Significance
This paper shows that a class of receptors known to modulate insulin release by pancreatic β cells also regulates the proliferation of these cells and restrains the perinatal β-cell expansion that establishes adult β-cell mass, suggesting that alterations in signaling by these receptors could contribute to the decreased β-cell numbers seen in patients with type 2 diabetes. Further, inhibition of signaling through these receptors potentially could be used to generate more β cells for people with diabetes.
Keywords: islet, β cell mass, perinatal, G-protein coupled receptors, diabetes mellitus
Abstract
Gi-GPCRs, G protein-coupled receptors that signal via Gα proteins of the i/o class (Gαi/o), acutely regulate cellular behaviors widely in mammalian tissues, but their impact on the development and growth of these tissues is less clear. For example, Gi-GPCRs acutely regulate insulin release from pancreatic β cells, and variants in genes encoding several Gi-GPCRs—including the α-2a adrenergic receptor, ADRA2A—increase the risk of type 2 diabetes mellitus. However, type 2 diabetes also is associated with reduced total β-cell mass, and the role of Gi-GPCRs in establishing β-cell mass is unknown. Therefore, we asked whether Gi-GPCR signaling regulates β-cell mass. Here we show that Gi-GPCRs limit the proliferation of the insulin-producing pancreatic β cells and especially their expansion during the critical perinatal period. Increased Gi-GPCR activity in perinatal β cells decreased β-cell proliferation, reduced adult β-cell mass, and impaired glucose homeostasis. In contrast, Gi-GPCR inhibition enhanced perinatal β-cell proliferation, increased adult β-cell mass, and improved glucose homeostasis. Transcriptome analysis detected the expression of multiple Gi-GPCRs in developing and adult β cells, and gene-deletion experiments identified ADRA2A as a key Gi-GPCR regulator of β-cell replication. These studies link Gi-GPCR signaling to β-cell mass and diabetes risk and identify it as a potential target for therapies to protect and increase β-cell mass in patients with diabetes.
The G protein-coupled receptors (GPCRs), including Gi-GPCRs (1), comprise the largest family of mammalian cell-surface receptors and the largest target group for Food and Drug Administration-approved drugs (2), including drugs used to treat diabetes (3). Gi-GPCR gene variants associated with human diseases are thought to influence disease risk by modifying acute cellular behaviors such as insulin release (3–9). However, diabetes also is associated with decreased pancreatic β-cell mass (10–13), and little is known about the role Gi-GPCR signaling plays in organ development or size or whether Gi-GPCR variants could impact disease risk by altering organ size.
Pancreatic β cells respond acutely to signaling through multiple GPCRs by altering insulin secretion. Examples include the gut incretins GIP and GLP1, which stimulate insulin secretion in a glucose-dependent manner through their cognate Gαs-linked GPC receptors, GIPR and GLP1R; acetylcholine, which stimulates insulin secretion in a glucose-independent manner through the Gαq-linked cholinergic receptor, muscarinic 3 (CHRM3); and catecholamines and somatostatin, which inhibit insulin secretion through the Gi-GPCRs α2A adrenergic receptor (ADRA2A) and SSTR3, respectively (3, 14). Variants in the genes encoding GIPR and ADRA2A alter the risk of type 2 diabetes (5, 6, 15).
Insulin secretory capacity depends on both the secretory capacity of individual β cells and total β-cell mass, which is reduced in both type 1 and type 2 diabetes (10–13). Two sources contribute to the pool of β cells in the pancreas: neogenesis from progenitor cells and proliferation of preexisting β cells. The β-cell population expands most dramatically during the perinatal and early postnatal period because of increased proliferation, which then falls markedly as adulthood approaches in both rodents and humans (16, 17).
Therefore, we asked whether Gi-GPCR signaling could modify diabetes risk by altering β-cell proliferation, especially during the perinatal expansion, when even modest changes in the high basal rates of proliferation potentially could have a large impact on final β-cell mass.
Results
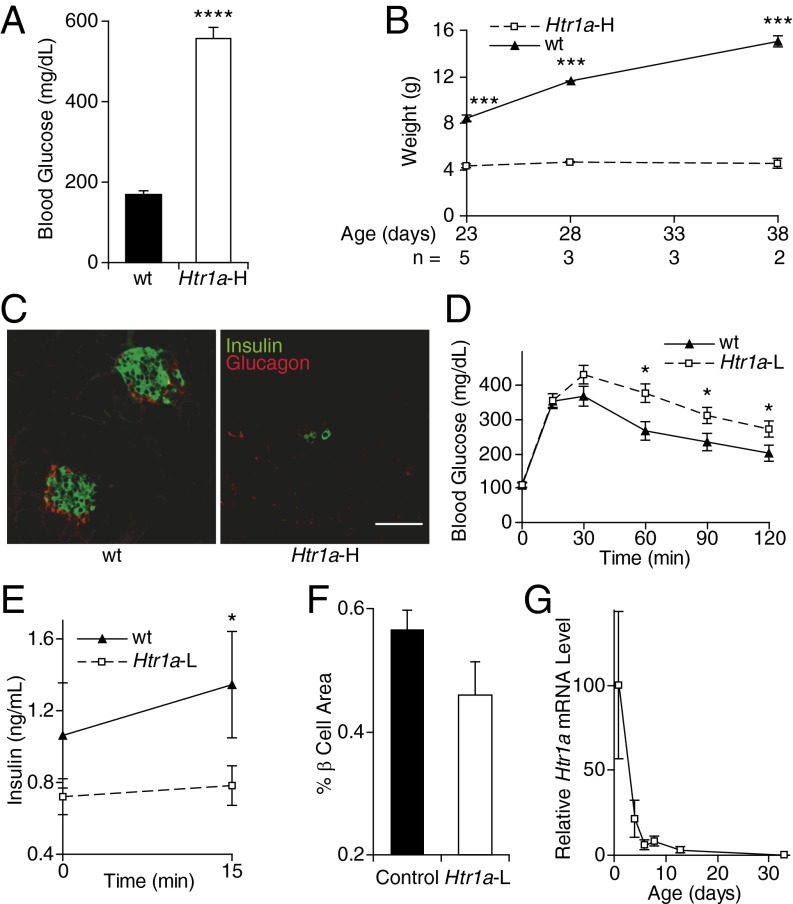
To examine this question, we manipulated perinatal β-cell Gi-GPCR signaling and then measured glucose homeostasis in adult animals. First, we expressed a well-studied Gi-GPCR, serotonin receptor HTR1A, in developing islet cells under the control of the ePet1 enhancer from the Fev gene (Fig. S1A) (18, 19). The transgenic progeny of one ePet1-Htr1a transgenic founder displayed marked hyperglycemia (Fig. 1A) and failure to thrive and gain weight (Fig. 1B), together with marked reductions in β-cell numbers (Htr1a-H; Fig. 1C).
Fig. 1.
Glucose metabolism in ePet1-Htr1a mice. (A) Blood glucose levels were measured in nonfasting Htr1a-H mice (n = 3) and nontransgenic control littermates (n = 4). (B) Weights of Htr1a-H mice and control littermates are shown. (C) Representative pancreatic sections from an Htr1a-H mouse and a control littermate at age P7 were stained for insulin (green) and glucagon (red). (Scale bar, 50 µm.) (D and E) Blood glucose (n = 19 Htr1a-L and 12 wild-type mice) (D) and plasma insulin (n = 19 Htr1a-L and 13 wild-type mice) (E) levels were measured at the indicated time points following i.p. glucose injection in adult mice. (F) β-Cell area was measured as a percent of total pancreatic area in adult control (n = 17) and Htr1a-L (n = 12) mice. (G) Htr1a mRNA levels were measured in the pancreata of Htr1a-L mice at the ages shown (n = 5–10 mice at each age). All data points represent the mean ± SEM. *P < 0.05, ***P < 0.001, and ****P < 0.0001 vs. wild-type animals by two-tailed Student’s t test.
We could not study this transgenic line further because of premature mortality. Animals from a second ePet1-Htr1a transgenic line with a lower transgene copy number (Htr1a-L; Fig. S1B) bred normally and displayed normal weight gain (Fig. S2A) but also exhibited impaired glucose tolerance (Fig. 1D) and glucose-stimulated insulin release (Fig. 1E). Blood glucose decreased normally in these mice after insulin injection (Fig. S2B), making impaired insulin sensitivity an unlikely cause of their impaired glucose tolerance. The Htr1a-L adults also had ∼30% decreased β-cell mass (Fig. 1F). After high perinatal expression, Htr1a expression shut off within 2 wk after birth in Htr1a-L animals (Fig. 1G), suggesting that HTR1A signaling during perinatal β-cell expansion led to persistent decreased β-cell mass and impaired glucose homeostasis in the adults.
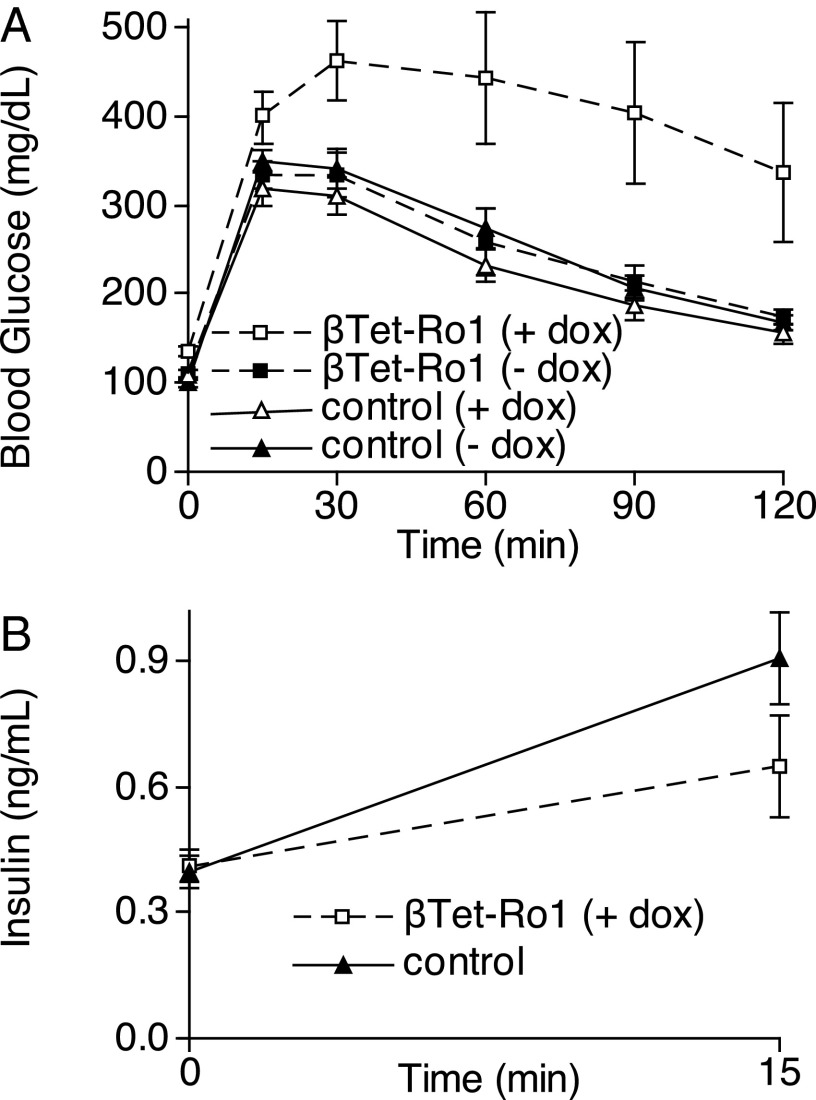
Next we examined whether these adult metabolic effects of expressing a Gi-GPCR were specific to HTR1A or could be generalized to other Gi-GPCRs when expressed on perinatal β cells. We used the reverse tetracycline transactivator under control of the rat insulin 2 gene promoter (RIP-rtTA) (20) to drive expression of the synthetic Gαi/o-coupled receptor activated solely by synthetic ligand (RASSL) Ro1 (21, 22) on β cells specifically during perinatal development (βTet-Ro1 mice). Ro1 can be activated by the synthetic ligand spiradoline but has basal Gαi/o signaling activity in the absence of ligand (23). Perinatal expression of Ro1 on β cells even without spiradoline administration led to worsened adult glucose homeostasis (Fig. 2A), with reduced insulin release after glucose challenge despite the higher glucose levels (Fig. 2B).
Fig. 2.
Glucose metabolism in βTet-Ro1 mice. (A) Glucose-tolerance tests were performed in adult βTet-Ro1 mice and control littermates whose mothers were treated with or without doxycycline from conception until day P7. (B) Plasma insulin levels were measured before and 15 min after i.p. glucose injection in βTet-Ro1 mice (n = 4) and controls (n = 17). All data points represent the mean ± SEM. See Table S1 for weights and Tables S2 and S3 for statistical analysis of A.
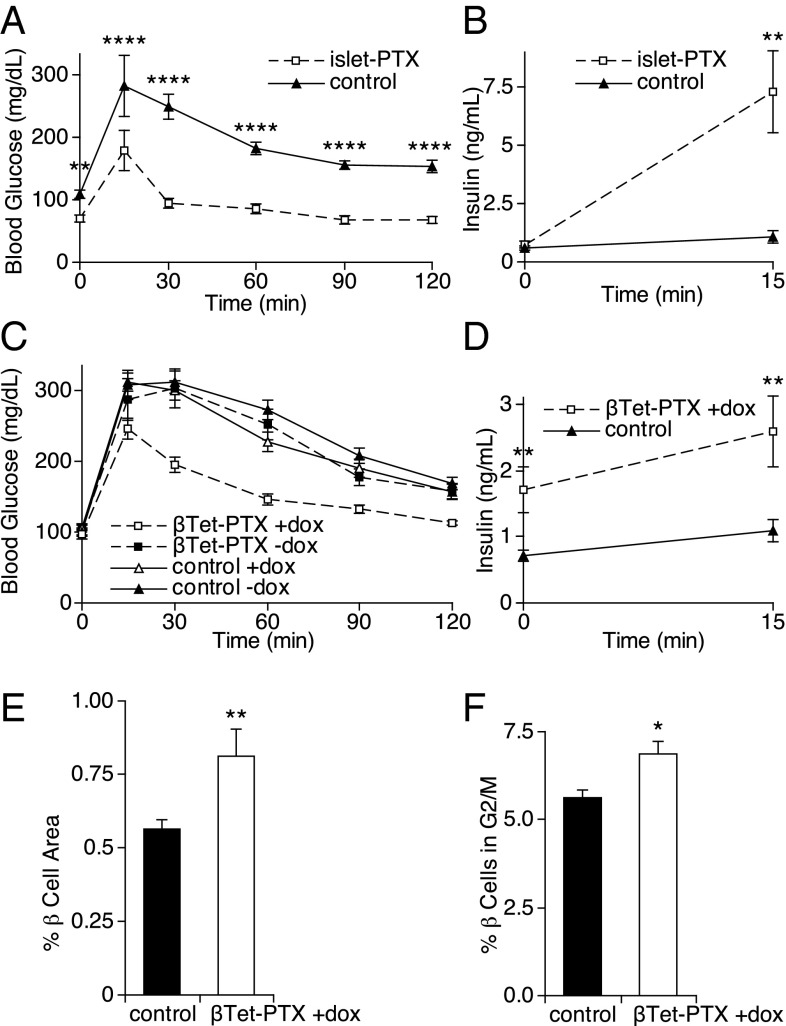
Because perinatal transgenic expression of Gi-GPCRs on β cells restricted β-cell expansion and impaired adult insulin secretion and glucose homeostasis, we asked whether endogenous Gi-GPCRs regulate perinatal β-cell development and subsequent adult glucose homeostasis. To answer this question, we expressed the Gαi/o signaling inhibitor pertussis toxin (PTX) in β cells by crossing ROSA-PTX knockin mice to ePet1-cre transgenic mice (islet-PTX mice) (18, 24). These animals responded to a glucose-tolerance test with lower blood glucose elevations and markedly increased glucose-stimulated insulin secretion (Fig. 3 A and B), consistent with previous data (24).
Fig. 3.
Blocking Gαi/o signaling with PTX in β-cells. (A) Glucose-tolerance tests were performed in adult islet-PTX (n = 7) and control (n = 7) mice. (B) Plasma insulin levels were measured at the indicated times after i.p. glucose injection in islet-PTX (n = 5) and control (n = 5) mice. (C) Glucose-tolerance tests were performed in adult βTet-PTX mice and control littermates whose mothers were treated with or without doxycycline from conception until day P7. (D) Plasma insulin levels were measured at the indicated times after i.p. glucose injection in βTet-PTX (n = 8) and control (n = 10) mice. (E) β-Cell area was measured as a percent of total pancreatic area in adult βTet-PTX (n = 7) and control (n = 17) mice. (F) Replication rate in β cells from βTet-PTX (n = 6) and control (n = 10) mice at age P1 was measured by determining the percentage of β cells in the G2 and M phases of the cell cycle by flow cytometry. All data points represent the mean ± SEM. *P < 0.05, **P < 0.01, and ****P < 0.0001 vs. control animals by two-tailed Student’s t test. See Tables S4 and S5 for weights for animals in A and C, respectively, and Tables S6 and S7 for statistical analysis of C.
Although we hypothesize that increased β-cell mass contributed to the improved glucose tolerance and insulin secretion observed in the islet-PTX mice, these improvements could result from decreased Gi-GPCR inhibition of insulin secretion alone, because PTX expression persists in adult β cells in this model. Therefore we blocked Gαi/o signaling selectively in developing β cells (Fig. S3) by treating mothers of RIP-rtTA/tetO-PTX double transgenic mice (βTet-PTX mice) with doxycycline until postnatal day 7. As adults, the βTet-PTX mice treated perinatally with doxycycline had improved glucose homeostasis (Fig. 3C), increased insulin release (Fig. 3D), and increased β-cell mass (Fig. 3E).
To determine how Gαi/o signaling modulates β-cell mass, we compared β-cell replication in FACS-purified β cells from MIP-GFP/βTet-PTX triple transgenic mice versus controls. We found an increased fraction of β cells in the G2/M phase of the cell cycle in neonates expressing PTX versus controls (Fig. 3F and Fig. S4). This result suggests that Gi-GPCRs suppress β-cell proliferation in a cell-autonomous manner during perinatal development, and this suppression in turn impacts adult β-cell mass and glucose homeostasis.
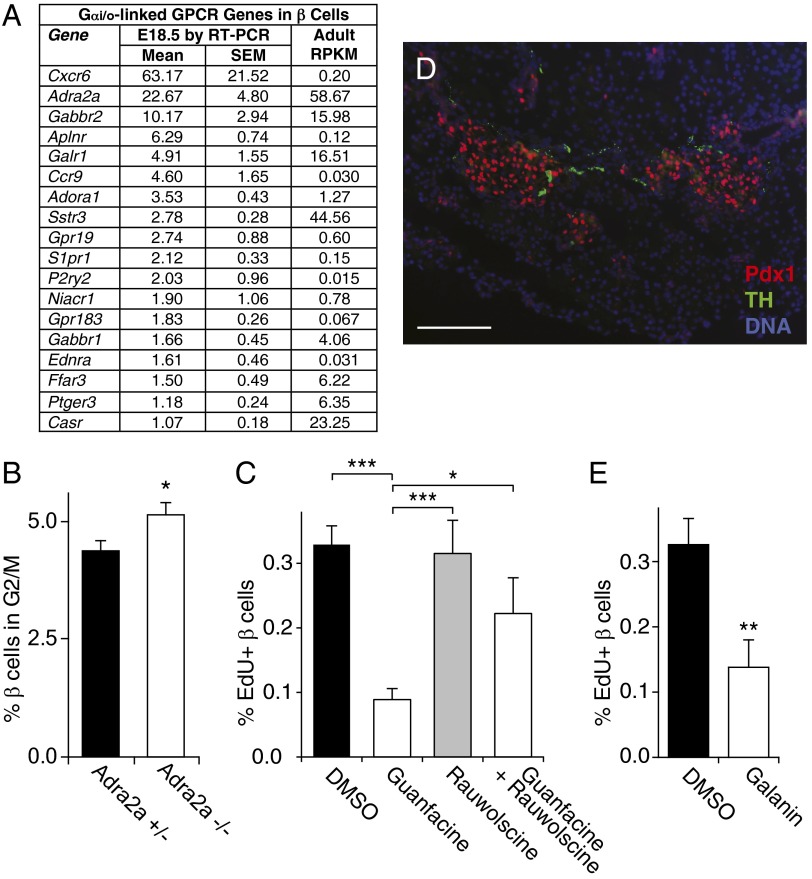
If Gαi/o signaling constrains β-cell replication, which endogenous Gi-GPCRs mediate this effect? To examine this question, we quantified the expression of all nonolfactory GPCRs in β cells isolated from mice at embryonic day 18.5 (E18.5) and postpartum day 4 (P4) by RT-PCR (Fig. 4A and Tables S8 and S9). We found multiple mRNAs encoding Gi-GPCRs and G protein subunits in these cells, and many also were expressed robustly in adult β cells (Fig. 4A and Tables S9 and S10). Among the most highly expressed Gi-GPCR mRNAs in both perinatal and adult β cells was Adra2a mRNA, which has been implicated in type 2 diabetes (5, 6). To examine whether ADRA2A might mediate part of the Gαi/o-mediated suppression of β-cell replication we observed in Fig. 3F, we measured β-cell replication in neonates lacking ADRA2A receptors. We found a 17% increase in neonatal β-cell replication in Adra2a-null animals, demonstrating that ADRA2A is an endogenous Gi-GPCR suppressor of neonatal β-cell replication (Fig. 4B and Fig. S5). Additionally, treatment with the ADRA2A agonist guanfacine reduced β-cell replication in isolated adult islets, and the ADRA2A antagonist rauwolscine blocked this effect (Fig. 4C and Fig. S6).
Fig. 4.
Inhibition of β-cell proliferation by ADRA2A. (A) mRNA encoding Gi-GPCRs expressed by mouse β cells at E18.5 are listed in order of expression level measured by RT-PCR relative to Actb/1000. The last column shows the expression level in adult mouse β cells as determined by sequencing expressed in RPKM (62). For complete data on expression of GPCR and related mRNAs, see Tables S8, S9, and S10. (B) The replication rate in β cells at day P1 from Adra2a+/− (n = 6) and Adra−/− (n = 6) mice was measured by determining the percentage of β cells in the G2 and M phases of the cell cycle by flow cytometry. (C and E) Adult mouse islets were cultured with the drugs shown and labeled with EdU before harvesting. The replication rate in β cells was calculated as the percent of cells costaining for the β-cell nuclear marker. (D) A pancreatic section from a mouse embryo at E17.5 was stained for tyrosine hydroxylase (TH, green), the β-cell nuclear marker Pdx1 (red), and the nuclear DNA stain DAPI (blue). (Scale bar, 50 µm.) *P < 0.05 and ***P < 0.001 vs. control Adra2a+/− animals (B) or vs. cells treated with guanfacine alone (C) by two-tailed Student’s t test.
To determine the source of ligand for the ADRA2A receptor in perinatal β cells, we stained for catecholamine-producing sympathetic neurons. We detected tyrosine hydroxylase-expressing sympathetic neurons innervating pancreatic islets as early as day E17.5 (Fig. 4D). Interestingly, β cells also robustly express Galr1 (Fig. 4A), which encodes a Gi-GPCR for galanin, another sympathetic neurotransmitter; and we found that galanin also inhibits β-cell replication (Fig. 4E).
Discussion
These studies shed light on a classic problem: How does a growing organ know when it has enough cells and should stop cell proliferation? Signals that restrain proliferation counter the more familiar forces driving proliferation and thereby set limits on organ growth. As in many biologic processes, this negative regulation is at least as important as positive regulation, and defects in negative regulation can have dire pathologic consequences. Limiting cell numbers may be particularly important for the β cell, as evidenced by the contraction in β-cell mass seen in settings such as starvation, insulinoma, or in maternal islets in the postpartum period (25–27). Our data demonstrate that Gαi/o-coupled signaling restricts β-cell replication, titrating cell number to provide for the organism’s metabolic needs.
Modulating β-cell Gi-GPCR signaling during the perinatal period, even just until 1 wk postpartum, had a lasting impact on adult β-cell mass and insulin secretory capacity. This perinatal sensitivity results from the high proliferation rates during the perinatal period. During fetal development, newly differentiated β cells initially are quiescent but enter a phase of rapid proliferation shortly before birth that lasts through the perinatal/early childhood period in both rodents and humans (16, 17, 28). Because the population size grows as an exponential function of the cellular replication rate, small changes in the replication rate have large effects on population size when the underlying replication rate is high. This sensitivity of adult β-cell mass to small perinatal changes in β-cell replication may explain how insults during the perinatal period, such as prematurity, intrauterine growth retardation, or nutrient restriction, increase the risk of diabetes as an adult in both animal models and humans (29–33).
Our results also suggest that the loss of sympathetic ligands working through their cognate Gi-GPCRs on β cells could explain the increased perinatal β-cell replication in mice lacking neurons in the pancreas (34) and may explain how activating variants in ADRA2A and stress contribute to diabetes risk in humans (5, 6, 35). Although important, sympathetic ligands alone likely do not explain all the PTX-sensitive inhibition of β-cell proliferation, and other Gi-GPCRs, such as Cnr1, along with non–cell-autonomous effects of Gαi/o signaling, surely contribute as well (24, 36–38).
Of course, Gi-GPCR inhibition is balanced by stimulators of β-cell proliferation, including Gαs- and Gαq-coupled GPCRs (39–41) such as the parasympathetic signaling target on β cells, CHRM3 (39, 42). Parasympathetic signaling through the vagus nerve also regulates β-cell mass (43–46) and has been implicated in the regulation of β-cell mass by the liver (47), although liver-specific secreted factors (48, 49) also may regulate β-cell proliferation directly (49).
Together, these data establish Gi-GPCR signaling in general and ADRA2A signaling in particular and implicate neural signaling and the CNS working through the autonomic nervous system as important determinants of β-cell proliferation and ultimately of β-cell population size, insulin production capacity, and metabolic control in the adult. These results have implications for understanding pancreatic islet development and the pathophysiology and pharmacotherapy of diabetes and could contribute to regenerative therapies for people with diabetes (50).
Experimental Procedures
Animals.
Mice were housed on a 12-h/12-h light/dark cycle in controlled-climate rooms (21.5–22.5 °C). All mouse lines were maintained on a C57BL/6J genetic background (Jackson Laboratories-West). All procedures were approved by the University of California, San Francisco (UCSF) Institutional Animal Care and Use Committee and were conducted in accordance with UCSF regulations. The RIP-rtTA (51), MIP-GFP (52), ePet1-cre (18), Ro1-RASSL (21), ROSA-PTX (24), and Adra2a+/− (53) mouse lines are described elsewhere. RIP-rtTA and tetO transgenic mice were given food containing 200 mg/kg doxycycline (BioServ) at the indicated times. Animals identified as sick by veterinary staff were not used for experiments.
Note that the expression pattern of ePet transgenics including ePet-cre (18, 19, 54) compare favorably with many other commonly used pancreas-, islet-, and β-cell–specific transgenic mouse lines, which, like ePet, also express in serotonergic brainstem nuclei as well as in the pancreas. However, unlike ePet, when used as cre drivers other pancreatic promoters frequently cause recombination in other CNS locations including the hypothalamus (Fig. S7) (55–57). (For a thorough discussion of the advantages and limitations of pancreatic cre driver lines, see ref. 58). Further, many of the most commonly used pancreas-specific transgenic lines were generated with a human growth hormone gene cassette that expresses along with the transgene, impacting pancreatic gene expression and islet function (59). The ePet transgenics do not include this cassette.
Generation of ePet1-Htr1a and tetO-PTX Transgenic Mice.
The plasmid pFLAG-Htr1a+iresGFP was generated by linking signal and FLAG epitope sequences to the 5′ end of the mouse HTR1A coding sequence followed by the IRES-eGFP sequence from pIRES-EGFP2 (Clontech Laboratories). The 4-kb pFLAG-5-Htr1a+iresGFP insert was inserted downstream of the mouse Pet1 enhancer and minimal β-globin promoter in pBAC-ePet (18) to generate the ePet1-Htr1a transgene (Fig. S1A). The plasmid ptetO-S1-PTX was generated by inserting the S1-PTX cDNA [provided by Eitan Reuveny, Weizmann Institute of Science, Rehovot, Israel (60)] downstream of the β-globin intron in the tTA-inducible pUHG 10-3 plasmid. Transgenes were injected into mouse oocyte pronuclei, and transgenic founders were identified by PCR analysis of genomic DNA. Transgene copy number was estimated by real-time PCR with genomic DNA. tetO-PTX mice are available through the Mutant Mouse Regional Resource Centers (https://www.mmrrc.org/catalog/sds.php?mmrrc_id=14241).
Glucose- and Insulin-Tolerance Tests.
After overnight fast, mice received 2 mg/kg of glucose for glucose-tolerance tests or 0.75 units/kg of insulin for insulin-tolerance tests via i.p. injection. Glucose was measured from tail blood at the indicated times with a OneTouch Ultra Glucometer and test strips (LifeScan, Inc.). Insulin was assayed in plasma from the tail vein by ELISA kit (Apco Diagnostics) at 0 and 15 min after glucose injection.
Pancreatic Histology.
Mice were anesthetized with Avertin and transcardiac perfused with 10 cm3 of 4 °C PBS, followed by 10 cm3 of 4% paraformaldehyde (PFA). The pancreas was isolated, fixed in 4% (wt/vol) PFA at 4 °C for 4 h, taken through an ethanol dehydration series, mounted in paraffin, cut into 6-μm-thick sections by a microtome at 4 °C, and mounted on glass slides. Sections were incubated with primary antibodies against insulin (rabbit, 1:1,000; EMD Millipore Corporation), glucagon (guinea pig, 1:2,000; EMD Millipore Corporation), pdx1 [guinea pig, 1:2,000 (61)], and tyrosine hydroxylase (rabbit, 1:500; EMD Millipore Corporation) in PBS with 5% (vol/vol) normal goat serum overnight at 4 °C. After washes in 4 °C PBS, slides were stained with secondary antibodies: Cy3-conjugated goat anti-guinea pig or anti-rabbit and FITC-conjugated goat anti-rabbit or anti-guinea pig (1:500; Jackson ImmunoResearch Laboratories) in PBS with 5% normal goat serum for 30 min. After washes in 4 °C PBS, stained slides were coverslipped with Vectastain mounting medium containing DAPI and were sealed with clear nail polish. Images were obtained with a Zeiss Axio Scope widefield fluorescence microscope and AxioVision software.
For measurement of the β-cell area, every 30th pancreatic section through the entire pancreas was imaged using a 10× objective. The area of fluorescent staining in β cells was quantified by circling stained cells using AxioVision. The total pancreatic section area was measured similarly using a 1.25× microscope objective. The percent β-cell area was defined as the β-cell area divided by pancreatic section area, multiplied by 100. Controls in Figs. 1F and 3E were combined for statistical power.
Pancreatic Gene-Expression Analysis.
Mice at the indicated ages were anesthetized with 4% (wt/vol) Avertin, and the pancreas was removed, minced immediately in RNAlater (Ambion), and incubated at 4 °C overnight. Pancreatic fragments were pelleted for 2 min at 400 × g at 4 °C in a microcentrifuge and then were resuspended in 5 mL of TRIzol and were homogenized with a pellet pestle motor using an RNase-free pestle (Fisher Scientific). GFP+ β cells were separated by FACS from digested pancreata, and RNA was purified as previously described (62).
cDNA was obtained by reverse transcription from these RNA samples, and TaqMan real-time PCR was performed with an Applied Biosystems 7300 Real-Time PCR System using 50 ng of cDNA per reaction in 96-well plates or 384-sample microfluidic plates (Applied Biosystems). Results were normalized to levels of Gapdh mRNA for Htr1a expression, to Gusb in Fig. 4, and to the average of Actb, Gapdh, and Ppia in Tables S8, S9, and S10. Data from four independent isolations were used for Fig. 4A. Primers and probe sequences are available on request.
Adult islet and β-cell RNA-sequencing data were derived from published data from massively parallel sequencing of cDNA purified from isolated 4-mo-old adult mouse islets and sorted β cells (62) and were expressed as reads per kilobase of exon model per million mapped reads (RPKM).
β-Cell Replication Rates.
β-Cell replication in MIP-GFP mice was measured by flow cytometry with gating and parameters as previously described (63).
For in vitro proliferation experiments, islets were isolated and cultured for 5 d as previously described (64), followed by 6 d of treatment with the drugs shown at 1 μm with 0.1% DMSO. On day 6 of treatment, islets were treated with 10 mM 5-ethynyl-2-deoxyuridine (EdU) for 3 h and then were fixed immediately in 4% PFA/10 mM PBS solution for 25 min. Fixed islets were washed three times with 10 mM PBS for 20 min, permeabilized with 0.3% Triton X-100 in 10 mM PBS for 3 h, blocked overnight at 4 °C in 5% goat serum/0.15% Triton-X 100/10 mM PBS, and then washed twice with antibody dilution buffer for 15 min at room temperature. Islets were stained with primary antibody, rabbit anti-human NKX6.1 (1:500; Sigma-Aldrich), and secondary antibody, Cy3-conjugated goat anti-rabbit (1:500; Sigma-Aldrich), diluted in 1% BSA/0.2% Triton X-100/10 mM PBS for 24 h at 4 °C. After immunostaining, EdU was labeled with the Click-iT EdU Alexa Fluor Imaging Kit (Invitrogen). Islets were imaged using a Leica SP5 confocal laser scanning microscope (Leica). The Volocity software (PerkinElmer) colocalization macro was used to count nuclei costaining for EdU and the unique β-cell nuclear marker Nkx6.1 (Fig. S3) (28). The percent of proliferating β cells was calculated by dividing the number of costaining nuclei by the total number of Nkx6.1+ nuclei and multiplying by 100.
Supplementary Material
Acknowledgments
We thank members of the M.S.G., E.S.D., and L.H.T. laboratories and Holly Ingraham, Pavel Koudria, Deborah Kurrasch, Greg Szot, and Hengameh Zahid for technical advice and assistance and Henry Bourne; and Steven Finkbeiner, Gerold Grodsky, William Rutter, David Warner, and members of the M.S.G. and L.H.T. laboratories for helpful discussions. This work was supported by Grant 2007/1B from the Larry L. Hillblom Foundation (to M.S.G.) and a grant from the Nora Eccles Treadwell Foundation (to M.S.G.); by Juvenile Diabetes Research Foundation Grants 16-2007-428 (to M.S.G.), 3-2007-721 (to T.M.), and 3-2007-187 and 10-2010-553 (to H.K.); American Diabetes Association Grant ADA-7-11-MN-22 (to M.S.G. and H.M.); National Institutes of Health Grants R01 DK021344 (to M.S.G.), U01 DK089541 (to M.S.G.), T32 GM07618 (to M.B.), F31 MH075708 (to M.B.), and P30 DK63720 (to M.S.G.); and by the University of California, San Francisco Sandler Program in Basic Science (M.S.G., G.H., and L.H.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319378112/-/DCSupplemental.
References
- 1.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85(4):1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 2.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 3.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8(5):369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 4.Thompson MD, Percy ME, McIntyre Burnham W, Cole DE. G protein-coupled receptors disrupted in human genetic disease. Methods Mol Biol. 2008;448:109–137. doi: 10.1007/978-1-59745-205-2_7. [DOI] [PubMed] [Google Scholar]
- 5.Rosengren AH, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327(5962):217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis J, et al. DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouatia-Naji N, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41(1):89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 8.Lyssenko V, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prokopenko I, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 11.MacLean N, Ogilvie RF. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes. 1955;4(5):367–376. doi: 10.2337/diab.4.5.367. [DOI] [PubMed] [Google Scholar]
- 12.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson MA, Gianani R. The pancreas in human type 1 diabetes: Providing new answers to age-old questions. Curr Opin Endocrinol Diabetes Obes. 2009;16(4):279–285. doi: 10.1097/MED.0b013e32832e06ba. [DOI] [PubMed] [Google Scholar]
- 14.Straub SG, Sharp GWG. Evolving insights regarding mechanisms for the inhibition of insulin release by norepinephrine and heterotrimeric G proteins. Am J Physiol Cell Physiol. 2012;302(12):C1687–C1698. doi: 10.1152/ajpcell.00282.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena R, et al. GIANT consortium; MAGIC investigators Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42(2):142–148. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44(3):249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 17.Meier JJ, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott MM, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102(45):16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohta Y, et al. Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes. 2011;60(12):3208–3216. doi: 10.2337/db10-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milo-Landesman D, et al. Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic beta cells controlled by the tet-on regulatory system. Cell Transplant. 2001;10(7):645–650. [PubMed] [Google Scholar]
- 21.Redfern CH, et al. Conditional expression and signaling of a specifically designed Gi-coupled receptor in transgenic mice. Nat Biotechnol. 1999;17(2):165–169. doi: 10.1038/6165. [DOI] [PubMed] [Google Scholar]
- 22.Coward P, et al. Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci USA. 1998;95(1):352–357. doi: 10.1073/pnas.95.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redfern CH, et al. Conditional expression of a Gi-coupled receptor causes ventricular conduction delay and a lethal cardiomyopathy. Proc Natl Acad Sci USA. 2000;97(9):4826–4831. doi: 10.1073/pnas.97.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regard JB, et al. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest. 2007;117(12):4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blume N, Skouv J, Larsson LI, Holst JJ, Madsen OD. Potent inhibitory effects of transplantable rat glucagonomas and insulinomas on the respective endogenous islet cells are associated with pancreatic apoptosis. J Clin Invest. 1995;96(5):2227–2235. doi: 10.1172/JCI118278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flatt PR, et al. Effects of transplantation and resection of a radiation-induced rat insulinoma on glucose homeostasis and the endocrine pancreas. Br J Cancer. 1986;54(4):685–692. doi: 10.1038/bjc.1986.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marynissen G, Aerts L, Van Assche FA. The endocrine pancreas during pregnancy and lactation in the rat. J Dev Physiol. 1983;5(6):373–381. [PubMed] [Google Scholar]
- 28.Sander M, et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127(24):5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 29.Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1297–R1305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- 30.Kajantie E, Osmond C, Barker DJP, Eriksson JG. Preterm birth—a risk factor for type 2 diabetes? The Helsinki birth cohort study. Diabetes Care. 2010;33(12):2623–2625. doi: 10.2337/dc10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaijser M, et al. Perinatal risk factors for diabetes in later life. Diabetes. 2009;58(3):523–526. doi: 10.2337/db08-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cutfield W. Short and sweet: The perinatal origins of type 2 diabetes mellitus. Pediatr Diabetes. 2004;5(3):113–116. doi: 10.1111/j.1399-543X.2004.00065.x. [DOI] [PubMed] [Google Scholar]
- 33.Barker DJP, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia. 1993;36(1):62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 34.Nekrep N, Wang J, Miyatsuka T, German MS. Signals from the neural crest regulate beta-cell mass in the pancreas. Development. 2008;135(12):2151–2160. doi: 10.1242/dev.015859. [DOI] [PubMed] [Google Scholar]
- 35.Surwit RS, Schneider MS, Feinglos MN. Stress and diabetes mellitus. Diabetes Care. 1992;15(10):1413–1422. doi: 10.2337/diacare.15.10.1413. [DOI] [PubMed] [Google Scholar]
- 36.Kim W, et al. Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes. 2011;60(4):1198–1209. doi: 10.2337/db10-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim W, et al. Cannabinoids induce pancreatic β-cell death by directly inhibiting insulin receptor activation. Sci Signal. 2012;5(216):ra23. doi: 10.1126/scisignal.2002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jourdan T, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19(9):1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guettier JM, et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA. 2009;106(45):19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drucker DJ. Glucagon-like peptide-1 and the islet β-cell: Augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144(12):5145–5148. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16(7):804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautam D, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3(6):449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Edvell A, Lindström P. Vagotomy in young obese hyperglycemic mice: Effects on syndrome development and islet proliferation. Am J Physiol. 1998;274(6 Pt 1):E1034–E1039. doi: 10.1152/ajpendo.1998.274.6.E1034. [DOI] [PubMed] [Google Scholar]
- 44.Lausier J, et al. Vagal control of pancreatic ß-cell proliferation. Am J Physiol Endocrinol Metab. 2010;299(5):E786–E793. doi: 10.1152/ajpendo.00202.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiba T. Relationships between the autonomic nervous system and the pancreas including regulation of regeneration and apoptosis: Recent developments. Pancreas. 2004;29(2):e51–e58. doi: 10.1097/00006676-200408000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Kiba T, et al. Ventromedial hypothalamic lesion-induced vagal hyperactivity stimulates rat pancreatic cell proliferation. Gastroenterology. 1996;110(3):885–893. doi: 10.1053/gast.1996.v110.pm8608899. [DOI] [PubMed] [Google Scholar]
- 47.Imai J, et al. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322(5905):1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- 48.Yi P, Park JS, Melton DA. Betatrophin: A hormone that controls pancreatic β cell proliferation. Cell. 2013;153(4):747–758. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.El Ouaamari A, et al. Liver-derived systemic factors drive β cell hyperplasia in insulin-resistant states. Cell Reports. 2013;3(2):401–410. doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halban PA, German MS, Kahn SE, Weir GC. Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab. 2010;95(3):1034–1043. doi: 10.1210/jc.2009-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Efrat S, Fusco-DeMane D, Lemberg H, al Emran O, Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci USA. 1995;92(8):3576–3580. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hara M, et al. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am J Physiol Endocrinol Metab. 2003;284(1):E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 53.Altman JD, et al. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol Pharmacol. 1999;56(1):154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- 54.Scott MM, Krueger KC, Deneris ES. A differentially autoregulated Pet-1 enhancer region is a critical target of the transcriptional cascade that governs serotonin neuron development. J Neurosci. 2005;25(10):2628–2636. doi: 10.1523/JNEUROSCI.4979-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honig G, Liou A, Berger M, German MS, Tecott LH. Precise pattern of recombination in serotonergic and hypothalamic neurons in a Pdx1-cre transgenic mouse line. J Biomed Sci. 2010;17:82. doi: 10.1186/1423-0127-17-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wicksteed B, et al. Conditional gene targeting in mouse pancreatic ß-Cells: Analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59(12):3090–3098. doi: 10.2337/db10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gannon M, Shiota C, Postic C, Wright CV, Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis. 2000;26(2):139–142. doi: 10.1002/(sici)1526-968x(200002)26:2<139::aid-gene12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 58.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18(1):9–20. doi: 10.1016/j.cmet.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brouwers B, et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab. 2014;20(6):979–990. doi: 10.1016/j.cmet.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vivaudou M, et al. Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the KACh channel, using functional homomeric mutants. J Biol Chem. 1997;272(50):31553–31560. doi: 10.1074/jbc.272.50.31553. [DOI] [PubMed] [Google Scholar]
- 61.Schwitzgebel VM, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127(16):3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 62.Ku GM, et al. Research resource: RNA-Seq reveals unique features of the pancreatic β-cell transcriptome. Mol Endocrinol. 2012;26(10):1783–1792. doi: 10.1210/me.2012-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyatsuka T, Kosaka Y, Kim H, German MS. Neurogenin3 inhibits proliferation in endocrine progenitors by inducing Cdkn1a. Proc Natl Acad Sci USA. 2011;108(1):185–190. doi: 10.1073/pnas.1004842108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szot GL, Koudria P, Bluestone JA. Murine pancreatic islet isolation. J Vis Exp. 2007;(7):255. doi: 10.3791/255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.