Abstract
Lenalidomide is an agent that has shown great activity in patients with multiple myeloma. However, studies have suggested that this drug negatively affects subsequent stem cell collection. To investigate whether lenalidomide impairs stem cell mobilization and collection, we reviewed data for patients with multiple myeloma who underwent mobilization with filgrastim. Predictors of mobilization failure were evaluated using logistic regression analysis. In 26 (9%) of 302 myeloma patients, stem cell mobilization failed. Mobilization failed in 25% of patients who had previously received lenalidomide, compared with 4% of patients who had not received lenalidomide (P < .001). In a multivariate analysis, prior lenalidomide use (odds ratio, 5.9; 95% CI, 2.4 to 14.3) and mobilization more than 1 year after diagnosis (odds ratio, 4.6; 95% CI, 1.9 to 11.1) were significantly associated with failed mobilization. Twenty-one of 26 patients in whom mobilization with filgrastim failed underwent remobilization with chemotherapy and filgrastim; in 18 (86%) of these 21 patients, stem cells were successfully mobilized and collected. In patients with multiple myeloma, prior lenalidomide therapy is associated with failure of stem cell mobilization with filgrastim. Remobilization with chemotherapy and filgrastim is usually successful in these patients.
INTRODUCTION
Thalidomide, bortezomib, and lenalidomide are changing therapeutic paradigms in patients with multiple myeloma (1, 2). Used as salvage therapy, these agents have been highly effective in increasing response rates, event-free survival and overall survival when standard therapies have failed (3–13). Increasingly, these agents are being used as frontline therapies (14–18). As initial therapy, Thalidomide plus dexamethasone has proven to be superior to dexamethasone alone (16). How these agents integrate with autologous stem cell transplantation, a standard therapy that improves survival (19–22), is being studied extensively, especially with regard to their impact on stem cell collection, a necessary step in the transplantation process.
Stem cell mobilization, or release of hematopoietic stem cells from the bone marrow to the peripheral blood in response to cytokines or chemotherapy, is dependent on several factors. These include patient age, bone marrow reserve, mobilizing regimen, and prior therapy (23–25). Melphalan use before stem cell collection has been associated with poor stem cell yield and mobilization failure (26); therefore, it is not used as first line therapy in patients who are candidates for autologous transplantation. Recently, concern has been raised about lenalidomide’s tendency to similarly decrease stem cell yield and increase the mobilization failure rate (27–29). Lenalidomide is myelosuppressive and alters stromal milieu and may indeed suppress stem cell mobilization. To confirm the hypothesis that lenalidomide impairs stem cell mobilization, we studied the impact of prior lenalidomide use on stem cell mobilization and collection with filgrastim. We also looked at other factors besides prior lenalidomide that affected mobilization outcome and whether remobilization attempts were successful in cases in which the first mobilization attempt failed.
PATIENTS AND METHODS
Included in this retrospective study were all patients with multiple myeloma referred to the Department of Stem Cell Transplantation and Cellular Therapy at The University of Texas M. D. Anderson Cancer Center between January 2005 and October 2007 who underwent stem cell mobilization with filgrastim. Detailed information about demographics, disease isotype, stage, prior treatment, interval from diagnosis, premobilization blood counts, and bone marrow cellularity was obtained from the departmental database and chart review. Data were updated and analyzed in January 2008. Permission for this retrospective review was obtained from the Institutional Review Board.
Of the 354 patients with multiple myeloma mobilized during this period, 302 patients underwent mobilization with filgrastim and are included in this study. Excluded were 52 patients who underwent mobilization with alternative strategies: Chemotherapy and filgrastim (n = 40), plerixafor plus filgrastrim (n = 4), and pegfilgrastim (n = 8). Chemotherapy was used at the discretion of the treating physician if the patient had disease that was unresponsive to previous therapy; 2 of 40 patients received cyclophosphamide, and the remaining 38 of 40 received CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone).
Leukapheresis
Patients received filgrastim at a dose of 10 µg/kg/day until completion of stem cell collection. Dose was adjusted at the discretion of the apheresis physician if the peripheral blood CD34 count or stem cell yield were suboptimal. Counts of peripheral blood circulating CD34+ progenitor cells were checked, and leukapheresis was started when the peripheral-blood CD34+ count exceeded 0.010 × 109/l. All patients underwent leukapheresis using the COBE Spectra cell separator (COBE BCT, Inc., Lakewood, CO). Three times the estimated blood volume was processed during each collection. Standard citrate dextrose solution was used as an anticoagulant. Daily leukapheresis was performed until the target cell doses reached at least 6 × 106 CD34+ cells/kg for patients who were to undergo tandem transplantation and reached at least 3 × 106/kg for patients who were to undergo a single transplantation procedure. If the collected CD34+ cell dose was <0.5 × 106/kg/d on two consecutive days, the apheresis was stopped.
Statistical Analysis
Mobilization failure was defined as the inability to collect at least 2 × 106 CD34+ cells/kg in 4 leukapheresis procedures. Risk factors predicting mobilization failure were determined using logistic regression analysis. Factors studied in univariate analysis included age, number of prior treatment regimens, prior radiation therapy, interval between diagnosis and mobilization, white cell count, platelet count, bone marrow cellularity, and prior lenalidomide treatment. Factors significant at a P value of .05 or less were evaluated in a multivariate model.
RESULTS
Patient Characteristics
During the study period, 302 patients with multiple myeloma underwent stem cell mobilization with filgrastim at our institution. Their median age was 58.5 years (range, 31 to 76 years). There were 185 men and 117 women. The majority of patients, 186 (62%), had received only one previous treatment regimen. Likewise, a majority of patients, 237 (78%), underwent mobilization within 12 months of diagnosis (Table 1). Lenalidomide was stopped at a median of 17.5 days (range 1–211 days) prior to initiation of filgrastim for mobilization.
Table 1.
Patient Characteristics (n = 302)
| Characteristic | Number (%) |
|---|---|
| Age | |
| Median (range) | 58.5 (38–76) |
| Sex | |
| Men | 185 (61) |
| Women | 117 (39) |
| Isotype | |
| IgG | 177 (59) |
| IgA | 63 (21) |
| Light chain only | 47 (15) |
| Other (IgD, IgM, Nonsecretory) | 15 (5) |
| Durie-Salmon stage at diagnosis | |
| I | 59 (20) |
| II | 127 (42) |
| III | 103 (34) |
| Unknown | 13 (4) |
| No. of previous treatment regimens | |
| 1 | 186 (62) |
| >1 | 115 (38) |
| Interval from diagnosis to mobilization | |
| ≤12 months | 237 (78) |
| >12 months | 65 (22) |
| Prior lenalidomide | |
| Yes | 64 (21) |
| No | 238 (79) |
Mobilization Failures
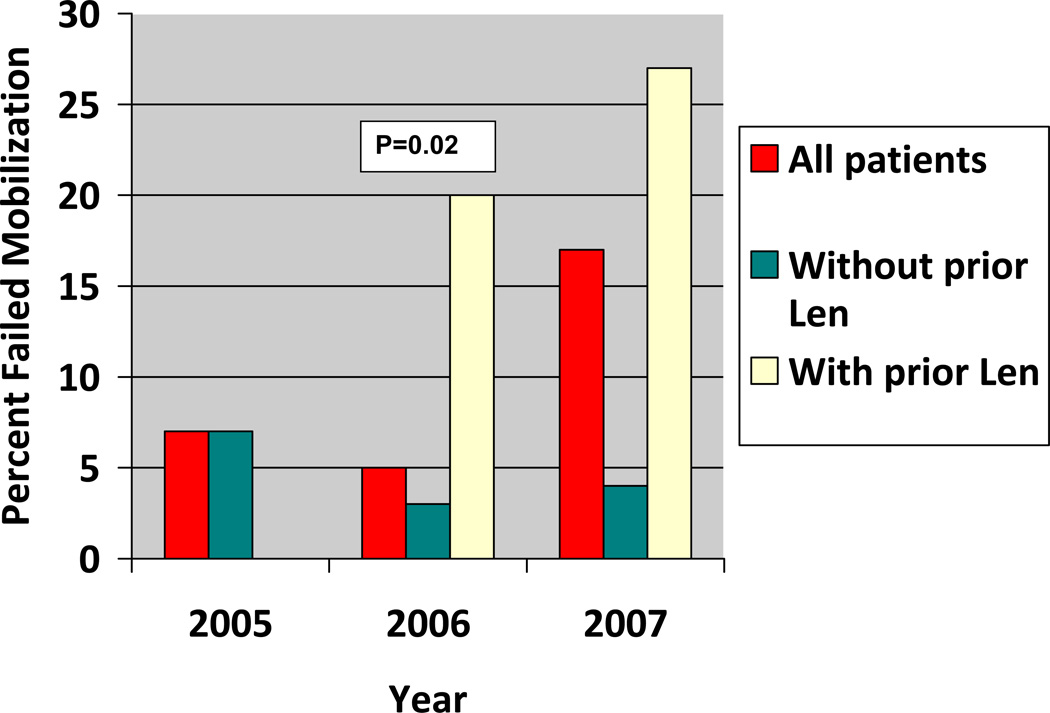
In 26 (9%) of 302 patients, mobilization of stem cells failed. Significant predictors of mobilization failure on univariate analysis (Table 2) included more than one prior chemotherapy regimens (P=0.004), an interval of more that one year after diagnosis of myeloma (P<0.001), prior use of lenalidomide (P<0.001) and transplants received in the year 2007 (P=0.02). The failure rate (fig 1) significantly increased in 2007 (14%) compared with rates in 2006 (5%) and 2005 (7%).
Table 2.
Risk Factors Predicting Mobilization Failure*
| Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | N failed |
% failed | OR | 95% CI | p | OR | 95% CI | p | |
| Total | 302 | 26 | 9% | ||||||
| Age | |||||||||
| ≤60 years | 171 | 11 | 6% | ||||||
| >60 years | 131 | 15 | 11% | 1.9 | 0.8–4.2 | 0.13 | |||
| Prior chemo regimens | |||||||||
| ≤1 | 186 | 9 | 5% | ||||||
| >1 | 115 | 17 | 15% | 3.4 | 1.5–7.9 | 0.004 | |||
| Previous radiation | |||||||||
| Yes | 90 | 9 | 10% | 1.3 | 0.5–2.9 | 0.6 | |||
| No | 212 | 17 | 8% | ||||||
| Interval between diagnosis and mobilization | |||||||||
| ≤12 months | 237 | 11 | 5% | ||||||
| >12 months | 65 | 15 | 23% | 6.1 | 2.7–14 | <0.001 | 4.6 | 1.9–11.1 | 0.001 |
| White blood cell count (× 109/Liter) | |||||||||
| ≤4 | 41 | 6 | 15% | ||||||
| >4 | 260 | 20 | 8% | 0.5 | 0.2–1.3 | 0.15 | |||
| ≤5.7 (median) | 152 | 17 | 11% | ||||||
| >5.7 | 149 | 9 | 6% | 0.5 | 0.2–1.2 | 0.1 | |||
| Platelet count (× 109/Liter) | |||||||||
| ≤262 (median) | 150 | 17 | 11% | ||||||
| >262 | 151 | 9 | 6% | 0.5 | 0.2–1.1 | 0.1 | |||
| ≤150 | 24 | 3 | 12% | ||||||
| >150 | 277 | 23 | 8% | 0.6 | 0.2–2.3 | 0.5 | |||
| Cellularity | |||||||||
| ≤30% | 137 | 11 | 8% | ||||||
| >30% | 164 | 14 | 8% | 1.1 | 0.5–2.4 | 0.9 | |||
| ≤35% (median) | 159 | 13 | 8% | ||||||
| >35% | 142 | 12 | 8% | 1 | 0.5–2.3 | 0.9 | |||
| Prior lenalidomide | |||||||||
| No | 238 | 10 | 4% | ||||||
| Yes | 64 | 16 | 25% | 7.6 | 3.2–18 | <0.001 | 5.9 | 2.4–14.3 | <0.001 |
| Year | |||||||||
| 2005 | 67 | 5 | 7% | ||||||
| 2006 | 137 | 7 | 5% | ||||||
| 2007 | 98 | 14 | 14% | 2.7 | 1.2–6 | 0.02 | |||
Totals vary because of unknown values of some characteristics for one patient
Fig 1.
Mobilization failure rate by calendar year with and without prior lenalidomide (len). (no patient received lenalidomide in 2005)
In a multivariate analysis evaluating the independent effects of these factors, only, prior lenalidomide use and stem cell mobilization more than one year after diagnosis of myeloma remained significant (P < .001), with odds ratios of 5.9 (95% CI, 2.4 to 14.3) and 4.6 (95% CI, 1.9 to 11.1), respectively (Table 2). It is noteworthy that the year was no longer significant on multivariate analysis. This was likely because higher proportion of patients in 2007 had pretransplant lenalidomide compared with previous years; 45% of 98 patients seen in 2007 had received premobilization lenalidomide compared to 11% of 204 patients in previous years.
Subgroup Analysis
We performed an univariate subgroup analysis in order to identify patients at high risk of mobilization failure among those who received and those who did not receive lenalidomide. The interval between diagnosis and mobilization was the only significant predictor of failure in both subgroups. In addition, in patients who were previously treated with lenalidomide, more than 3 cycles of lenalidomide (P=0.001), and more than 1 prior treatment regimen (P=0.04) were associated with significantly higher risk. Mobilization failed in 78% of patients (7 of 9) who received more than 3 cycles of lenalidomide, compared with 16% of patients (9 of 55) who received 1 to 3 cycles of lenalidomide. It is noteworthy that the interval between the last day of lenalidomide therapy and filgrastim initiation did not significantly affect the success rate of stem cell mobilization. In addition, our data showed a trend for higher risk of failure for older patients (P=0.05) who had received lenalidomide. Because of sample size limitations we could not perform a multivariate analysis to evaluate the independent effects of the interval since diagnosis, number of prior chemotherapy regimens, number of lenalidomide cycles and age in the subgroup of patients who had received lenalidomide.
Remobilization
The majority of patients in whom mobilization failed, including those who received lenalidomide, successfully underwent remobilization with chemotherapy. Remobilization with chemotherapy and filgrastim was attempted in 21 of 26 patients for whom mobilization failed on the first attempt. Chemotherapy regimens used included CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone) in 16 patients, cyclophosphamide alone in 4 patients, and ifosfamide plus etoposide in 1 patient. Remobilization was successful in 18 (86%) of 21 patients; a median of 7 × 106 CD34+ cells/kg (range, 2.2 to 15 × 106 CD34+ cells/kg) was collected. This group included 10 (77%) of 13 patients who had previously received lenalidomide. All patients underwent autologous transplantation. Times to neutrophil and platelet engraftment were similar to those for the patients whose stem cells mobilized on the first attempt with filgrastim alone (data not shown). Two patients for whom less than 2 × 106 CD34+ cells/kg were collected in 4 leukapheresis procedures underwent additional leukapheresis, and adequate doses of stem cells were collected after a total of 7 procedures in 1 patient and 8 procedures in the other. One patient underwent bone marrow harvest. Repeat mobilization was not attempted in two patients.
Outcome of patients mobilized with alternative strategies
Thirty nine of 40 patients given chemotherapy and filgrastim were successfully mobilized, with a median yield of 13.2 × 106 CD34+ cells/kg (range 2.1 – 70 × 106 CD34+ cells/kg). This included 12 patients who had previously received lenalidomide. One patient died prior to stem cell collection. All 4 patients who received plerixafor and filgrastim and 7 of 8 patients mobilized with Peg filgrastim were successfully collected; none of these 12 patients had received lenalidomide previously.
DISCUSSION
In this study, we suggest that lenalidomide use before transplantation impairs stem cell mobilization in response to filgrastim, but this effect is overcome by using chemotherapy and filgrastim. Mobilization with filgrastim failed in 25% of patients who had previously received lenalidomide, compared with 4% of patients who had not. In the majority of these patients (77%), remobilization with chemotherapy and filgrastim was successful, and these patients could successfully undergo autologous stem cell transplantation.
Although our study is limited by its retrospective nature, several lines of reasoning support our conclusions. It could be argued that biases inherent in this design may explain our findings and that patients who received lenalidomide had advanced disease and therefore were more susceptible to mobilization failure. However, this conclusion was not supported by the persistence of a significant lenalidomide effect after adjusting for the number of prior chemotherapy regimens in multivariate analysis. Among patients who had previously received only one chemotherapy regimen, a significantly higher mobilization failure rate was seen in patients who received lenalidomide as part of that regimen (13%) than was seen in patients who had not received lenalidomide (3%). In fact, higher failure rates were seen in all subgroups of patients who received lenalidomide (Table 3). Moreover, the higher the number of course of lenalidomide, the higher the failure rate; the failure rate was 78% in patients who had more than 3 courses of lenalidomide, compared with 16% in patients who received 3 courses or less.
Table 3.
Risk Factors Predicting Mobilization Failure In Patients Who Received And Those Who Did Not Receive Lenalidomide
|
Lenalidomide used |
Lenalidomide not used |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | N failed | % failed |
P | N | N failed |
% failed |
P | |
| 64 | 16 | 25% | 238 | 10 | 4% | |||
| Age | ||||||||
| ≤60 years | 34 | 5 | 15% | 137 | 6 | 4% | ||
| >60 years | 30 | 11 | 37% | 0.05 | 101 | 4 | 4% | 0.9 |
| Prior chemo regimens | ||||||||
| ≤1 | 31 | 4 | 13% | 155 | 5 | 3% | ||
| >1 | 33 | 12 | 36% | 0.04 | 82 | 5 | 6% | 0.3 |
| Previous radiation | ||||||||
| Yes | 24 | 5 | 21% | 66 | 4 | 6% | ||
| No | 40 | 11 | 28% | 0.5 | 172 | 6 | 3% | 0.4 |
| Interval between diagnosis and mobilization | ||||||||
| ≤12 months | 40 | 6 | 15% | 197 | 5 | 3% | ||
| >12 months | 24 | 10 | 42% | 0.02 | 41 | 5 | 12% | 0.01 |
| White blood cell count (× 109/Liter) | ||||||||
| ≤4 | 14 | 3 | 21% | 27 | 3 | 11% | ||
| >4 | 50 | 13 | 26% | 0.7 | 210 | 7 | 3% | 0.07 |
| ≤5.7 (median) | 38 | 9 | 24% | 114 | 8 | 7% | ||
| >5.7 | 26 | 7 | 27% | 0.8 | 123 | 2 | 2% | 0.06 |
| Platelet count (× 109/Liter) | ||||||||
| ≤262 (median) | 36 | 11 | 31% | 114 | 6 | 5% | ||
| >262 | 28 | 5 | 18% | 0.2 | 123 | 4 | 3% | 0.4 |
| ≤150 | 8 | 2 | 25% | 16 | 1 | 6% | ||
| >150 | 56 | 14 | 25% | 0.9 | 221 | 9 | 4% | 0.7 |
| Cellularity | ||||||||
| ≤30% | 27 | 6 | 22% | 110 | 5 | 5% | ||
| >30% | 36 | 9 | 25% | 0.8 | 128 | 5 | 4% | 0.8 |
| ≤35% (med) | 32 | 8 | 25% | 127 | 5 | 4% | ||
| >35% | 31 | 7 | 23% | 0.8 | 111 | 5 | 5% | 0.8 |
| Lenalidomide cycles | ||||||||
| 1–3 | 55 | 9 | 16% | NA | NA | NA | ||
| >3 | 9 | 7 | 78% | 0.001 | NA | NA | NA | |
| Interval between stopping lenalidomide and mobilization | ||||||||
| ≤9 days | 18 | 5 | 28% | NA | NA | NA | ||
| 10–17 days | 15 | 4 | 27% | 0.9 | NA | NA | NA | |
| 18–46 days | 13 | 2 | 15% | 0.4 | NA | NA | NA | |
| >46 days | 17 | 4 | 24% | 0.8 | ||||
Further supporting our findings are other reports of lower stem cell yields and increased mobilization failure rates in patients who had received prior lenalidomide (27–29). Kumar et al (27) reported a significant decrease in stem cell yield in patients who had been previously treated with lenalidomide compared with those who had not received it beforehand; moreover, among all patients who underwent mobilization with filgrastrim, stem cells could not be collected in 3 (7%) of 43 patients who had previously received lenalidomide, compared with only 2 (1%) of 199 who had not received lenalidomide. All 3 lenalidomide-treated patients in whom mobilization failed had received more than 6 months of this agent. Although confirming the trend of impaired stem cell mobilization after lenalidomide, the study of Kumar and colleagues had a lower failure rate than did our study, possibly because Kumar et al included only patients who were within 12 months of diagnosis. In our study, an interval of more than 12 months between diagnosis of myeloma and collection of stem cells was a significant (P < .001) factor in predicting mobilization failure, a finding that has previously been documented as well (25).
Results of two other small case series (28, 29) are in agreement with our findings. The first showed a mobilization failure rate of 45% in 20 lenalidomide-treated patients, compared with a rate of 7% in 41 patients who had not received lenalidomide. The second study showed a failure rate of 43% in 28 patients previously treated with lenalidomide. As in our study described here, stem cell remobilization in these patients was successfully induced with chemotherapy and filgrastim. The failure rate of 43%–45% observed in these studies is higher then the rate of 25% observed in the current study. It is possible that this may be due to differing patient characteristics. But it is also possible that because of the design of our study, we may have underestimated the incidence of mobilization failure. Patients were mobilized with filgrastim or chemotherapy at the discretion of their treating physicians. Patients with high disease burden who had failed previous treatments were more likely to be mobilized with chemotherapy. Likewise the realization of increased failure rate with filgrastim may have prompted upfront use of chemotherapy to mobilize stem cells in some of the patients who had previously received lenalidomide in the later period of the study.
Our study includes a large number of patients who were treated similarly at a single institution over a short period of time, so it provides the necessary power for identifying potential risk factors for mobilization failure. What this study does not do is provide a reason or a mechanism that explains the effect of lenalidomide on stem cell trafficking.
The role of prior melphalan therapy in impairing subsequent stem cell collection has been well known, and frontline use of this agent is discouraged in patients who are suitable candidates for autologous transplantation (26). The implications of this finding for lenalidomide are more uncertain, particularly because it is such an active agent, with a response rate of more than 90% (17). It has been recommended that stem cells should be collected within 6 months of starting lenalidomide (27). Our data, however, indicate that the failure rate is very high (78%) if patients have received more than 3 cycles of this therapy.. Mobilization with chemotherapy and filgrastim rather than filgrastim alone might be considered in patients receiving more than 3 cycles of lenalidomide prior to mobilization. Certainly, for patients in whom a first mobilization attempt with filgrastim fails, it would be reasonable to make a second mobilization attempt with chemotherapy and filgrastim, a strategy that was successful in more than three quarters of our patients. The impact of newer mobilizing agents such as plerixafor for initial mobilization or remobilization in patients who have previously received lenalidomide is uncertain and needs to be studied.
To conclude, lenalidomide hampers subsequent stem cell mobilization and collection with filgrastim, and remobilization with chemotherapy and filgrastim is successful in most patients in whom stem cell mobilization with filgrastim alone has failed. The mechanism for this effect largely remains speculative and will certainly be a subject of further studies. This study does, however, have significant implications for how we should integrate this highly effective drug within our therapeutic armamentarium against myeloma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reece DE. Management of multiple myeloma: the changing landscape. Blood Rev. 2007;21:301–314. doi: 10.1016/j.blre.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostopoulos A, Weber D, Rankin K, Delasalle K, Alexanian R. Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol. 2003;121:768–771. doi: 10.1046/j.1365-2141.2003.04345.x. [DOI] [PubMed] [Google Scholar]
- 4.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, Anagnostopoulos A, Weber D. Treatment of plasma cell dyscrasias with thalidomide and its derivatives. J Clin Oncol. 2003;21:4444–4454. doi: 10.1200/JCO.2003.07.200. [DOI] [PubMed] [Google Scholar]
- 6.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee CK, Barlogie B, Munshi N, et al. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003;21:2732–2739. doi: 10.1200/JCO.2003.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Palumbo A, Ambrosini MT, Benevolo G, et al. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007;109:2767–2772. doi: 10.1182/blood-2006-08-042275. [DOI] [PubMed] [Google Scholar]
- 9.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 12.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 13.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 14.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 15.Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005;129:755–762. doi: 10.1111/j.1365-2141.2005.05519.x. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21:16–19. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 19.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 20.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- 21.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 22.Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052–3057. doi: 10.1182/blood-2004-02-0408. [DOI] [PubMed] [Google Scholar]
- 23.Bensinger W, Appelbaum F, Rowley S, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–2555. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 24.Cottler-Fox MH, Lapidot T, Petit I, et al. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- 25.Morris CL, Siegel E, Barlogie B, et al. Mobilization of CD34+ cells in elderly patients (>/= 70 years) with multiple myeloma: influence of age, prior therapy, platelet count and mobilization regimen. Br J Haematol. 2003;120:413–423. doi: 10.1046/j.1365-2141.2003.04107.x. [DOI] [PubMed] [Google Scholar]
- 26.Boccadoro M, Palumbo A, Bringhen S, et al. Oral melphalan at diagnosis hampers adequate collection of peripheral blood progenitor cells in multiple myeloma. Haematologica. 2002;87:846–850. [PubMed] [Google Scholar]
- 27.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 28.Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia. 2007 doi: 10.1038/sj.leu.2405035. [DOI] [PubMed] [Google Scholar]
- 29.Paripati H, Stewart AK, Cabou S, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia. 2008 doi: 10.1038/sj.leu.2405100. [DOI] [PubMed] [Google Scholar]