Significance
While CD8+ T cells are essential for antitumor immunity, tumors often evade CD8+ T cell surveillance by immunosuppression. Recent study has shown that tumor-induced β-catenin activation in DCs suppresses CD8+ T cell immunity by inhibiting cross-priming, suggesting that activation of β-catenin in DCs might be a key mechanism tumors use to achieve immunosuppression. This report identified mTOR/IL-10 signaling as a previously unidentified mechanism for β-catenin–dependent inhibition of cross-priming. Surprisingly, our study also revealed that β-catenin in DCs was required for CD8+ T cell maintenance post-clonal expansion, suggesting that β-catenin exerts opposite functions during different stages of CD8+ T cell responses. Based on these findings, we have demonstrated selectively manipulating β-catenin signaling as a feasible strategy to improve DC vaccine efficacy.
Keywords: dendritic cells, beta-catenin, IL-10, mTOR, CD8+ T-cell immunity
Abstract
Recent studies have demonstrated that β-catenin in DCs serves as a key mediator in promoting both CD4+ and CD8+ T-cell tolerance, although how β-catenin exerts its functions remains incompletely understood. Here we report that activation of β-catenin in DCs inhibits cross-priming of CD8+ T cells by up-regulating mTOR-dependent IL-10, suggesting blocking β-catenin/mTOR/IL-10 signaling as a viable approach to augment CD8+ T-cell immunity. However, vaccination of DC–β-catenin−/− (CD11c-specific deletion of β-catenin) mice surprisingly failed to protect them against tumor challenge. Further studies revealed that DC–β-catenin−/− mice were deficient in generating CD8+ T-cell immunity despite normal clonal expansion, likely due to impaired IL-10 production by β-catenin−/− DCs. Deletion of β-catenin in DCs or blocking IL-10 after clonal expansion similarly led to reduced CD8+ T cells, suggesting that β-catenin in DCs plays a positive role in CD8+ T-cell maintenance postclonal expansion through IL-10. Thus, our study has not only identified mTOR/IL-10 as a previously unidentified mechanism for β-catenin–dependent inhibition of cross-priming, but also uncovered an unexpected positive role that β-catenin plays in maintenance of CD8+ T cells. Despite β-catenin’s opposite functions in regulating CD8+ T-cell responses, selectively blocking β-catenin with a pharmacological inhibitor during priming phase augmented DC vaccine-induced CD8+ T-cell immunity and improved antitumor efficacy, suggesting manipulating β-catenin signaling as a feasible therapeutic strategy to improve DC vaccine efficacy.
As the initiators of antigen-specific immune responses, dendritic cells (DCs) play a central role in regulating both T-cell immunity and tolerance (1). β-Catenin, a major component in Wnt signaling pathway, has emerged as a key factor in DC differentiation and function (2). Previous studies have shown that β-catenin regulates DC-mediated CD4+ T-cell responses and promotes CD4+ T-cell tolerance in murine models of autoimmune diseases (3, 4). Consistently, activation of β-catenin in DCs has recently been shown to suppress CD8+ T-cell immunity in a DC-targeted vaccine model (5), suggesting that β-catenin in DCs might similarly serve as a tolerizing signal that shifts the balance between CD8+ T-cell immunity and tolerance. Although the underlying mechanisms of how β-catenin mediates CD8+ T-cell tolerance remain largely unclear, we have shown that activation of β-catenin in DCs genetically or induced by tumors suppresses CD8+ T-cell immunity by inhibiting cross-priming (5). Exploiting their ability to potentiate host effector and memory CD8+ T-cell responses, DC vaccines have emerged as a leading strategy for cancer immunotherapy (6). However, one major obstacle for their success is host DC-mediated immunosuppression (7–9). Given that cross-priming plays a major role in generating antitumor CD8+ T-cell immunity (7, 10), activation of β-catenin in DCs might be a key mechanism for tumors to achieve immunosuppression. Thus, manipulating β-catenin function in cross-priming might be a viable approach to overcome DC-mediated immunosuppression and improve DC vaccine efficacy. However, The underlying mechanisms of how β-catenin in DCs achieves immunosuppression, in particular how β-catenin negatively regulates cross-priming to suppress CD8+ T-cell immunity, remain poorly understood.
Although the mechanisms for DC-mediated priming of antitumor CD8+ T cells through cross-presentation remain incompletely understood, DC subsets, DC maturation status and cytokines have been shown to possibly affect their capacity in cross-priming (7, 10, 11). Although the role of cytokines in cross-priming has not been directly tested, cytokines as “signal 3” have been shown in principal to play a critical role in priming and effector differentiation of antitumor CD8+ T cells (12). β-Catenin in DCs has been shown to play a critical role in regulating cytokine induction (3, 4), thus suggesting that β-catenin might regulate DC cytokine production to achieve its effects on cross-priming.
In this report we have identified mTOR/IL-10 signaling as a mechanism for β-catenin–dependent inhibition of cross-priming. Activation of β-catenin in DCs inhibited cross-priming of CD8+ T cells by up-regulating mTOR-dependent IL-10, and blocking mTOR or IL-10 led to restored cross-priming by β-cateninactive DCs. Surprisingly, mice with DC-specific deletion of β-catenin (DC–β-catenin−/− mice) exhibited reduced antitumor immunity upon vaccination, despite the fact that deletion of β-catenin in DCs abrogated tumor-induced inhibition of cross-priming. Further studies showed that DC–β-catenin−/− mice were deficient in generating CD8+ T-cell immunity despite normal clonal expansion, and β-catenin in DCs was required to maintain primed CD8+ T cells postclonal expansion. Thus, β-catenin in DCs exerts negative and positive functions in cross-priming and maintenance of CD8+ T cells, respectively. Importantly, we have demonstrated blocking β-catenin selectively at priming phase as a feasible strategy to improve DC vaccine efficacy.
Results
IL-10 Mediates β-Catenin–Dependent Inhibition of Cross-Priming.
As β-catenin regulates DC maturation and differentiation (3, 13), we asked whether they could contribute to impaired cross-priming observed in DC–β-cateninactive mice that express constitutively active β-catenin in their DCs (5). Surprisingly, although the percentages of total CD11c+ cells, CD8+, and CD4+ conventional DCs (CD11c+B220−) were similar in skin-draining lymph nodes, DC–β-cateninactive mice exhibited significantly higher percentage of splenic CD8+ DCs, which have been shown to be especially efficient at cross-presentation (14) (Fig. S1A). No significant differences in the expression of CD80, CD86, MHCII and MHCI were observed between steady state WT and β-cateninactive DCs, and no difference in apoptosis was observed (Fig. S1B). β-Cateninactive DCs also exhibited no deficiency in phagocytosis (Fig. S1C). Taken together, these findings suggest that DC frequency, composition, maturation, apoptosis and phagocytosis unlikely account for impaired cross-priming observed in DC–β-cateninactive mice.
We next asked whether β-catenin inhibited cross-priming through the regulation of cytokines, as previous studies have shown that β-catenin regulates DC cytokine production (3, 4). Both splenic and LN DCs from DC–β-cateninactive mice produced significantly higher IL-10 than WT DCs upon CpG treatment, whereas the production of Th1 cytokine IL-12 was similarly induced (Fig. 1A). The lack of difference in IL-12 was not entirely surprising, as we have shown that DC-produced IL-12 is not required for BMDC vaccine-induced CD8+ T-cell priming (15). Treatment with a neutralizing antibody against IL-10 restored cross-priming in DC–β-cateninactive mice, resulting in significantly increased percentages of IFN-γ–producing effectors compared with untreated DC–β-cateninactive mice (Fig. 1B), suggesting that increased IL-10 contributes to impaired cross-priming in DC–β-cateninactive mice. Further analysis showed that IFN-γ+ OTI cells from WT mice or anti–IL-10-treated DC–β-cateninactive mice exhibited significantly higher mean fluorescence intensity (MFI) of IFN-γ (Fig. S2A), suggesting that β-catenin in DCs negatively regulates IFN-γ+ OTI cells quantitatively and qualitatively. Consistent with our previous report (5), proliferation of Thy1.1+ OTI cells was not significantly different among all three groups (Fig. S2 B and C). Both anti–IL-10-treated and nontreated DC–β-cateninactive mice exhibited significantly higher Foxp3+ regulatory T cells than WT mice (Fig. S2 D and E), suggesting that β-catenin in DCs unlikely regulates cross-priming through Foxp3+ regulatory T cells. The deficiency in producing IFN-γ is not due to their differentiation into type 2 (Tc2) CD8+ T cells (16), as primed Thy1.1+ OTI cells from all three groups did not produce IL-4 or IL-10 (Fig. S2 F–I). We also examined the expression of activation/differentiation markers CD44, CD62L, CD127, and KLRG1, and observed no significant differences (Fig. S3 A and B). However, IFN-γ+ OTI effector cells were significantly reduced in DC–β-cateninactive mice compared with WT mice or anti–IL-10 treated DC–β-cateninactive mice when examined 15 d after immunization (Fig. S3C), suggested that impaired cross-priming of Thy1.1+ OTI cells in DC–β-cateninactive mice results in reduced production of IFN-γ+ OTI effectors, consistent with our previous study (5).
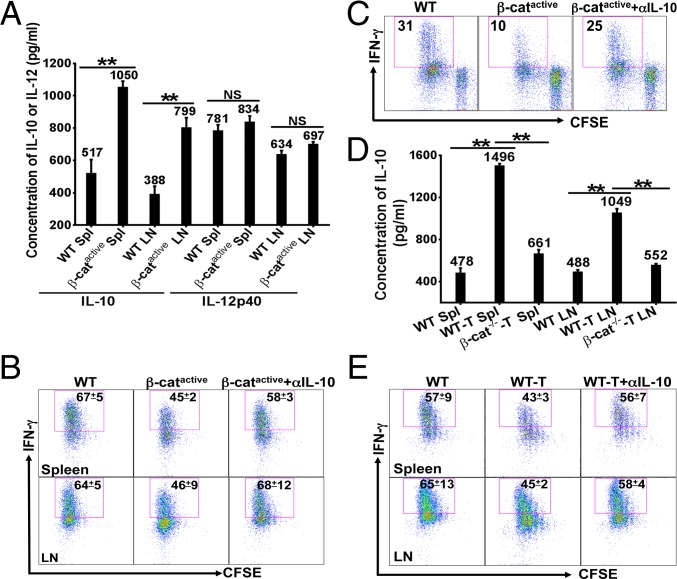
Fig. 1.
β-Catenin in DCs inhibits cross-priming through IL-10. (A) β-Cateninactive DCs produced significantly higher IL-10. DCs were treated with CpG and cytokines were measured by ELISA after 36 h. (B) Blocking IL-10 in vivo during priming phase restored cross-priming in DC–β-cateninactive mice. WT and DC–β-cateninactive mice (n = 4) were treated with anti–IL-10 or PBS and cross-priming was examined. Mean and SD of the percentages of IFN-γ+ cells out of total Thy1.1+CD8+ cells are shown. (C) Anti–IL-10 treatment restored cross-priming by β-cateninactive DCs in vitro. DCs were pulsed with anti–DEC-205-OVA plus CpG, and cross-priming was examined as in B. The percentages of IFN-γ+ cells out of total Thy1.1+CD8+ cells are shown. (D) Deletion of β-catenin in DCs significantly reduced tumor-induced IL-10. DCs isolated from immunized mice at day 3 were cultured with CpG, and IL-10 was measured after 36 h. (E) Blocking IL-10 during priming phase restored cross-priming in tumor-bearing mice. Cross-priming was examined in tumor-free and B16OVA-bearing WT mice (n = 4–5) and data were presented as in B. Data shown are representative of at least two experiments. NS = P > 0.05, *P < 0.05 and **P < 0.01.
To determine whether increased IL-10 in β-cateninactive DCs is directly responsible for the impaired cross-priming, we assessed DCs’ capacity in cross-priming with an in vitro DC-OTI cell coculture system. As expected, cocultures with β-cateninactive DCs produced significantly more IL-10 than cocultures with WT DCs (Fig. S4). Anti–IL-10 treatment largely restored cross-priming by β-cateninactive DCs (Fig. 1C), suggesting that β-catenin in DCs negatively regulates cross-priming by enhancing IL-10 production.
As we have shown that tumors activate β-catenin in DCs to inhibit cross-priming (5), we asked whether IL-10 was similarly involved in tumor-induced inhibition of cross-priming. We first investigated whether DCs in B16-bearing mice produced higher IL-10 upon immunization and if so whether tumor-induced IL-10 production in DCs was regulated by β-catenin. DCs isolated from tumor-free WT, B16OVA-bearing WT and DC–β-catenin−/− mice immunized with anti–DEC-205-OVA plus CpG were further stimulated with CpG to mimic the presence of CpG under in vivo condition. As shown in Fig. 1D, DCs from B16OVA-bearing WT mice produced significantly higher IL-10 compared with DCs from tumor-free WT mice, consistent with increased IL-10 by DCs from tumor-bearing mice in the Ret transgenic melanoma model (17). DCs from tumor-bearing DC–β-catenin−/− mice, however, produced significantly less IL-10 compared with DCs from tumor-bearing WT mice (Fig. 1D), suggesting that tumors up-regulate IL-10 in DCs through β-catenin. Not surprisingly, anti–IL-10 treatment restored cross-priming in tumor-bearing mice, significantly increasing the percentages of IFN-γ-producing effectors (Fig. 1E), indicating that increased IL-10 plays a critical role in tumor-induced inhibition of cross-priming. Thus, IL-10 mediates β-catenin–dependent inhibition of cross-priming in both DC–β-cateninactive and tumor-bearing mice.
β-Catenin Positively Regulates mTOR Activation to Enhance IL-10 Production in DCs.
As recent studies have shown that Mammalian Target of Rapamycin complex 1 (mTORC1) positively regulates IL-10 in DCs (18, 19), we asked whether β-catenin enhanced IL-10 induction through mTOR pathway. β-Cateninactive DCs expressed substantially elevated mTOR than WT DCs (Fig. 2A and Fig. S5A). We next asked whether β-cateninactive DCs would also exhibit enhanced mTOR activation under immunization condition. Both splenic and LN DCs from immunized DC–β-cateninactive mice exhibited elevated mTOR activation compared with WT DCs, as measured by phosphorylation of S6, a downstream target of mTORC1 (Fig. 2 B and C). Consistently, DCs from immunized DC–β-catenin−/− mice exhibited reduced phosphorylated S6 compared with DCs of WT mice (Fig. S5 B and C). Thus, β-catenin positively regulates mTOR activation in DCs under immunization condition. To determine whether activation of β-catenin in DCs is directly responsible for enhanced mTOR activation, we examined mTOR activation in purified primary DCs treated with CpG in vitro. Indeed, increased phosphorylation of S6 was observed in β-cateninactive DCs (Fig. 2D and Fig. S5D), suggesting that β-catenin directly enhances mTOR activation in DCs. Increased mTOR activation by β-cateninactive DCs was also confirmed when we examined the phosphorylation and activation of p70S6K and 4E-BP1, two immediate downstream targets of mTORC1 (Fig. S5E). As expected, treatment with mTOR inhibitor rapamycin greatly reduced mTOR activation in β-cateninactive DCs (Fig. 2D and Fig. S5D).
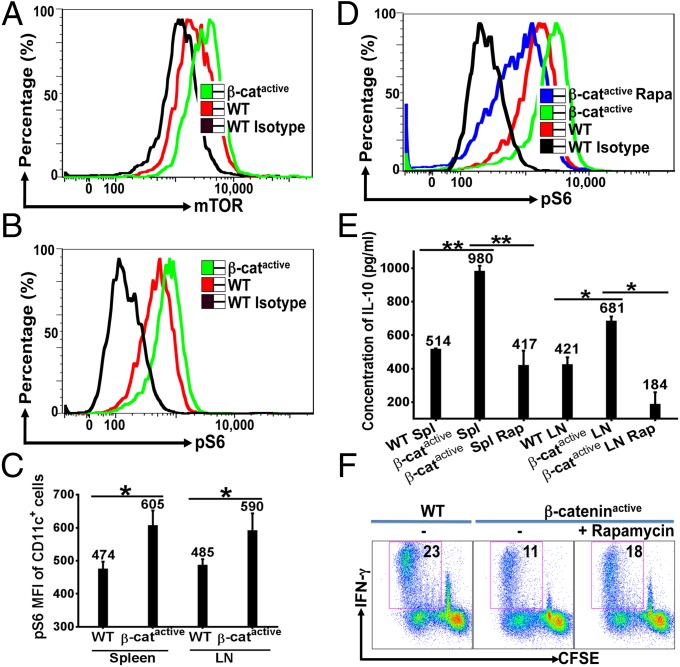
Fig. 2.
β-Catenin positively regulates IL-10 via mTOR signaling pathway. (A) β-Catenin up-regulates mTOR in DCs. Splenocytes were subjected to flow cytometry and gated on CD11c+ cells. (B and C) DCs from immunized DC–β-cateninactive mice exhibited enhanced mTOR activity. Spleen and LN cells from immunized mice (n = 4) were isolated and subjected to flow cytometry as in A. Expression of phosphorylated S6 (pS6) of CD11c+ cells is shown as histogram overlays in B and Mean Fluorescent Intensity (MFI) in C. (D) β-Cateninactive DCs exhibited augmented CpG-induced mTOR activation in vitro. Splenic DCs from naive mice were stimulated by CpG with or without rapamycin. (E) Inhibition of mTOR led to reduced IL-10 by β-cateninactive DCs. DCs were treated as indicated and IL-10 was measured by ELISA. (F) Inhibition of mTOR led to increased cross-priming by β-cateninactive DCs in vitro. Splenic DCs were treated as indicated, and were subjected to cross-priming assays. Data shown are representative of three or more experiments. *P < 0.05 and **P < 0.01.
We next asked whether inhibition of mTOR by rapamycin affected IL-10 induction and cross-priming of β-cateninactive DCs. Although CpG-stimulated β-cateninactive DCs produced significantly higher IL-10 than WT DCs, rapamycin treatment led to substantially reduced IL-10 (Fig. 2E), suggesting that mTOR activation is required for β-catenin–mediated regulation of IL-10. The induction of IL-12, however, was not significantly changed by rapamycin treatment (Fig. S5F). Treatment with rapamycin also led to increased cross-priming by β-cateninactive DCs (Fig. 2F). The partial rescue might be due to the fact that we were only able to treat DCs with rapamycin before coculture with OTI CD8+ T cells, as rapamycin has profound effects on CD8+ T cells (20). Taken together, these data support a model that activation of β-catenin in DCs inhibits cross-priming through regulation of mTOR-dependent IL-10.
DC–β-Catenin−/− Mice Failed to Maintain Primed Antigen-Specific CD8+ T Cells Despite Normal Clonal Expansion.
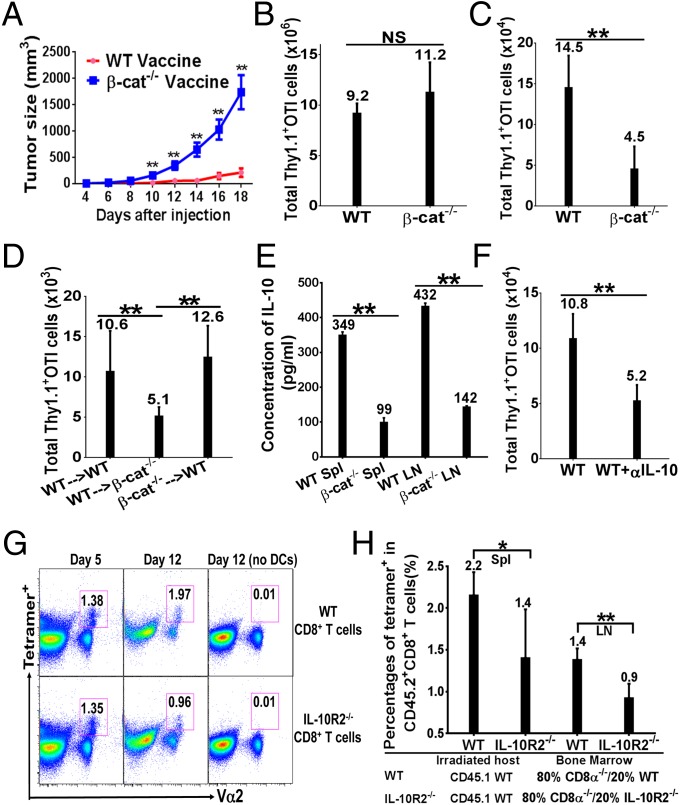
As deletion of β-catenin in DCs significantly reduced tumor-induced IL-10 production (Fig. 1D) and abrogated tumor-induced inhibition of cross-priming (5), we asked whether inhibition of β-catenin in DCs would augment antitumor immunity. To our surprise, B16OVA-bearing DC–β-catenin−/− and WT mice exhibited no significant difference in tumor growth (Fig. S6A). We thus asked whether deletion of β-catenin in DCs augmented vaccination-induced antitumor immunity. Although vaccinated WT mice were protected against B16OVA challenge, vaccinated DC–β-catenin−/− mice still exhibited significant tumor growth (Fig. 3A), suggesting that DC–β-catenin−/− mice generated reduced antitumor immunity upon vaccination. Thus, β-catenin in DCs likely plays an additional role in regulating antitumor immunity.
Fig. 3.
β-Catenin in DCs is required for CD8+ T-cell maintenance after clonal expansion. (A) DC–β-catenin−/− mice exhibited reduced antitumor immunity. WT and DC–β-catenin−/− mice (n = 8) were challenged with 2 × 106 B16OVA cells 20 d after immunization. (B) DC–β-catenin−/− mice exhibited normal primary CD8+ T-cell responses. LN cells from immunized mice (n = 5) were analyzed at day 4 after immunization. (C) DC–β-catenin−/− mice failed to maintain Thy1.1+ OTI cells. LN cells from immunized mice (n = 4–5) were analyzed at day 15. (D) β-Catenin in DCs is required postclonal expansion to maintain primed CD8+ T cells. Thy1.1+ OTI cells purified at day 4 after immunization were transferred into immunized WT and DC–β-catenin−/− mice (n = 4–5), and LN cells were analyzed 8 d after transfer. (E) DCs from immunized DC–β-catenin−/− mice produced significantly less IL-10. DCs isolated from immunized mice at day 7 were cultured with CpG (75 ng/mL) and IL-10 was measured by ELISA. (F) Blocking IL-10 after priming phase led to reduced OTI CD8+ T cells. Immunized WT mice were treated with anti–IL-10 antibody or PBS (n = 5), and LN cells were analyzed at day 15 as in B. (G) IL-10R-mediated signaling in CD8+ T cells was required for their maintenance. WT or IL-10R2−/− CD8+ T cells were cultured with antigen-pulsed WT DCs, and OVA-specific CD8+ T cells were detected by tetramer staining at day 5 and 12. Percentages of tetramer+ cells of gated CD8+ T cells are shown. (H) IL-10R2−/− bone marrow chimeras exhibited significantly lower OVA-specific CD8+ T cells upon vaccination. Chimeras (n = 5) were analyzed at day 12 after vaccination with anti–DEC-205-OVA plus CpG, and percentages of tetramer-positive cells of CD45.2+CD8+ T cells are shown. Data are representative of two or three experiments. NS = P > 0.05, *P < 0.05 and **P < 0.01.
Because CD8+ T cells play a major role in antitumor immunity in the B16 model (21), we asked how deletion of β-catenin in DCs affected vaccination-induced CD8+ T-cell responses. We first examined primary CD8+ T-cell responses in DC–β-catenin−/− mice. When examined at day 4 after immunization, Thy1.1+ OTI cells in LN were slightly but not significantly higher in DC–β-catenin−/− mice compared with WT mice (Fig. 3B). The percentages of IFN-γ+, IL-2+, Granzyme B+ and TNF-α+ OTI cells were not significantly different with the exception of higher TNF-α+ cells in DC–β-catenin−/− mice (Fig. S6B). Thus, DC–β-catenin−/− mice are not deficient in the initial clonal expansion of antigen-specific CD8+ T cells. We thus asked whether primed CD8+ T cells were similarly maintained after clonal expansion. To our surprise, total Thy1.1+ OTI cells were significantly reduced in DC–β-catenin−/− mice compared with WT mice 15 d after immunization (Fig. 3C). Thus, although DC–β-catenin−/− mice exhibited no defect in vaccination-induced clonal expansion of CD8+ T cells, β-catenin in DCs is required for maintenance of primed CD8+ T cells. To determine at which stage β-catenin in DCs is required for CD8+ T-cell maintenance, we carried out adoptive transfer experiments. When Thy1.1+ OTI cells primed in WT and DC–β-catenin−/− mice were adoptively transferred into WT recipients, similar numbers of Thy1.1+ OTI cells were recovered, suggesting that deletion of β-catenin during priming phase does not cause the deficiency in CD8+ T-cell maintenance (Fig. 3D). In contrast, when Thy1.1+ OTI cells primed in WT mice were transferred into DC–β-catenin−/− hosts, recovered Thy1.1+ OTI cells were significantly reduced (Fig. 3D), suggesting that β-catenin in DCs is required to maintain primed CD8+ T cells after clonal expansion. Reduced Thy1.1+ OTI cells were similarly observed in WT mice treated with β-catenin inhibitor XAV939 after clonal expansion (Fig. S6C).
As β-catenin positively regulates IL-10 in DCs upon immunization, we asked whether β-catenin–regulated IL-10 was required for maintenance of CD8+ T cells. We first examined whether DCs from immunized WT and DC–β-catenin−/− mice differed in their ability to produce IL-10 postclonal expansion. DCs were isolated from WT and DC–β-catenin−/− mice 7 d after immunization, and treated with lower concentrations of CpG to mimic the presence of CpG under in vivo condition, as a previous study on tissue distribution of phosphorothioate oligonucleotides (e.g., CpG) has shown that their levels in spleen decreased slightly from 3 to 10 d (22). DCs from DC–β-catenin−/− mice produced substantially lower IL-10 than WT DCs (Fig. 3E and Fig. S6D), suggesting that impaired IL-10 production by β-catenin−/− DCs might contribute to the deficiency in CD8+ T-cell maintenance. We thus tested whether blocking IL-10 after clonal expansion could lead to reduced CD8+ T cells. Indeed, total Thy1.1+ OTI cells in LN were significantly diminished in mice treated with anti–IL-10 after 5 d postimmunization (Fig. 3F). Taken together, our data suggest that β-catenin–regulated IL-10 is required for maintenance of CD8+ T cells after clonal expansion.
The role of IL-10 in CD8+ T-cell immunity has remained controversial, as both positive and negative roles of IL-10 have been reported (23–32). We thus asked whether IL-10R-mediated signaling in CD8+ T cells was required for their maintenance. Indeed, cocultures of OVA antigen-pulsed DCs with IL-10R2−/− CD8+ T cells exhibited significantly lower percentages of OVA-specific CD8+ T cells compared with cocultures with WT CD8+ T cells at day 12, whereas similar percentages of OVA-specific CD8+ T cells were observed at day 5 (Fig. 3G), suggesting that IL-10R-mediated signaling in CD8+ T cells plays a positive role in the maintenance of primed CD8+ T cells. Supporting this notion, mixed bone marrow chimeras expressing IL-10R2−/− CD8+ T cells exhibited significantly lower OVA-specific CD8+ T cells upon vaccination compared with WT chimeras (Fig. 3H). However, antigen-specific CD8+ T cells primed in DC–β-catenin−/− mice were not deficient in IL-10R-mediated signaling, as Thy1.1+ OTI cells from immunized WT and DC–β-catenin−/− mice exhibited no significant difference in IL-10R signaling as measured by STAT3 phosphorylation (Fig. S6 E and F). Taken together, our data support a model that deletion of β-catenin negatively regulates DCs’ IL-10 production to reduce IL-10/IL-10R signaling in primed CD8+ T cells, leading to impaired maintenance of CD8+ T cells.
Blocking β-Catenin Pharmacologically During Priming Phase Augmented CD8+ T-Cell Immunity and Improved DC Vaccine Efficacy.
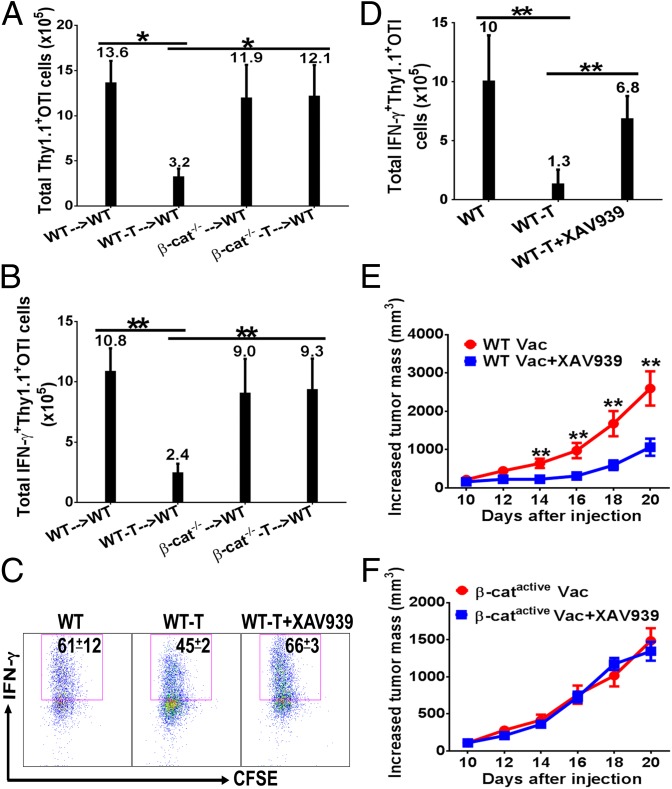
Our data have established that β-catenin in DCs plays a negative role in cross-priming but is required for maintenance of CD8+ T cells, therefore the question becomes whether β-catenin functions could be selectively manipulated to augment CD8+ T-cell immunity. As deletion of β-catenin abrogates tumor-induced inhibition of cross-priming (5), and OTI cells primed in DC–β-catenin−/− mice do not exhibit impaired maintenance (Fig. 3D), we reasoned that blocking β-catenin’s function in cross-priming might be a feasible approach to enhance CD8+ T-cell immunity. We thus examined whether OTI cells primed in tumor-bearing DC–β-catenin−/− mice were functional in generating memory CD8+ T-cell responses. Although Thy1.1+ OTI cells primed in B16OVA-bearing WT mice were deficient in generating memory responses (Fig. 4 A and B, left two bars), Thy1.1+ OTI cells primed in tumor-bearing DC–β-catenin−/− mice functioned similarly in generating antitumor memory CD8+ T cells compared with OTI cells primed in tumor-free WT and DC–β-catenin−/− mice, producing similar number of IFN-γ–producing OTI cells (Fig. 4B, right bars). Thus, inhibition of β-catenin during priming phase in tumor-bearing mice restores the function of primed antigen-specific CD8+ T cells in generating memory responses.
Fig. 4.
Blocking β-catenin with XAV939 augmented antitumor CD8+ T-cell immunity. (A and B) β-Catenin deletion in DCs during priming phase abrogated tumor-induced suppression of memory CD8+ T-cell responses. Primed Thy1.1+ OTI cells were transferred into naïve WT mice (n = 4–5), and recalled at day 40 with OVA in CFA. Total numbers of Thy1.1+ (A) and IFN-γ+Thy1.1+ (B) OTI cells in LN are depicted. (C) Blocking β-catenin with XAV939 restored cross-priming in tumor-bearing mice. Tumor-free and B16OVA-bearing WT mice (n = 5) were treated as indicated and cross-priming was examined as in Fig. 1B. (D) Blocking β-catenin during priming phase rescued tumor-suppressed CD8+ T-cell immunity. Tumor-free and B16OVA-bearing WT mice (n = 4–5) were treated as indicated and were recalled at day 15 and analyzed as in B. (E) Blocking β-catenin with XAV939 during priming phase enhanced vaccine efficacy for WT mice. B16OVA-bearing WT mice (n = 7) were immunized with DEG, and treated with XAV939 as in C, but no Thy1.1+OTI cells were transferred. (F) Blocking β-catenin with XAV939 did not improve vaccine efficacy in DC–β-cateninactive mice. B16OVA-bearing DC–β-cateninactive mice were treated as in E, but a lower dose of B16OVA cells were inoculated. No significant difference was observed on all time points. Data are representative of at least two experiments. *P < 0.05, **P < 0.01.
We next asked whether blocking β-catenin pharmacologically during priming phase could similarly augment antitumor CD8+ T-cell immunity to improve DC vaccine efficacy. We have chosen XAV939, which belongs to a class of β-catenin inhibitors that stimulate the degradation of β-catenin (33). DCs from immunized WT mice treated with XAV939 exhibited significantly reduced phosphorylation of S6, and reduced production of IL-10 compared with mice without XAV939 treatment (Fig. S7 A–C), similarly to the phenotypes observed with β-catenin−/− DCs (Fig. S5 B and C). Treatment with XAV939 greatly enhanced cross-priming in B16OVA-bearing WT mice compared with untreated ones, leading to percentages of IFN-γ+ OTI cells comparable to tumor-free WT mice (Fig. 4C). Thus, blocking β-catenin with XAV939 during priming phase could restore vaccine-induced cross-priming in tumor-bearing mice. To determine whether tumor-suppressed CD8+ T-cell immunity is similarly restored by XAV939 treatment during priming phase, recall responses were examined. Indeed, total Thy1.1+ OTI and IFN-γ+ Thy1.1+ OTI cells were largely restored in B16OVA-bearing mice treated with XAV939 compared with nontreated mice (Fig. S7D and Fig. 4D).
We then asked whether blocking β-catenin during priming phase could improve DC vaccine efficacy. Vaccinated B16OVA-bearing mice treated with XAV939 exhibited significantly slower tumor growth compared with mice without XAV939 treatment (Fig. 4E). However, XAV939 treatment alone without vaccination had no effects on tumor growth (Fig. S7E), suggesting that XAV939 treatment did not directly affect tumor growth to improve vaccine efficacy. Similarly, XAV939 did not inhibit proliferation of B16OVA cells in vitro (Fig. S7F). As DC–β-cateninactive mice express mutated nondegradable β-catenin specifically in their DCs, β-catenin is blocked by XAV939 in all other cells except DCs. No difference in tumor growth was observed between vaccinated B16OVA-bearing DC–β-cateninactive mice with or without XAV939 treatment (Fig. 4F), suggesting that inhibition of β-catenin in DCs likely play a major role in improving vaccine efficacy.
Discussion
Here we have identified mTOR/IL-10 signaling pathway as a previously unidentified mechanism for β-catenin in DCs to inhibit cross-priming. DC-produced IL-10, a multifunctional antiinflammatory cytokine (34), has been implicated in CD8+ T-cell priming (35), although its role in cross-priming has not been directly examined. In this report, we were able to demonstrate that blocking IL-10 restored cross-priming by β-cateninactive DCs in vivo and in vitro. We further demonstrated that tumors enhanced IL-10 production in DCs through β-catenin, and IL-10 mediated tumor-induced inhibition of cross-priming. Taken together, our data indicate that activation of β-catenin in DCs either genetically or induced by tumors inhibits cross-priming by up-regulating IL-10. mTOR, a serine/threonine kinase that exerts its effects through two complexes, mTOR complex 1 (mTORC1) and mTORC2, plays a critical role in DC development and function (36–38). Rapamycin-sensitive mTORC1 has emerged as a key regulator of IL-10 in DCs (18, 19, 36). Our data indicate that mTORC1 mediates β-catenin–dependent up-regulation of IL-10 to inhibit cross-priming, as inhibition of mTORC1 by rapamycin reduced IL-10 production and restored cross-priming by β-cateninactive DCs. These findings are consistent with a recent study showing that vaccination with rapamycin-treated BMDCs led to increased antitumor immunity (39).
Currently, studies on β-catenin have supported its role in promoting tolerance function of DCs (2–5, 40–43). Given that deletion of β-catenin in DCs abrogated tumor-induced inhibition of cross-priming (5), we were surprised that DC–β-catenin−/− mice generated reduced antitumor immunity upon vaccination. By blocking β-catenin genetically or with pharmacological inhibitor XAV939, we were able to demonstrate that β-catenin in DCs was required for CD8+ T-cell maintenance after clonal expansion. Thus, our study has uncovered, to our knowledge for the first time, a positive role of β-catenin in regulating DC-mediated CD8+ T-cell responses. Interestingly, blocking β-catenin in DCs significantly reduced their IL-10 production under vaccination condition, and anti–IL-10 treatment after clonal expansion similarly led to reduced CD8+ T cells, suggesting that β-catenin in DCs exerts its positive function in CD8+ T-cell maintenance also through IL-10. Supporting the positive role of β-catenin–regulated IL-10 in DCs, we have shown that IL-10R-mediated signaling in CD8+ T cells plays a positive role in the maintenance of primed CD8+ T cells. Intriguingly, a recent study has shown that although IL-10−/− mice exhibited threefold increase in IFN-γ+ CD8+ T cells compared with WT mice at day 9 after an acute virus infection, similar memory CD8+ T-cell responses were observed at day 45 (30), suggesting that IL-10 might play a similarly positive role in the maintenance of virus-specific CD8+ T cells (from day 9–45). Taken together, our data suggest that β-catenin in DCs exerts both positive and negative functions through IL-10 in regulating CD8+ T-cell responses. Whether IL-10 plays a positive or negative role in CD8+ T-cell immunity has remained controversial (23–32). Especially for antitumor immunity, IL-10 has been shown to both inhibit and promote antitumor CD8+ T-cell immunity (24, 25, 31). Thus, our findings might provide a potential explanation for the opposite roles of IL-10 in regulating CD8+ T-cell responses.
Whether β-catenin in DCs adversely affects antitumor efficacy of human DC vaccines has not been investigated. Interestingly, in a clinical trial of a DC cancer vaccine against melanoma in combination with regulatory T-cell depletion with unexpected reduced efficacy, DCs were shown to exhibit increased expression of β-catenin and a tolerogenic phenotype (44), suggesting that activation of β-catenin in human DCs might similarly suppress their function and therefore blocking β-catenin could potentially provide therapeutic benefit. Our findings that β-catenin in DCs exerts opposite functions during different phases of CD8+ T-cell responses, however, suggest that β-catenin must be specifically manipulated to augment CD8+ T-cell immunity. Indeed, we have demonstrated that selectively blocking β-catenin pharmacologically during priming phase augments vaccine-induced CD8+ T-cell immunity and improves antitumor efficacy. Given that β-catenin signaling functions similarly in human CD34+-derived DCs (3) that are currently used in clinical trials (6), our findings support the application of β-catenin inhibitors in combination with DC vaccines to improve their efficacy.
Materials and Methods
Mice and Treatment.
DC–β-cateninactive (CD11c-Cre+β-cateninExon3/Exon3) and DC–β-catenin−/− (CD11c-Cre+β-catenindel/del) mice were generated and maintained as described (5). C57BL/6 and IL-10R2−/− mice were from Jackson Laboratory, and CD45.1 mice were from Taconic Biosciences. CD8α−/− mice were maintained at the Blood Center of Wisconsin. For the generation of mixed bone marrow chimeras, lethally irradiated CD45.1 B6 mice were reconstituted with 4–5 × 106 bone marrow cells containing CD8α−/− bone marrow cells (CD45.1 or CD45.1/CD45.2) mixed with either WT or IL-10R2−/− bone marrow (CD45.2) at a 80:20 ratio. Immunization (vaccination) with anti–DEC-205-OVA plus CpG, and adoptive transfer of naïve OTI Thy1.1+ CD8+ T cells were carried out as described previously (5). Primary and recall responses were examined as described (5). Anti–IL-10 (200 μg per mouse), XAV939 (30 μg per mouse) and DMSO were injected intraperitoneally in PBS, at day −1, 0, 1, 3, and 5 after immunization. For maintenance experiments, immunized mice were treated with anti–IL-10 every other day after 5 d postimmunization. All procedures on animals followed protocols approved by the Institutional Animal Care and Use Committee at Roswell Park Cancer Institute and Blood Center of Wisconsin.
Cross-Priming Assays and DC–T-Cell Cocultures.
In vivo cross-priming assays were described previously (5). Purified CD11c+ DCs were pulsed with anti–DEC-205-OVA plus CpG, and were cultured with naïve Thy1.1+ OTI cells or total CD8+ T cells. Thy1.1+ OTI cells were stimulated and assayed for cross-priming as described (5). In some experiments, anti–IL-10 (10 μg/mL) was added to the cocultures and during in vitro restimulation; rapamycin (10 ng/mL) was added to DCs 20 min before CpG treatment. Recombinant IL-10 (1 ng/mL) was added to cocultures with total CD8+ T cells at day 6, and the cocultures were stained with H-2Kb SIINFEKL tetramer according to the manufacturer's protocols (MBL International).
ELISA.
Purified CD11c+ DCs were stimulated with CpG (50–100 ng/mL) for 36 h, and cytokine were measured by ELISA according to the manufacturer's protocols.
Tumor Cell Lines and Treatment of Tumor-Bearing Mice.
B16OVA melanoma cells were inoculated by s.c. injection and tumor sizes were calculated as (0.5 × short length × long length2) (5). For inhibitor treatment, increased tumor volume was calculated as tumor volume on the day of measurement minus the tumor volume at the time of immunization.
Statistical Analysis.
The statistical significance of experimental results was evaluated with Excel or GraphPad Prism 6 using two-tailed unpaired two-sample Student’s t test.
Supplementary Material
Acknowledgments
This work was supported by a grant from the American Asthma Foundation and was supported by an award from the Roswell Park Alliance Foundation. B.E.C. was a fellow of the Landsteiner Foundation of Blood Transfusion Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414167112/-/DCSupplemental.
References
- 1.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Van den Bossche J, Malissen B, Mantovani A, De Baetselier P, Van Ginderachter JA. Regulation and function of the E-cadherin/catenin complex in cells of the monocyte-macrophage lineage and DCs. Blood. 2012;119(7):1623–1633. doi: 10.1182/blood-2011-10-384289. [DOI] [PubMed] [Google Scholar]
- 3.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27(4):610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329(5993):849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang X, et al. β-catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8⁺ T cells. J Leukoc Biol. 2014;95(1):179–190. doi: 10.1189/jlb.0613330. [DOI] [PubMed] [Google Scholar]
- 6.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39(1):38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. 2010;127(2):77–84. doi: 10.1016/j.imlet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 11.Wagner CS, Grotzke JE, Cresswell P. Intracellular events regulating cross-presentation. Front Immunol. 2012;3:138. doi: 10.3389/fimmu.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J Immunol. 2007;178(11):6752–6760. doi: 10.4049/jimmunol.178.11.6752. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity. 2009;30(6):845–859. doi: 10.1016/j.immuni.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234(1):18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 15.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27(15):2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodland DL, Dutton RW. Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol. 2003;15(3):336–342. doi: 10.1016/s0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhao F, et al. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res. 2009;15(13):4382–4390. doi: 10.1158/1078-0432.CCR-09-0399. [DOI] [PubMed] [Google Scholar]
- 18.Haidinger M, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185(7):3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani M, et al. Cutting edge: mTORC1 in intestinal CD11c+ CD11b+ dendritic cells regulates intestinal homeostasis by promoting IL-10 production. J Immunol. 2012;188(10):4736–4740. doi: 10.4049/jimmunol.1200069. [DOI] [PubMed] [Google Scholar]
- 20.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23(6):707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonifaz LC, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199(6):815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal S, Temsamani J, Galbraith W, Tang J. Pharmacokinetics of antisense oligonucleotides. Clin Pharmacokinet. 1995;28(1):7–16. doi: 10.2165/00003088-199528010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160(7):3188–3193. [PubMed] [Google Scholar]
- 24.Fujii S, Shimizu K, Shimizu T, Lotze MT. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood. 2001;98(7):2143–2151. doi: 10.1182/blood.v98.7.2143. [DOI] [PubMed] [Google Scholar]
- 25.Dercamp C, Chemin K, Caux C, Trinchieri G, Vicari AP. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumor CD8 T-cell responses. Cancer Res. 2005;65(18):8479–8486. doi: 10.1158/0008-5472.CAN-05-1319. [DOI] [PubMed] [Google Scholar]
- 26.Kang SS, Allen PM. Priming in the presence of IL-10 results in direct enhancement of CD8+ T cell primary responses and inhibition of secondary responses. J Immunol. 2005;174(9):5382–5389. doi: 10.4049/jimmunol.174.9.5382. [DOI] [PubMed] [Google Scholar]
- 27.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J Immunol. 2006;177(4):2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 28.Biswas PS, et al. Pathogen-specific CD8 T cell responses are directly inhibited by IL-10. J Immunol. 2007;179(7):4520–4528. doi: 10.4049/jimmunol.179.7.4520. [DOI] [PubMed] [Google Scholar]
- 29.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205(3):533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci USA. 2010;107(7):3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mumm JB, et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011;20(6):781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35(5):792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 34.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 35.Banchereau J, et al. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood. 2012;119(24):5742–5749. doi: 10.1182/blood-2011-08-371245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Chi H. mTOR signaling and dendritic cell biology. J Immunol Clin Res. 2014;2(1):1015. [Google Scholar]
- 38.Wang Y, et al. Tuberous sclerosis 1 (Tsc1)-dependent metabolic checkpoint controls development of dendritic cells. Proc Natl Acad Sci USA. 2013;110(50):E4894–E4903. doi: 10.1073/pnas.1308905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amiel E, et al. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol. 2012;189(5):2151–2158. doi: 10.4049/jimmunol.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vander Lugt B, et al. TGF-β suppresses β-catenin-dependent tolerogenic activation program in dendritic cells. PLoS ONE. 2011;6(5):e20099. doi: 10.1371/journal.pone.0020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orr SJ, et al. LAB/NTAL facilitates fungal/PAMP-induced IL-12 and IFN-γ production by repressing β-catenin activation in dendritic cells. PLoS Pathog. 2013;9(5):e1003357. doi: 10.1371/journal.ppat.1003357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian C, et al. Fas signal promotes the immunosuppressive function of regulatory dendritic cells via the ERK/β-catenin pathway. J Biol Chem. 2013;288(39):27825–27835. doi: 10.1074/jbc.M112.425751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shan M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342(6157):447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baur AS, et al. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood. 2013;122(13):2185–2194. doi: 10.1182/blood-2012-09-456988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.