Significance
Plants have undergone repeated rounds of whole-genome duplication, followed by gene degeneration and loss. Using whole-genome resequencing, we examined the origins of the recent tetraploid Capsella bursa-pastoris and the earliest stages of genome evolution after polyploidization. We conclude the species had a hybrid origin from two distinct Capsella lineages within the past 100,000–300,000 y. Our analyses suggest the absence of rapid gene loss but provide evidence that the species has large numbers of inactivating mutations, many of which were inherited from the parental species. Our results suggest that genome evolution following polyploidy is determined not only by genome redundancy but also by demography, the mating system, and the evolutionary history of the parental species.
Keywords: polyploidy, population genomics, speciation, gene loss
Abstract
Whole-genome duplication (WGD) events have occurred repeatedly during flowering plant evolution, and there is growing evidence for predictable patterns of gene retention and loss following polyploidization. Despite these important insights, the rate and processes governing the earliest stages of diploidization remain poorly understood, and the relative importance of genetic drift, positive selection, and relaxed purifying selection in the process of gene degeneration and loss is unclear. Here, we conduct whole-genome resequencing in Capsella bursa-pastoris, a recently formed tetraploid with one of the most widespread species distributions of any angiosperm. Whole-genome data provide strong support for recent hybrid origins of the tetraploid species within the past 100,000–300,000 y from two diploid progenitors in the Capsella genus. Major-effect inactivating mutations are frequent, but many were inherited from the parental species and show no evidence of being fixed by positive selection. Despite a lack of large-scale gene loss, we observe a decrease in the efficacy of natural selection genome-wide due to the combined effects of demography, selfing, and genome redundancy from WGD. Our results suggest that the earliest stages of diploidization are associated with quantitative genome-wide decreases in the strength and efficacy of selection rather than rapid gene loss, and that nonfunctionalization can receive a “head start” through a legacy of deleterious variants and differential expression originating in parental diploid populations.
Following whole-genome duplication (WGD) events, genomes undergo a process of diploidization, where duplicate genes are lost, and only a minority of genes are retained as duplicates for extended periods of evolutionary time (1, 2). Although this process has been well characterized (3), the dominant evolutionary forces driving gene degeneration and loss, and the rate at which diploidization occurs, remain unclear.
Large-scale genome projects have provided a number of important insights into genome evolution in ancient polyploids (1, 4, 5). First, gene retention and loss are nonrandom with respect to gene function, with signs of strong convergence in which genes are lost (1). This observation raises an open question about the extent to which gene loss proceeds solely via a process of relaxed purifying selection, or whether there is also positive selection for gene loss. Second, gene loss is often biased toward particular chromosomes, such that genes from one chromosome duplicated by WGD (homeolog) are preferentially lost (4), or become expressed at lower levels than those genes on the other homeolog (5). This process is termed “biased fractionation” (6), and although the underlying mechanism remains unresolved, it could result from differences in epigenetic regulation or selective history of the parental species (7) and may occur rapidly upon polyploid formation or following a longer time scale of genome rearrangement and evolution.
Studying the early stages of gene degeneration and loss should provide important insights into the causes of genome evolution in polyploids (8, 9) for several reasons. First, transitions to polyploidy are often accompanied by strong demographic bottlenecks and shifts to higher selfing rates (10), which can limit the efficacy of natural selection regardless of ploidy. Examination of population genomic patterns in recent polyploids can disentangle the impact of demography and gene redundancy on the efficacy of selection. Second, genomic studies of recent polyploids also allow inference about the extent of early gene degeneration following WGD. Rapid genome rearrangements and deletions have previously been identified in certain neopolyploids within even a single generation (11), and past work examining a small set of genes indicated that gene loss may be extremely rapid (9). Rapid divergence in expression between homeologous genes (“transcriptomic shock”) has also been identified following polyploidy (12, 13), although the relationship between transcriptomic shock and permanent genomic changes remains to be resolved. Overall, these studies imply that derived changes occur in the earliest stages of ploidy transitions; however, the genome-wide extent of such changes remains unclear. Finally, whether the fate of genes in polyploid lineages is influenced by the evolutionary history before WGD or solely through de novo changes following polyploidization remains a key open question (14). Comparative population genomics and expression analyses of early polyploids and their recent progenitor species provide an outstanding opportunity to understand the dynamics associated with the early stages of polyploid evolution better.
The genus Capsella includes the primarily self-fertilizing tetraploid Capsella bursa-pastoris (2n = 4x = 32), as well as three diploid species: the self-incompatible outcrosser Capsella grandiflora and two self-compatible species, Capsella rubella and Capsella orientalis (2n = 2x = 16). These species differ greatly in their geographical distribution. The outcrosser C. grandiflora is limited to northwestern Greece and Albania, whereas the self-compatible C. rubella has a broader Mediterranean/central European distribution and self-compatible C. orientalis is found from Eastern Europe to Central Asia (15). In contrast to its diploid congeners, the selfing tetraploid C. bursa-pastoris has a worldwide distribution that is, at least in part, anthropogenic (16, 17).
We have previously shown that the self-fertilizing diploid C. rubella is derived from the outcrossing, self-incompatible diploid C. grandiflora and that these species split relatively recently, within the past 50,000–100,000 y (18–21). There is reason to believe that outcrossing is the ancestral state in this system, because C. grandiflora shows transspecific shared polymorphism with Arabidopsis lyrata and Arabidopsis halleri at the self-incompatibility locus (19, 22, 23). The self-compatible C. orientalis is therefore also thought to be derived from an outcrossing, C. grandiflora-like ancestor, although further back in time than C. rubella (15).
The origin of the widespread tetraploid C. bursa-pastoris has proven difficult to determine, however (17, 24). Various hypotheses on the origin of this species have been put forward (15, 16, 24–28), and, most recently, C. bursa-pastoris has been suggested to be an autopolyploid of C. orientalis (15) or C. grandiflora (16), or an allopolyploid (28). Despite the hypotheses of autopolyploidy, C. bursa-pastoris appears to have strict disomic inheritance, with two separate homeologous genomes segregating independently (26).
In this study, we investigate the mode of formation and genomic consequences of polyploidization in C. bursa-pastoris using high-coverage massively parallel genomic sequence data. We first evaluate the origins of C. bursa-pastoris and conclude that the species has recent, hybrid, allopolyploid origins from the C. orientalis and C. grandiflora/C. rubella lineages. By comparing genome-wide patterns of nucleotide polymorphism and gene inactivation between C. bursa-pastoris and its two close relatives, C. grandiflora and C. orientalis, we then quantify the rate and sources of early gene inactivation following polyploid formation. Our results suggest that the earliest stages of degeneration and gene silencing have been strongly influenced by selection and expression patterns in the diploid parents, highlighting a strong “parental legacy” (14) in early polyploid genome evolution. Furthermore, relaxed selection in this early polyploid is driven not only by genomic redundancy due to ploidy but also, in large part, by demographic effects and the transition to selfing. The results are important for an improved understanding of the consequences of polyploidization for the strength and direction of selection in plant genomes.
Results and Discussion
De Novo Assemblies and Whole-Genome Alignment Across the Capsella Genus.
We generated de novo assemblies for C. bursa-pastoris and C. orientalis (29) using Illumina genomic sequence (Materials and Methods and SI Text). Scaffolds from these assemblies were then aligned to the C. rubella reference genome (21) using whole-genome alignment, along with assemblies of C. grandiflora and the outgroup species Neslia paniculata (21). With the exception of C. bursa-pastoris, only a single orthologous chain was retained for each genomic region, whereas for C. bursa-pastoris, we allowed up to two chains, based on the expectation of assembling sequences from the two homeologous chromosomes. In total, our C. bursa-pastoris assembly spans ∼102 megabases of the 130-megabase C. rubella assembly, of which ∼40% (42 megabases) is covered by two homeologous sequences, whereas the remainder is covered by a single sequence.
Although it is possible that regions covered by a single C. bursa-pastoris sequence reflect large-scale gene deletions, it is more likely that our assembly method often collapses the two homeologous sequences into a single assembled sequence, given the relatively low pairwise differences between the subgenomes (average of 3.7% differences in assembled subgenomes). For investigating the genomic relationships across the Capsella genus, we therefore focus on the 42 megabases of the genome that contain two C. bursa-pastoris subgenomes and provide further support that these regions are representative of genome-wide patterns in follow-up polymorphism analyses.
Evolutionary Origin of C. bursa-pastoris.
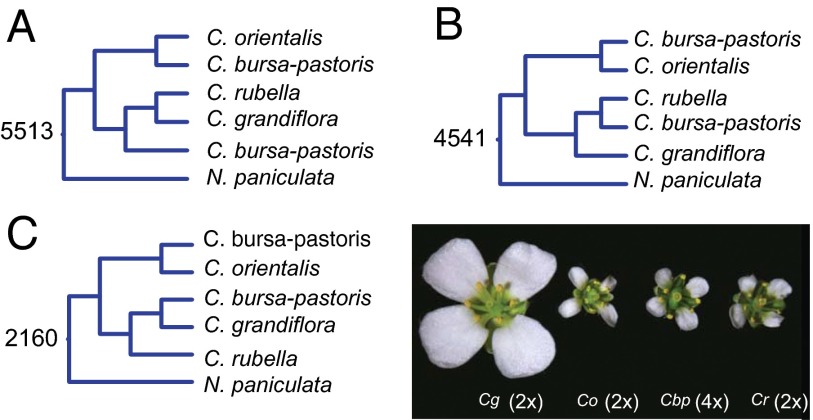
We constructed independent phylogenetic trees for each alignment from genomic fragments that included two homeologous regions of C. bursa-pastoris and one each from C. grandiflora, C. orientalis, C. rubella, and the outgroup sequence N. paniculata. We obtained 17,258 trees where all branches had at least 80% bootstrap support. The total alignment length across these trees represents 13.6 megabases of sequence across the genome. Of these trees, 70% showed clustering of one subgenome with the C. grandiflora/C. rubella lineage (Fig. 1 A–C; hereafter, the “A” subgenome) and a second subgenome with C. orientalis (Fig. 1 A–C; hereafter, the “B” subgenome), providing strong support for a hybrid origin of C. bursa-pastoris. Thus, we conclude that C. bursa-pastoris is an allopolyploid resulting from a hybridization event between these two lineages, and these conclusions have also been supported by Sanger sequencing of a set of genes (SI Text and Fig. S1). Because C. bursa-pastoris clusters with C. orientalis for maternally inherited chloroplast DNA (15), we conclude that the maternal parent of C. bursa-pastoris came from the C. orientalis lineage and the paternal contribution came from an ancestral population from the C. grandiflora/C. rubella lineage. Given the current disjunct distribution of C. grandiflora and C. orientalis (15), the ancestral ranges must have been overlapping in the past to allow for hybridization to occur.
Fig. 1.
Results of RAxML tree analysis from whole-genome alignments of the four Capsella species, using N. paniculata as an outgroup. The three most common topologies are shown in A–C. Numbers represent the number of trees with greater than 80% bootstrap support, showing each most common topology (of 17,258 total), from genome-wide alignments.
Overall, our comparative four-species genome analysis provides strong support for an allopolyploid origin of C. bursa-pastoris. To verify that these conclusions are not biased by a focus on genomic regions that have two subgenomes assembled, and to investigate polyploid origins in more detail, we resequenced nine additional genomes of C. bursa-pastoris. We compared SNP sharing and divergence across these samples by mapping all reads to the reference C. rubella genome, and incorporated genome resequencing data from 13 C. grandiflora accessions (25, 30) and 10 C. orientalis accessions (29) obtained previously. Because we are mapping our tetraploid sequences to a diploid, we expect to see high levels of apparent “heterozygosity”; because C. bursa-pastoris is highly selfing, we expect the vast majority of these heterozygous sites to represent homozygous polymorphic differences between the two homeologous copies. Across the genome, both higher order heterozygosity and depth appear to be quite uniform (Fig. S2A), suggesting the general absence of large-scale deletions and/or recombination events between homeologous chromosomes.
One category of particular interest is sites that exhibit “fixed heterozygosity” across all of our C. bursa-pastoris samples, because these sites represent fixed differences between the subgenomes that arose either because of initial sequence differences between the parental species or due to subsequent mutations that have spread through the tetraploid species. We find that 58% of the 105,082 fixed heterozygous positions are fixed SNP differences between C. orientalis and C. grandiflora. Of the remainder, 30% represent sites that are segregating within C. grandiflora, only 0.2% are segregating within the highly selfing C. orientalis, 0.06% are segregating in both species, and 12% of fixed heterozygous sites are not found segregating in our diploid samples. Conversely, 95.4% of the 77,524 fixed differences identified between C. orientalis and C. grandiflora have both alleles in C. bursa-pastoris, and 83% of these fixed differences show fixed heterozygosity. The high proportion of fixed differences between diploids found within C. bursa-pastoris argues against widespread gene conversion having occurred between homeologous genes. However, there is spatial clustering of the 4.6% of sites fixed for only one of the alleles fixed between the diploids, suggesting a small degree of gene conversion between the subgenomes may have occurred (Fig. S2B). Finally, we examined patterns of transposable element insertion polymorphism sharing across the three species. As expected, principal component analysis places C. bursa-pastoris intermediately between the two diploid species (Fig. S3A), and 72% (2,415) of the insertions shared between two of the three species are shared between C. bursa-pastoris and C. grandiflora, 20% (675) are shared between C. bursa-pastoris and C. orientalis, and only 7% are shared uniquely between C. grandiflora and C. orientalis. These patterns are highly consistent with our earlier conclusions about hybrid origins, with the vast majority of fixed differences between subgenomes having been sampled either from the differences between C. orientalis and C. grandiflora or segregating variation from within the highly polymorphic outcrossing C. grandiflora.
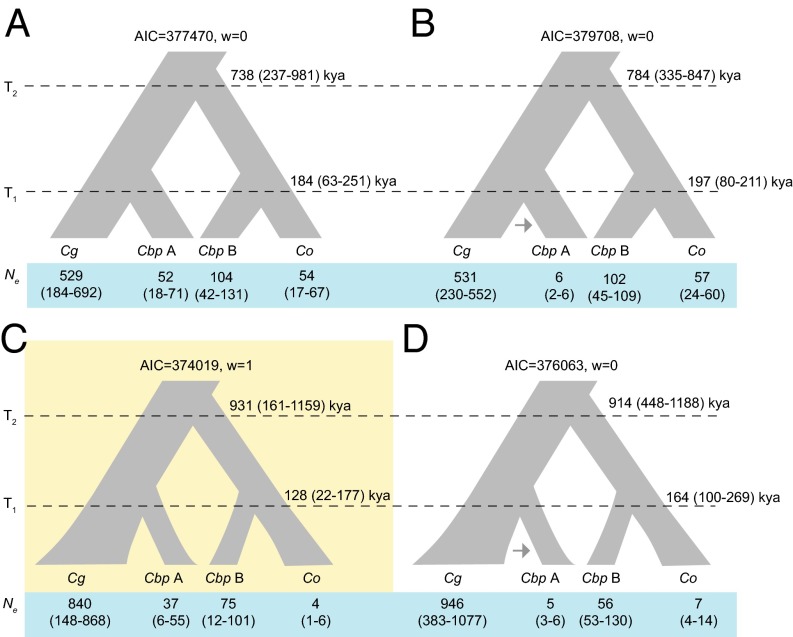
To estimate the timing of origin of C. bursa-pastoris, SNPs from each individual were phased and identified as segregating on the C. grandiflora or C. orientalis descended subgenome (Materials and Methods and SI Text). Principal component analysis of these phased SNPs shows strong clustering of one subgenome (hereafter, the “A subgenome”) with C. grandiflora and C. rubella, whereas the alternate subgenome (hereafter, the “B subgenome”) clusters with C. orientalis (Fig. S3 B and C). We used coalescent simulations applying the composite likelihood method implemented in fastsimcoal2.1 (31) to fit models of speciation to the joint site frequency spectra data from 60,225 phased SNPs at intergenic nonconserved regions (7) and fourfold synonymous sites from the C. bursa-pastoris A subgenome, C. bursa-pastoris B subgenome, C. grandiflora, and C. orientalis. We compared four different models using Akaike’s information criterion, including models with either a stepwise or exponential change in population size and with or without postpolyploidization gene flow. We assumed a mutation rate of 7 × 10−9 per base pair per generation and a generation time of 1 y when converting estimates to units of years and individuals. The models show general agreement in terms of timing of origin, but the best-fit model is one without migration but with exponential growth following speciation (Fig. 2 and SI Text). These analyses suggest that C. bursa-pastoris formed very recently, between 100 and 300 kya, whereas the divergence time between the parental lineages of C. orientalis and C. grandiflora was considerably older, at ∼900 kya. Based on our simulations incorporating exponential population size changes, we estimate that the current effective population size (Ne) of C. bursa-pastoris (∼37,000–75,000) is considerably larger than the Ne of the highly selfing, nearly invariant diploid C. orientalis (4,000) but much smaller than the Ne of the highly outcrossing C. grandiflora (840,000). Given the shared variation between C. bursa-pastoris and both parental species, we can clearly rule out a single polyploid origin from two haploid gametes, although there is likely to be a high level of uncertainty about the precise number of founding lineages (20). Our best-fitting model implies that the Ne of the founding population may be as large as 40,000. The reductions in Ne in C. bursa-pastoris and C. orientalis likely reflect the combined effects of demography and selfing, including the action of greater background selection in highly selfing populations.
Fig. 2.
Demographic parameter estimates with 95% confidence intervals for four models of allopolyploid speciation in Capsella. Four models were investigated: stepwise population size change, no gene flow (A); stepwise population size change, asymmetrical gene flow (B); exponential population size change, no gene flow (C); and exponential population size change, asymmetrical gene flow (D). Model C was preferred based on Akaike’s information criterion (AIC) and Akaike’s weight of evidence (w). Estimates of the Ne for C. grandiflora (Cg), C. orientalis (Co), and C. bursa-pastoris [subgenome A (Cbp A) and subgenome B (Cbp B)] are given in thousands of individuals, and estimates of the timing of the origin of C. bursa-pastoris [T1(Cbp)] and the split between C. grandiflora and C. orientalis [T1(Cg-Co)] are given in thousand years before present (kya). Note that for models with exponential population size change, Ne corresponds to the current Ne. Confidence intervals are given in parentheses.
Identification and Characterization of Putatively Deleterious Mutations.
To examine evidence for gene loss, we looked for genes with significant reductions in normalized coverage in our 10 C. bursa-pastoris genomes compared with the diploid parental species, C. grandiflora and C. orientalis. We find only 76 genes with statistical evidence of gene loss specific to C. bursa-pastoris, suggesting that massive gene loss events have not yet occurred over ∼200,000 y of divergence (Dataset S1). The identified deletions averaged 5,015 bp and spanned 1,067–19,643 bp in length, consistent with conclusions from ancient polyploids that most gene losses are mediated by short deletion events (32). Although some of these deletions are in annotated genes that often show the presence/absence of polymorphism even in diploids, such as F-box proteins and disease resistance genes (33), others appear to be in essential genes (Dataset S1).
Because the coverage analysis requires significant reductions in read number across our entire set of C. bursa-pastoris individuals from a broad geographic range, including Europe and Asia, it is likely to be highly enriched for deletions that are at high frequency or fixed across the species. To examine the potential for sample-specific deletions, structural variants were called using Pindel (34), and we identified deletions spanning 80% or more of a gene. In C. bursa-pastoris, 150 gene deletions unique to the species were identified, whereas 155 and 26 were found in C. grandiflora and C. orientalis, respectively. The relative number of gene deletions compared with all other polymorphic deletions was moderately higher in C. bursa-pastoris than in the diploids (χ2, P < 0.05).
Given the apparently low levels of whole-gene loss in C. bursa-pastoris, we were interested in examining whether more subtle signs of gene degeneration were apparent. We therefore assessed the numbers and frequencies of derived genic loss-of-function SNPs and insertion/deletion events (indels) causing frameshift mutations in coding regions. Possible compensatory mutations, including indels restoring frame and neighboring SNPs with opposite effects (35), were also accounted for and removed from this analysis (SI Text).
We identified a large number of putatively deleterious SNPs in the C. bursa-pastoris genome (Table 1). Interestingly, a large proportion of these SNPs are fixed between subgenomes, implying that one of the duplicate copies may be inactive in all individuals. Nevertheless, we observed an excess of rare variants relative to fourfold degenerate sites among the putatively inactivating mutations still segregating in C. bursa-pastoris, suggesting the continued action of weak purifying selection on at least some of these mutations (Fig. S4). To understand the origins of the putatively deleterious mutations, we examined the frequency and abundance of putatively inactivating mutations occurring in the diploid Capsella genomes (Table 1). Strikingly, the majority of fixed deleterious SNPs between the subgenomes in C. bursa-pastoris were also found in the diploid species; in particular, depending on the effect type, 40–60% of these fixed deleterious SNPs were found in C. orientalis, 5–17% were found in C. grandiflora, and 10–20% were found in both species.
Table 1.
Counts of the different categories of SNPs segregating in C. bursa-pastoris
| SNP effect | Unique to C. bursa-pastoris | Shared with C. grandiflora | Shared with C. orientalis | Shared with both |
| Fourfold synonymous | 10,767 (1,071) | 9,203 (6,875) | 9,325 (5,027) | 4,106 (2,755) |
| Start codon lost | 347 (22) | 75 (11) | 199 (71) | 125 (21) |
| Stop codon gained | 2,829 (105) | 650 (55) | 1,093 (348) | 485 (122) |
| Stop codon lost | 312 (18) | 138 (25) | 252 (105) | 220 (37) |
| Splice site lost | 1,652 (74) | 394 (56) | 743 (271) | 454 (77) |
Values in parentheses indicate fixed heterozygous SNPs likely reflecting fixed differences between homeologs.
Nearly all (98%) of the putatively deleterious SNPs in C. orientalis also found in C. bursa-pastoris are fixed in C. orientalis, reflecting its very low levels of polymorphism. Because C. orientalis is highly selfing and has a low Ne, slightly deleterious mutations could have been fixed in C. orientalis and passed on to C. bursa-pastoris. In strong contrast, many of the putatively deleterious SNPs from C. grandiflora are found at low frequencies in this highly polymorphic outcrosser, and less than 1% are fixed in C. grandiflora. Furthermore, there is evidence that the frequencies of these mutations have increased in C. bursa-pastoris compared with C. grandiflora. Thus, although there is an apparent bias toward C. orientalis being a source of deleterious SNPs, it is likely that additional “unique” deleterious SNPs in C. bursa-pastoris originated from rare SNPs from the C. grandiflora-like ancestor. We may thus be underestimating the proportion of deleterious SNPs inherited from C. grandiflora and overestimating the number of unique deleterious SNPs in C. bursa-pastoris. In either case, it appears that the beginnings of gene inactivation in C. bursa-pastoris may have had a “head start” from both fixed, slightly deleterious inactivations in C. orientalis and low-frequency, possibly recessive deleterious variation from the outcrossing C. grandiflora-like progenitor.
Biased Gene Loss.
To investigate bias in the types of genes being inactivated in all three Capsella species, we examined the functional categories of Arabidopsis orthologs using the Virtual Plant online server (36). Consistent with analyses of convergent ancient gene loss events (1), there was an enrichment of C. bursa-pastoris SNPs affecting stop codon gain and splice site loss in genes related to several functions associated with DNA (Table S1). Interestingly, segregating C. grandiflora stop codon-gained SNPs are enriched for similar functional categories related to DNA repair and metabolism. These shared enrichments of functional categories are for mutations unique to each species, as well as those mutations shared between Capsella species, suggesting the similarities are not trivially due to shared polymorphisms. In contrast, putative loss-of-function mutations in C. orientalis were not enriched for any gene ontology (GO) category. Thus, genes for which inactivating mutations segregate in the outbred diploid ancestor also tend to show independent derived mutations causing gene inactivation in the recently formed tetraploid, but not in the highly homozygous selfing C. orientalis. However, genes within these enriched GO categories also tend to have larger coding regions on average (mean difference of 416 base pairs; Wilcoxon rank sum test, P < 2.2 × 10−12) and, as a result, harbor more mutations in general. Because several of these GO categories have also been shown to be overrepresented for gene losses in ancient polyploids (1), mutational target size could be an underappreciated deciding factor in which genes are recurrently and rapidly lost following WGD. It is also possible that mutations in these genes are tolerated in both the outcrossing diploid and allotetraploid, perhaps due to their predominantly recessive effects. This latter idea is consistent with the hypothesis that dosage-insensitive genes are more likely to experience gene loss following WGD (37), because these genes should also be more tolerated as polymorphisms in outbred diploid populations.
To test whether positive selection is driving the fixation of loss-of-function point mutations, we scanned for signs of “selective sweeps” or dips in neutral diversity surrounding fixed inactivating mutations. Average nucleotide diversity at fourfold synonymous sites was assessed in 50-kb windows of coding regions upstream and downstream of fixed putatively deleterious point mutations, separately for homeologous chromosomes. These windows show a large amount of variation but no clear trend of reduced neutral diversity near focal mutations in comparison to synonymous substitutions (Fig. S5). Thus, there is no clear evidence that inactivating mutations were fixed by positive selection.
No Evidence for Homeolog-Specific Loss of Expression.
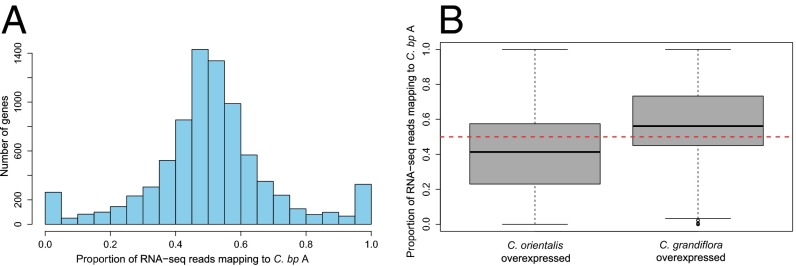
Genomic patterns suggest that there has not been rapid gene loss and that the early stages of gene degeneration in C. bursa-pastoris may be driven, in large part, by ancestral variation from its diploid progenitors. However, it is still possible that de novo changes in gene expression associated with epigenetic modification are widespread. To investigate this possibility, we examined adult leaf gene expression patterns for the two homeologous genomes of one C. bursa-pastoris strain from our population genomic analyses and compared expression with replicate genotypes of the diploid progenitors (SI Text). In general, expression levels were comparable on the two subgenomes, with the median relative expression of 0.5 (Fig. 3A) showing no evidence of general expression biases toward one of the two homeologous genomes. We investigated if ancestral differences, or “legacies,” in gene expression could have contributed to differences in subgenome expression (14, 38). Overall, there was conservation in levels of leaf gene expression between the diploids, but of the 8,164 genes assayed, we identified 241 as differentially expressed (false discovery rate-adjusted P < 0.01). Genes overexpressed by at least twofold in either diploid are also more highly expressed on the corresponding descendent subgenome (Fig. 3B), confirming that ancestral differences in gene expression predict differential expression in C. bursa-pastoris. Thus, as with our genomic analyses, we conclude that the early stages of homeologous gene silencing are determined, at least in part, by the gene expression history of diploid progenitors.
Fig. 3.
Transcriptome analyses testing for homeolog-specific expression. (A) Proportion of reads mapping to Cbp A for all genes assayed. (B) Proportion of reads mapping to Cbp A in genes overexpressed within each diploid progenitor. The dashed red line indicates where an equal proportion of reads maps to both C. bursa-pastoris subgenomes. RNA-seq, RNA-sequencing.
Quantifying Relaxation of Purifying Selection.
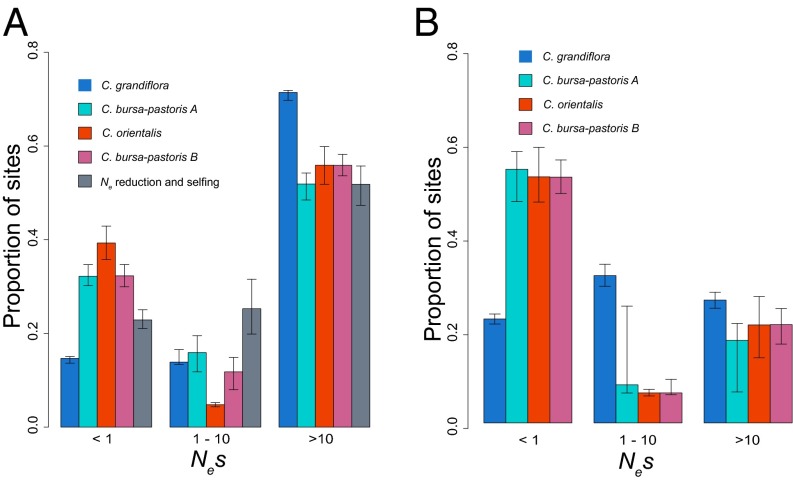
Overall, we detected small numbers of gene loss events and signs that many major-effect mutations were inherited from parental species. Therefore, the earliest stages of polyploid gene degeneration may be subtle and reflect a global shift in selection pressures genome-wide rather than rampant gene loss. To investigate the strength of selection acting on C. bursa-pastoris compared with its close diploid relatives, we estimated the distribution of fitness effects (DFE) of deleterious mutations (39) for all three Capsella species using allele frequency spectra and separating the two homeologous genomes of C. bursa-pastoris. The method explicitly incorporates nonequilibrium population size changes, and thus should account for between-species differences in demographic history. We estimated the DFE at both zerofold nonsynonymous sites and conserved noncoding sites, as previously defined through conservation between the Capsella reference and nine additional Brassicaceae species (30). Between C. bursa-pastoris subgenomes, there was no significant difference in the DFE for either site type, providing no evidence for early signs of biased fractionation in terms of the efficacy of selection on the two subgenomes (Fig. 4). However, genes showing homeolog-specific gene silencing (SI Text) are under significantly less purifying selection on the silenced subgenome (P < 0.05), suggesting that the ancestral differences in gene expression inherited in C. bursa-pastoris can help drive the early degeneration of lower expressed homeologous genes.
Fig. 4.
Estimates of the DFE of deleterious mutations at zerofold nonsynonymous sites (A) and conserved noncoding sites (B), based upon the allele frequency spectra of SNPs at these sites. The simulated DFE for zerofold nonsynonymous sites under Ne reduction and a shift to selfing is also shown in A. The strength of selection is measured as Nes, where s is the strength of selection. Error bars correspond to 95% confidence intervals of 200 bootstrap replicates of 10-kb blocks.
As predicted, the C. bursa-pastoris A subgenome showed a significantly elevated proportion of effectively neutral mutations (Nes < 1, where s is the strength of selection against deleterious mutations) compared with C. grandiflora for both nonsynonymous and conserved noncoding sites, consistent with the prediction that the transition to polyploidy has led to genome-wide relaxed selection (Fig. 4A). In contrast, however, C. orientalis shows a significantly elevated proportion of effectively neutral nonsynonymous mutations compared with the C. bursa-pastoris B subgenome (Fig. 4A), and there is no difference between the two for conserved noncoding sites (Fig. 4B). Given the severe reduction in Ne and the selfing mating system in C. orientalis (Fig. 2), demographic effects and mating system changes could have similar effects on the strength of purifying selection as the ploidy transition.
To what extent is relaxed selection in C. bursa-pastoris driven by the shift to self-fertilization and reductions in Ne, rather than the change in ploidy? To address this question, we conducted forward simulations, modeling a diploid population that has experienced the mating system transition and reduction in Ne over the time scale inferred for C. bursa-pastoris (Fig. 2 and SI Text). In these simulated populations, we observe a considerable increase in the proportion of effectively neutral mutations (Fig. 4A), implying that the shift in the strength of purifying selection is due, in part, to greater effects of drift caused by demography and selfing. However, the extent of relaxed selection due to the simulated reduction in Ne is considerably smaller than what we observe (Fig. 4). Specifically, based on the bootstrapped 95% confidence interval, the contributions of demography and changes in mating system account for 30–66% of the shift in purifying selection in C. bursa-pastoris. Thus, the transition to polyploidy and the corresponding redundancy in gene function are also likely contributing to the changes in selection.
Conclusions
Our results provide comprehensive evidence for recent allopolyploid origins of C. bursa-pastoris from two parental lineages ancestral to present-day C. orientalis and C. grandiflora. Given the patterns of polymorphism and expression, our results suggest that gene degradation and silencing following allopolyploidization can get a head start from standing variation in progenitors. By contrasting patterns of functional polymorphism and divergence genome-wide, we find evidence for significant relaxation of purifying selection driven by both increased drift and relaxed selection due to gene redundancy, but no evidence for massive gene loss rapidly upon polyploidization. Demographic and historical factors that accompanied polyploidy were responsible for a large proportion of the inferred relaxation of purifying selection, suggesting that masking due to gene redundancy is only one of several contributors to early genome evolution following polyploidy. Taken together, and in contrast to patterns inferred in other systems (9, 11), our results suggest that the early stages of allopolyploid evolution in C. bursa-pastoris were characterized by relaxed purifying selection rather than large-scale “genomic shock” and rapid gene loss.
Materials and Methods
Seeds from 10 C. bursa-pastoris plants were collected from one individual per population across the species range in Eurasia (SI Text). Nuclear genomic DNA was extracted from leaf material for the 10 C. bursa-pastoris individuals using a modified CTAB protocol (40). Paired-end 108-bp and 150-bp reads were generated for C. bursa-pastoris, with a median depth coverage of 20 reads per site using Illumina GAII and Illumina HiSeq 2000 systems. Sanger sequencing of a more extensive sample of C. bursa-pastoris was used in combination with published sequences from other Capsella species (SI Text) to validate the conclusions about hybrid origins. For the transcriptome analyses, we extracted RNA from adult leaf tissue with a Qiagen Plant RNEasy Plant Mini Kit from one C. bursa-pastoris individual (SE14), six C. grandiflora individuals, and three biological replicates each of two C. orientalis individuals. RNA-sequencing libraries were generated using the TruSeq RNA version 2 protocol (Illumina) and sequenced on an Illumina HiSeq 2000 system.
De novo fragment assemblies for C. bursa-pastoris were generated using Ray version 1.4 (41). Read mapping of Illumina genomic reads to the C. rubella reference genome (21) was conducted using Stampy version 13 (42), and phasing of read-mapped samples was conducted using HapCUT (43). SNP calling and polymorphism analyses were conducted using the Genome Analysis Toolkit (GATK) (44). We constructed phylogenies using RAxML’s (45) rapid bootstrap algorithm to find the best-scoring maximum likelihood (ML) tree. For demographic inferences, we used fastsimcoal2.1 (31) to infer demographic parameters based on the multidimensional site frequency spectrum for C. grandiflora, C. orientalis, and the two C. bursa-pastoris homeologous genomes. To evaluate the fit of the best demographic model (Table S2), simulated datasets were compared with the observed site frequency spectra (Fig. S6). The DFE was estimated for each species using the ML approach designed by Keightley and Eyre-Walker (39). We conducted forward simulations using SLiM software (46). SnpEff (47) was used to predict the effects of SNPs called using the GATK. Detailed methods on de novo assembly, expression analysis, population genomics analyses, and validation are provided in SI Text.
Supplementary Material
Acknowledgments
We thank Vitor Sousa (University of Bern) for help with fastsimcoal analyses. DNA and RNA sequencing was performed by the Genome Quebec Innovation Centre, and RNA sequencing was performed by the SNP&SEQ Technology Platform, Science for Life Laboratory (Uppsala University), a national infrastructure supported by the Swedish Research Council (VR-RFI) and the Knut and Alice Wallenberg Foundation. This project was funded by a Natural Sciences and Engineering Research Council (NSERC) Discovery grant and a Genome Quebec/Genome Canada grant (to S.I.W.) and by grants from the Swedish Research Council (to T.S. and M.L.). G.M.D. was supported by an NSERC scholarship, and E.B.J. was supported by a National Science Foundation graduate fellowship. The fastsimcoal2.1 computations were performed on resources provided by the Swedish National Infrastructure for Computing through the Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project b2012190.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the Sequence Read Archive, www.ncbi.nlm.nih.gov/sra [accession nos. PRJNA268827 (Capsella bursa-pastoris genomic data), PRJNA268847 (C. bursa-pastoris RNA-sequencing), and PRJNA268848 (Capsella grandiflora RNA-sequencing)], and the European Bioinformatics Institute, www.ebi.ac.uk [accession no. PRJEB7879 (Capsella orientalis RNA-sequencing data)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412277112/-/DCSupplemental.
References
- 1.De Smet R, et al. Convergent gene loss following gene and genome duplications creates single-copy families in flowering plants. Proc Natl Acad Sci USA. 2013;110(8):2898–2903. doi: 10.1073/pnas.1300127110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290(5494):1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 3.Otto SP, Whitton J. Polyploid incidence and evolution. Annu Rev Genet. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. [DOI] [PubMed] [Google Scholar]
- 4.Sankoff D, Zheng C. Fractionation, rearrangement and subgenome dominance. Bioinformatics. 2012;28(18):i402–i408. doi: 10.1093/bioinformatics/bts392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnable JC, Springer NM, Freeling M. Differentiation of the maize subgenomes by genome dominance and both ancient and ongoing gene loss. Proc Natl Acad Sci USA. 2011;108(10):4069–4074. doi: 10.1073/pnas.1101368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langham RJ, et al. Genomic duplication, fractionation and the origin of regulatory novelty. Genetics. 2004;166(2):935–945. doi: 10.1093/genetics/166.2.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodhouse MR, et al. Origin, inheritance, and gene regulatory consequences of genome dominance in polyploids. Proc Natl Acad Sci USA. 2014;111(14):5283–5288. doi: 10.1073/pnas.1402475111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang PL, Dilkes BP, McMahon M, Comai L, Nuzhdin SV. Homoeolog-specific retention and use in allotetraploid Arabidopsis suecica depends on parent of origin and network partners. Genome Biol. 2010;11(12):R125. doi: 10.1186/gb-2010-11-12-r125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buggs RJA, et al. Rapid, repeated, and clustered loss of duplicate genes in allopolyploid plant populations of independent origin. Curr Biol. 2012;22(3):248–252. doi: 10.1016/j.cub.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst. 1998;29:467–501. [Google Scholar]
- 11.Chen ZJ, Ni Z. Mechanisms of genomic rearrangements and gene expression changes in plant polyploids. BioEssays. 2006;28(3):240–252. doi: 10.1002/bies.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams KL, Cronn R, Percifield R, Wendel JF. Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. Proc Natl Acad Sci USA. 2003;100(8):4649–4654. doi: 10.1073/pnas.0630618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buggs RJA, et al. Transcriptomic shock generates evolutionary novelty in a newly formed, natural allopolyploid plant. Curr Biol. 2011;21(7):551–556. doi: 10.1016/j.cub.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Buggs RJA, et al. The legacy of diploid progenitors in allopolyploid gene expression patterns. Philos Trans R Soc Lond B Biol Sci. 2014;369(1648):20130354. doi: 10.1098/rstb.2013.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurka H, Friesen N, German DA, Franzke A, Neuffer B. ‘Missing link’ species Capsella orientalis and Capsella thracica elucidate evolution of model plant genus Capsella (Brassicaceae) Mol Ecol. 2012;21(5):1223–1238. doi: 10.1111/j.1365-294X.2012.05460.x. [DOI] [PubMed] [Google Scholar]
- 16.St Onge KR, et al. Coalescent-based analysis distinguishes between allo- and autopolyploid origin in Shepherd’s Purse (Capsella bursa-pastoris) Mol Biol Evol. 2012;29(7):1721–1733. doi: 10.1093/molbev/mss024. [DOI] [PubMed] [Google Scholar]
- 17.Hurka H, Neuffer B. Evolutionary processes in the genus Capsella (Brassicaceae) Plant Syst Evol. 1997;206(1-4):295–316. [Google Scholar]
- 18.Foxe JP, et al. Recent speciation associated with the evolution of selfing in Capsella. Proc Natl Acad Sci USA. 2009;106(13):5241–5245. doi: 10.1073/pnas.0807679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y-L, et al. Recent speciation of Capsella rubella from Capsella grandiflora, associated with loss of self-incompatibility and an extreme bottleneck. Proc Natl Acad Sci USA. 2009;106(13):5246–5251. doi: 10.1073/pnas.0808012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandvain Y, Slotte T, Hazzouri KM, Wright SI, Coop G. Genomic identification of founding haplotypes reveals the history of the selfing species Capsella rubella. PLoS Genet. 2013;9(9):e1003754. doi: 10.1371/journal.pgen.1003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slotte T, et al. The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nat Genet. 2013;45(7):831–835. doi: 10.1038/ng.2669. [DOI] [PubMed] [Google Scholar]
- 22.Paetsch M, Mayland-Quellhorst S, Hurka H, Neuffer B. Evolution of the mating system in the genus Capsella (Brassicaceae) In: Glaubrecht M, editor. Evolution in Action. Springer; Berlin: 2010. pp. 77–100. [Google Scholar]
- 23.Paetsch M, Mayland-Quellhorst S, Neuffer B. Evolution of the self-incompatibility system in the Brassicaceae: Identification of S-locus receptor kinase (SRK) in self-incompatible Capsella grandiflora. Heredity (Edinb) 2006;97(4):283–290. doi: 10.1038/sj.hdy.6800854. [DOI] [PubMed] [Google Scholar]
- 24.Slotte T, Ceplitis A, Neuffer B, Hurka H, Lascoux M. Intrageneric phylogeny of Capsella (Brassicaceae) and the origin of the tetraploid C. bursa-pastoris based on chloroplast and nuclear DNA sequences. Am J Bot. 2006;93(11):1714–1724. doi: 10.3732/ajb.93.11.1714. [DOI] [PubMed] [Google Scholar]
- 25.Haudry A, et al. An atlas of over 90,000 conserved noncoding sequences provides insight into crucifer regulatory regions. Nat Genet. 2013;45(8):891–898. doi: 10.1038/ng.2684. [DOI] [PubMed] [Google Scholar]
- 26.Hurka H, Freundner S, Brown AH, Plantholt U. Aspartate aminotransferase isozymes in the genus Capsella (Brassicaceae): Subcellular location, gene duplication, and polymorphism. Biochem Genet. 1989;27(1-2):77–90. doi: 10.1007/BF00563019. [DOI] [PubMed] [Google Scholar]
- 27.Mummenhoff K, Hurka H. Evolution of the tetraploid Capsella bursa-pastoris (Brassicaceae): Isoelectric-focusing analysis of Rubisco. Plant Syst Evol. 1990;172(1-4):205–213. [Google Scholar]
- 28.Roux C, Pannell JR. Inferring the mode of origin of polyploid species from next-generation sequence data. Mol Ecol. 2015 doi: 10.1111/mec.13078. [DOI] [PubMed] [Google Scholar]
- 29.Ågren JA, et al. Mating system shifts and transposable element evolution in the plant genus Capsella. BMC Genomics. 2014;15:602. doi: 10.1186/1471-2164-15-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson RJ, et al. Evidence for widespread positive and negative selection in coding and conserved noncoding regions of Capsella grandiflora. PLoS Genet. 2014;10(9):e1004622. doi: 10.1371/journal.pgen.1004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Excoffier L, Dupanloup I, Huerta-Sánchez E, Sousa VC, Foll M. Robust demographic inference from genomic and SNP data. PLoS Genet. 2013;9(10):e1003905. doi: 10.1371/journal.pgen.1003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodhouse MR, et al. Following tetraploidy in maize, a short deletion mechanism removed genes preferentially from one of the two homologs. PLoS Biol. 2010;8(6):e1000409. doi: 10.1371/journal.pbio.1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark RM, et al. Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science. 2007;317(5836):338–342. doi: 10.1126/science.1138632. [DOI] [PubMed] [Google Scholar]
- 34.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: A pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25(21):2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long Q, et al. Massive genomic variation and strong selection in Arabidopsis thaliana lines from Sweden. Nat Genet. 2013;45(8):884–890. doi: 10.1038/ng.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katari MS, et al. VirtualPlant: A software platform to support systems biology research. Plant Physiol. 2010;152(2):500–515. doi: 10.1104/pp.109.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conant GC, Birchler JA, Pires JC. Dosage, duplication, and diploidization: Clarifying the interplay of multiple models for duplicate gene evolution over time. Curr Opin Plant Biol. 2014;19:91–98. doi: 10.1016/j.pbi.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb LD. Plant polyploidy: Gene expression and genetic redundancy. Heredity (Edinb) 2003;91(2):91–92. doi: 10.1038/sj.hdy.6800317. [DOI] [PubMed] [Google Scholar]
- 39.Keightley PD, Eyre-Walker A. Joint inference of the distribution of fitness effects of deleterious mutations and population demography based on nucleotide polymorphism frequencies. Genetics. 2007;177(4):2251–2261. doi: 10.1534/genetics.107.080663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 41.Boisvert S, Laviolette F, Corbeil J. Ray: Simultaneous assembly of reads from a mix of high-throughput sequencing technologies. J Comput Biol. 2010;17(11):1519–1533. doi: 10.1089/cmb.2009.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lunter G, Goodson M. Stampy: A statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 2011;21(6):936–939. doi: 10.1101/gr.111120.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bansal V, Bafna V. HapCUT: An efficient and accurate algorithm for the haplotype assembly problem. Bioinformatics. 2008;24(16):i153–i159. doi: 10.1093/bioinformatics/btn298. [DOI] [PubMed] [Google Scholar]
- 44.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Messer PW. SLiM: Simulating evolution with selection and linkage. Genetics. 2013;194(4):1037–1039. doi: 10.1534/genetics.113.152181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6(2):80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.