Significance
Current genomic and biochemical analysis revealed mutations in isocitrate dehydrogenase (IDH) genes associated with several neoplasms and a novel enzymatic activity of IDH mutations to catalyze α-ketoglutarate to d-2-hydroxyglutarate, contributing to tumorigenesis. We identified a broad range of IDH1 mutations, including a previously unidentified IDH1-R132Q mutation, in cartilage tumors. Cartilage-specific Col2a1-Cre/ERT2;Idh1-R132 mutant knock-in mice developed multiple enchondroma-like lesions. These data show that mutant Idh in growth-plate cells causes persistence of chondrocytes, giving rise to enchondromas adjacent to the growth cartilage in bone.
Keywords: isocitrate dehydrogenase, cartilage tumor, hedgehog
Abstract
Enchondromas are benign cartilage tumors and precursors to malignant chondrosarcomas. Somatic mutations in the isocitrate dehydrogenase genes (IDH1 and IDH2) are present in the majority of these tumor types. How these mutations cause enchondromas is unclear. Here, we identified the spectrum of IDH mutations in human enchondromas and chondrosarcomas and studied their effects in mice. A broad range of mutations was identified, including the previously unreported IDH1-R132Q mutation. These mutations harbored enzymatic activity to catalyze α-ketoglutarate to d-2-hydroxyglutarate (d-2HG). Mice expressing Idh1-R132Q in one allele in cells expressing type 2 collagen showed a disordered growth plate, with persistence of type X-expressing chondrocytes. Chondrocyte cell cultures from these animals or controls showed that there was an increase in proliferation and expression of genes characteristic of hypertrophic chondrocytes with expression of Idh1-R132Q or 2HG treatment. Col2a1-Cre;Idh1-R132Q mutant knock-in mice (mutant allele expressed in chondrocytes) did not survive after the neonatal stage. Col2a1-Cre/ERT2;Idh1-R132 mutant conditional knock-in mice, in which Cre was induced by tamoxifen after weaning, developed multiple enchondroma-like lesions. Taken together, these data show that mutant IDH or d-2HG causes persistence of chondrocytes, giving rise to rests of growth-plate cells that persist in the bone as enchondromas.
Enchondromas, one of the most common benign tumors occurring in bone, are present in more than 3% of the population (1, 2). They are composed of cells derived from chondrocytes and occur as solitary lesions or multiple lesions in enchondromatosis syndromes (Ollier disease or Maffucci syndrome—in the latter, enchondromas are associated with vascular malformations). Clinical problems caused by enchondromas include pain, fractures, and skeletal deformity. There is a potential for malignant progression to chondrosarcoma that may be greater than 50% in some cases of multiple enchondromatosis (i.e., Maffucci syndrome) (3–7). Many chondrosarcomas are thought to derive from enchondromas, and such sarcomas are termed central chondrosarcomas (3).
The hedgehog (Hh) signaling pathway is constitutively active in enchondromas and chondrosarcomas (8, 9). Hh is important in growth-plate chondrocyte differentiation, where it cooperates with parathyroid hormone-like hormone in a negative feedback loop to inhibit the differentiation of proliferative growth-plate chondrocytes (6, 10–14). Disruption of this feedback loop can result in either skeletal dysplasias with abnormal bone growth or enchondromas; 5% of enchondromas harbor mutation in parathyroid hormone-like hormone receptor (PTHR1), resulting in activation of Hh signaling (6, 10–14), and expression of a mutant PTHR1 or overexpression of the Hh-regulated transcription factor Gli2 under the Col2a1 promoter causes enchondroma-like cartilage lesions to develop adjacent to the growth-plate cartilage in mice (8).
The majority of enchondromas and chondrosarcomas harbor somatic isocitrate dehydrogenase 1 (IDH1) or IDH2 mutations (15–18). Mutations in IDH genes are common in several other neoplasms, including glioma, glioblastoma, acute myeloid leukemia, angioimmunoblatic T-cell lymphoma, and intrahepatic cholangiocarcinomas (19–22). Biochemical studies in these cancer types identified a neomorphic enzymatic activity of the mutant IDH that converts α-ketoglutarate (α-KG) to d-2-hydroxyglutarate (d-2HG), which builds up to high concentrations in IDH mutant cells. d-2HG can competitively inhibit the function of a large group of α-KG–dependent enzymes and thereby, modulate a number of cellular processes, including DNA methylation, histone methylation, and activity of hypoxia-inducible factor 1α (23–25). However, how IDH mutations contribute to enchondroma and chondrosarcoma tumorigenesis is unclear.
We previously established tissue-specific Idh1 mutant knock-in (KI) mice and reported the pathological phenotypes in the hematopoietic system and the brain. Specifically, the Idh1-KI mutant mouse lines showed brain hemorrhage, increased numbers of early hematopoietic progenitors, and anemia with extramedullary hematopoiesis, which is known to progress to acute myeloid leukemia (26, 27). Here, we generated cartilage-specific Idh1-KI mice to examine the effect of mutant Idh1 on the differentiation of chondrocytes and the development of cartilaginous neoplasia.
Results
Human Enchondromas and Chondrosarcomas Have a Broad Variety of IDH Mutations That Results in d-2HG Production.
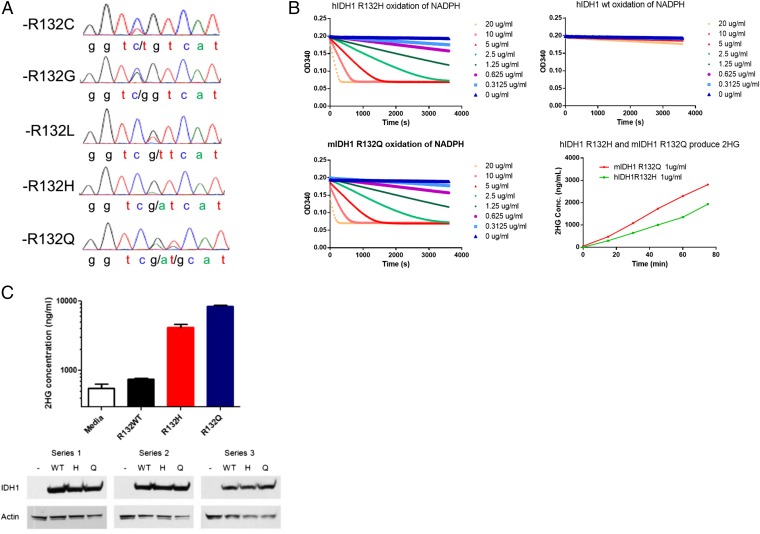
To identify the range of IDH mutations in cartilage tumors, we assessed the presence of mutations in a cohort of 43 chondrosarcomas and 13 enchondromas. Sanger sequencing was used to genotype the tumor samples. Sequencing showed that 20 of the chondrosarcomas and 8 of the enchondromas harbored an IDH mutation. We confirmed the somatic nature of the mutations by comparing with the germ line of the patients, finding a wild-type (WT) sequence in the germ line in all cases. The most frequent mutation in IDH1 was R132C followed by R132G, R132L, R132H, and R132Q (Fig. 1A and Tables 1 and 2). This distribution is a broader range of IDH1 mutations than previously identified, although all of the mutations change the same amino acid residue. We further examined whether this IDH1 mutation results in the acquisition of the altered enzymatic activity capable of converting α-KG to d-2HG before conducting experiments in a genetically engineered mouse model. To quantify the d-2HG–producing neoactivity compared with a known human oncogenic mutation, nicotinamide adenine dinucleotide phosphate (NADPH) consumption was measured using recombinant mouse IDH1-R132Q, the well-characterized human IDH1-R132H, and the WT IDH1 protein. The results revealed that the R132Q mutation confers the same neoactive ability to produce d-2HG as previously reported for R132H and other IDH1 and IDH2 oncogenic mutations (Fig. 1B and Table 3) (28). To further examine this mutation in a cell-based assay, HEK293T cells were transfected with mouse R132H, R132Q, and WT IDH1. d-2HG was greatly elevated in the culture media of both IDH1 R132Q and IDH1 R132H but not in WT IDH1-overexpressing HEK293T cells (Fig. 1C). Thus, for all tumor-associated amino acid substitutions described to date, including the previously undescribed R132Q, a mutation of IDH1 at R132 causes a gain of function that produces d-2HG.
Fig. 1.
IDH1 R132Q mutations confer an enzymatic activity that converts α-KG to d-2HG. (A) Chromatograms generated by Sanger sequencing at the locus coding IDH1-R132 in human chondrosarcoma samples. (B) NADPH consumption and d-2HG production by IDH1 mutant proteins. Recombinant R132Q, R132H, and WT IDH1 proteins were assayed for NADPH consumption activity at different protein concentrations. This neoactivity is acquired by known IDH1 driver mutations. MS identified the production of d-2HG by R132Q and R132H mutant proteins. Liquid chromatography (LC) /MS was used to identify and measure the concentration of d-2HG in enzymatic assays of recombinant protein by comparing spectra with a d-2HG standard. Time-dependent d-2HG production was detected in the reactions catalyzed by IDH1 R132Q and IDH1 R132Q proteins but not WT protein. (C) Cellular production of d-2HG by R132Q and R132H mutant IDH1. HEK293 cells were transiently transfected with expression plasmids containing WT IDH1 or R132H or R132Q mutations. After 48 h, d-2HG concentration in the culture media was measured by LC/MS and compared with a media alone control, and protein expression was assessed by Western blot.
Table 1.
Sequence results of chondrosarcoma (n = 43)
| Gene | Mutation | n (%) of Mutation-positive cases |
| IDH1 | R132C | 12 (60) |
| IDH1 | R132G | 5 (25.0) |
| IDH1 | R132L | 1 (5.0) |
| IDH1 | R132H | 1 (5.0) |
| IDH1 | R132Q | 1 (5.0) |
| Total | 20 (100) |
Table 2.
Sequence results of enchondroma (n = 13)
| Gene | Mutation | n (%) of Mutation-positive cases |
| IDH1 | R132C | 2 (25.0) |
| IDH1 | R132S | 1 (12.5) |
| IDH2 | R172S | 2 (25.0) |
| IDH2 | R172M | 1 (12.5) |
| IDH2 | R172C | 1 (12.5) |
| IDH2 | R172K | 1 (12.5) |
| Total | 8 (100) |
Table 3.
Kinetic parameters of IDH1 R132Q and R132H
| mIDH1 R132Q | hIDH1 R132H | |
| Km α-KG (mM) | 41 | 800 |
| kcat (1/s) | 1.67 × 103 | 1.50 × 103 |
| kcat/Km | 40.7 | 1.89 |
Mutant Idh1 Disrupts Growth-Plate Structure.
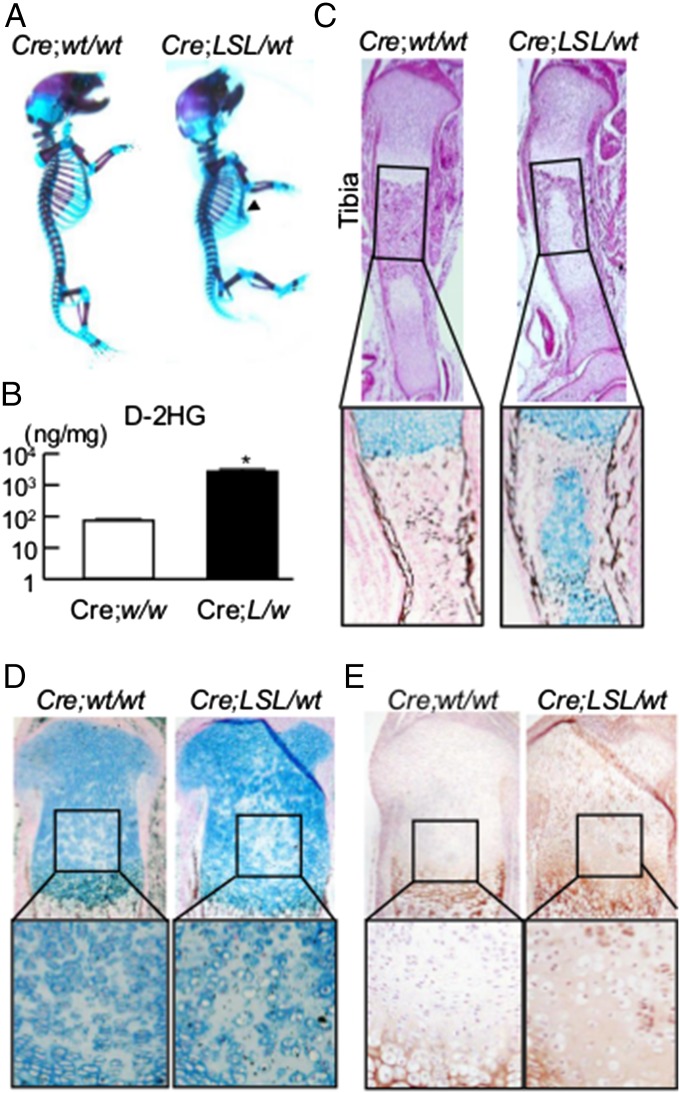
To determine how mutant Idh alters chondrocyte function in vivo, we generated conditional Idh1-KI mutant mice. A conditional Idh1-KI mouse line previously generated (29) was crossed with Col2a1-Cre transgenic mice to generate the KI mice. Unexpectedly, Col2a1-Cre;Idh1-KI pups were rarely found alive after birth. Even if the mutant mice did survive after birth, they died before weaning (Table S1). Because other cartilage-specific mutant mice are known to have dysfunction of the respiratory system, rib cage and tracheal cartilage were examined by whole-mount skeletal staining with alcian blue and alizarin red. This approach showed dwarfism in Idh1 mutant mice as well as pectus excavatum characterized by a caved-in or sunken appearance of the chest and dysplasia of tracheal cartilage (Fig. 2A and Fig. S1). These changes likely caused the early deaths in these mutant mice.
Fig. 2.
Col2a1-Cre;Idh1-KI mice showed impaired chondrocyte differentiation. (A) Whole-mount skeletal staining with alcian blue and alizarin red in Col2a1-Cre;Idh1-KI mutant and WT littermates. Arrowhead shows pectus excavatum in the mutant mice. (B) d-2HG levels determined by liquid chromatography/MS in 10 mg limbs harvested from WT and Idh1 mutant littermates at E16.5. (C) Representative (Upper) H&E and (Lower) alcian blue and von Kossa staining of tibias at E16.5 from both genotypes. (D and E) Representative alcian blue and von Kossa staining and Col10a1 staining in proximal tibias at postnatal day 0 from both genotypes.
The Col2a1-Cre;Idh1-KI mice showed high levels of d-2HG in analysis of their limbs, whereas control mice did not (Fig. 2B). Histological analysis of the tibia showed reduced cartilage mineralization in the middle section (Fig. 2C). These mice also exhibited a disrupted columnar structure of proliferative chondrocytes, especially in the middle of the growth plate, with ectopic expression of Col10a1 (Fig. 2 D and E). This latter phenotype could be detected from embryonic day (E) 16.5 to day 0 after birth (Fig. S2). This region of the growth plate is reported to be hypoxic, and similar disruption of columnar structure has been found in Vhlh-deficient mice (30). Thus, Idh mutation has a cell-autonomous effect on chondrocytes, resulting in a cartilaginous dysplasia of the long bones, ribs, and tracheal cartilage.
Idh1 Mutations and 2HG Regulate Proliferation and Expression of Markers of Hypertrophic Differentiation.
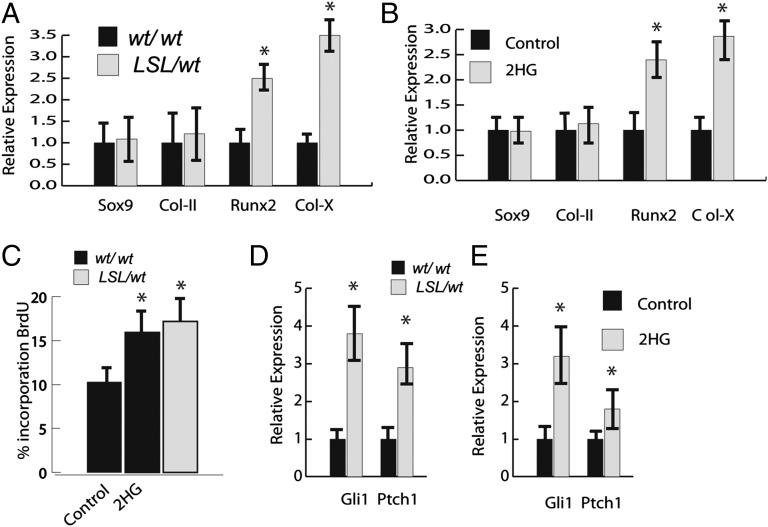
Because the fetal limbs showed a persistence of cells expressing type X collagen, we examined how mutant Idh might alter the expression of genes characteristically produced by hypertrophic chondrocytes. Chondrocytes from the mice were grown as primary cell cultures for 4 d. Cells were examined for the expression of Sox9, type 2 collagen, Runx2, and type X collagen or bromodeoxyuridine (BrdU) incorporation over the last 12 h of culture. They were also examined for the expression of the Hh target genes Gli1 and Ptch1, because these genes are known to be expressed in enchondromas (8). There was increased expression of genes expressed by hypertrophic chondrocytes, Runx2, and type X collagen (Fig. 3A) in the cells from the mice expressing the mutation. In contrast, there was no difference in expression of Sox9 or type 2 collagen. There was also a mild increase in proliferation as detected by BrdU incorporation in the mutant cultures (Fig. 3D). WT chondrocyte cultures were then treated with 10 μM Octyl-d-2HG or l-2HG, an enantiomer of d-2HG. Treatment with either agent caused the same effect. In both cases, this treatment caused an up-regulation of Runx2 and collagen X expressions to levels similar to those observed in the mutant cultures as well as an increase in BrdU incorporation (Fig. 3 B and D). In addition, there was an increase in expression of Hh target genes in chondrocytes from mice expressing the mutant Idh1 or control chondrocytes treated with 2HG (Fig. 3E). Thus, mutant Idh1 as well as 2HG treatment result in an increase in expression of Hh target genes (genes characteristic of hypertrophic chondrocytes) and an increase in cell proliferation.
Fig. 3.
Idh1-R132Q and 2HG increase the expression of hypertrophic chondrocyte markers and increase cell proliferation. (A) Expression of markers of hypertrophic chondrocytes is significantly higher in chondrocytes from mice expressing Idh1-R132Q. Relative expression is shown, with levels in WT cells arbitrarily set to one. (B) Treatment of WT cells with 2HG has a similar effect on gene expression as observed in cells expressing Idh1-R132Q. (C) Percentage of cells incorporating BrdU in mutant and WT cells treated with 2-HD or AGI-5198. (D and E) Cells expressing the mutant Idh or cells from mice expressing WT Idh treated with 2HG up-regulate expression of Hh target genes. Data are given as means, and error bars represent SDs. *P < 0.05 vs. WT control.
Conditional Idh Mutant Mice Develop Enchondroma-Like Cartilage Lesions.
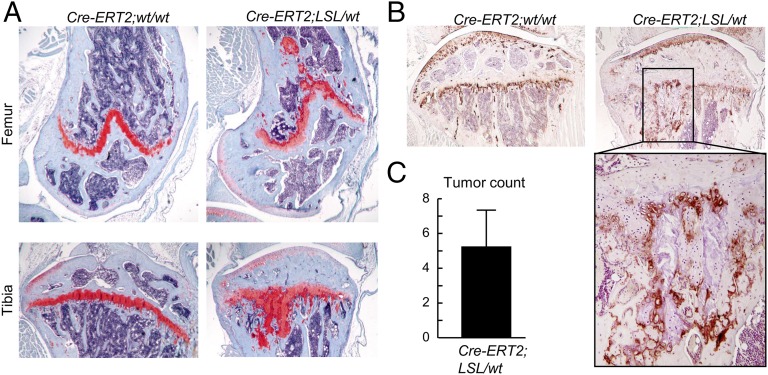
Because Col2a1-Cre;Idh1-KI mice could not survive after weaning, a tamoxifen-inducible Col2a1-Cre/ERT2 transgenic mouse line was crossed with the Idh1-KI mouse line to examine the effect of mutant Idh1 on the postnatal mouse growth plate. Tamoxifen was injected into mutant and WT littermates at 4 wk of age for 10 doses at 100 mg/kg body weight, and their skeletons were harvested 3 mo after the injection was completed. Histological analysis of their knees showed multiple enchondroma-like cartilage lesions adjacent to the growth plates (Fig. 4A). Between four and eight cartilage lesions were detected in the growth plates of each mutant knee (Fig. 4C). No lesions were observed in the control mice. Immunohistochemistry showed patchy expression of Col10a1 in the cartilage lesions (Fig. 4B), also suggesting dysregulation of chondrocyte differentiation. To determine the longer-term effect of mutant Idh1 on growth-plate cartilage, mutant mice were observed 6 mo after induction of the conditional allele. Multiple cartilage lesions were also found in knees of older Col2a1-Cre/ERT2;Idh1-KI mutant mice, although no obvious difference in size or numbers of such lesions was observed (Fig. S3).
Fig. 4.
Col2a1-Cre/ERT2;Idh1-KI mice develop multiple enchondroma-like cartilage lesions with dysregulated chondrocyte differentiation. (A and B) Safranin O staining and Col10 immunohistochemistry in (A, Upper) femurs and (A, Lower) tibias of WT and Col2-Cre/ERT2;Idh1-KI littermates. Multiple cartilage lesions were detected adjacent to growth plates in mutant mice. Col10 immunohistochemistry in the high-power field showed patchy expression of Col10 within the cartilage lesions. (C) Tumor counts in Idh1 mutant mice (n = 5). Data are given as means, and error bars represent SDs.
Discussion
Here, we show that there is a high variability in the types of IDH mutations present in enchondromas and chondrosarcomas at IDH1 R132 and that these mutations result in the production of d-2HG. Both mutant IDH and treatment with d-2HG increase cell proliferation and the expression of genes associated with hypertrophic chondrocytes. In vivo, this mutation also results in the persistence of type X collagen-producing chondrocytes. Conditional expression of a mutant Idh in chondrocytes inhibits growth-plate chondrocyte differentiation, and driving the mutation postnatally results in an enchondroma-like phenotype, showing that the presence of the Idh mutation alone is sufficient to cause enchondromatosis.
We found a broad range of IDH1 mutations in enchondromas and chondrosarcomas, including the IDH1-R132Q mutation, which has not been previously reported to our knowledge. As has been described for all other IDH1 driver mutations, this mutation resulted in increased production of d-2HG. As such, it seems that any substitution at R132 observed in human tumors samples functions to produce d-2HG. In our cell culture studies, either a mutant Idh or treatment with d-2HG caused the same changes in gene expression and an increase in cell proliferation.
Data from enchondromas in humans and mice overexpressing Gli2 or a mutant PTHR1 in chondrocytes show that these tumors consist of rests of cartilage that persist in the ends of bone. These cells proliferate slowly as the animals mature but then do not enlarge over time after skeletal maturity (8). This phenotype is similar to what we observed in the Idh mutant mice. As such, persistence of hypertrophic chondrocytes likely allows rests of growth-plate cells to remain near the end of the bone, resulting in enchondromas. The mild proliferative advantage that we observed is likely enough to maintain these chondrocytes, preventing their normal replacement by bone, but does not result in a lesion that grows with time.
Previous studies found IDH mutations in a variety of benign and malignant musculoskeletal tumors, including osteosarcomas and giant cell tumors of bone (16, 31, 32). Furthermore, the mouse mesenchymal cell line C3H10T1/2 expressing the IDH2 R172K mutation can give rise to poorly differentiated sarcomas in xenograft models (17). Although IDH mutations can be found in a number of neoplastic conditions, they may not be causative in all. Because IDH mutations are present in both enchondromas and chondrosarcomas and expression of mutant Idh in mice results in the development of enchondroma-like lesions, not chondrosarcomas, it is likely that the mutation is causative for the benign precursor lesion, and additional mutations are needed for malignant progression. This notion is supported by the overall low frequency of genes harboring mutations in chondrosarcomas (33). This finding is consistent with previous data from mice overexpressing the Gli2 transcription factor, which develop enchondroma-like lesions but when crossed with mice deficient in p53, develop chondrosarcomas (34). Indeed, we found reregulation of Hh target genes with expression of the mutant receptor, and the phenotype that we observed was identical to that seen with overexpression of Gli2 in chondrocytes. The fatal nature of driving the mutation during development supports the concept that a somatic event during growth causes this lesion.
We found that a mutant IDH and 2HG result in a persistence of hypertrophic chondrocytes. This finding is consistent with data from a genetically modified mouse, in which Gli2 transcriptional activation inhibits chondrocyte differentiation, causing enchondromas (8), which show a similar phenotype. 2HG causes persistence of growth-plate chondrocytes, resulting in enchondromas, which then could undergo additional genetic events to become chondrosarcomas. This etiology raises the possibility that therapies to drive terminal differentiation could be used to treat these tumors, for which there are no current universally effective therapies. Because IDH mutants produce d-2HG that inhibits differentiation, IDH-targeted therapy is one such potential approach. Future experiments will evaluate the effects of mutant IDH inhibitors in this model and others of IDH-driven disease.
Materials and Methods
Sequencing.
All human chondrosarcoma samples were coded and handled according to the ethical guidelines of the host institutions. Genomic DNA from frozen tumors was isolated using the DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer’s protocol. Capillary Sanger sequencing was performed to analyze the samples for IDH1 and IDH2 mutations after PCR amplification on exon 4 of IDH1 and IDH2. Standard cloning followed by Sanger sequencing was used to confirm sequencing results for the R132Q mutation.
Animals.
Idh1-KI (27), Col2a1-Cre (35), and Col2a1-Cre/ERT2 (36) have been described previously. The Idh1-KI mouse used in this study is identical to the animal previously described (27) but bears an R132Q mutation rather than an R132H mutation. A mouse protocol describing the above experimental procedures was approved by the animal care committee of the Hospital for Sick Children. For analysis of perinatal skeletons, mice from E15.5 to postnatal day 1 were examined, and at least three mice of each genotype were analyzed. The tumor phenotypes between at least five littermates of each genotype and age group were compared. Tamoxifen was injected into mutant and WT littermates at 4 wk of age for 10 doses at 100 mg/kg body weight, and their skeletons were harvested 3 mo after the injection was completed.
Chondrocyte Cultures.
The chondrocyte isolation protocol was modified from previously published methods. Growth plates of hind limbs from 16.5-d postcoitum embryos were isolated and incubated in collagenase type 4 solution (3 mg/mL; Worthington) for 45 min at 37 °C under 5% CO2 in a Petri dish (37). Cells were cultured for 4 d with or without l-2HG (Toronto Research Chemicals) or octyl-d-2HG ester (Toronto Research Chemicals). BrdU was added for the last 12 h in culture and detected immunohistochemically as previously reported (38).
Histological Analysis.
Samples were fixed in 4% (wt/vol) paraformaldehyde, embedded in paraffin, and sectioned for histological evaluation. Adult mouse bones were treated with Immunocal (Decal) for decalcification before embedding. Safranin O, H&E, von Kossa, and alcian blue staining was performed using standard techniques. Col10 staining was used as a marker for chondrocyte-terminal differentiation by incubating the sections with a 1:50 dilution of anti-human recombinant Col10 (Quartett Immunodiagnostika Biotechnologie GMBH) at 4 °C overnight. For whole-mount alizarin red and alcian blue staining, embryos were stained after they were fixed in 100% ethanol and transferred to acetone as described previously (39).
Real-Time Quantitative PCR.
Total RNA was extracted and purified using the RNeasy Kit (Qiagen) according to the manufacturer’s protocol. Purified RNA was reverse-transcribed with QuantiTect Reverse Transcription (Qiagen). cDNAs were subjected to quantitative PCR using SYBR green PCR Master Mix (Applied Biosystems). The primer sequences are available on request.
IDH1 Enzymology.
WT and mutant IDH1 vectors were created in the pET30A plasmid by standard PCR-directed mutagenesis; 6× His-tagged recombinant protein was produced in Escherichia coli and purified using a standard nickel column. The spectrophotometric assay for NADPH consumption was performed under the following conditions: 20 mM Tris (pH 7.5), 150 mM NaCl, 10 mM MgCl2, 0.05% BSA, 2 mM α-KG, and 0.05 mM NADPH. The reaction was initiated by adding recombinant protein at the indicated concentration. Spectrophotometric measurement of NADPH was made directly at 340 nm over time; for determination of kinetic parameters with regard to α-KG, NADPH concentration was held constant, and α-KG was varied from 0.1 to 10 mM. For in-cell assays, HEK293T cells (ATCC) were transfected with pcDNA3.1 mammalian expression vectors as indicated and allowed to grow for 24 h. Transfected cells were collected to assess protein expression by Western blot and plated to 96-well plates to measure cellular d-2HG production. After another 48 h, media were collected to measure d-2HG production by the transfected cells.
Metabolite Determinations.
For d-2HG measurement, metabolites were extracted using 80% aqueous methanol as previously described (17). Briefly, 10–15 mg limb cartilage at E16.5 or 200 μL culture medium was immersed in 80% methanol at −80 °C and homogenized. Extracts were subjected to ion-paired, reverse-phase liquid chromatography coupled to negative-mode electrospray triple-quadrupole MS using multiple reaction monitoring. Integrated elution peaks were compared with metabolite standard curves for absolute quantification.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health Grant R01AR06676501.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424400112/-/DCSupplemental.
References
- 1.Hong ED, Carrino JA, Weber KL, Fayad LM. Prevalence of shoulder enchondromas on routine MR imaging. Clin Imaging. 2011;35(5):378–384. doi: 10.1016/j.clinimag.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Walden MJ, Murphey MD, Vidal JA. Incidental enchondromas of the knee. AJR Am J Roentgenol. 2008;190(6):1611–1615. doi: 10.2214/AJR.07.2796. [DOI] [PubMed] [Google Scholar]
- 3.Bovee JV, Hogendoorn PC, Wunder JS, Alman BA. Cartilage tumours and bone development: Molecular pathology and possible therapeutic targets. Nature Rev Cancer. 2010;10(7):481–488. doi: 10.1038/nrc2869. [DOI] [PubMed] [Google Scholar]
- 4.Levesque J, Bell RS, Marx R, Wunder JS. University of Toronto Division of Orthopaedic Surgery . A Guide to Orthopaedic Tumors. Division of Orthopaedic Surgery, Department of Surgery, University of Toronto; Toronto: 1996. p. 132. [Google Scholar]
- 5.Schwartz HS, et al. The malignant potential of enchondromatosis. J Bone Joint Surg Am. 1987;69(2):269–274. [PubMed] [Google Scholar]
- 6.Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37. doi: 10.1186/1750-1172-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdegaal SH, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: An international multicenter study of 161 patients. Oncologist. 2011;16(12):1771–1779. doi: 10.1634/theoncologist.2011-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopyan S, et al. A mutant PTH/PTHrP type I receptor in enchondromatosis. Nat Genet. 2002;30(3):306–310. doi: 10.1038/ng844. [DOI] [PubMed] [Google Scholar]
- 9.Tiet TD, et al. Constitutive hedgehog signaling in chondrosarcoma up-regulates tumor cell proliferation. Am J Pathol. 2006;168(1):321–330. doi: 10.2353/ajpath.2006.050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couvineau A, et al. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum Mol Genet. 2008;17(18):2766–2775. doi: 10.1093/hmg/ddn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T, et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129(12):2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 12.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 13.Lanske B, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273(5275):663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 14.Vortkamp A, et al. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273(5275):613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 15.Amary MF, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224(3):334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 16.Amary MF, et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet. 2011;43(12):1262–1265. doi: 10.1038/ng.994. [DOI] [PubMed] [Google Scholar]
- 17.Lu C, et al. Induction of sarcomas by mutant IDH2. Genes Dev. 2013;27(18):1986–1998. doi: 10.1101/gad.226753.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pansuriya TC, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011;43(12):1256–1261. doi: 10.1038/ng.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borger DR, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17(1):72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cairns RA, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012-1903;119(8):1901–1903. doi: 10.1182/blood-2011-11-391748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mardis ER, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361(11):1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan H, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figueroa ME, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noushmehr H, et al. Cancer Genome Atlas Research Network Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward PS, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki M, et al. D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev. 2012;26(18):2038–2049. doi: 10.1101/gad.198200.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki M, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488(7413):656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465(7300):966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfander D, et al. Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development. 2004;131(10):2497–2508. doi: 10.1242/dev.01138. [DOI] [PubMed] [Google Scholar]
- 31.Kato Kaneko M, et al. Isocitrate dehydrogenase mutation is frequently observed in giant cell tumor of bone. Cancer Sci. 2014;105(6):744–748. doi: 10.1111/cas.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, et al. Isocitrate dehydrogenase 2 mutation is a frequent event in osteosarcoma detected by a multi-specific monoclonal antibody MsMab-1. Cancer Med. 2013;2(6):803–814. doi: 10.1002/cam4.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarpey PS, et al. Frequent mutation of the major cartilage collagen gene COL2A1 in chondrosarcoma. Nat Genet. 2013;45(8):923–926. doi: 10.1038/ng.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho L, et al. Gli2 and p53 cooperate to regulate IGFBP-3- mediated chondrocyte apoptosis in the progression from benign to malignant cartilage tumors. Cancer Cell. 2009;16(2):126–136. doi: 10.1016/j.ccr.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26(2):145–146. [PubMed] [Google Scholar]
- 36.Chen M, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45(1):44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3(8):1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 38.Alman BA, Greel DA, Ruby LK, Goldberg MJ, Wolfe HJ. Regulation of proliferation and platelet-derived growth factor expression in palmar fibromatosis (Dupuytren contracture) by mechanical strain. J Orthop Res. 1996;14(5):722–728. doi: 10.1002/jor.1100140507. [DOI] [PubMed] [Google Scholar]
- 39.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.