Significance
Naive CD4+ T cells differentiate into diverse effector and regulatory subsets to orchestrate immunity and tolerance. Whereas the mechanism of T-cell intrinsic signals has been extensively studied, how T-cell lineage differentiation is controlled by innate immune signals remains unknown. Here we used loss-of-function mouse systems, combined with other complementary approaches and models, to define the role of dendritic cell (DC) sirtuin 1 (SIRT1) as a key regulator in orchestrating the orientation of T-cell differentiation via HIF1α signaling in a mammalian target of rapamycin–independent manner. DC-expressed SIRT1, a type III histone deacetylase, programmed reciprocal T helper 1 (TH1) and regulatory T-cell (Treg) differentiation by modulating IL-12–STAT4 and TGF-β1–SMAD3 axes and cytokine receptor expressions at the DC–T-cell interface.
Keywords: dendritic cells, SIRT1, innate immunity, T-cell differentiation, HIF1α
Abstract
The differentiation of naive CD4+ T cells into distinct lineages plays critical roles in mediating adaptive immunity or maintaining immune tolerance. In addition to being a first line of defense, the innate immune system also actively instructs adaptive immunity through antigen presentation and immunoregulatory cytokine production. Here we found that sirtuin 1 (SIRT1), a type III histone deacetylase, plays an essential role in mediating proinflammatory signaling in dendritic cells (DCs), consequentially modulating the balance of proinflammatory T helper type 1 (TH1) cells and antiinflammatory Foxp3+ regulatory T cells (Treg cells). Genetic deletion of SIRT1 in DCs restrained the generation of Treg cells while driving TH1 development, resulting in an enhanced T-cell–mediated inflammation against microbial responses. Beyond this finding, SIRT1 signaled through a hypoxia-inducible factor-1 alpha (HIF1α)-dependent pathway, orchestrating the reciprocal TH1 and Treg lineage commitment through DC-derived IL-12 and TGF-β1. Our studies implicates a DC-based SIRT1–HIF1α metabolic checkpoint in controlling T-cell lineage specification.
CD4+ T cells are essential components of the adaptive immune system that regulate immune responses against foreign antigen. Upon antigen recognition, naive CD4+ T cells undergo activation and expansion, and, depending on inflammatory contexts and cytokine milieus, differentiate into functional and phenotypic T helper (TH) subsets characterized by distinct cytokine production profile and function (1–3). TH1 cells produce IFN-γ and elicit cellular immunity in responding to intracellular pathogens; TH2 cells produce IL-4 and IL-5 and promote humoral immunity in responding to extracellular bacteria and helminthes; and TH17 cells produce IL-17 and mediate antifungal defense and inflammation (4, 5). Additionally, regulatory T cells, often known as “induced regulatory T cells” (iTreg cells), which act in synergy with naturally occurring Treg cells (nTreg cells), produce IL-10 and TGF-β1 and dampen immune responses elicited from TH1, TH2, and TH17 (6–9).
Dendritic cells (DCs), an essential component in the innate immune system, play a critical role in initiating front-line primary immune responses and directing subsequent pathogen-specific adaptive immune responses (2). In addition to presenting antigens and modulating cell surface costimulatory molecules, DC-derived cytokines and chemokines can result in either a proinflammatory or antiinflammatory environment, engaging distinct T-cell differentiation programs on naive CD4+ T cells (1, 10–16). For example, DC-producing IL-12 can support TH1 development, whereas DC-producing IL-10 or TGF-β1 can support Treg development. Recent studies from us and others have shown that innate signaling in DCs mediated by G protein-coupled receptor S1P1, the mitogen-activated kinases (MAPKs), and Wnt–β-catenin plays important roles in stimulating adaptive immune responses through directing native CD4+ T-cell differentiation (17–20). However, other critical signaling components in DCs that may play a role in shaping T-cell lineage engagement remain to be identified.
SIRT1 is a mammalian homolog of the yeast NAD+-dependent protein deacetylase Sirt2, and plays a role in a variety of essential biological processes, including cell cycle progression, apoptosis, cell survival, gene silencing, heterochromatin formation, tumorigenesis, metabolism, and development (21, 22). SIRT1 has also been implicated in regulating immune responses. In T cells, SIRT1 is required to maintain T-cell tolerance (23, 24) and also play a role in inhibiting the function of Treg cells in allograft survival (25). In myeloid cells, SIRT1 limits the inflammatory process by inhibiting the expression of proinflammatory cytokines (26, 27), while promoting DC maturation and TH2 response in airway allergy (28). However, whether SIRT1 is involved in bridging the innate immune signal to adaptive immune response remains unexplored.
Here, we found that SIRT1 plays a critical role in determining the T-cell lineage fate by directing DC-derived cytokine production, which links innate and adaptive immune modulation. Largely through a HIF1α–dependent signaling pathway, SIRT1 is required for the reciprocal production of IL-12 and TGF-β1 production in DCs as well as the expression of IL-12Rβ2 and TGF-βR2 in responding T cells, resulting in a differential lineage engagement of TH1 and iTreg in the microbial-induced inflammation.
Results
SIRT1 Is Highly Expressed in DCs and the Deficiency of SIRT1 in CD11c+ Cells Does Not Change DC Homeostasis.
To systematically profile SIRT1 expression in the immune system, we purified the mouse immune cells based on surrogate cell surface markers: macrophages (CD11b+F4/80+ cells), DCs (CD11c+MHCII+F4/80−Ly6G−NK1.1−CD19−TCR− cells), neutrophils (CD11b+ Ly6G+ cells), natural killer (NK) cells (NK1.1+ CD49b+ cells), B cells (CD19+ cells), and T cells (TCR+ cells) (SI Appendix, Fig. S1A). Subsequent qPCR and immunoblot analysis showed that SIRT1 is highly expressed in DC cells (SI Appendix, Fig. S1 B–D). To gain insights into the role of SIRT1 in DCs, we generated a CD11c+ cell-specific SIRT1-deficient mouse model by crossing SIRT1fl/fl with CD11c-Cre (referred to as SIRT1CD11c−/− hereafter). In addition to DCs, the expression of CD11c in monocytes/macrophages, neutrophils, and some B cells has also been reported (29, 30). To examine the specificity and efficacy of SIRT1 deletion, we isolated various cell populations (indicated in SI Appendix, Fig. S1A) from wild-type (WT) and SIRT1CD11c−/− mice and examined the expression of SIRT1. We revealed a reduction of SIRT1 protein in DCs but not in other immune cells (SI Appendix, Fig. S1C). Also, we observed a reduction of SIRT1 mRNA expression in CD11c+ DC cells but not in other immune cells isolated from SIRT1CD11c−/− mice compared with their WT counterparts (SI Appendix, Fig. S1D). Notably, the deletion of SIRT1 in DCs is incomplete (about 5-fold reduction in protein level and about 2.5-fold reduction in mRNA level). We reason that both the potential limited efficacy of CD11c-Cre and impurity of DCs (95% in purity, SI Appendix, Fig. S1A) may contribute to the residue level of SIRT1 in our experiment. Nevertheless, these data suggested that the CD11c-Cre activity is largely restrained in DCs with a relatively limited efficacy.
Next, we examined the composition of DC subsets in vivo and revealed a comparable percentage and cell number of CD11c+ cells as well as CD8α+, CD11b+, and mPDCA1+ subsets in the spleen isolated from WT and SIRT1CD11c−/− mice (SI Appendix, Fig. S2A). Also, the expression of costimulatory molecules: CD80, CD86, CD40, and CD54 in CD11c+ cells isolated from WT and Sirt1CD11c−/−mice were comparable (SI Appendix, Fig. S2B). Moreover, acute deletion of SIRT1 in bone marrow cells (Sirt1CreER model described in detail below) resulted in a similar number of bone marrow-derived DCs (BMDCs) in vitro (SI Appendix, Fig. S3). These data suggested that SIRT1 deficiency does not affect DC development and homeostasis.
SIRT1 Signaling in DCs Is Involved in Regulating T-Cell Differentiation.
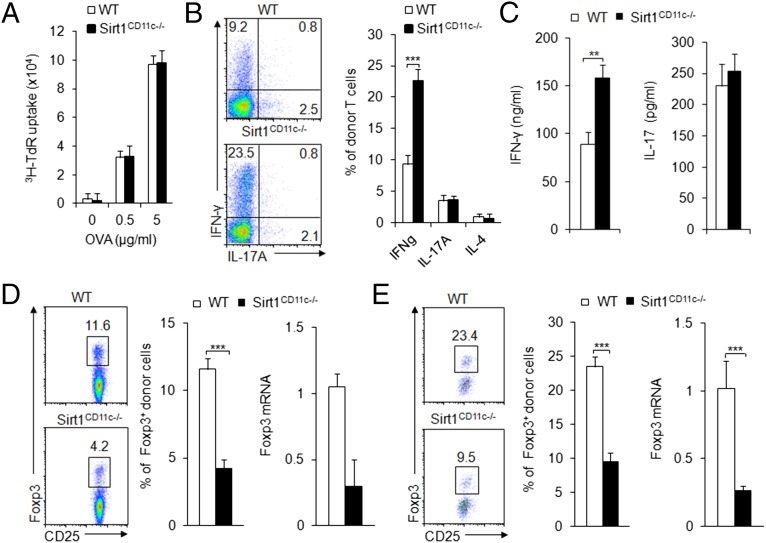
As professional antigen-presenting cells (APCs), DCs present foreign antigens with major histocompatibility complexes (MHCs) to T cells and modulate T-cell immune function. We then examined whether SIRT1 regulates antigen presentation in DCs. For the analysis of antigen presentation in DC, we transferred naive CD4+ T cells isolated from OT-II TCR-transgenic donors into WT and Sirt1CD11c−/− recipients, which were subsequently immunized with ovalbumin (OVA) and complete Freund’s adjuvant (CFA). At day 7 after immunization, draining lymph node (DLN) cells were isolated and stimulated with OVA ex vivo for 3 d. CD4+ T donor cells isolated from WT and Sirt1CD11c−/− recipients display a comparable proliferation rate, indicating a similar capability of antigen presentation in both groups (Fig. 1A). However, our parallel assays on T-cell differentiation markers revealed increased IFN-γ+ but not IL-17A+ or IL-4+ donor CD4+ T cells isolated from Sirt1CD11c−/− recipients (Fig. 1B). Also, the amount of secreted IFN-γ but not IL-17 was higher in donor T cells isolated from the immunized Sirt1CD11c−/− recipients than from WT recipients, indicative of enhanced TH1 differentiation (Fig. 1C). By contrast, we observed fewer Foxp3+ cells and lower level of Foxp3 mRNA expression in T cells from Sirt1CD11c−/− recipients compared with those in WT recipients, indicative of decreased Treg differentiation (Fig. 1D). Also, we observed similar alterations on Treg differentiation in mesenteric lymph nodes (MLNs) from a model of oral antigen-induced iTreg generation, whereby Sirt1CD11c−/− recipients were fed the antigen in the drinking water following adoptive transfer of OT-II T cells (Fig. 1E). Together, these results indicate that Sirt1 signaling in DCs may restrain inflammatory responses through modulating T-cell differentiation.
Fig. 1.
DC SIRT1 is required for regulating T-cell differentiation. (A–D) Naive OT-II (Thy1.1+) T cells were transferred into WT and SIRT1CD11c−/− mice and immunized with OVA + CFA. DLN cells were analyzed at 7 d after immunization. (A) Proliferation of DLN cells after ex vivo stimulation with OVA for 72 h. (B) Expression of IFN-γ and IL-17A in donor-derived T cells after PMA + ionomycin stimulation. (Right) The proportion of IFN-γ+ population among donor cells. (C) Secreted IFN-γ and IL-17A after stimulation with 5 μg/mL OVA for 72 h. (D, Left) The intracellular expression of Foxp3. (Middle) The proportion of Foxp3+ population among donor T cells. (Right) The mRNA expression of Foxp3 in donor-derived CD4+T cells. (E) Naive OT-II (Thy1.1+) T cells were transferred into WT and SIRT1CD11c−/− mice and then fed with OVA in the drinking water for 5 d. (Left) Foxp3+ population in donor-derived CD4+ T cells from MLN. (Middle) the proportion of Foxp3+ population among donor T cells. Right, the mRNA expression of Foxp3 in donor-derived CD4+ T cells. Data are shown as mean ± SD, n = 4, from one of three (A–D) or four (E) independent experiments. **P < 0.01 and ***P < 0.001 compared with the indicated groups.
DC SIRT1 Deletion Enhances Microbial Infection-Induced Inflammation.
Next, we examined the expression of SIRT1 in DCs in responding to various proinflammatory or antiinflammatory stimuli such as LPS, IFN-γ, TNFα, TGF-β1, and IL-10 (7). The proinflammatory and antiinflammatory stimuli readily suppress and promote SIRT1 expression, respectively (Fig. 2A and SI Appendix, Fig. S4).
Fig. 2.
Dendritic cell SIRT1 alters the differentiation of CD4+T-cell lineage against microbial-induced inflammation. (A) The protein level of SIRT1 in the DC cells following stimulation with LPS (10 ng/mL), TNFα (100 ng/mL), IL-12 (10 ng/mL), IFN-γ (50 ng/mL), IL-10 (20 ng/mL) and TGF-β1 (5 ng/mL). (B and C) SIRT1CD11c−/− mice were infected with L. monocytogenes. The intracellular staining of IFN-γ, IL-17A and Foxp3 in the spleen was determined following LLO189–201 stimulation at day 7. (D–F) T cells (CD4+TCR+CD45RbhiCD25−) were transferred into Rag1−/− and SIRT1CD11c−/−Rag1−/− mice, and body weight was measured weekly (D) and representative colon histology (E). The intracellular staining of IFN-γ, IL-17A and Foxp3 in donor cells (CD45.1+ cells) isolated from the MLNs (F). Data (B–F) are shown as mean ± SD, from one of four (B–F) independent experiments (at least four mice per group). **P < 0.01 and ***P < 0.001 compared with the indicated groups.
To further explore the role of SIRT1 in relevant in vivo contexts, we examined the pathological progression and T-cell differentiation of WT and SIRT1CD11c−/− mice in an inflammatory model. We challenged mice with Gram-positive bacteria Listeria monocytogenes, a prototypic model eliciting a strong TH1 cell-polarized response (31). In this model, we found L. monocytogenes infection resulted in more TH1 cells, comparable TH17 cells, but fewer Treg cells in splenic CD4+ T cells isolated from Sirt1CD11c−/− mice compared with T cells isolated from WT mice, as demonstrated by FACS, qPCR, and ELISA analysis of IFN-γ, IL-17A, and Foxp3 (Fig. 2 B and C and SI Appendix, Fig. S5 A and B).
Furthermore, we tested the role of DC–SIRT1 in a colitis model, the pathogenic progression of which is largely determined by the balance of TH1 and iTreg in vivo (32–34). For this test, we transferred naive WT T cells (CD4+TCR+CD45RbhiCD25−) into Rag1−/− or Sirt1CD11c−/−Rag1−/− mice. Sirt1CD11c−/−Rag1−/− recipients exhibited markedly accelerated weight loss (Fig. 2D) and pathological inflammatory injuries (Fig. 2E) compared with Rag1−/− recipients. Moreover, we observed more IFN-γ+ cells, comparable IL-17+ cells, and fewer Treg cells in Sirt1CD11c−/−Rag1−/− recipients than their WT counterparts (Fig. 2F). Altogether, our data from two different in vivo models indicate that the ablation of SIRT1 in DCs results in an altered balance of TH1 and Treg cells.
The Role of SIRT1 Signaling in DCs in Directing Antigen-Specific TH1 and Treg Differentiation.
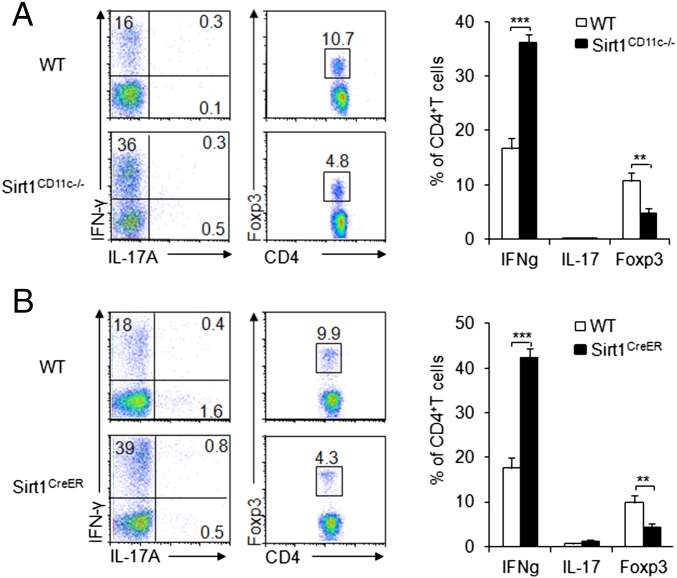
To determine how DC–SIRT1 directs antigen-specific T-cell differentiation, we first primed DCs from WT and Sirt1CD11c−/− with OVA and LPS, and subsequently injected these cells into two groups of recipient mice, which previously received naive OT-II T cells. Then, we analyzed the intracellular expression of T-cell differentiation markers in donor T cells 7 d after DC transfer. Evidently, Sirt1CD11c−/− DC transfer induced more IFN-γ+, comparable IL-17A+, and fewer iTreg donor T cells than those in the WT DC transferred group (Fig. 3A). Next, we extended experiments in the CD11c-Cre model to the acute deletion model. We generated Sirt1fl/flRosa26-Cre-ERT2 mice (referred to as Sirt1CreER mice hereafter) by crossing Sirt1fl/fl mice with Rosa26-Cre-ERT2 mice (CreERT2 fusion gene in the ubiquitously expressed Rosa26 locus). This model allows us to acutely delete targeted gene in a wide range of cell lineages by treating mice in vivo or treating isolated cells in vitro with tamoxifen. Also, the acute deletion of SIRT1 may avoid any potential adaptive and compensatory effects caused by CD11c-Cre–mediated deletion, a chronic process started at early development stage. In addition, the assessment of phenotypes following the deletion of the SIRT1 in CreER system will allow us to determine whether the phenotypes in CD11c-CRE are due to a CRE-specific effect. Both WT and Sirt1CreER mice were pretreated with tamoxifen for 3 d, which resulted in an efficient deletion of SIRT1 in DCs (SI Appendix, Fig. S6). Then DCs from Sirt1CreER and WT mice were isolated and treated, and these cells were injected into recipient mice as described above. Acute deletion of SIRT1 in DCs promotes TH1 but suppresses Treg differentiation as demonstrated by IFN-γ, IL-17A, and Foxp3 intracellular staining of donor T cells (Fig. 3B). Collectively, these results suggested that SIRT1 deficiency in DCs alters antigen-specific TH1 and iTreg cell differentiation in a reciprocal manner in vivo.
Fig. 3.
SIRT1 is required for adoptively transferred DCs to direct TH1 and iTreg immunity. Indicated splenic DCs were pulsed with LPS and OVA and injected s.c. into SIRT1CD11c−/− (A) or Sirt1CreER (B) recipient mice, which previously received naive OT-II (Thy1.1+) T cells 24 h before, as described in Materials and Methods. The intracellular staining of IFN-γ and IL-17A was determined in donor-derived CD4+T cells. (Right) The proportion of IFN-γ+, IL-17+, and Foxp3+ population among donor cells. Data are shown as mean ± SD, from one of three independent experiments (at least five mice per group). **P < 0.01 and ***P < 0.001 compared with the indicated groups.
SIRT1 Is Involved in a DC-Dependent Regulation of TH1 and Treg Cell Differentiation in Vitro.
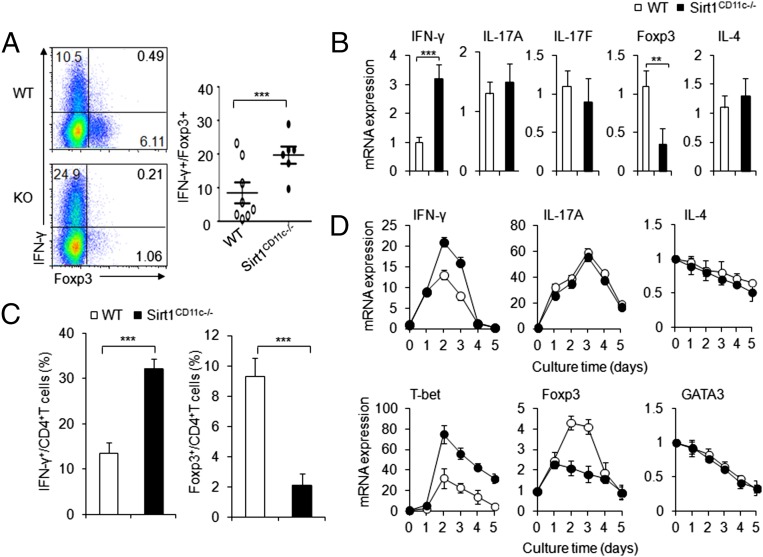
Next, we applied an in vitro coculture system (composed of purified naive OT-II T cells, WT, or Sirt1CD11c−/− splenic DCs and stimuli LPS and OVA) to determine the functional interaction between antigen-specific OTII T cells and DCs. We observed more IFN-γ+ T cells, higher expression of IFN-γ mRNA, fewer Foxp3+ T cells, and lower expression of Foxp3 mRNA in T cells cocultured with Sirt1CD11c−/− DCs than in T cells cocultured with WT DCs (Fig. 4 A and B). Also, polyclonal T cells activated by α-CD3 and SIRT1-deficient DCs displayed similar alterations in TH1 and iTreg differentiation (Fig. 4 C and D). Notably, the expression of TH2 and TH17 cell differentiation markers are comparable in both groups (Fig. 4 B and D). Thus, SIRT1 signaling in DCs directly inhibits TH1, whereas it instructs iTreg cell differentiation in vitro.
Fig. 4.
DC–SIRT1 signaling directs TH1 and Treg differentiation in vitro. (A and B) Naive OT-II T cells were cocultured with WT or SIRT1CD11c−/− DCs in the presence of antigen for 5 d. (A) Intracellular staining of IFN-γ and Foxp3 expression in cultured T cells. (Right) The proportions of IFN-γ+ and Foxp3+ proportion in CD4+ T cells. (B) The mRNA expression of indicated genes in T cells restimulated with α-CD3 for 5 h. (C and D) Naive T cells from C57BL/6 mice were cocultured with WT or SIRT1CD11c−/− DCs in the presence of α-CD3 for 5 d. (C) The proportions of IFN-γ+ and Foxp3+ proportion in CD4+ T cells. (D) The mRNA expression of indicated genes in OT-II T cells. Data are representative of two (A and B) and three (C and D) independent experiments (three to five mice per group), **P < 0.01 and ***P < 0.001 compared with the indicated groups.
SIRT1 Modulates DC-Derived T-Cell Polarizing Cytokines.
We next sought to measure DC-derived cytokines that are known to regulate TH1 and iTreg cell differentiation, including IL-12 and TGF-β1. LPS stimulation of SIRT1CD11c−/− DCs in vitro resulted in an increased intracellular Il-12p40 protein but decreased TGF-β1 protein compared with WT DCs (Fig. 5A). Also, a similar alteration of IL-12 (IL-12p70) and TGF-β1 in mRNA and protein level was observed in DCs after LPS stimulation in vitro (Fig. 5B) and in vivo (Fig. 5C and SI Appendix, Fig. S7). On the other hand, the expression of IL-12p35 and IL-23p19, two known partners of IL-12p40, was not significantly altered in SIRT1CD11c−/− DCs (SI Appendix, Fig. S8A). Also, the expression of TGF-β2 and TGF-β3 is comparable in SIRT1CD11c−/− DCs and WT DCs after LPS stimulation in vitro (SI Appendix, Fig. S8B). Moreover, we were unable to find any significant changes of IL-12 and TGF-β1 in other immune cells isolated from Sirt1CD11c−/− or WT mice following in vitro LPS stimulation (SI Appendix, Fig. S9).
Fig. 5.
SIRT1-dependent IL-12 and TGF-β1 production in DCs regulates TH1 and Treg cell differentiation. (A) The intracellular staining of indicated genes in WT and SIRT1CD11c−/− splenic DCs 5 h following LPS stimulation in vitro. (Right) The proportion of IL-12p40+ and intracellular TGF-β1 expression (mean fluorescence intensity, MFI) among CD11c+ cells. (B and C) The mRNA expression of IL-12p70 and TGF-β1 in WT and SIRT1CD11c−/− splenic DCs following LPS stimulation in vitro (B) and in vivo (C) were detected. (D) The intracellular staining of IFN-γ and Foxp3 in T cells cocultured with WT or SIRT1CD11c−/− splenic DCs with indicated treatment. (Right) The proportion of IFN-γ+ and Foxp3+ proportion in CD4+ T cells. Data are representative of four independent experiments (three to five mice per group). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the indicated groups.
Next, we applied a DC–T-cell coculture system (as described above) to determine whether SIRT1 signaling in DCs modulates T-cell differentiation through intercellular cytokine signaling. In T cells cocultured with SIRT1CD11c−/− DCs, we observed an increased STAT4 phosphorylation and decreased SMAD3 phosphorylation compared with T cells cocultured with WT DCs (SI Appendix, Fig. S10). The alteration of these phosphorylation events is consistent with the fact that STAT4 and SMAD3 are downstream signaling targets of IL-12 and TGF-β1, respectively. Moreover, we were able to reverse the impact of SIRT1CD11c−/− on TH1 cell differentiation and Treg differentiation in our coculture system by adding anti–IL-12 neutralizing antibodies and adding TGF-β1, respectively (Fig. 5D). Together, our data suggested that enhanced IL-12 and diminished TGF-β1 production in SIRT1CD11c−/− DCs contributes to the alteration of TH1 and Treg differentiation.
HIF1α Mediates SIRT1-Dependent Regulation of IL-12 and TGF-β1 in DCs.
To gain more mechanistic insights on SIRT1-dependent regulation of DC-derived cytokines, we assessed the predominant signaling molecules in DCs that are involved in LPS-mediated proinflammatory signaling pathways. DCs isolated from SIRT1CD11c−/− and WT display a comparable level of phosphorylation on ErK, JNK, p38, and AKT, indicative of unaltered signaling. By contrast, SIRT1 deficiency in DCs results in an enhanced S6 phosphorylation (pS6), indicative of the mammalian target of rapamycin (mTOR) signaling, and an accumulated transcriptional factor, HIF1α (Fig. 6A). Conversely, pharmacological stimulation of SIRT1 activity by SRT1720 (an activator of SIRT1) significantly decreases HIF1α expression (Fig. 6A).
Fig. 6.
SIRT1 regulates IL-12 and TGF-β1 production through HIF1α but independent of mTOR. (A) Intracellular staining and immunoblot of indicated proteins in DCs following LPS stimulation in vitro. (B) Intracellular staining of IL-12p40 and TGF-β1 in LPS-stimulated WT and SIRT1CD11c−/− splenic DCs pretreated with rapamycin (100 μM) for 1 h. (Left) The proportions of IL-12p40+ and intracellular TGF-β1 expression (MFI) in DCs. (Right) Intracellular staining of phosphorylation of S6 and HIF1α. (C–E) The intracellular staining of IL-12p40 and TGF-β1 in DCs isolated from indicated mice 4 h following i.p. injection of 10 mg/kg LPS (C). The proportion of IL-12p40 and MFI of TGF-β1 in DCs (D). The serum IL-12 and TGF-β1 levels indicated in mice (E). Data are representative of four (A and E) and two (B–D) independent experiments (three to five mice per group). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the indicated groups.
To determine whether mTOR signaling is involved in SIRT1-dependent regulation on DC-derived cytokines, we applied a pharmacological approach (rapamycin) to block mTOR activity in DCs. Whereas rapamycin treatment is sufficient to reduce the level of pS6 in SIRT1CD11c−/− DCs to a comparable level as in WT DCs following LPS stimulation, it failed to reverse the cytokine production and HIF1α level (Fig. 6B). Thus, our results suggested that mTOR is unlikely involved in mediating SIRT1-dependent regulation on cytokines and HIF1α in DCs.
Next, we sought to assess the role of HIF1α in DCs. The treatment of LPS-stimulated DCs with CoCl2, which resulted in an accumulation of HIF1α, readily increases IL-12 and decreases TGF-β1 (SI Appendix, Fig. S11A). Conversely, 2-ME2, an inhibitor of HIF1α, significantly reversed the up-regulated IL-12 expression and down-regulated TGF-β1 expression, which resulted from SIRT1 deficiency in DCs (SI Appendix, Fig. S11B). Furthermore, the genetic deletion of HIF1α in SIRT1CD11c−/− DCs (DKO) reversed the alterations on IL-12 and TGF-β1 caused by SIRT1 deficiency following LPS stimulation (Fig. 6 C–E). Together, our results suggested that HIF1α signaling mediates SIRT1 effects on IL-12 and TGF-β1 in DCs.
DC SIRT1 Signaling Modulates IL-12Rβ2 and TGF-βR2 Expression in T Cells.
T-cell polarizing cytokines often induce the expression of their corresponding cytokine receptors on T cells, resulting in a robust programming of cell fate determination (1, 16, 20, 35). This led us to investigate the expression of cytokine receptors of IL-12 and TGF-β in T cells activated by WT and SIRT1CD11c−/− DCs. Expression of IL-12Rβ1, TGF-βR1, and TGF-βR3 protein and mRNA is comparable in T cells induced by WT or SIRT1CD11c−/− DCs (SI Appendix, Fig. S12). In contrast, SIRT1CD11c−/− DCs induced more IL-12Rβ2 but less TGF-βR2 in T cells compared with WT DCs. Also, either the genetic deletion of HIF1α (SI Appendix, Fig. S13) and the IL-12 neutralizing antibodies or TGF-β1 treatment could reverse the changes of IL-12Rβ2 or TGF-βR2 in T cells cocultured with SIRT1CD11c−/− DCs, respectively (SI Appendix, Fig. S14).
Next, we sought to determine whether the alteration of cytokine receptors mediates the change of TH1 and Treg differentiation. For this inquiry, we applied shRNA approaches to knockdown IL-12Rβ2 and TGF-βR2 expression in OTII T cells (SI Appendix, Fig. S15), which were subsequently stimulated by either WT or SIRT1CD11c−/− DCs. Then, we determined mRNA expression of IFN-γ and Foxp3, indicative of TH1 and iTreg differentiation. Knockdown of IL-12Rβ2 or TGF-βR2 in OT II T cells selectively reversed the alteration of TH1 or iTreg differentiation caused by SIRT1 deficiency in DCs, respectively (SI Appendix, Fig. S16). Collectively, IL-12Rβ2 and TGF-βR2 expression in T cells is required for the alteration of TH1 and iTreg cell differentiation induced by SIRT1CD11c−/− DCs.
Pharmacologically Targeting SIRT1 in Mouse and Human DCs Modulates T-Cell Differentiation.
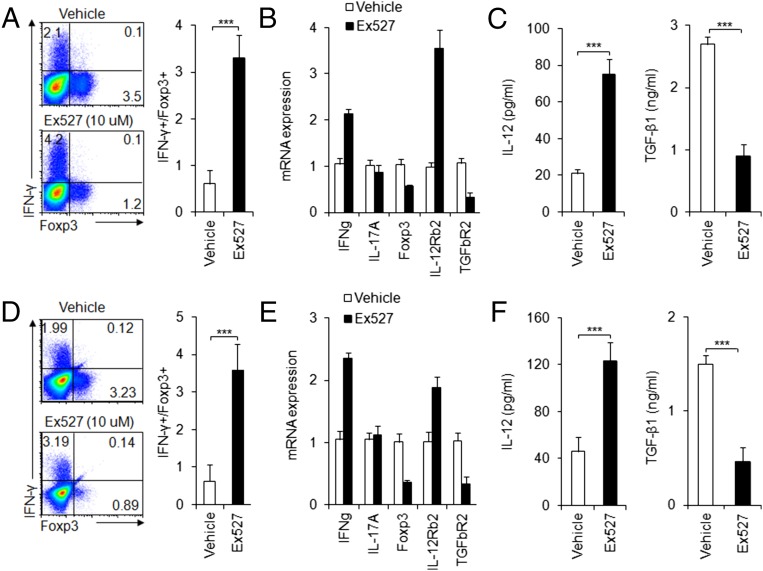
Next, we sought to apply a pharmacological approach to target SIRT1 in both mouse and human DCs and determine whether we can recapitulate our finding in genetic targeting SIRT1. We first applied Ex527, a selective inhibitor for SIRT1 but not for other histone deacetylases (36, 37) to the mouse DC–T-cell coculture system. In consistent with the results from genetic targeting SIRT1, we observed that SIRT1 inhibitor significantly enhanced TH1 but suppressed iTreg differentiation (Fig. 7 A–C). Then, we extended our inhibitor experiment to the human DC–T-cell coculture system, whereby DCs were derived from human peripheral blood monocytes and T cells were isolated from human cord blood. The pharmacological inhibition of SIRT1 in human DCs largely recapitulated what we observed in genetic and pharmacological targeting of mouse DCs in terms of the alterations of human T-cell IFN-γ, Foxp3, IL-12, TGF-β1, IL-12Rβ2, and TGF-βR2 expression (Fig. 7 D–F). Thus, our data indicated that SIRT1 mediated an evolutionarily conserved signaling pathway in both mouse and human DCs.
Fig. 7.
Pharmacologically targeting SIRT1 in mouse and human DCs. As described in Materials and Methods, mouse DCs (A–C) or human DCs (D–F) pulsed with LPS (100 ng/mL) were cocultured with mouse or human T cells, respectively, in the absence or presence of EX-527 (10 μM). The intracellular staining of IFN-γ and Foxp3 in T cells (A or D); the mRNA expression of indicated genes in T cells (B or E); the level of IL-12 and TGF-β1 in indicated supernatant (C or F) were detected. Data are shown as mean ± SD, n = 3–5, from one of two independent experiments. ***P < 0.001 compared with the indicated groups.
Discussion
DCs play a central role in initiating front-line innate immunity and inducing subsequent adaptive immunity in the process of host defense against infection (38, 39). Particularly, DCs shape antigen-specific adaptive immune response through presenting antigens, modulating cell surface costimulatory molecules, and producing cytokines and chemokines (40, 41). Fine tuning a myriad of DC intrinsic signaling pathways is required for eliciting an effective adaptive immune response without triggering inflammation-induced host damage (41, 42). Our current study revealed that an integrated SIRT1–HIF1α signaling axis in DCs directs the generation of two particular subsets of T cells, TH1 and iTreg cells, under infectious inflammation. Whereas SIRT1 is not involved in regulating antigen presentation in DCs, SIRT1–HIF1α axis in DCs instructs TH1 and iTreg differentiation through modulating the production of DC-derived T-cell polarizing cytokines, including IL-12 and TGF-β1. The altered IL-12Rβ2/TGF-βR2 expression and downstream STAT4/SMAD3 signaling in responding T cells further confer a robust DC–T-cell cross-talk, dictating the programming of TH1 and iTreg differentiation (SI Appendix, Fig. S17).
Recent studies suggested that SIRT1 is involved in regulating immune response in various inflammatory models (23–28, 43, 44). Mechanistically, transcriptional factor has been implicated as a critical proinflammatory-signaling module in myeloid leukocytes (45–50). In consistent with recent findings that SIRT1 is responsible for the deacetylation and destabilization of HIF1α (51, 52), we found that HIF1α is not only regulated by SIRT1 but also is involved in mediating the effects of SIRT1 deficiency on DCs.
Metabolic regulation and cell signaling are tightly and ubiquitously linked with immune responses (53–55). The effective immune response requires DCs to function in various conditions, including the alteration of extracellular or intracellular metabolic states due to the migration to a nutrient and/or oxygen-deficient environment (tumor microenvironment and inflammatory sites) or an ongoing metabolic reprogramming (resulted from inflammatory stimulation), respectively. The adaptation of DCs to changing metabolic states resulted from a mechanism of “metabolic checkpoint,” an active signaling process involved in sensing metabolic alteration and subsequently signaling transaction and execution (56). Recent studies indicated that a Toll-like receptor signaling-mediated metabolic reprogramming is required for DC maturation and antigen presentation (57–59). Moreover, the dysregulated mTORC1 or the adenosine monophosphate (AMP)-activated protein kinase (AMPK) activity results in an impaired DC development and maturation, indicating a metabolic checkpoint sensing amino acids and intracellular ATP during DC development and maturation (58, 60). Our current study further implicated a metabolic checkpoint in DCs requiring the interplay of SIRT1 and HIF1α, two metabolic sensors of redox and oxygen states, respectively (61–63). Finally, the above scenarios indicate that the DC-directed adaptive immunity requires the coupling of two evolutionarily conserved stress responses, inflammatory response, and metabolic stress response.
Materials and Methods
Mice and Bacterial Infection Model.
All animal experiments were performed with the approval of the Animal Ethics Committee of Fudan University. C57BL/6 Sirt1fl/fl, CD11c-Cre, Rosa26-Cre-ERT2, and HIF1αfl/fl mice were obtained from The Jackson Laboratory. Thy1.1 and OT-II TCR-transgenic mice were obtained from the Center of Model Animal Research at Nanjing University. Rag1−/− and CD45.1 mice were obtained from the Beijing University Experimental Animal Center. C57BL/6 mice were obtained from the Fudan University Experimental Animal Center. All mice had been backcrossed to the C57BL/6 background for at least eight generations and were used at an age of 6–12 wk. WT control mice were of the same genetic background and, where relevant, included Cre+ mice to account for the effects of Cre (no adverse effects due to Cre expression itself were observed in vitro or in vivo). For bacterial infection, mice were injected i.v. with 4 × 104 L. monocytogenes-expressing OVA. After 7 d, splenocytes were harvested and stimulated with listeriolysin O189–120 (LLO189–120) for 5 h for intracelluar staining.
Cell Adoptive Transfer.
Naive T cells 2 × 106 (CD4+CD62LhiCD44loCD25−) from OT-II TCR-transgenic mice were sorted and transferred into Sirt1fl/fl-CD11c-Cre− (WT) and Sirt1fl/fl-CD11c-Cre+ (Srit1CD11c−/−) mice. After 24 h, they were injected s.c. with OVA323–339 in the presence of CFA (Difco) and LPS (Sigma). At day 7–8 after immunization, DLN cells were harvested and stimulated with the cognate peptide for 2–3 d for cytokine mRNA and secretion analyses, or pulsed with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 5 h for intracellular staining from donor-derived T cells. For oral antigen stimulation, after adoptive transfer of 2 × 106 naive OT-II T cells, mice were fed with water supplemented with 20 mg/mL OVA protein (grade VI; Sigma-Aldrich) for 5 d. For T-cell transfer-induced colitis, 3 × 105 T cells (CD4+TCR+CD45RbhiCD25−) from WT mice were transferred into Rag1−/− or SIRT1CD11c−/−Rag1−/− mice and body weight was measured weekly. For DC transfer, splenic DCs (2 × 105) were pulsed with 50 μg/mL OVA and 500 ng/mL LPS for 8 h, washed, and injected s.c. into C57BL/6 mice that had received 2 × 106 naive OT-II T cells 24 h before. Mice were killed 7–8 d later for analysis.
Cell Cultures and Flow Cytometry.
Spleens were digested with collagenase D, and DCs (CD11c+TCR−CD19−NK1.1−F4/80−Ly6G−) were sorted on a FACSAria II (Becton Dickinson). Lymphocytes were sorted to enrich for naive T cells. For DC–T-cell cocultures, DCs and T cells (1:10) were mixed in the presence of 1 μg/mL OVA323–339 peptide and 100 ng/mL LPS. After 5 d of culture, live T cells were stimulated with PMA and ionomycin for intracellular cytokine staining or with plate-bound α-CD3 to measure cytokine secretion and mRNA expression. T-cell proliferation was determined by pulsing of cells with 3H-thymidine for the final 12–16 h of culture. For drug treatments, cells were incubated with vehicle, CoCl2 (200 μM; Calbiochem), 2-ME2 (2 μM; Calbiochem), rapamycin (100 μM; Calbiochem), or EX-527 (10 μM; Sigma) for 0.5–1 h before stimulation. For antibody or cytokine treatment, cultures were supplemented with 5 ng/mL TGF-β1 (R&D Systems), anti–IL-12 mAb (R&D Systems). Flow cytometry was performed with antibodies from eBioscience or BD Biosciences. Anti-mouse TGF-βR3 (ab78421) is from Abcam. Anti-mouse HIF1α mAb (241812), anti-mouse TGF-β1 mAb (9016), anti-mouse TGF-βR1 mAb (141231), anti-mouse TGF-βR2 (21813), anti-mouse IL-12Rβ1(16161), and anti-mouse IL-12Rβ2 (16162) are from R&D Systems. Flow cytometry data were acquired on a FACSCalibur (Becton Dickinson) or an Epics XL bench-top flow cytometer (Beckman Coulter) and data were analyzed with FlowJo (Tree Star). For inducible deletion of loxP-flanked alleles in vivo, mice were treated with 2 mg tamoxifen (Sigma-Aldrich) daily for 3 d.
RNA and Protein Analysis.
RNA was extracted with an RNeasy kit (Qiagen), and cDNA was synthesized using SuperScrip III reverse transcriptase (Invitrogen). An ABI 7900 real-time PCR system was used for quantitative PCR, with primer and probe sets obtained from Applied Biosystems. Results were analyzed using SDS 2.1 software (Applied Biosystems). The cycling threshold value of the endogenous control gene (Hprt1, encoding hypoxanthine guanine phosphoribosyl transferase) was subtracted from the cycling threshold (ΔCT). The expression of each target gene is presented as the fold change relative to that of control samples (2-ΔΔCT), as described previously (64). For detection of phosphorylated signaling proteins, purified cells were activated with LPS (Sigma), immediately fixed with Phosflow Perm buffer (BD Biosciences), and stained with phycoerythrin or allophycocyanin directly conjugated to antibody to Erk phosphorylated at Thr202 and Tyr204 (20A; BD Biosciences), p38MAPK phosphorylated at Thr180 and Thr182 (D3F9; Cell Signaling Technology), JNK phosphorylated at Thr183 and Tyr185 (G9; Cell Signaling Technology), STAT4 phosphorylated at Tyr701 and Ser727 (58D6; Cell Signaling Technology), SMAD3 phosphorylated at Tyr705 and Ser727 (D3A7; Cell Signaling Technology), AKT phosphorylated at Ser473 and Thr308 (587F11; Cell Signaling Technology), or S6 phosphorylated at Ser235 and Ser236 (D57.2.2E; Cell Signaling Technology), as described previously (9). Immunoblot analysis was performed as described (64) using anti-HIF1α (H1α67; Sigma-Aldrich), anti-Sirt1 (D60e1; Cell Signaling Technology), and anti–β-actin (AC-15; Sigma-Aldrich) antibodies.
IL-12Rβ2 and TGF-βR2 Knockdown with RNA Interference.
A gene-knockdown lentiviral construct was generated by subcloning gene-specific short hairpin RNA (shRNA) sequences into lentiviral shRNA expression plasmids (pMagic4.1) as described before (65). Lentiviruses were harvested from culture supernatant of 293T cells transfected with shRNA vector. Sorted OT-II CD4+ T cells were infected with recombinant lentivirus, and green fluorescent protein expressing cells were isolated using fluorescence sorting 48 h later. The IL-12Rβ2 and TGF-βR2 expression was confirmed using real-time PCR. The sorted T cells with either control or shRNA vectors were used for functional assay.
Human DC and T-Cell Cultures.
For assays of human DC-mediated T-cell activation and differentiation, normal human DCs (CC-2701; Lonza) were cultured and their populations were expanded for 5 d with human granulocyte-macrophage colony-stimulating factor and IL-4 (R&D), followed by treatment with EX-527 and stimulation for 24 h with LPS. DCs were washed extensively and cultured with human cord blood CD4+ T cells (2C-200; Lonza) at a ratio of 1:10. After 7 d of culture, live T cells were purified and then stimulated either with PMA and ionomycin for intracellular cytokine staining for 5 h or with plate-bound anti-CD3 for analysis of mRNA expression.
Statistical Analysis.
All data are presented as the mean ± SD. Student’s unpaired t test was applied for comparison of means and to compare differences between groups. Comparison of the survival curves was performed using the log-rank (Mantel–Cox) test. A P value (alpha-value) of less than 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
The authors’ research is supported by the National Natural Science Foundation for General Programs of China Grants 31171407 and 81273201 (to G.L.) and Grant 81271907 (to Y.B.), Key Basic Research Project of the Science and Technology Commission of Shanghai Municipality Grant 12JC1400900 (to G.L.), Innovation Program of Shanghai Municipal Education Commission Grant 14Z Z009 (to G.L.), and Excellent Youth Foundation of Chinese Academy of Sciences Grant KSCX2-EW-Q-7-1 (to G.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420419112/-/DCSupplemental.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28(1):445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21(1):685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30(5):616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27(1):485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9(2):194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 7.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler SF. FOXP3: Of mice and men. Annu Rev Immunol. 2006;24(1):209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 9.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10(7):769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy KM, Stockinger B. Effector T cell plasticity: Flexibility in the face of changing circumstances. Nat Immunol. 2010;11(8):674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinstry KK, Strutt TM, Swain SL. Regulation of CD4+ T-cell contraction during pathogen challenge. Immunol Rev. 2010;236(1):110–124. doi: 10.1111/j.1600-065X.2010.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li MO, Flavell RA. TGF-beta: A master of all T cell trades. Cell. 2008;134(3):392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stockinger B, Veldhoen M, Martin B. Th17 T cells: Linking innate and adaptive immunity. Semin Immunol. 2007;19(6):353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20(1):4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, et al. Targeting S1P1 receptor protects against murine immunological hepatic injury through myeloid-derived suppressor cells. J Immunol. 2014;192(7):3068–3079. doi: 10.4049/jimmunol.1301193. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11(11):1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329(5993):849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang G, Wang Y, Shi LZ, Kanneganti TD, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity. 2011;35(1):45–58. doi: 10.1016/j.immuni.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11(6):443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao B, Kong Q, Kemp K, Zhao YS, Fang D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc Natl Acad Sci USA. 2012;109(3):899–904. doi: 10.1073/pnas.1118462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119(10):3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beier UH, et al. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol. 2011;31(5):1022–1029. doi: 10.1128/MCB.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schug TT, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30(19):4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez Y, et al. Sirtuin 1 is a key regulator of the interleukin-12 p70/interleukin-23 balance in human dendritic cells. J Biol Chem. 2012;287(42):35689–35701. doi: 10.1074/jbc.M112.391839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legutko A, et al. Sirtuin 1 promotes Th2 responses and airway allergy by repressing peroxisome proliferator-activated receptor-γ activity in dendritic cells. J Immunol. 2011;187(9):4517–4529. doi: 10.4049/jimmunol.1101493. [DOI] [PubMed] [Google Scholar]
- 29.Karmaus PW, Chi H. Genetic dissection of dendritic cell homeostasis and function: Lessons from cell type-specific gene ablation. Cell Mol Life Sci. 2014;71(10):1893–1906. doi: 10.1007/s00018-013-1534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burt BM, et al. CD11c identifies a subset of murine liver natural killer cells that responds to adenoviral hepatitis. J Leukoc Biol. 2008;84(4):1039–1046. doi: 10.1189/jlb.0408256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11(1):83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haribhai D, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182(6):3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136(4):1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Napper AD, et al. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J Med Chem. 2005;48(25):8045–8054. doi: 10.1021/jm050522v. [DOI] [PubMed] [Google Scholar]
- 37.Solomon JM, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26(1):28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medzhitov R, et al. Highlights of 10 years of immunology in Nature Reviews Immunology. Nat Rev Immunol. 2011;11(10):693–702. doi: 10.1038/nri3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinman RM. Decisions about dendritic cells: Past, present, and future. Annu Rev Immunol. 2012;30(1):1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 40.Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227(1):234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soderberg KA, et al. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc Natl Acad Sci USA. 2005;102(45):16315–16320. doi: 10.1073/pnas.0506190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purushotham A, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sequeira J, et al. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314(16):3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Cramer T, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rius J, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453(7196):807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandau KB, Fandrey J, Brüne B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001;97(4):1009–1015. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- 48.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao S, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324(5924):261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McInturff AM, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 α. Blood. 2012;120(15):3118–3125. doi: 10.1182/blood-2012-01-405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim JH, et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38(6):864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 52.Laemmle A, et al. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1α protein under hypoxic conditions. PLoS ONE. 2012;7(3):e33433. doi: 10.1371/journal.pone.0033433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunol Rev. 2012;249(1):14–26. doi: 10.1111/j.1600-065X.2012.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol. 2012;33(4):168–173. doi: 10.1016/j.it.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: Insights into metabolism and lymphocyte function. Science. 2013;342(6155):1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13(10):907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 57.Pantel A, et al. Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol. 2014;12(1):e1001759. doi: 10.1371/journal.pbio.1001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ando T, Ito H, Ohtaki H, Seishima M. Toll-like receptor agonists and alpha-galactosylceramide synergistically enhance the production of interferon-gamma in murine splenocytes. Sci Rep. 2013;3(1):2559. doi: 10.1038/srep02559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, et al. Tuberous sclerosis 1 (Tsc1)-dependent metabolic checkpoint controls development of dendritic cells. Proc Natl Acad Sci USA. 2013;110(50):E4894–E4903. doi: 10.1073/pnas.1308905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8(11):865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 62.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 64.Liu G, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF1a-dependent glycolysis. Cancer Res. 2014;74(3):727–737. doi: 10.1158/0008-5472.CAN-13-2584. [DOI] [PubMed] [Google Scholar]
- 65.Liu G, et al. Phosphatase Wip1 negatively regulates neutrophil development through p38 MAPK-STAT1. Blood. 2013;121(3):519–529. doi: 10.1182/blood-2012-05-432674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.