Abstract
Purpose of review
The field of VCA to achieve its full potential will require induction of tolerance. This review will introduce a new method of potential inducing tolerance in hand transplantation.
Recent Findings
Hand transplantation is never a life-extending transplant. This fact resulted in considerable debate both for and against the use of immunosuppression for non-life-extending transplants. There is considerable debate about the ethics of hand transplantation (1–8). There is now consensus that non-life-extending transplants are acceptable in properly selected patients. However, ideally hand transplants should not receive life-long immunosuppression. Therefore, attempts to achieve drug free tolerance through non-life endangering therapies are warranted. To this end we propose implementation of tolerizing therapy long after peri-inflammation has subsided and drug minimization has proven successful. Evidence that short term treatment with low doses of IL-2 or a long lived IL-2.Ig can tilt the balance of immunity from tissue destructive to tolerance come from pre-clinical demonstrations in mouse and nonhuman primate models of autoimmunity and/or transplantation and even more recent clinical trials (9–20).
Summary
We believe that with the proper use of low dose IL-2 given at an opportune time in the inflammatory process of transplant that reduce immunosuppression and even tolerance can be induced in hand transplantation. We propose that tolerance can be inducted after a long-period of conventional treatment to avoid “tolerance-hindering” adverse inflammation that occurs in the post transplant period. With abatement of post transplant inflammation and with time, we will institute low dose IL-2 based therapy to support the proliferation, viability and functional phenotype of regulatory T cells.
Keywords: Composite tissue allografts, Ischemia-reperfusion, Inflammation, Tolerance induction
Introduction
As skin transplants evoke very powerful rejection in preclinical transplant models, it came as a surprise to many, including several co-authors of this review, that composite tissue allografts, e.g., limb and face allografts, can be engrafted using immunosuppressive protocols that were developed for use in recipients of conventional kidney, liver, heart and pancreas transplants. Rejection, while frequent, is not nearly as formidable a barrier for success as many had predicted. The development of the multi-disciplined infrastructure required to perform the surgery and post-transplant care has been the greater challenge to success (21, 22).
A few “wounded warriors”, victims of severe combat injuries, are recipient-pioneers of hand transplants (23). Should young, otherwise healthy individuals be subject to life-long immunosuppression to save a limb, particularly, a hand allograft? It is reasonable, therefore, to attempt to foster a state of donor specific transplant tolerance as a means to avoid the inevitable toxicity of life- long immunosuppression. Taking into consideration that limb transplants, while technically amazing and providing profound rehabilitation, do not save lives, we must ask which potentially tolerizing protocols are the best fit for limb transplant recipients? The following is an attempt to address these all-important issues.
Ethical Considerations For Tolerance Induction In Hand Transplant Recipients
Life-long maintenance immunotherapy cannot be easily justified as a means to preserve the engraftment of hand transplants since hand transplants are not “life- saving” and prosthetic hands provide acceptable, albeit not perfect, function. Successful application of tolerizing strategies would lessen the risk for opportunistic infectious disease and cancer, avoid off target non-immune system drug toxicity (e.g., kidney toxicity by calcineurin inhibitors) and prevent chronic rejection, thereby improving graft and patient survival.
Hence, many in the field regard tolerance induction as not merely an interesting exercise but instead crucial to the ability to avoid life-long immunosuppression and thus a necessity for wider application of hand transplantation. Efforts to achieve tolerance must be safe without undue risk for toxicity or loss of graft function although it seems unlikely that not every hand transplant recipient will be rendered tolerant using “safe” therapeutic tools currently in hand. It is notable that some transplant patients, such as select liver transplant recipients, can undergo drug withdrawal and yet maintain transplant function indefinitely (24–34).
For example, drug withdrawal can be successful with little risk of graft loss. Hover monitoring for liver fibrosis is necessary. Withdrawal is not always tolerated. It is possible that properly supervised hand transplant recipients can also be withdrawn from immunosuppression, if treated with low dose IL-2 at the proper time, and by monitoring of skin rejection.
Should treatment to induce tolerance fail short- or long-term in hand transplant recipients, the diagnosis of early hand transplant rejection can be made far more readily than in recipients of conventional kidney, heart, lung or liver transplants and the opportunity for prompt restoration of immunosuppressive drug therapy is obvious. The diagnosis of rejection of conventional kidney, liver and other transplants is made in the course of a work up for graft injury/dysfunction or through random surveillance biopsies. Thus, rejection is not readily detected at an early stage. Early rejection of hand transplants involves the skin and rejection related changes are obvious upon physical examination (Figure 1). However, monitoring of deep tissues such as arteries will also be mandatory. Skin is not always a perfect marker of rejection (35).
Figure 1. Rejection of the hand transplant at 16 months post bilateral hand allograft transplantation.
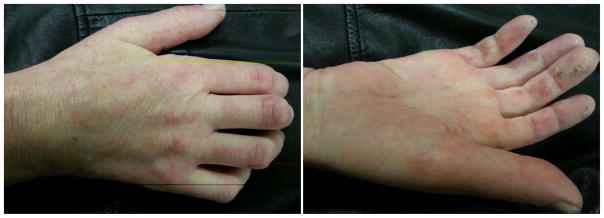
Patient was treated with topic tacrolimus, as well as increases in maintenance immunosuppression. Rejection resolved after 4–6 weeks.
The diagnosis of rejection can often be confirmed or refuted through pathologic analysis of small skin biopsies. As a consequence, anti-rejection therapy can be quickly re-instituted enabling salvage of the hand transplant. Interestingly, hand transplant patients often experience many more rejection episodes than recipients of conventional transplants yet graft loss is uncommon in hand transplant as compared to conventional transplant recipients. This issue is discussed in companion articles in this issue.
Potential tolerizing therapies
Transplant tolerance can be induced through one of two broad mechanisms, either total deletion of anti-donor effector T cells or donor specific dominant immunoregulation. Perhaps the most durable form of transplant tolerance is created through selective total and durable deletion of recipient anti-donor T cell clones. In many mouse models in which total deletion of anti-donor T cell clones can be obtained and in human recipients of successful hematopoietic cell allograft who have been treated for hematopoietic malignancies, the recipients can later be successfully transplanted with organ allografts from the hematopoietic stem cell donor in the absence of maintenance immunosuppressive therapy. To date, clinical protocols that employ donor hematopoietic stem cells to achieve stable multi-lineage mixed hematopoietic cell chimerism have shown promise for the induction of kidney allograft tolerance. (36); (37))
The appropriateness of this approach for hand transplants is diminished by the risk for graft versus host disease. The use of donor hematopoietic stem cells as the facet of treatment inherently carries this risk in the immunosuppressed hand transplant recipient, a complication that may not be consistent with the mandate for safe treatment in recipients of a transplant that is not life-saving or life-sustaining. We acknowledge that this is an opinion, not a fact. A secondary consideration inherent in tolerance produced by hematopoietic stem cell protocols is the difficulty of achieving tolerance in HLA mismatched individuals.
Another means of producing transplant tolerance is to produce dominant donor- directed immunoregulation, a state in which overall recipient anti-donor immunity is marked by the functional superiority of donor reactive regulatory T cells over donor reactive effector T cells. Donor reactive regulatory T cells hold donor reactive effector T cells in check and thereby the immune system protects not attacks the transplant. Paradoxically this state might be responsible for the ability to withdraw immunosuppression in recipients who have been given donor hematopoietic stem cells but transient and modest rather than durable and robust mixed chimerism has been established. (36)) in preclinical models other therapeutic strategies such co-stimulation blockade have been used to achieve this end and evolution of this state (38)) with convention treatment is almost certainly responsible for the ability to successfully withdraw immunosuppressive from select transplant recipients long-term.
Barriers To Immune Tolerance In Patients With Organ/Tissue Transplants
Memory B and T Cells
Donor reactive memory-type immunity can result from immunization with microbes, a previous transplant or transfusion when the new transplant shares antigens or antigenic epitopes with these previous antigenic exposures. Following transplantation the ability to detect early rejection of conventional (kidney, liver, heart, pancreas or lung) transplants is compromised by the lack of early clinical signs of rejection. As a consequence, memory type anti-donor immunity is well established by the time that a clinical diagnosis of rejection is established and treated. While treatment may suppress the action of donor reactive T cells, they can be reactivated if an inflammatory state develops. There are only a few studies in which surveillance biopsies of kidney transplants have been undertaken. These studies demonstrate that it is not uncommon for transplant biopsies to reveal typical histopathologic evidence of “subclinical” rejection despite an absence of conventional clinical evidence or even suspicion of ongoing rejection. In recipients with “subclinical rejection”, long-term outcomes are compromised. Unlike conventional transplants, the hand transplant can be visually inspected and early signs of rejection are clearly evident and the diagnosis can often be confirmed or refuted by biopsy. Surprisingly, the incidence of diagnosis is frequent yet long term outcomes are excellent (39). Frequent rejections in recipients of conventional transplant are ominous and associated with poor outcomes. As surveillance biopsies reveal that “subclinical rejection” episodes are missed in conventional transplants and that this situation leads to poor outcomes, it seems reasonable to speculate that the ease of diagnosis of rejection in hand transplants leads to a better response to treatment long term.
Early diagnosis also should reduce the number of donor reactive memory type immune cells that accrue in the recipient. As memory cells are far less responsive to treatment or the tolerizing effects of regulatory T cells than naïve immune cells, a reduction in the burden of anti-donor directed memory cells leads to excellent long-term outcomes and almost certainly enhances the opportunities for successful minimization and withdrawal of immunosuppressive drug therapy.
The peri-transplant inflammatory response
Implications for tolerance induction
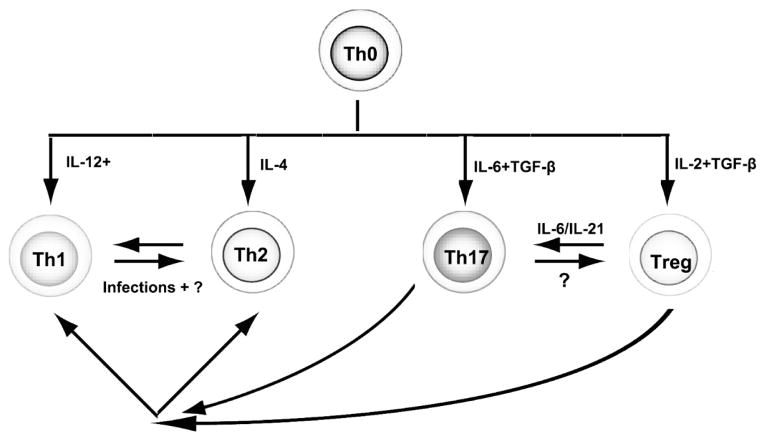
Ischemia-reperfusion injury is an inherent component of the recipient response to organ or tissue transplants and is evident even in syngeneic transplants. Of course this situation is particularly problematic in deceased donor transplants. It seems certain that ischemia-perfusion injury will be a problem for tolerance induction in limb transplants (40). As a consequence of ischemia-reperfusion injury expression of pro-inflammatory cytokines and chemokines, complement activation, a pro-thrombotic state and very rapid influx of innate immune cells ensues. This situation exerts an adverse ripple effect upon the nature of recipient anti-donor immunity. It is now abundantly clear that the phenotype assumed by adaptive immunity is strongly influenced by the state of innate immunity. (41); (42) Naïve T cells that recognize antigen within a microenvironment devoid of pro- inflammatory cytokines are prone to become regulatory T cells through the unopposed action of TGF-β, a constitutively expressed cytokine. (43); (44). With ischemia- reperfusion injury expression of IL-6 or IL-21 and other pro-inflammatory cytokines is induced. The presence of IL-6 or IL-21 negate the ability of TGF-β promote polarization of naïve T cells to the regulatory T cell phenotype and also promote polarization of antigen activated naïve CD4+T cells to the highly inflammatory and cytodestructive Th17 phenotype. (45) (46) (47) The negation of induction to regulatory T cells is likely more detrimental than polarization to the Th17 phenotype for induction of transplant tolerance. Other pro-inflammatory molecules impact upon the nature of T cell polarization occurring with antigen stimulation (Figure 2). IL-12, a monokine, and IL-4 promote polarization of naïve CD4+T cells following antigen activation to the Th1 and Th2 phenotypes respectively. The development of drug-free immune tolerance in the absence of complete eradication of antigen specific clones is dependent upon an enduring functional supremacy of antigen specific regulatory T cells over conventional antigen reactive immune cells. Hence, the microenvironment created by ischemia- reperfusion injury and inflammatory damage to the transplant in the peri-transplant period is not at all conducive to the creation of transplant tolerance because of the adverse influence of this environment upon polarization of T cells to the regulatory T cell phenotype.
Figure 2. The differentiation and conversion of CD4+ T cells is determined by the cytokines present in the environment.
Naive CD4+ T cells have plasticity to differentiate into Th1, Th2, Th17, and Treg (regulatory T cells). Upon antigen activation, naïve CD4 T cells respond to cytokines produced by innate immune cells, such as IL-12 and IFN-γ, which are important for Th1 cell differentiation, and IL-4, which is crucial for Th2 cell differentiation. TGF-β together with IL-6 induces Th17 cell differentiation, whereas Treg differentiation is induced by TGF-β and IL-2. It is important to note that these T differentiated cell phenotypes also have plasticity. When IL-6/IL-21 is present, such as in the episode of autoimmune inflammation, Treg phenotype can change to Th17, therefore losing the immunoregulatory functions. Recently, it was also found that Treg can become Th1 and Th2 in certain conditions. Th17, on the other hand, can become Th1 and Th2 respective in the milieu of IL-12 and IL-4.
The duration of a tolerance unfriendly post-transplant inflammatory state in a living donor non-human primate model as evidenced by expression of pro- inflammatory cytokines, chemokines and resistance to tolerance induction lasts for months (48). Yet, time-relate resolution of the tolerance-unfriendly pro- inflammatory state achieved through delayed application of tolerizing protocols affords a far better opportunity to establish more enduring mixed chimerism and tolerance than application in the immediate post-transplant period (48). Similarly liver transplants have been long known to be far more amenable to tolerance induction than other conventional organ transplants and it is possible to discontinue immunosuppression in a substantial number of patients who have safely undergone dose minimization of their immunosuppressive drugs (49, 50). More recently it has become apparent that the ability to successfully withdraw immunosuppressive drugs in liver transplant recipients is related to the duration of engraftment at which drug withdrawal is attempted (50). In order to induce transplant tolerance in patients lacking detrimental inflammation, a strategy that we will embrace in our hand transplant program, we will utilize standard therapy initially and wait until detrimental, tolerance-negating inflammation resolves before trying to induce tolerance. Recall that the presence of IL-6 in microenvironment in which naïve T cells recognize antigen negates the ability to generate regulatory T cells. Moreover, expression of IL-6 and other pro- inflammatory cytokines, often expressed during early acute rejection episodes, disrupts the phenotype of established regulatory T cells and turns these former regulatory cells into aggressive effector T cells. Waiting to create tolerance at a time point beyond the early period in which hand transplants are rejection prone may also prove beneficial. Again, this will be our core strategy to maximize the possibilities to generate antigen specific regulatory T and hence donor-specific tolerance. Our patients will be carefully monitored for expression of pro- inflammatory cytokines during and after the withdrawal of conventional immunosuppressive therapy. Should robust inflammation accompany drug withdrawal we will consider adding therapy to selectively neutralize these cytokines. Corticosteroids, agents that broadly block inflammation, will not be used for lack of specificity in this regard.
What else can be done to maximize the potential for generating and maintaining the enduring supremacy of donor reactive regulatory T cells over donor reactive effector T cells as a means to achieve durable transplant tolerance? In our effort we will use short-term low dose IL-2 therapy to bridge the period between the total withdrawal of conventional immunosuppression and absolute freedom from drugs. Our strategy is informed by the knowledge that the meta-stable phenotype of regulatory T cells and their survival is IL-2 dependent. IL-2 treatment should, if properly administered, strengthen the molecular and functional phenotype and durability of regulatory T cells and hence help tilt the balance of immunity more firmly toward tolerance.
Nonetheless, IL-2 therapy will cause unnecessary pro-inflammatory side effects and as a consequence fail to tilt immunity toward tolerance unless such therapy is nuanced and carefully conceived (11, 51, 52). Low-, not high-, doses of IL-2 are required to achieve selective targeting of regulatory T cells and thereby achieve the best outcomes. Regulatory T cells express the tri-molecular, high affinity IL-2 receptor. The high-affinity IL-2 receptor consists of the JAK/STAT signaling common chain, a trans-membrane protein that is an all-important component of receptors for each of the T cell growth factors including IL-2, IL-4, IL-7, IL-15 and IL-21 (11, 51). The IL-2 receptor as well as the tri-molecular IL-15 receptor includes a beta chain (11, 51, 53). Recalling that regulatory T cells are CD25 high, the 3rd component of the high affinity IL-2 receptor also includes the IL-2 receptor specific chain also known as CD25. The tri-molecular, high affinity IL-2 receptor responds to IL-2 concentrations as low as 10−11 to 10−12 M. Newly activated T cells transiently express trimolecular high affinity IL-2 receptors and IL-2 serves as an apparent death factor for these cells (54). Significantly higher concentrations of IL-2, i.e., 10−9 M, stimulate a variety of innate immune cells and memory T cells. These features result in highly undesirable side effects resulting from an out-pouring of inflammatory cytokines and tissue infiltration by activated innate immune cells. Indeed high doses of IL-2 probably act to inhibit tolerance induction.
Recent findings
Evidence that short term treatment with low doses of IL-2 or a long lived IL-2.Ig can tilt the balance of immunity from tissue destructive to tolerance come from pre-clinical demonstrations in the NOD mouse model of type 1 diabetes (55), islet transplantation in the daunting model in which NOD mice are transplanted with allogeneic islets (56) and in the cynomolgus islet allograft model (52). More recently low dose IL-2 therapy has achieved remarkably favorable effects in humans with drug resistant graft versus host disease and hepatitis virus induced vasculitis (57, 58).
Conclusion
In this review, we have described the current status of composite tissue allografts in a donor specific transplant tolerance. Special circumstances inherent in hand transplantation favor attempts to induce tolerance. Considering the limited success of conventional treatments, low dose IL-2 based therapy is likely a viable option.
Key points.
Hand transplant recipients should not receive life-long immunosuppressive therapy as the transplants are not life-sustaining.
Tolerance induction not maintenance immunosuppressive therapy is the goal in this patient population.
To this end and recognizing the adverse influence of post transplant inflammation on tolerance we will employ delayed induction of tolerizing protocols.
Low not high dose IL-2 will serve as the linchpin of our tolerizing regimen.
Acknowledgments
The authors would like to acknowledge that Dr. Ericka Bueno have provided valuable inputs into this review.
Furthermore we would like to acknowledge funding support from the National Institutes of Health (MK and TBS receive support from NIH grant U01 AI102427-02) and the U.S. Department of Defense (BP receives support from Department of Defense Biomedical Translational Initiative research contract #W911QY-09-C-0216 and W81XWH-12-2-0037).
Footnotes
Conflicts of interest
All authors have no conflicts of interest in the manuscript, including financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
References and recommended reading
* of special interest
** of outstanding interest
- 1.Lubbe AS. Successful hand transplantation or too early to tell? Transplantation. 2003;75(11):1916–7. doi: 10.1097/01.TP.0000065290.60470.9E. Epub 2003/06/18. [DOI] [PubMed] [Google Scholar]
- 2.Rohrich RJ, Longaker MT, Cunningham B. On the ethics of composite tissue allotransplantation (facial transplantation) Plastic and reconstructive surgery. 2006;117(6):2071–3. doi: 10.1097/01.prs.0000201309.72162.38. Epub 2006/05/03. [DOI] [PubMed] [Google Scholar]
- 3.Siegler M. Ethical issues in innovative surgery: should we attempt a cadaveric hand transplantation in a human subject? Transplantation proceedings. 1998;30(6):2779–82. doi: 10.1016/s0041-1345(98)00807-0. Epub 1998/09/24. [DOI] [PubMed] [Google Scholar]
- 4.Tobin GR, Breidenbach WC, Klapheke MM, Bentley FR, Pidwell DJ, Simmons PD. Ethical considerations in the early composite tissue allograft experience: a review of the Louisville Ethics Program. Transplantation proceedings. 2005;37(2):1392–5. doi: 10.1016/j.transproceed.2004.12.179. Epub 2005/04/26. [DOI] [PubMed] [Google Scholar]
- 5.Chang J, Mathes DW. Ethical, financial, and policy considerations in hand transplantation. Hand clinics. 2011;27(4):553–60. xi. doi: 10.1016/j.hcl.2011.07.006. Epub 2011/11/05. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez J, Latorre LF, Moreno C, DeBedout R. Hand transplantation: is it an ethical decision, a bioethical one, or both? Transplantation proceedings. 2011;43(9):3512–5. doi: 10.1016/j.transproceed.2011.09.046. Epub 2011/11/22. [DOI] [PubMed] [Google Scholar]
- 7.Jones NF. Concerns about human hand transplantation in the 21st century. The Journal of hand surgery. 2002;27(5):771–87. doi: 10.1053/jhsu.2002.34373. Epub 2002/09/20. [DOI] [PubMed] [Google Scholar]
- 8.Mathes DW, Schlenker R, Ploplys E, Vedder N. A survey of north american hand surgeons on their current attitudes toward hand transplantation. The Journal of hand surgery. 2009;34(5):808–14. doi: 10.1016/j.jhsa.2009.01.021. Epub 2009/05/05. [DOI] [PubMed] [Google Scholar]
- 9.Shibasaki S, Yamashita K, Goto R, Oura T, Wakayama K, Hirokata G, et al. NK026680 inhibits T-cell function in an IL-2-dependent manner and prolongs cardiac allograft survival in rats. Transplant immunology. 2012;26(1):42–9. doi: 10.1016/j.trim.2011.10.002. Epub 2011/10/25. [DOI] [PubMed] [Google Scholar]
- 10.Fujimura K, Oyamada A, Iwamoto Y, Yoshikai Y, Yamada H. CD4 T cell-intrinsic IL-2 signaling differentially affects Th1 and Th17 development. Journal of leukocyte biology. 2013;94(2):271–9. doi: 10.1189/jlb.1112581. Epub 2013/05/30. [DOI] [PubMed] [Google Scholar]
- 11**.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. Epub 2013/01/29 This paper reviews the biology of IL-2 and the high and intermediate affinity IL-2 receptor with particular reference to the interaction and tolerance promoting consequences of IL-2 with the high affinity IL-2 receptor expressed upon regulatory T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyman O, Krieg C, Letourneau S, Webster K, Surh CD, Sprent J. Selectively expanding subsets of T cells in mice by injection of interleukin-2/antibody complexes: implications for transplantation tolerance. Transplantation proceedings. 2012;44(4):1032–4. doi: 10.1016/j.transproceed.2012.01.093. Epub 2012/05/09. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi T, Kishi A, Osaki M, Morikawa H, Prieto-Martin P, Wing K, et al. Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(23):E2116–25. doi: 10.1073/pnas.1307185110. Epub 2013/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, Tey SK, Koyama M, Kuns RD, Olver SD, Lineburg KE, et al. Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. J Immunol. 2013;191(10):5291–303. doi: 10.4049/jimmunol.1301181. Epub 2013/10/15. [DOI] [PubMed] [Google Scholar]
- 15.Satake A, Schmidt AM, Archambault A, Leichner TM, Wu GF, Kambayashi T. Differential targeting of IL-2 and T cell receptor signaling pathways selectively expands regulatory T cells while inhibiting conventional T cells. Journal of autoimmunity. 2013;44:13–20. doi: 10.1016/j.jaut.2013.06.009. Epub 2013/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham S, Pahwa R, Ye C, Choi JG, Pahwa S, Jaggaiahgari S, et al. Long-term engraftment of human natural T regulatory cells in NOD/SCID IL2rgammac(null) mice by expression of human IL-2. PloS one. 2012;7(12):e51832. doi: 10.1371/journal.pone.0051832. Epub 2012/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amado IF, Berges J, Luther RJ, Mailhe MP, Garcia S, Bandeira A, et al. IL-2 coordinates IL-2-producing and regulatory T cell interplay. The Journal of experimental medicine. 2013;210(12):2707–20. doi: 10.1084/jem.20122759. Epub 2013/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhunjhunwala S, Balmert SC, Raimondi G, Dons E, Nichols EE, Thomson AW, et al. Controlled release formulations of IL-2, TGF-beta1 and rapamycin for the induction of regulatory T cells. Journal of controlled release : official journal of the Controlled Release Society. 2012;159(1):78–84. doi: 10.1016/j.jconrel.2012.01.013. Epub 2012/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009;206(4):751–60. doi: 10.1084/jem.20082824. Epub 2009/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia G, He J, Leventhal JR. Ex vivo-expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(2):298–306. doi: 10.1111/j.1600-6143.2007.02088.x. Epub 2008/01/15. [DOI] [PubMed] [Google Scholar]
- 21*.Dahlborg EJ, Diaz-Siso JR, Bueno EM, Sisk GC, Pomahac B. The value of innovation: face and hand transplantation programs at Brigham and Women’s Hospital. Plastic and reconstructive surgery. 2014;134(1):178e–9e. doi: 10.1097/PRS.0000000000000464. Epub 2014/07/17. Development of a successful hand and face transplant program requires innovation since an accurate roadmap for success has not yet been drafted. [DOI] [PubMed] [Google Scholar]
- 22.Dubernard JM, Owen E, Herzberg G, Lanzetta M, Martin X, Kapila H, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353(9161):1315–20. doi: 10.1016/S0140-6736(99)02062-0. Epub 1999/04/28. [DOI] [PubMed] [Google Scholar]
- 23.Schneeberger S, Gorantla VS, Brandacher G, Zeevi A, Demetris AJ, Lunz JG, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Annals of surgery. 2013;257(2):345–51. doi: 10.1097/SLA.0b013e31826d90bb. Epub 2012/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE. Immunosuppressive therapy and tolerance of organ allografts. The New England journal of medicine. 2008;358(4):407–11. doi: 10.1056/NEJMe0707578. Epub 2008/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Murase N, Demetris AJ, Trucco M, Abu-Elmagd K, Gray EA, et al. Lessons of organ-induced tolerance learned from historical clinical experience. Transplantation. 2004;77(6):926–9. doi: 10.1097/01.tp.0000117780.74133.74. Epub 2004/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donckier V, Craciun L, Lucidi V, Buggenhout A, Troisi R, Rogiers X, et al. Acute liver transplant rejection upon immunosuppression withdrawal in a tolerance induction trial: potential role of IFN-gamma-secreting CD8+ T cells. Transplantation. 2009;87(9 Suppl):S91–5. doi: 10.1097/TP.0b013e3181a2dee6. Epub 2009/05/14. [DOI] [PubMed] [Google Scholar]
- 27.Tisone G, Orlando G, Cardillo A, Palmieri G, Manzia TM, Baiocchi L, et al. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. Journal of hepatology. 2006;44(4):702–9. doi: 10.1016/j.jhep.2005.11.047. Epub 2006/02/14. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Fueyo A. Hot-topic debate on tolerance: immunosuppression withdrawal. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17 (Suppl 3):S69–73. doi: 10.1002/lt.22421. Epub 2011/08/19. [DOI] [PubMed] [Google Scholar]
- 29.Londono MC, Rimola A, O’Grady J, Sanchez-Fueyo A. Immunosuppression minimization vs. complete drug withdrawal in liver transplantation. Journal of hepatology. 2013;59(4):872–9. doi: 10.1016/j.jhep.2013.04.003. Epub 2013/04/13. [DOI] [PubMed] [Google Scholar]
- 30.Pons JA, Ramirez P, Revilla-Nuin B, Pascual D, Baroja-Mazo A, Robles R, et al. Immunosuppression withdrawal improves long-term metabolic parameters, cardiovascular risk factors and renal function in liver transplant patients. Clinical transplantation. 2009;23(3):329–36. doi: 10.1111/j.1399-0012.2008.00944.x. Epub 2009/02/13. [DOI] [PubMed] [Google Scholar]
- 31.Tryphonopoulos P, Ruiz P, Weppler D, Nishida S, Levi DM, Moon J, et al. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation. 2010;90(12):1556–61. doi: 10.1097/TP.0b013e3182003db7. Epub 2010/11/19. [DOI] [PubMed] [Google Scholar]
- 32.Girlanda R, Rela M, Williams R, O’Grady JG, Heaton ND. Long-term outcome of immunosuppression withdrawal after liver transplantation. Transplantation proceedings. 2005;37(4):1708–9. doi: 10.1016/j.transproceed.2005.03.070. Epub 2005/05/28. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Lee SK, Lee HJ, Seo JM, Joh JW, Kim SJ, et al. Withdrawal of immunosuppression in pediatric liver transplant recipients in Korea. Yonsei medical journal. 2009;50(6):784–8. doi: 10.3349/ymj.2009.50.6.784. Epub 2010/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Garza RG, Sarobe P, Merino J, Lasarte JJ, D’Avola D, Belsue V, et al. Trial of complete weaning from immunosuppression for liver transplant recipients: factors predictive of tolerance. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19(9):937–44. doi: 10.1002/lt.23686. Epub 2013/06/21. [DOI] [PubMed] [Google Scholar]
- 35.Hautz T, Brandacher G, Engelhardt TO, Pierer G, Lee WP, Pratschke J, et al. How reconstructive transplantation is different from organ transplantation--and how it is not. Transplantation proceedings. 2011;43(9):3504–11. doi: 10.1016/j.transproceed.2011.08.044. Epub 2011/11/22. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(7):1599–611. doi: 10.1111/ajt.12731. Epub 2014/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Sarwal M, Millan MT, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(5):1133–45. doi: 10.1111/j.1600-6143.2012.03992.x. Epub 2012/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilat N, Baranyi U, Klaus C, Jaeckel E, Mpofu N, Wrba F, et al. Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(4):751–62. doi: 10.1111/j.1600-6143.2010.03018.x. Epub 2010/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonastre J, Landin L, Diez J, Casado-Sanchez C, Casado-Perez C. Factors influencing acute rejection of human hand allografts: a systematic review. Annals of plastic surgery. 2012;68(6):624–9. doi: 10.1097/SAP.0b013e318255a411. Epub 2012/05/31. [DOI] [PubMed] [Google Scholar]
- 40.Caterson EJ, Lopez J, Medina M, Pomahac B, Tullius SG. Ischemia-reperfusion injury in vascularized composite allotransplantation. The Journal of craniofacial surgery. 2013;24(1):51–6. doi: 10.1097/SCS.0b013e31827104e1. Epub 2013/01/17. [DOI] [PubMed] [Google Scholar]
- 41.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nature reviews Immunology. 2012;12(6):459–71. doi: 10.1038/nri3215. Epub 2012/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asgari E, Farrar CA, Sacks SH. Control of innate immunological mechanisms as a route to drug minimization. Current opinion in organ transplantation. 2014;19(4):342–7. doi: 10.1097/MOT.0000000000000094. Epub 2014/06/07. [DOI] [PubMed] [Google Scholar]
- 43.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179(7):4685–93. doi: 10.4049/jimmunol.179.7.4685. Epub 2007/09/20. [DOI] [PubMed] [Google Scholar]
- 44.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 − precursors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(10):1614–27. doi: 10.1111/j.1600-6143.2004.00566.x. Epub 2004/09/16. [DOI] [PubMed] [Google Scholar]
- 45.Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–85. doi: 10.1182/blood-2009-07-233437. Epub 2010/04/16. [DOI] [PubMed] [Google Scholar]
- 46.Ma X, Robin C, Ottersbach K, Dzierzak E. The Ly-6A (Sca-1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem Cells. 2002;20(6):514–21. doi: 10.1634/stemcells.20-6-514. Epub 2002/11/29. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Liu C, Zhu F, Liu F, Zhang P, Guo C, et al. Reoxygenation of hypoxia-differentiated dentritic cells induces Th1 and Th17 cell differentiation. Molecular immunology. 2010;47(4):922–31. doi: 10.1016/j.molimm.2009.09.038. Epub 2009/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2):330–40. doi: 10.1111/j.1600-6143.2011.03795.x. Epub 2011/11/08. Induction of transplant tolerance is expedited through elimination of C8+ memory cells and delayed introduction, i.e., following resolution of peri-transplant inflammation, via a mixed chimerism approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Sanchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140(1):51–64. doi: 10.1053/j.gastro.2010.10.059. Epub 2010/11/16. This paper reviews the experience of successful delayed withdrawal of maintenance immunosuppressive therapy in select liver transplant patient. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Benitez C, Londono MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58(5):1824–35. doi: 10.1002/hep.26426. Epub 2013/03/28. This paper is an update and refinement for selection of liver transplant patients who can successfully be withdrawn from immunosuppressive therapy. [DOI] [PubMed] [Google Scholar]
- 51.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33(2):153–65. doi: 10.1016/j.immuni.2010.08.004. Epub 2010/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Koulmanda M, Sampathkumar RS, Bhasin M, Qipo A, Fan Z, Singh G, et al. Prevention of nonimmunologic loss of transplanted islets in monkeys. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(7):1543–51. doi: 10.1111/ajt.12723. Epub 2014/06/11. The tolerance promoting action of a short-term treatment regimen that includes a long-lived IL-2 molecule (IL-2.Ig) is studied in the difficult nonhuman primate islet allograft model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunological reviews. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. Epub 2004/11/18. [DOI] [PubMed] [Google Scholar]
- 54.Li XC, Li Y, Dodge I, Wells AD, Zheng XX, Turka LA, et al. Induction of allograft tolerance in the absence of Fas-mediated apoptosis. J Immunol. 1999;163(5):2500–7. Epub 1999/08/24. [PubMed] [Google Scholar]
- 55.Koulmanda M, Budo E, Bonner-Weir S, Qipo A, Putheti P, Degauque N, et al. Modification of adverse inflammation is required to cure new-onset type 1 diabetic hosts. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(32):13074–9. doi: 10.1073/pnas.0705863104. Epub 2007/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19(4):503–14. doi: 10.1016/s1074-7613(03)00259-0. Epub 2003/10/18. [DOI] [PubMed] [Google Scholar]
- 57**.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. The New England journal of medicine. 2011;365(22):2055–66. 56–57. doi: 10.1056/NEJMoa1108188. Epub 2011/12/02. A powerful therapeutic effect of IL-2 is demonstrated in patients with drug resistant GVHD and HCV-induced vasculitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. The New England journal of medicine. 2011;365(22):2067–77. doi: 10.1056/NEJMoa1105143. Epub 2011/12/02. [DOI] [PubMed] [Google Scholar]