Significance
Moderate or severe traumatic brain injury (TBI) damages white matter, thereby contributing to long-term neurological deficits. Currently, there are no satisfactory therapies to mitigate this white matter injury (WMI). Here we show that inhibition of histone deacetylases (HDACs) exerts robust structural and functional protection of white matter in a murine model of TBI/WMI by polarizing microglia/macrophages toward the beneficial M2 phenotype. HDAC inhibition shifted microglia/macrophage phenotype by up-regulating glycogen synthase kinase 3 beta (GSK3β), which inactivated phosphatase and tensin homologue (PTEN) through phosphorylation, thereby promoting PI3K/Akt signaling. The GSK3β-dependent M2 phenotype exerted potent anti-inflammatory effects that protected myelin-forming oligodendrocytes and diminished WMI. These results reveal a previously unexplored role for GSK3β/PTEN/PI3K signaling in the regulation of microglia/macrophages and demonstrate the promise of HDAC inhibition in the treatment of TBI/WMI.
Keywords: traumatic brain injury, oligodendrocyte, microglial polarization, myelination, inflammation
Abstract
Severe traumatic brain injury (TBI) elicits destruction of both gray and white matter, which is exacerbated by secondary proinflammatory responses. Although white matter injury (WMI) is strongly correlated with poor neurological status, the maintenance of white matter integrity is poorly understood, and no current therapies protect both gray and white matter. One candidate approach that may fulfill this role is inhibition of class I/II histone deacetylases (HDACs). Here we demonstrate that the HDAC inhibitor Scriptaid protects white matter up to 35 d after TBI, as shown by reductions in abnormally dephosphorylated neurofilament protein, increases in myelin basic protein, anatomic preservation of myelinated axons, and improved nerve conduction. Furthermore, Scriptaid shifted microglia/macrophage polarization toward the protective M2 phenotype and mitigated inflammation. In primary cocultures of microglia and oligodendrocytes, Scriptaid increased expression of microglial glycogen synthase kinase 3 beta (GSK3β), which phosphorylated and inactivated phosphatase and tensin homologue (PTEN), thereby enhancing phosphatidylinositide 3-kinases (PI3K)/Akt signaling and polarizing microglia toward M2. The increase in GSK3β in microglia and their phenotypic switch to M2 was associated with increased preservation of neighboring oligodendrocytes. These findings are consistent with recent findings that microglial phenotypic switching modulates white matter repair and axonal remyelination and highlight a previously unexplored role for HDAC activity in this process. Furthermore, the functions of GSK3β may be more subtle than previously thought, in that GSK3β can modulate microglial functions via the PTEN/PI3K/Akt signaling pathway and preserve white matter homeostasis. Thus, inhibition of HDACs in microglia is a potential future therapy in TBI and other neurological conditions with white matter destruction.
Traumatic brain injury (TBI) often leads to catastrophic neurological disabilities and sometimes ends in death (1). TBI results not only in gray matter damage, but also in severe white matter injury (WMI), thereby disrupting signal transmission and eliciting poor functional outcomes (2, 3). WMI in TBI patients is strongly correlated with neurological deficits, and diffusion tensor imaging of white matter offers prognostic value for neurological status (2–4). At present, there are no satisfactory therapies to protect TBI patients against either gray matter injury or WMI. Furthermore, most preclinical TBI studies greatly emphasize gray matter over white matter, which may contribute to the many disappointing results in clinical trials to date (5).
Previous studies have shown that histone deacetylase (HDAC) inhibitors mitigate WMI after ischemia (6, 7). HDACs allow DNA to be wrapped more tightly around histones, thereby blocking gene transcription and acting in opposition to histone acetyltransferases that promote gene transcription (8, 9). Some HDAC inhibitors preferentially promote the transcription of neuroprotective genes. We recently reported that Scriptaid, a novel inhibitor of class I/II HDACs, protects gray matter against experimental TBI (10). These findings are consistent with reports indicating that other HDAC inhibitors can attenuate gray matter injury in TBI models (11–14). Whether Scriptaid can protect white matter is unclear, however. Therefore, we tested whether Scriptaid would preserve white matter in a well-established murine model of controlled cortical impact (CCI).
WMI is thought to be potentiated by strong, persistent inflammatory responses after TBI (15). During the cerebral inflammatory response, resident microglia and peripheral macrophages migrate to the site of injury and release factors that recruit additional immune cells, thereby initiating a self-perpetuating loop (16). Furthermore, microglia/macrophages assume a number of phenotypes, including the two polarized phenotypes, M1 and M2 (16, 17). The proinflammatory M1 phenotype favors the production and release of cytokines that exacerbate neural injury (18, 19). In contrast, the M2 phenotype promotes the release of neurotrophic factors that promote neurorepair (18, 20). We recently showed that M1 conditioned medium (CM) enhances oligodendrocyte cell death in vitro following oxygen glucose deprivation (OGD), whereas M2 CM is protective (19, 21). In the same study, we found that microglia/macrophages respond to TBI with a transient M2 phenotype, followed by a shift to M1, and that the number of M1 cells is strongly correlated with the severity of WMI (21). Thus, therapies that prime microglia/macrophages toward the beneficial M2 phenotype after TBI may offer new anti-inflammatory strategies to protect against WMI.
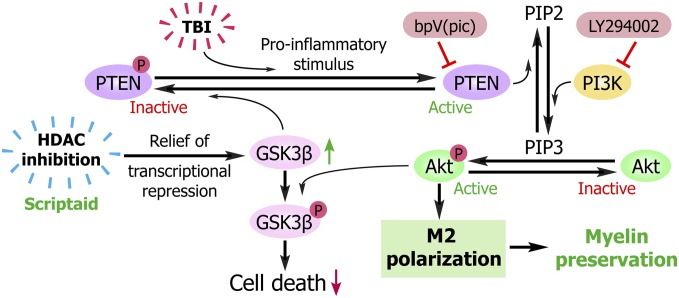
Previous studies have reported the anti-inflammatory properties of HDAC inhibition (7); therefore, we analyzed the effects of Scriptaid on phenotypic polarization of microglia/macrophages and their effects on cocultured oligodendrocytes. Here we report that HDAC inhibition increases microglial glycogen synthase kinase 3 beta (GSK3β) expression, which serves to inactivate phosphatase and tensin homologue (PTEN) via phosphorylation (Fig. 1). PTEN inactivation then enhances phosphatidylinositide 3-kinases (PI3K)/Akt signaling and polarizes microglia toward M2, which helps protect oligodendrocytes.
Fig. 1.
Proposed mechanism underlying the preservation of white matter by HDAC inhibition. Microglia/macrophages respond to TBI with proinflammatory changes that increase active, dephosphorylated PTEN. Active PTEN then converts phosphatidylinositol (3,5)-triphosphate (PIP3) to phosphatidyl (4,5)-biphosphate (PIP2), thereby inactivating the prosurvival kinase Akt and leading to microglial and oligodendroglial toxicity. HDAC inhibition counterbalances this inflammatory effect by increasing GSK3β, perhaps by relieving transcriptional repression of its mRNA. Active GSK3β then phosphorylates PTEN at Thr366, leading to a loss of PTEN activity. This loss of PTEN function results in activation of Akt, because PIP3 is no longer converted to PIP2. Activated Akt can now shift microglia from the destructive M1 phenotype toward the beneficial M2 phenotype and thereby elicit the protection of neighboring oligodendrocytes. Activated Akt also phosphorylates GSK3β, thereby disabling its pro-death effect. Knockdown of GSK3β or the PI3K/Akt inhibitor LY294002 each blocks the protective effects of HDAC inhibition, whereas the PTEN inhibitor bpV(pic) fails to provide any additional effect over that of HDAC inhibition.
Results
HDAC Inhibition Confers Robust and Long-Term Preservation of WM Following TBI.
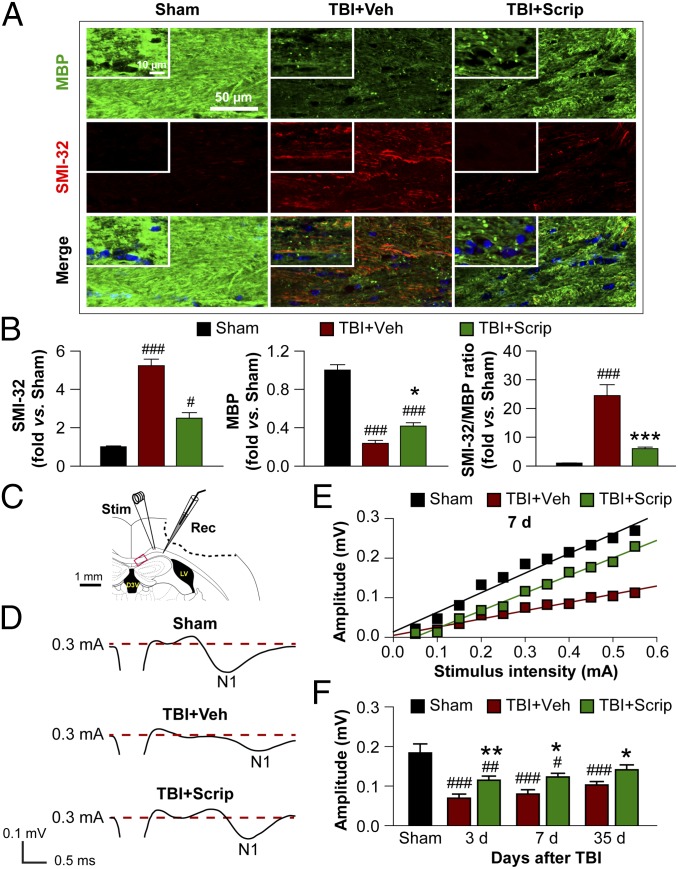
CCI on the right hemisphere resulted in motor deficits (as demonstrated by the rotarod and wire hang tests) at 1–11 d after injury. The CatWalk gait analysis detected sensorimotor deficits in TBI mice at 1–4 wk after injury. Scriptaid treatment (3.5 mg/kg, injections at 2, 26, and 50 h after CCI) facilitated and enhanced recovery of motor functions after CCI (Fig. S1). We also examined damage to axons and the myelin sheath in the corpus callosum (CC; Fig. 2 A and B) and striatum (Fig. S2A) by assessing the loss of myelin basic protein (MBP) and the increase in abnormally dephosphorylated neurofilament protein (detected using the SMI-32 antibody), and by measuring the loss of myelin with Luxol fast blue staining. As expected, Scriptaid significantly reduced the SMI-32:MBP ratio at 35 d postinjury (Fig. 2 A and B) and partially protected (or restored) Luxol fast blue staining (Fig. S2 B and C), suggesting long-term preservation (or remyelination) of myelinated axons.
Fig. 2.
Long-term preservation of white matter after TBI is promoted by Scriptaid. (A) Double immunofluorescent staining for dephosphorylated neurofilament protein (SMI-32) and MBP in the ipsilesional CC at 35 d after TBI or sham treatment. Nuclei are labeled with DAPI (blue). (Insets) High-power images. (B) Degree of WMI, expressed as the fold increases of SMI-32, fold decreases of MBP, and the ratio of SMI-32 to MBP. (C) Illustration of the CCI lesion (dashed line) and regions for immunohistochemistry and electron microscropy (red box). Stimulating and recording electrodes were positioned at the CC as shown to measure the evoked CAPs. (D) Representative traces of the evoked CAPs in the CC (stimulus, 0.3 mA; 0.48 mm lateral to the stimulating electrode) at 7 d post-TBI. (E) Signal conduction along nerve fibers, as measured by the amplitude of the N1 component of the CAPs in response to increasing stimulus strength (0.05–0.55 mA) at 7 d post-TBI. (F) N1 amplitude in response to a 0.3-mA stimulus at 3, 7, and 35 d post-TBI. Shown are the mean ± SEM values from six mice per group. #P ≤ 0.05; ##P ≤ 0.01, ###P ≤ 0.001 vs. sham; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. TBI + vehicle.
To verify the protective effects of Scriptaid on white matter at the ultrastructural level, we examined the CC by electron microscopy at 7 and 35 d postinjury (Fig. S3). After TBI, the number of axons was decreased, and the majority of remaining axons had lost their myelin sheaths; these changes were significantly attenuated by Scriptaid treatment (Fig. S3 A–C). Along with anatomic preservation of myelinated axons, we also examined functional changes in nerve conduction in the CC at 3, 7, and 35 d after TBI. The setup for evoking compound action potentials (CAPs) is shown in Fig. 2C. TBI decreased the amplitude of the N1 segment, as would be expected after injury to myelinated axons (Fig. 2D). Consistent with the ultrastructural and histological findings, Scriptaid partially prevented the reduction in magnitude of the N1 segment and protected nerve conduction (Fig. 2 E and F).
HDAC Inhibition Protects Oligodendrocytes Indirectly Through Microglia.
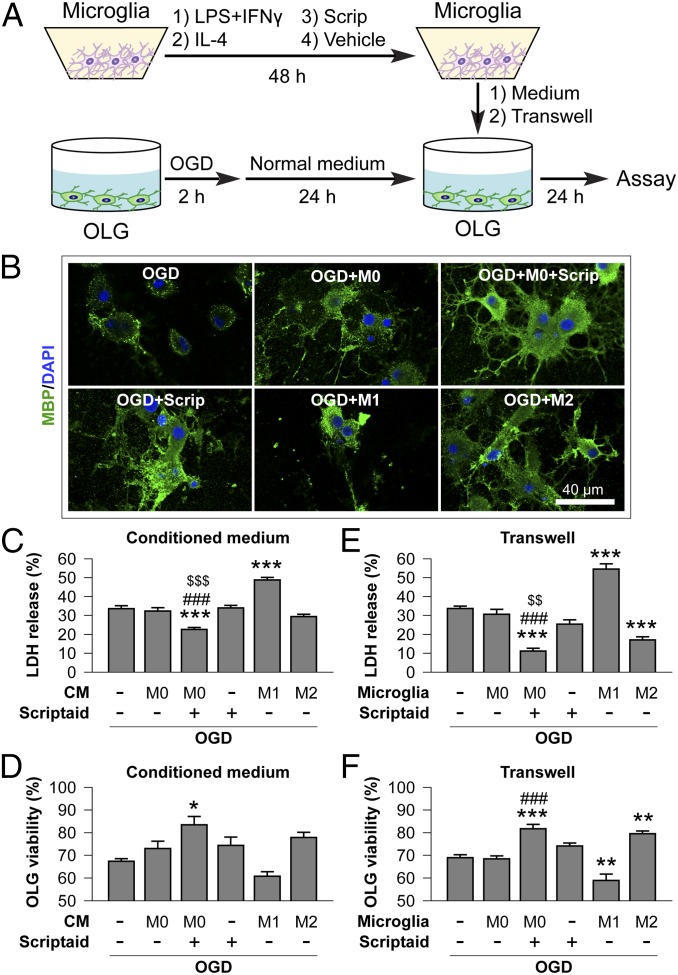
Our in vivo results demonstrated that Scriptaid preserves the myelin sheath and axonal function after TBI; thus, we tested whether Scriptaid specifically protects the myelin-producing oligodendrocytes in cultures. We found that Scriptaid elicited little direct protection of primary oligodendrocytes against OGD (Fig. 3). This finding suggests that the in vivo protection of white matter by Scriptaid may be indirect, perhaps mediated by other cell types in the brain; for example, HDAC inhibition is known to mitigate the proinflammatory actions of microglia (22). Thus, we hypothesized that Scriptaid protects oligodendrocytes by regulating microglia. Consistent with this hypothesis, CM collected from Scriptaid-treated microglia elicited significantly greater protection against OGD in oligodendrocyte cultures compared with CM from vehicle-treated microglia or with Scriptaid treatment without CM (Fig. 3). More profound protection by Scriptaid was observed using the Transwell system of cocultured microglia and oligodendrocytes (Fig. 3 E and F), likely resulting from a continuous supply of diffusible (but short-lived) oligodendrocyte-protecting factors from microglia. Moreover, supporting the role of different microglial phenotypes in modulating oligodendrocyte viability after injury, M1 and M2 phenotypes were induced in microglia before the experiments described above (SI Materials and Methods). Whereas M1 microglia reduced oligodendrocyte viability after OGD, M2 microglia increased oligodendrocyte viability (Fig. 3). In the absence of OGD, none of the treatments affected oligodendrocyte viability or lactate dehydrogenase (LDH) release (Fig. S4).
Fig. 3.
HDAC inhibition indirectly reduces oligodendrocyte injury by modulating microglia/macrophages. (A) In vitro experiments using a CM transfer system or a Transwell system. Microglia cultures (M0) were incubated with Scriptaid (1 μM) or vehicle for 48 h; as positive controls, microglia were primed toward M1 or M2 using LPS (100 ng/mL) plus IFN-γ (20 ng/mL) or IL-4 (20 ng/mL), respectively, for 48 h (18). Cultured oligodendrocytes were exposed to 2-h OGD or control, non-OGD conditions and returned to normal medium. Twenty-four hours later, microglia CM was applied to oligodendrocytes for 24 h by pipetting or by a Transwell system. OLG, oligodendrocytes. (B) MBP and DAPI staining of oligodendrocytes exposed to OGD and cultured with CM from microglia exhibiting M0 (with or without Scriptaid), M1, or M2 phenotypes. (C–F) Oligodendrocyte survival and cell death were quantified by LDH release and MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay, respectively, after exposure to CM (C and D) or in Transwells (E and F). M0 phenotype in conjunction with Scriptaid or the M2 phenotype protected oligodendrocyte viability after OGD, whereas the M1 phenotype decreased oligodendrocyte viability. Shown are the mean ± SEM values from three independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. OGD + OLG; ###P ≤ 0.001 vs. OGD + OLG + M0 microglia without Scriptaid; $$P ≤ 0.01, $$$P ≤ 0.001 vs. OGD + OLG + Scriptaid.
HDAC Inhibition Shifts Microglia/Macrophage Polarization Toward M2 and Mitigates Cerebral Inflammation After TBI.
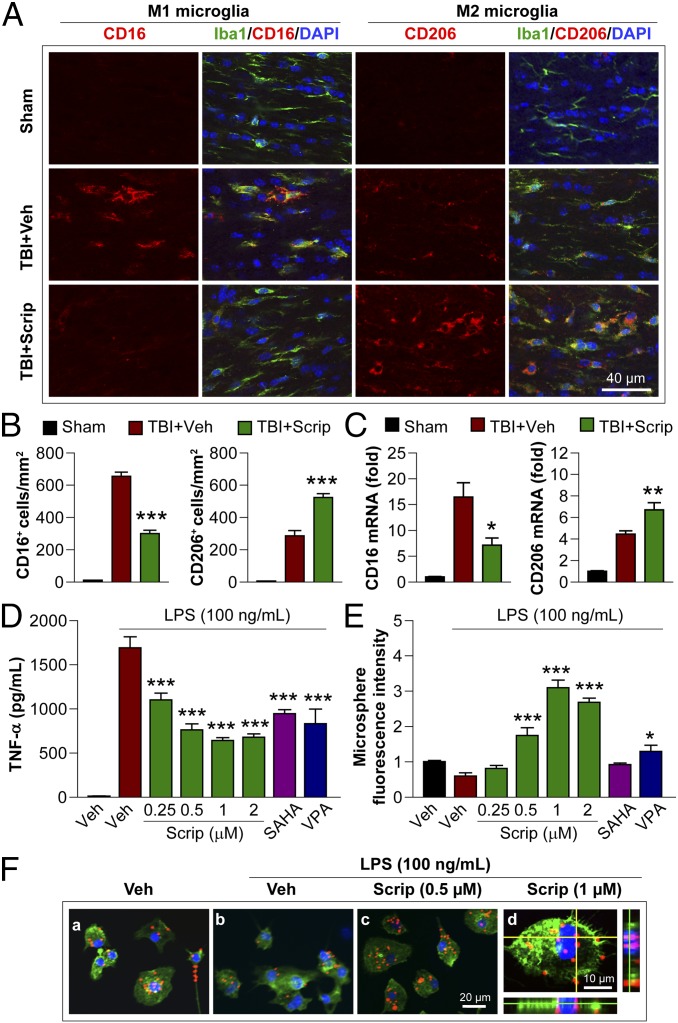
The in vitro results presented thus far suggest that Scriptaid exerts anti-inflammatory effects, perhaps by polarizing microglia toward M2. To test this hypothesis in vivo, we performed double staining for the microglia/macrophage marker Iba1 and M1-associated or M2-associated marker proteins in the CC at 7 d after TBI (Fig. 4A). As expected, cells labeled with the M1 marker CD16 were increased after TBI but significantly decreased by Scriptaid, whereas the M2 marker CD206 was further increased by Scriptaid (Fig. 4B). RT-PCR in the ipsilateral striatum showed that Scriptaid significantly decreased the expression of M1-type genes [CD16 and inducible nitric oxide synthase (iNOS) genes] and increased that of M2-type genes (CD206 and IL-10 genes; Fig. 4C and Fig. S5A).
Fig. 4.
HDAC inhibition primes microglia toward the M2 phenotype in vitro and in vivo. (A) Double immunofluorescent staining for M1 marker CD16 or M2 marker CD206 with Iba1 marker for activated microglia in the ipsilesional CC at 7 d following TBI (vehicle and Scriptaid groups) or sham surgery (sham group). Shown is immunofluorescent staining for CD16 or CD206 (first or third column) and colabeling of the same sections for Iba1 and nuclei (DAPI; second and fourth columns). (B) Blinded cell counts of microglia immunolabeled for CD16 and CD206 from the same animals as shown in A. (C) Messenger RNA levels of CD16 and CD206 in the ipsilateral striatum at 7 d after TBI with or without Scriptaid. Scriptaid was injected at 3.5 mg/kg at 2 h after CCI, and this was repeated daily for the next 2 d (at 26 and 50 h after injury). (D) HDAC inhibitors Scriptaid, SAHA (2.5 µM), and VPA (4 μM) reduced LPS-induced production of TNF-α by microglia. (E) Microglial cultures were treated with LPS alone or in combination with Scriptaid, SAHA, or VPA for 48 h. Fluorescent microspheres were added for 3 h, and intramicroglial fluorescence intensity was measured. Scriptaid was more potent than SAHA or VPA in enhancing microsphere uptake by microglia. (F) Microglia were treated with LPS with or without Scriptaid as above and stained with phalloidin (green) to visualize F-actin. After a 3-h incubation, phagocytosed microspheres appeared red, and DAPI-stained nuclei appeared blue. Shown are the mean ± SEM values from four independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. LPS + vehicle.
We also examined the impact of HDAC inhibition on proinflammatory microglial responses to lipopolysaccharide (LPS) in vitro. In these experiments, the HDAC inhibitors Scriptaid, suberoylanilide hydroxamic acid (SAHA, 2.5 μM), and valproic acid (VPA; 4 μM) all suppressed the production of proinflammatory markers TNF-α, NO, and IL-6 in LPS-treated microglia (Fig. 4D and Fig. S5 B and C). Furthermore, Scriptaid, but not SAHA, promoted microglial phagocytosis of fluorescent microspheres, a characteristic feature of the M2 phenotype (Fig. 4 E and F). These findings suggest that HDAC inhibition promotes microglia polarization toward M2 and suppresses inflammation.
HDAC Inhibition Modulates Microglial Polarization Through the PI3K/Akt Pathway.
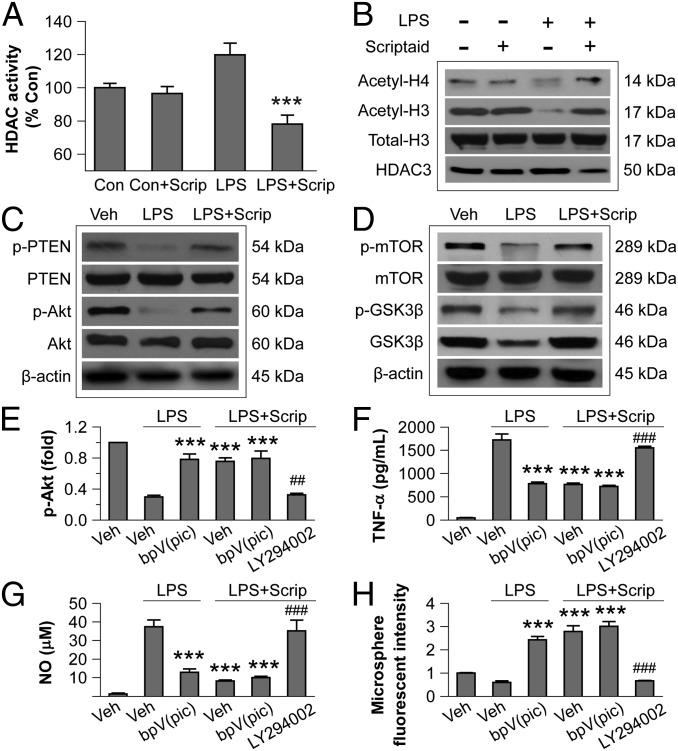
We previously showed that Scriptaid activates the PI3K/Akt signaling pathway in neurons by inhibiting the phosphatase PTEN (21); thus, we tested the hypothesis that HDAC inhibition modulates microglial polarization through PTEN and PI3K/Akt signaling (Fig. 1). First, we ensured that Scriptaid was inhibiting HDAC activity in this cell type. Indeed, Scriptaid reduced HDAC enzymatic activity in primary microglia to below baseline levels under LPS challenge (Fig. 5A), although it had no effect on the basal HDAC activity (in the absence of LPS). Scriptaid also prevented LPS-induced decreases in acetylated histones H3 and H4 (Fig. 5B), and again, Scriptaid had no effect on the basal levels of acetylated histone H3 or H4 (Fig. 5B).
Fig. 5.
HDAC inhibition modulates microglial polarization through the PI3K/Akt pathway. (A) LPS-induced HDAC activity in primary microglia cultures was reduced by Scriptaid. (B) Scriptaid prevented decreases in acetylated-histone 3 (H3) and acetylated H4 in primary microglia at 3 h after LPS, as measured by Western blot analysis. (C) Scriptaid prevented loss of p-PTEN (Ser380/Thr382/383) and p-Akt (Ser473) in LPS-challenged microglia, as measured by Western blot analysis. (D) Scriptaid largely prevented the decrease in p-mTOR (Ser2488) and p-GSK3β (Ser9) levels in LPS-treated microglia. (E) LPS reduced p-Akt levels, an effect largely blocked by the PTEN inhibitor dipotassium bisperoxo (picolinato) oxovanadate [bpV(pic)] (1 μM). Scriptaid partially preserved p-Akt levels in LPS-treated microglia alone or in the presence of bpV(pic), but not in the presence of the PI3K/Akt inhibitor LY294002 (10 µM). (F and G) LPS treatment of microglia increased TNF-α and NO production, an effect reduced by bpV(pic) and by Scriptaid. The effect or Scriptaid was reduced by LY294002, but not by bpV(pic). (H) Microsphere uptake was reduced by LPS alone, but increased by LPS + bpV(pic) or LPS + Scriptaid +/− bpV(pic); the effect of Scriptaid on LPS was blocked by LY294002. Thus, all LPS effects were opposed by Scriptaid, and the effects of Scriptaid were abolished by LY294002 and unchanged by [bpV(pic)]. Data are mean ± SEM values from four independent experiments. ***P ≤ 0.001 vs. LPS alone; ##P ≤ 0.01, ###P ≤ 0.001 vs. LPS + Scriptaid.
We also tested whether HDAC inhibition modulates the PTEN/PI3K/Akt axis in LPS-treated microglia. LPS induced a considerable decrease in phosphorylated PTEN (p-PTEN) and Akt (p-Akt) at 3 h (Fig. 5C). Dephosphorylated PTEN is the active form that inhibits PI3K/Akt signal transduction by dephosphorylating PIP3 to form PIP2 (Fig. 1) (23). Of note, a 30-min pretreatment with Scriptaid attenuated the decreases in p-PTEN and p-Akt in LPS-treated microglia (Fig. 5C). In addition, Scriptaid prevented LPS-induced decreases of p-mTOR, p-GSK3β (Ser9), and total GSK3β expression in microglia (Fig. 5D).
To further test the role of PTEN/Akt signaling, we used LY294002 to block PI3K. This treatment abolished the Scriptaid-induced partial protection of p-Akt in LPS-challenged microglia (Fig. 5E). As expected, LY294002 abolished the inhibition of LPS-induced TNF-α and NO production by Scriptaid (Fig. 5 F and G). LY294002 also hindered the increase in microglial phagocytic activity by Scriptaid (Fig. 5H). In contrast, the PTEN inhibitor dipotassium bisperoxo (picolinato) oxovanadate V [bpV(pic)] had no additional effects, probably because Scriptaid already inhibited PTEN maximally. These results suggest that PI3K/Akt signaling contributes to the anti-inflammatory and prophagocytic effects of Scriptaid in microglia.
GSK3β Mediates the Anti-Inflammatory and Protective Effects of HDAC Inhibition.
Given the robust preservation of p-Akt levels by Scriptaid in LPS-challenged microglia, we further tested the effects of Scriptaid on Akt-related signaling molecules p-mTOR and GSK3β. To this end, we infected primary microglia cultures with lentiviral vectors (Ln) bearing shRNA targeting mTOR or GSK3β, or bearing a scrambled control (Fig. S6). Scriptaid prevented LPS-induced decreases in p-mTOR, p-Akt, and GSK3β (Fig. S7A, third column). The preservation of p-mTOR by Scriptaid was blocked by LY294002 (Fig. S7A, fourth column), suggesting that phosphorylation of mTOR lies downstream of PI3K/Akt signaling. Furthermore, GSK3β knockdown reduced Scriptaid-mediated protection of p-Akt in LPS, suggesting that GSK3β lies upstream of PI3K/Akt signaling in this model (Fig. 1 and Fig. S7A, seventh column). In contrast, neither LY294002 nor mTOR knockdown affected the Scriptaid-mediated preservation of GSK3β levels in LPS-challenged microglia (Fig. S7 A, fourth and sixth columns, and B, a). These results suggest that GSK3β increases PI3K/Akt signal transduction, and that p-Akt may activate (phosphorylate) mTOR in Scriptaid-treated, LPS-challenged microglia.
We also examined the effect of GSK3β knockdown on proinflammatory, M1-like responses in LPS-challenged microglia. As expected, both GSK3β knockdown and LY294002 abolished the Scriptaid-mediated decrease in TNF-α and NO after LPS stimulation (Fig. S7 B, b and c). In contrast, knockdown of mTOR was less effective. Furthermore, GSK3β knockdown and LY294002 both abolished the enhancing effects of Scriptaid on phagocytosis by LPS-challenged microglia, whereas knockdown of mTOR again was ineffective (Fig. S7 B, d). Taken together, these results support the notion that GSK3β-mediated activation of PI3K/Akt polarizes microglial activation toward M2 following HDAC inhibition.
Thus far, we have shown that Scriptaid increases GSK3β in LPS-challenged microglia but not in control, unchallenged microglia (Fig. S8B), leading to PI3K/Akt activation and reduces proinflammatory responses to LPS. We also have shown that Scriptaid increases PTEN phosphorylation in LPS-treated microglia without altering PTEN protein expression (Fig. 5C). Scriptaid might affect PTEN phosphorylation through the regulation of genes that code for kinases, such as GSK3β and casein kinase II (CK2). Ser-370 and Ser-385 in the PTEN C-terminal tail domain are thought to be phosphorylated by CK2, whereas Thr-366 is phosphorylated by GSK3β (Fig. S7C). Therefore, we performed real-time PCR for GSK3β and CK2 mRNA in primary microglia treated with LPS with or without Scriptaid. LPS modestly reduced GSK3β mRNA levels, whereas Scriptaid elicited a large increase inthese levels (Fig. S7D). There was little effect on CK2α mRNA. As expected, LPS decreased, but Scriptaid increased, phosphorylation of PTEN at Thr366 in LPS-challenged microglia (Fig. S7 E and F). Notably, knockdown of GSK3β abolished Scriptaid-mediated phosphorylation of PTEN at Thr366 in LPS-challenged microglia, confirming that inactivation of PTEN by Scriptaid is dependent on GSK3β. Although LPS also decreased phosphorylation of p-PTEN at Ser385, Scriptaid and GSK3β knockdown had no effect on this measure, or on phosphorylation at Ser370 (Fig. S7F).
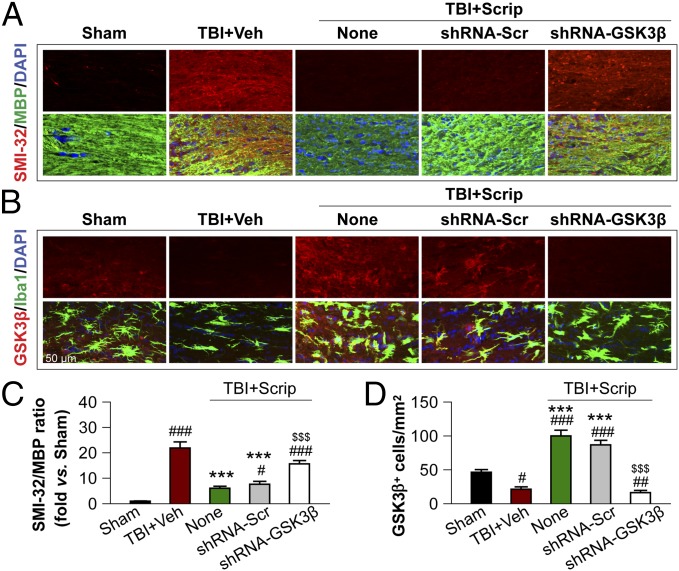
To examine GSK3β-dependent protection against WMI in vivo, we infused lentiviral vectors containing GSK3β-targeting shRNA directly into the CC of mice at 7 d before TBI. The protective effects of Scriptaid against WMI, as reflected by decreases in the SMI-32:MBP ratio at 7 d after TBI, were attenuated by GSK3β knockdown before TBI (Fig. 6 A and C). Scriptaid markedly increased the number of GSK3β-immunoreactive cells in the CC, and this effect was prevented by GSK3β shRNA (Fig. 6 B and D). These results support the hypothesis that microglial GSK3β mediates the protective effects of Scriptaid against WMI.
Fig. 6.
In vivo GSK3β knockdown attenuates Scriptaid-afforded protection against WMI. (A and C) Immunofluorescent staining for SMI-32 (upper row in A) and colabeling of the same sections for MBP and nuclei (DAPI) in the second row. Images were taken from the CC of animals at 7 d post-TBI. Lentiviral shRNA for GSK3β (shRNA-GSK3β) or scrambled shRNA (shRNA-Scr) was infused into the CC 7 d before TBI; Scriptaid was injected at 2, 26, and 50 h after TBI. WMI was expressed as the ratio of SMI-32 to MBP in the CC normalized to sham (C). (B and D) Immunofluorescent staining for GSK3β and Iba-1 in the CC at 7 d post-TBI as in A and C. Quantification of GSK3β+ cells is illustrated in D. Shown are the mean ± SEM values from six mice per group. #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 vs. sham; ***P ≤ 0.001 vs. vehicle; $$$P < 0.001 vs. Scriptaid.
To further investigate the effects of Scriptaid on GSK3β, we examined the spatiotemporal kinetics of this protein in LPS- and Scriptaid-treated primary microglial cultures. Scriptaid prevented the acute loss of GSK3β protein in LPS-treated microglia at 3 h, but within 12 h of LPS treatment, LPS itself also increased GSK3β expression, and in all groups, expression returned to baseline by 24 h (Fig. S8A). Scriptaid alone had no effect on the level of total GSK3β (Fig. S8B). In addition, GSK3β was predominantly cytosolic in untreated microglia, but was decreased in the cytoplasm and increased in the nucleus after LPS treatment (Fig. S8C). Scriptaid significantly increased cytosolic GSK3β levels relative to the control and LPS-only groups, and almost completely reversed the LPS-induced increase in nuclear GSK3β. These findings suggest that Scriptaid maintains cytosolic GSK3β levels in activated microglia by preventing GSK3β translocation to the nucleus.
Discussion
At present, there are no therapies that can cure the cognitive and motor deficits in TBI patients (5, 15); however, an increasing number of studies show that HDAC inhibition can ameliorate injury in experimental models of TBI and other diseases, perhaps by modulating gene expression in a cell type-dependent manner (11–14, 24). Taken together with previously reported findings, our present study supports the view that Scriptaid is a suitable therapeutic candidate for trauma-induced mechanical injury and secondary inflammation-induced cell death in both gray and white matter. Given that the secondary impact of microglia/macrophages in TBI unfolds well after the original insult, HDAC inhibition may even exert restorative or protective effects when administered in a delayed fashion. Consistent with this notion, our previous study demonstrated that Scriptaid protected against TBI even when delivered up to 12 h after injury (10). These observations and the effects of HDAC inhibitors on multiple cell types bode well for their eventual clinical translation.
Despite its significant contribution to functional outcomes, the pathophysiology underlying WMI remains relatively underexplored (25). Nonetheless, it is known that oligodendrocytes are especially vulnerable to mechanical trauma (10, 26, 27), and that neuroinflammation may exacerbate WMI following TBI (15, 21). The present study improves our understanding of white matter pathophysiology by showing that microglia/macrophages exert protective or destructive effects on oligodendrocytes depending on M2 or M1 polarization status, respectively. Whereas HDAC inhibition by Scriptaid in oligodendrocyte cultures showed little direct protection, Scriptaid appeared to hinder destructive M1 responses and promote protective M2 responses in microglia/macrophages, thereby protecting neighboring oligodendrocytes indirectly.
Consistent with our findings, previous reports have shown that HDAC inhibitors protect against ischemia-induced loss of oligodendrocytes and suppress the proinflammatory actions of microglia/macrophages (6, 22, 28). We and other investigators also have shown that HDAC inhibition in experimental TBI modulates the PTEN/PI3K/Akt axis (13, 21). The present study indicates that microglial GSK3β-dependent signal transduction cascades, which involve the PTEN/PI3K/Akt axis, may mediate the protection of oligodendrocytes by HDAC inhibition. We have identified GSK3β as a potential HDAC-regulated gene product, because its mRNA and protein levels were both increased with Scriptaid. Furthermore, HDAC inhibition promoted the cytosolic retention of GSK3β, where it may be more likely to inactivate cytoplasmic PTEN. PTEN is a negative regulator of PI3K/Akt signal transduction (23); therefore, GSK3β-mediated phosphorylation/inactivation of PTEN may lead to disinhibition of PI3K/Akt signaling, which in turn may lead to anti-inflammatory and prophagocytic microglial effects.
Under most conditions involving severe injury, Akt-dependent phosphorylation of GSK3β is known to inhibit its prodeath functions (29). Microglial inflammation is a sublethal stimulus, however, and the induction of GSK3β appears to play a unique role in M2 polarization by promoting PI3K/Akt activity through PTEN inactivation. Some of these interpretations, illustrated in Fig. 1, are consistent with the view that p-Akt improves survival and microglial metabolic function (30). Nonetheless, we cannot rule out the possibility that LY294002 prevents M2 polarization via PI3K substrates other than Akt. Fully establishing the role of Akt in microglial polarization and its downstream consequences on oligodendrocytes will require specific manipulation of Akt levels by RNA interference or transgenic constructs.
Consistent with our present findings, recent studies have shown that M2 microglia/macrophages encourage oligodendrocyte differentiation from resident precursor cells and remyelination processes (31). Furthermore, our data also support the new view that the role of GSK3β in brain repair is quite subtle (32), and that inhibitors of GSK3β, as have been proposed for neuroprotection (33), could have unintended, negative consequences as well.
In conclusion, we have demonstrated that HDAC inhibition promotes oligodendrocyte survival indirectly through a GSK3β/PI3K/Akt-mediated phenotypic shift in microglia. The anti-inflammatory effects of HDAC inhibition and the preservation of oligodendrocytes also may improve the environmental milieu surrounding nearby neurons and indirectly exert neuroprotective effects. Thus, further efforts using HDAC inhibitors to modulate the GSK3β/PTEN/PI3K/Akt axis are warranted to combat TBI and neurological diseases characterized by white matter as well as gray matter destruction, such as stroke, neurodegenerative disorders, and drug/alcohol overdose.
Materials and Methods
This study used the well-established CCI model of TBI in mice (10, 21). Scriptaid was injected at 3.5 mg/kg at 2 h after TBI, and this was repeated daily for the next 2 d. Animals were assigned at random to groups, and all measurements were performed by investigators blinded to the experimental group assignments. Methodological details beyond the descriptions below are provided in SI Materials and Methods.
Intracerebral Lentiviral shRNA Infection.
Lentiviral particles containing shRNA for GSK3β or nontargeting sequences were infused into the right CC at 7 d before TBI. To confirm efficient infection, viral particles containing GFP were infused as described above, and GFP expression in mouse brains was verified 7 d later.
Primary Microglia and Oligodendrocyte Cultures.
Primary microglia and oligodendrocytes were collected from mixed cultures harvested from 1-d-old postnatal mice (18). LPS or LPS plus IFN-γ was added to microglia for 48 h for M1 induction. IL-4 was added for 48 h for M2 induction. A Transwell system and a simple CM transfer were used to assess the interactions between oligodendrocytes and microglia.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grants NS36736, NS43802, and NS45048 (to J.C.) and NS45287 (to M.V.L.B.); US Department of Veterans Affairs Research Career Scientist Award (to J.C.); Chinese Natural Science Foundation Grants 81020108021, 81171149, and 81371306 (to Y.G.), 81000497 and 81471257 (to G.W.), and 81228008 (to J.C. and P.Z.), and PhD Training Grant 20120071110042 (to J.C.); Ministry of Education of the People’s Republic of China Talent Training Fellowship Grant J1210041 (to B.T.); and State Administration of Foreign Experts Affairs High-End Distinguished Professorship Grant GDW20133100069 (to M.V.L.B.). R.K.L. is supported by a Hillman Foundation Award.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501441112/-/DCSupplemental.
References
- 1.Rosenfeld JV, et al. Early management of severe traumatic brain injury. Lancet. 2012;380(9847):1088–1098. doi: 10.1016/S0140-6736(12)60864-2. [DOI] [PubMed] [Google Scholar]
- 2.Spitz G, Maller JJ, O’Sullivan R, Ponsford JL. White matter integrity following traumatic brain injury: The association with severity of injury and cognitive functioning. Brain Topogr. 2013;26(4):648–660. doi: 10.1007/s10548-013-0283-0. [DOI] [PubMed] [Google Scholar]
- 3.Kinnunen KM, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(Pt 2):449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betz J, Zhuo J, Roy A, Shanmuganathan K, Gullapalli RP. Prognostic value of diffusion tensor imaging parameters in severe traumatic brain injury. J Neurotrauma. 2012;29(7):1292–1305. doi: 10.1089/neu.2011.2215. [DOI] [PubMed] [Google Scholar]
- 5.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31(12):596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu XS, et al. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience. 2012;220:313–321. doi: 10.1016/j.neuroscience.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adcock IM. HDAC inhibitors as anti-inflammatory agents. Br J Pharmacol. 2007;150(7):829–831. doi: 10.1038/sj.bjp.0707166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sleiman SF, et al. Putting the “HAT” back on survival signalling: The promises and challenges of HDAC inhibition in the treatment of neurological conditions. Expert Opin Investig Drugs. 2009;18(5):573–584. doi: 10.1517/13543780902810345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazo-Gómez R, Ramírez-Jarquín UN, Tovar-Y-Romo LB, Tapia R. Histone deacetylases and their role in motor neuron degeneration. Front Cell Neurosci. 2013;7:243. doi: 10.3389/fncel.2013.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G, et al. Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway: Scriptaid protects against TBI via AKT. Neurotherapeutics. 2013;10(1):124–142. doi: 10.1007/s13311-012-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu F, et al. Posttrauma cotreatment with lithium and valproate: Reduction of lesion volume, attenuation of blood-brain barrier disruption, and improvement in motor coordination in mice with traumatic brain injury. J Neurosurg. 2013;119(3):766–773. doi: 10.3171/2013.6.JNS13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dash PK, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS ONE. 2010;5(6):e11383. doi: 10.1371/journal.pone.0011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shein NA, et al. Histone deacetylase inhibitor ITF2357 is neuroprotective, improves functional recovery, and induces glial apoptosis following experimental traumatic brain injury. FASEB J. 2009;23(12):4266–4275. doi: 10.1096/fj.09-134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, et al. HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res. 2008;1226:181–191. doi: 10.1016/j.brainres.2008.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson VE, et al. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(Pt 1):28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Ishii H, Bai Z, Itokazu T, Yamashita T. Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS ONE. 2012;7(7):e41892. doi: 10.1371/journal.pone.0041892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 19.Boche D, Perry VH, Nicoll JA. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39(1):3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 20.Hu X, et al. Microglial and macrophage polarization: New prospects for brain repair. Nat Rev Neurol. 2015;11(1):56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33(12):1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannan V, et al. Histone deacetylase inhibitors suppress immune activation in primary mouse microglia. J Neurosci Res. 2013;91(9):1133–1142. doi: 10.1002/jnr.23221. [DOI] [PubMed] [Google Scholar]
- 23.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 24.Dash PK, Orsi SA, Moore AN. Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience. 2009;163(1):1–8. doi: 10.1016/j.neuroscience.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellyer PJ, Leech R, Ham TE, Bonnelle V, Sharp DJ. Individual prediction of white matter injury following traumatic brain injury. Ann Neurol. 2013;73(4):489–499. doi: 10.1002/ana.23824. [DOI] [PubMed] [Google Scholar]
- 26.Borgens RB, Liu-Snyder P. Understanding secondary injury. Q Rev Biol. 2012;87(2):89–127. doi: 10.1086/665457. [DOI] [PubMed] [Google Scholar]
- 27.Wang GH, et al. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma. 2011;28(10):2123–2134. doi: 10.1089/neu.2011.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HJ, Chuang DM. HDAC inhibitors mitigate ischemia-induced oligodendrocyte damage: Potential roles of oligodendrogenesis, VEGF, and anti-inflammation. Am J Transl Res. 2014;6(3):206–223. [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen MV, Downey JM. Ischemic postconditioning: From receptor to end-effector. Antioxid Redox Signal. 2011;14(5):821–831. doi: 10.1089/ars.2010.3318. [DOI] [PubMed] [Google Scholar]
- 30.Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB. Characterization of fractalkine in rat brain cells: Migratory and activation signals for CX3CR-1–expressing microglia. J Immunol. 1999;163(3):1628–1635. [PubMed] [Google Scholar]
- 31.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seira O, Del Río JA. Glycogen synthase kinase 3 beta (GSK3β) at the tip of neuronal development and regeneration. Mol Neurobiol. 2014;49(2):931–944. doi: 10.1007/s12035-013-8571-y. [DOI] [PubMed] [Google Scholar]
- 33.Cohen P, Goedert M. GSK3 inhibitors: Development and therapeutic potential. Nat Rev Drug Discov. 2004;3(6):479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.