Abstract
Leukemic transformation (LT) from myelofibrosis has a very poor prognosis with the current treatment strategies. We hypothesized that allogeneic stem cell transplantation (ASCT) can improve outcomes for patients with LT, and reviewed 55 consecutive patients that were treated for myelofibrosis with ASCT at our institution. Fourteen patients (25%) were identified to have LT. Thirteen of these patients received induction chemotherapy and 6 achieved remission at the time of transplant. Conditioning regimen was melphalan (Mel)-based in 9 patients. All patients engrafted and achieved remission after transplant, whereas 4 subsequently relapsed. After a median follow-up of 31 months, 6 patients (49%) survived long term. Although limited by a small number of patients, this study suggests that patients with myelofibrosis and LT may achieve long-term remission after induction chemotherapy and ASCT.
Keywords: Myelofibrosis, Leukemic transformation, Allogeneic stem cell transplantation
INTRODUCTION
Primary myelofibrosis (PMF) is a clonal myelogenous disease of unknown etiology characterized by neo-plastic megakaryocyte proliferation, extensive bone marrow (BM) fibrosis, massive splenomegaly, mobilization of stem cells in circulation, and extramedullary hematopoiesis [1-3]. Secondary myelofibrosis (SMF) is the consequence of progression of polycythemia vera (PV) or essential thrombocythemia (ET) to a disease indistinguishable from PMF [4]. The disease evolves to a phase of extensive tumor burden, progressive cytopenias, followed eventually by a myelogenous blast phase that resembles acute myelogenous leukemia (AML), categorized by the World Health Organization (WHO) asmyeloid leukemia evolvingfrom a myeloproliferative neoplasm [1,5].
Leukemictransformation (LT)has a dismalprognosis with the current treatment strategies [6]. Allogeneic stem cell transplantation (ASCT) is a curative approach for patients with MF [7,8]; however, the outcomes of patients with LT are currently unknown. Here, we hypothesized that patients with myelofibrosis and LT can achieve durable long-term remission after ASCT.
PATIENTS AND METHODS
Selection of Patients
Of 55 consecutive patients with myelofibrosis who received an ASCT at the University of Texas M.D. Anderson Cancer Center between August 1994 and November 2008, 14 patients (25%) were identified to have LT (defined as >20% blasts in the BM or peripheral blood [PB]]). Patients with myelofibrosis and <20% blasts and patients with other myeloproliferative diseases transformed to AML were not included in this study. A retrospective study protocol, which included a waiver of informed consent, was approved by the M.D. Anderson Cancer Center institutional review board.
Characteristics of these patients are presented in Table 1. Thirteen patients received induction chemo-therapy for LT, 8 with “3+7” regimen (idarubicin or daunorubicin plus cytarabine), 3 with cytarabine and either fludarabine (Flu) or azacytidine, and 2 with other agents. Six patients achieved complete remission (CR), 6 had a reduction in the percentage of BM blasts (median final percentage of BM blasts prior to transplant was 7%, range: 0%-36%), and 1 had progressive disease (86% blasts) at the time of transplantation. Three patients had prior autologous (1) or ASCT (2) for myelofibrosis at different institutions.
Table 1.
Patient Characteristics
| Number of patients | 14 |
| Age (median, range) | 59 (50-67) |
| Sex | |
| Male | 9 |
| Diagnosis | |
| PMF | 11 |
| SMF | 4 |
| Lille Score at diagnosis of the time of LT | |
| 0 | 4 |
| 1 | 7 |
| 2 | 3 |
| Median time from MF to LT (months) (range) | 38 (10-144) (n = 13) |
| Number of patients with prior splenectomy | 5 |
| Prior therapy for myelofibrosis | |
| Hydroxyurea | 9 |
| Thalidomide/lenalidomide | 4 |
| Interferon | 3 |
| Azacitidine/decitabine | 2 |
| Anabolic steroids | 2 |
| Erythropoietin | 1 |
| Bortezomib | 1 |
| Number of patients who received cytoreductive chemotherapy prior to transplant | 13 |
| Number of patients with LT in CR at transplant | 6 |
| Cytogenetics at LT | |
| Normal | 3 |
| CRS 1 abnormalities (+1, 1q-, t(1;6), der (1;19), t(1;17), dup(1), del(1)) | 7 |
| CRS 7 abnormalities (del 7, 7q-, t(7;10)) | 3 |
| CRS 8 abnormalities (+8, −8, t(3;8)) | 4 |
| Complex karyotype | 4 |
PMF indicates primary myelofibrosis; SMF, secondary myelofibrosis; LT, leukemic transformation; CR, complete remission from acute myelogenous leukemia; CRS, chromosome; MF, myelofibrosis.
Transplantation for acute leukemia was performed from matched siblings, unrelated or 1 antigen mismatched related donors (Table 2). Conditioning included reduced-intensity conditioning (RIC) preparative regimens in 9 patients using melphalan (Mel), Flu 6 gemtuzumab ozogamicin, and myeloablative (MA) conditioning using busulfan (Bu)-based conditioning [9,10]. Although there are limitations to these terms, the conditioning regimens that included Bu 520 mg/m2 total dose were considered MA conditioning whereas those including Mel 140 mg/m2 or less were considered RIC. Antithymocyte globulin (ATG) was administered to patients who received a matched unrelated or mismatched related graft.
Table 2.
Transplant Characteristics
| Number | |
|---|---|
| Donor | |
| MSD | 8 |
| MUD | 4 |
| 1 antigen mismatched related | 2 |
| Cell type | |
| G-CSF mobilized peripheral blood stem cells | 10 |
| Bone marrow | 4 |
| Conditioning | |
| Reduced-intensity (Melphalan-based) | |
| Melphalan 140 mg/m2 + fludarabine 120 mg/m2 + Gemtuzumab (2, 4, 6, 9 or 16 mg/m2) | 8 |
| Melphalan 100 mg/m2 + fludarabine 120 mg/m2 | 1 |
| Myeloablative (Busulfan-based) | |
| i.v. Busulfan 520 mg/m2 + fludarabine 160 mg/m2 | 3 |
| i.v. Busulfan 520 mg/m2 + clofarabine 120 mg/m2 + fludarabine 40 mg/m2 | 1 |
| p.o. Busulfan 1 mg/kg × 10 doses + cyclophosphamide 120 mg/kg + thiotepa 750 mg/m2 | 1 |
| GVHD prophylaxis | |
| Tacrolimus + methotrexate | 13 |
| Cyclosporine + methotrexate | 1 |
MSD indicates matched sibling donor; MUD, matched unrelated donor; i.v., intravenous; p.o., oral; GVHD, graft-versus-host disease; G-CSF, granulocyte colony-stimulating factor.
Definitions
Engraftment was defined as achieving an absolute neutrophil count (ANC) >0.5 × 109/L for at least 3 consecutive days before day 30, with donor derived cells detected by DNA microsatellite analysis. Platelet recovery was defined as the first day on which the platelet count was >20 × 109/L unsupported by plate let transfusions for 7 days. Acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD) were defined and graded according to previously described criteria [11,12].
Statistical Methods
Progression-free survival (PFS) and overall survival (OS) were estimated by the Kaplan-Meier method [13]. Days to engraftment for patients with or without splenectomy were compared using the Wilcoxon rank-sum test [14]. The incidence of disease progression, nonre-lapse mortality (NRM), and GVHD was estimated using the cumulative incidence method to account for competing events. Death in the absence of disease progression, disease progression, and death without GVHD were considered as competing events for the respective outcomes. Statistical significance was defined at the .05 level.
RESULTS
Transplant outcomes are summarized in Table 2. Briefly, all patients engrafted after a median of 13 days for neutrophils and 21.5 days for platelets. Of 13 patients who had PB chimerism performed by PCR on day 30 after transplant, 10 had 100% donor myeloid and T cells. Three patients had a mixed chimerism (90% donor cells) on day 30, subsequently increased to 100% in 2 patients and decreased in 1 who subsequently relapsed.
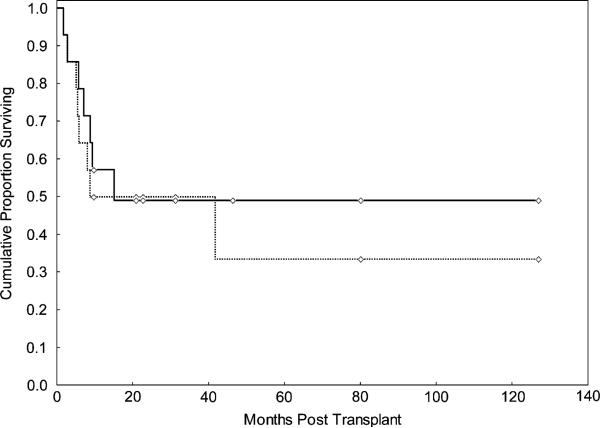
Seven patients died after a median follow-up of 1 year, 3 of relapsed disease, 2 of infection, 1 of GVHD, and 1 secondary to decompensated liver disease, whereas 7 patients survived. The median follow-up for survivors was 31 months (range: 10-127 months). Causes of death included disease relapse in 2 patients and NRM in 3 patients (2 pneumonia and 1 decompensated liver disease) for the patients who received Mel-based conditioning, and 1 because of relapse disease and 1 NRM (GVHD) in the Bu-based conditioning group. OS was 49% at 2 years and at last follow-up (Figure 1). The cumulative incidence of NRM was 29% (95% confidence interval [CI] 12-65) at 2 years and overall.
Figure 1.
OS (continuous line) and DFS (interrupted line) for 14 consecutive patients with myelofibrosis and leukemic transformation treated with ASCT.
All patients achieved remission after transplant while 4 relapsed. One patient relapsed 42 months after a matched sibling donor transplant and received a second allogeneic transplant using a 4/6 allele matched cord blood unit (CBU) added to the sibling PB stem cells (PBSCs) to augment graft-vesus-tumor effect, part of a separate clinical trial. The cumulative incidence of disease progression was 23% (95% CI 9-63) at 2 years and 38% (95% CI 16-89) at last follow-up, whereas PFS was 49% (95% CI 20-70) at 2 years and 33% (95% CI 7-63) at the last follow-up (Table 3).
Table 3.
Outcomes of 14 Patients with Myelofibrosis and Leukemic Transformation Treated with Allogeneic Stem Cell Transplantation
| Primary engraftment (%)* | 100% |
| Engraftment (median, range) | |
| Day to ANC 500 (N = 14) | 13 (8-21) |
| Days to platelet count 20,000 (N = 11) | 21.5 (13-40) |
| Chimerism at day 30 by PCR* (N = 14) | |
| 100% | 11 |
| 85%-99% | 2 |
| Not tested | 1 |
| Median follow-up survivors, months (range) | 31 (10-127) |
| aGVHD, n | |
| Grade II-IV | 5 |
| Grade III-IV | 2 |
| cGVHD, n | |
| All grades | 5 |
| Extensive | 2 |
| Complete remission after transplant, n | 14 |
| Disease progression | |
| n (% CI) | 4 (38%) (16-89)† |
| NRM, Overall | |
| n (% CI) | 4 (29%) (12-65)† |
| PFS, Overall | |
| n (% CI) | 6 (33%) (7-63)† |
| OS, Overall | |
| n (% CI) | 7 (49%) (22-72)† |
ANC indicates absolute neutrophil count; PCR, polymerase chain reaction; aGVHD, acute graft-versus-host-disease; cGVHD, chronic graft-versus-host disease; NRM, nonrelapse mortality; PFS, progression-free survival; OS, overall survival; CI, cumulative incidence.
Donor myeloid and T cells.
95% Confidence interval.
Five patients developed grade II-IV aGVHD and 5 patients developed cGVHD (2 de novo, whereas 3 progressed after aGVHD). The cumulative incidence was 39% (95% CI 19-77) for each event.
JAK2V617F was positive in 6 of 8 tested patients, with 71% median allele burden (range: 13%-86.7%). This percentage decreased after induction chemotherapy to a median of 46% with a wide variation, ranging from no change in 1 patient (71%), to a 7-fold decrease in another patient (from 72%–10%). All JAK2V617F-positive patients became negative on day 30 after transplant. The only 2 patients with JAK2V617F disease who relapsed were again positive for JAK2V617F.
Cytogenetic abnormalities were present in 11 of 14 patients with LT; clonal evolution occurred in almost all these patients at the time of progression to acute leukemia. Interestingly, 7 of 11 patients (64%) had abnormalities of chromosome 1 at the time of LT (Table 1). Three patients with normal cytogenetics did not acquire any cytogenetic abnormalities at the time of transformation. All JAK2V617F-positive patients with LT had clonal evolution except 1. Prior splenectomy did not appear to favor the acquisition of new cytogenetic abnormalities or hasten progression to LT (median time to progression 37.5 months in splenectomized patients, n = 4).
It is likely that patients with low disease burden at transplant do better; however, this could not be demonstrated by this report; 3 of 6 patients in remission and 4 of 8 patients with relapsed disease at transplant survived long term. Two patients with persistent high tumor burden after induction chemotherapy (>30% blasts) fared poorly. No other factors associated with outcomes including conditioning chemotherapy were identified.
DISCUSSION
LT has been reported to occur after a median of 31 months from the diagnosis of myelofibrosis, at a median age of 66 years [6]. Factors predicting of its development were leukocytosis, increase percentage of blasts in the PB, progressive anemia, or increased number of karyotypic abnormalities (>2) [15-17].
The mechanisms of LT are unclear. It is controversial whether JAK2V617F or allele burden accumulation is associatedwith LT or not [18,19]. Ina small numberof patients, we have found that the allele burden decreased with disease progression, suggesting that the evolution to an accelerated phase may originate in a clone independent of the one carrying this mutation [20].
With the available treatment, the median survival for patients with LT is approximately 3 months [6,21]. The outcomes of these patients were similar among those treated with high or low intensity chemo-therapy, or no treatment at all [6]. Approximately 40% of patients who received induction chemotherapy for acute leukemia achieved a temporary remission; however, this was evidently not enough to obtain long-term disease control in these patients [6].
Jurado and colleagues [22] previously reported on a series of 19 PV or ET patients (median age of 43 years) who received an SCT for progression to myelofibrosis, myelodysplastic syndrome (MDS), or AML. These patients were treated with MA total body irradiation (TBI)-based conditioning regimen. All patients transformed to AML except one had a very poor outcome, predominantly because of a high treatment-related mortality (TRM) [22]. Improved outcomes for patients with LT in the present study could be because of, at least in part, the use of non-TBI, RIC (RIC) for transplantation.
We have previously found that a small number of patients who received an ASCT after induction chemotherapy survived long term [20]. Here, we report the largest experience to date of patients with LT evolved from myelofibrosis treated with ASCT. Transplantation was performed mostly from sibling donors using Flu-Mel-based RIC chemotherapy. Remarkably, approximately half of these patients achieved survival and long-term remission of their disease. The median age was slightly younger than the median age of patients with LT reported in previous studies suggesting that at least a subgroup of patients can be salvaged with transplantation [6]. As expected in patients with myelofibrosis, all patients with JAK2V617F-positive LT became negative for this mutation after transplant [23]. This reoccurred in 2 patients who subsequently relapsed, suggesting that this mutation could be used to monitor the disease status after transplant.
In conclusion, this report suggests for the first time that long-term survival can be achieved in patients with myelofibrosis and LT. Induction chemotherapy followed by transplantation for patients with good performance status and possible low tumor burden may be required to achieve this goal.
Footnotes
Financial disclosure: The authors declare no conflict of interest.
Authorship Statement
S.O.C. designed the study, collected and analyzed the results, and wrote the paper. M.L. and S.G. contributed to patient care and data collection, reviewed, and coauthored the manuscript. R.M.S. performed the statistical analysis, and reviewed and approved the manuscript. C.B.R. reviewed the pathology slides, and reviewed and approved the manuscript. B.S.A., C.H., S.V., and R.E.C. contributed to patient accrual, and reviewed and approved the manuscript. U.P. contributed to study design, patient accrual, data analysis and interpretation, and manuscript writing.
REFERENCES
- 1.Tefferi A. Pathogenesis of myeloid metaplasia with myelofibrosis. J Clin Oncol. 2005;23:8520–8530. doi: 10.1200/JCO.2004.00.9316. [DOI] [PubMed] [Google Scholar]
- 2.Ciurea SO, Merchant D, Mahmud N, et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood. 2007;110:986–993. doi: 10.1182/blood-2006-12-064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barosi G, Viarengo G, Pecci A, et al. Diagnostic and clinical relevance of the number of circulating CD34(1) cells in myelofibrosis with myeloid metaplasia. Blood. 2001;98:3249–3255. doi: 10.1182/blood.v98.12.3249. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman R, Prchal JT, Samuelson S, Ciurea SO, Rondelli R. Philadelphia chromosome-negative myeloproliferative disorders: biology and treatment. Biol Blood Marrow Transplant. 2007;13:64–72. doi: 10.1016/j.bbmt.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the WHO classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 6.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson R, Tefferi A. Leukemic transformation in myeloid metaplasia with myelofibrosis: a single institution experience with 91 cases. Blood. 2005;105:973–977. doi: 10.1182/blood-2004-07-2864. [DOI] [PubMed] [Google Scholar]
- 7.Guardiola P, Anderson JE, Bandini G, et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Soci et e Fran-çaise de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood. 1999;93:2831–2838. [PubMed] [Google Scholar]
- 8.Rondelli D, Barosi G, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning in intermediate- or high-risk patients with myelofibrosis with myeloid metaplasia. Blood. 2005;105:4115–4119. doi: 10.1182/blood-2004-11-4299. [DOI] [PubMed] [Google Scholar]
- 9.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of myeloablative, reduced-intensity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 10.de Lima M, Champlin RE, Thall PF, et al. Phase I/II study of gemtuzumab ozogamicin added to fludarabine, melphalan and allogeneic hematopoietic stem cell transplantation for high-risk CD33 positive myeloid leukemias and myelodysplastic syndromes. Leukemia. 2008;22:258–264. doi: 10.1038/sj.leu.2405014. [DOI] [PubMed] [Google Scholar]
- 11.Przepiorka D, Martin P, Klingmann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 12.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bull. 1945;1:80–83. [Google Scholar]
- 15.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 16.Dupriez B, Morel P, Demory JL, et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood. 1996;88:1013–1018. [PubMed] [Google Scholar]
- 17.Huang J, Li CY, Mesa RA, et al. Risk factors for leukemic transformation in patients with primary myelofibrosis. Cancer. 2008;112:2726–2732. doi: 10.1002/cncr.23505. [DOI] [PubMed] [Google Scholar]
- 18.Barosi G, Bergamaschi G, Marchetti M, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110:4030–4036. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- 19.Tefferi A, Lasho TL, Schwager SM, et al. The JAK2(V617F) tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: lineage specificity and clinical correlates. Br J Haematol. 2005;131:320–328. doi: 10.1111/j.1365-2141.2005.05776.x. [DOI] [PubMed] [Google Scholar]
- 20.Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood. 2008;112:1628–1637. doi: 10.1182/blood-2008-02-138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervantes F, Tassies D, Salgado C, Rovira M, Pereira A, Rozman C. Acute transformation in nonleukemic chronic myeloproliferative disorders: actuarial probability and main characteristics in a series of 218 patients. Acta Haematol. 1991;85:124–127. doi: 10.1159/000204873. [DOI] [PubMed] [Google Scholar]
- 22.Jurado M, Deeg H, Gooley T, et al. Haemopoietic stem cell transplantation for advanced polycythemia vera or essential thrombocytopenia. Br J Haematol. 2001;112:392–396. doi: 10.1046/j.1365-2141.2001.02584.x. [DOI] [PubMed] [Google Scholar]
- 23.Kroger N, Badbaran A, Holler E, et al. Monitoring of the JAK2-V617F mutation by highly sensitive quantitative real-time PCR after allogeneic stem cell transplantation in patients with myelofibrosis. Blood. 2007;109:1316–1321. doi: 10.1182/blood-2006-08-039909. [DOI] [PubMed] [Google Scholar]