK886, FCCP, and/or DNP were preincubated with cells for 1 h before the activation of cells for NETosis.
Statistical Analysis.
All data are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism statistical analysis software (Version 5.0a for Mac OS X). Student’s t test was used for comparing two groups. When comparing more than two groups, ANOVA with Bonferroni post test or Dunnett’s test was used where appropriate. A P value of 0.05 or less was considered to be statistically significant.
Supplementary Material
Acknowledgments
We thank Mr. Chongbo Yang for providing assistance with immunoblot assays. D.N.D. was supported by an Ontario Graduate Scholarship, the Ontario Student Opportunity Trust Fund (SickKids Restracomp, Dr. Goran Enhorning Award in Pulmonary Research, and Peterborough K. M. Hunter Graduate Studentship), and a University of Toronto Doctoral Thesis Completion Grant. The study was funded by SickKids Research Institute's Trainee Start Up Fund (to D.N.D.), Canadian Institutes of Heath Research (MOP-111012) and Cystic Fibrosis Canada (2619) Grants (to N.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414055112/-/DCSupplemental.
References
- 1.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng OZ, Palaniyar N. NET balancing: A problem in inflammatory lung diseases. Front Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J Cell Biol. 2012;198(5):773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remijsen Q, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21(2):290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi M, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114(13):2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilsczek FH, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185(12):7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 7.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 2012;92(4):841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 8.Krause KH, Welsh MJ. Voltage-dependent and Ca2(+)-activated ion channels in human neutrophils. J Clin Invest. 1990;85(2):491–498. doi: 10.1172/JCI114464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause KH, Demaurex N, Jaconi M, Lew DP. Ion channels and receptor-mediated Ca2+ influx in neutrophil granulocytes. Blood Cells. 1993;19(1):165–173; discussion 173–175. [PubMed] [Google Scholar]
- 10.Essin K, et al. Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am J Physiol. 2007;293(1):C45–C54. doi: 10.1152/ajpcell.00450.2006. [DOI] [PubMed] [Google Scholar]
- 11.Fay AJ, Qian X, Jan YN, Jan LY. SK channels mediate NADPH oxidase-independent reactive oxygen species production and apoptosis in granulocytes. Proc Natl Acad Sci USA. 2006;103(46):17548–17553. doi: 10.1073/pnas.0607914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves EP, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416(6878):291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 13.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakkim A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7(2):75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 15.Douda DN, Yip L, Khan MA, Grasemann H, Palaniyar N. Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood. 2014;123(4):597–600. doi: 10.1182/blood-2013-09-526707. [DOI] [PubMed] [Google Scholar]
- 16.Keshari RS, Verma A, Barthwal MK, Dikshit M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J Cell Biochem. 2013;114(3):532–540. doi: 10.1002/jcb.24391. [DOI] [PubMed] [Google Scholar]
- 17.Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J Immunol. 2011;187(4):1856–1865. doi: 10.4049/jimmunol.1004201. [DOI] [PubMed] [Google Scholar]
- 18.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180(3):1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 19.Neeli I, Radic M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol. 2013;4:38. doi: 10.3389/fimmu.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock JT, Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987;242(1):103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulua AC, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208(3):519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy MP. Modulating mitochondrial intracellular location as a redox signal. Sci Signal. 2012;5(242):pe39. doi: 10.1126/scisignal.2003386. [DOI] [PubMed] [Google Scholar]
- 23.Rimini R, Rimland JM, Terstappen GC. Quantitative expression analysis of the small conductance calcium-activated potassium channels, SK1, SK2 and SK3, in human brain. Brain Res Mol Brain Res. 2000;85(1-2):218–220. doi: 10.1016/s0169-328x(00)00255-2. [DOI] [PubMed] [Google Scholar]
- 24.Arai Y, et al. Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem Biophys Res Commun. 2014;443(2):556–561. doi: 10.1016/j.bbrc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Li P, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinod K, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA. 2013;110(21):8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Femling JK, et al. The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels. J Gen Physiol. 2006;127(6):659–672. doi: 10.1085/jgp.200609504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl- secretion by benzimidazolones. I. Direct activation of a Ca(2+)-dependent K+ channel. Am J Physiol. 1996;271(5 Pt 1):L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan MJ, Radic M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J Immunol. 2012;189(6):2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabriel C, McMaster WR, Girard D, Descoteaux A. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J Immunol. 2010;185(7):4319–4327. doi: 10.4049/jimmunol.1000893. [DOI] [PubMed] [Google Scholar]
- 32.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: Evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37(16):5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 33.Traore K, et al. Redox-regulation of Erk1/2-directed phosphatase by reactive oxygen species: Role in signaling TPA-induced growth arrest in ML-1 cells. J Cell Physiol. 2008;216(1):276–285. doi: 10.1002/jcp.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51(7):1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797(6-7):897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 36.Nazarewicz RR, Dikalova AE, Bikineyeva A, Dikalov SI. Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress. Am J Physiol Heart Circ Physiol. 2013;305(8):H1131–H1140. doi: 10.1152/ajpheart.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szewczyk A, Jarmuszkiewicz W, Kunz WS. Mitochondrial potassium channels. IUBMB Life. 2009;61(2):134–143. doi: 10.1002/iub.155. [DOI] [PubMed] [Google Scholar]
- 38.Dolga AM, et al. Subcellular expression and neuroprotective effects of SK channels in human dopaminergic neurons. Cell Death Dis. 2014;5:e999. doi: 10.1038/cddis.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolga AM, et al. Mitochondrial small conductance SK2 channels prevent glutamate-induced oxytosis and mitochondrial dysfunction. J Biol Chem. 2013;288(15):10792–10804. doi: 10.1074/jbc.M113.453522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia XM, et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395(6701):503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 41.Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554(Pt 2):255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bildl W, et al. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron. 2004;43(6):847–858. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 43.Itoh T, Kanmura Y, Kuriyama H. A23187 increases calcium permeability of store sites more than of surface membranes in the rabbit mesenteric artery. J Physiol. 1985;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cudd L, Clarke C, Clinkenbeard K. Contribution of intracellular calcium stores to an increase in cytosolic calcium concentration induced by Mannheimia haemolytica leukotoxin. FEMS Microbiol Lett. 2003;225(1):23–27. doi: 10.1016/S0378-1097(03)00471-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.