Abstract
A method for aminoquinoline-directed, cobalt-promoted dimerization of benzamides has been developed. Reactions proceed in ethanol solvent in the presence of Mn(OAc)2 cocatalyst and Na2CO3 base and use oxygen as a terminal oxidant. Bromo, iodo, nitro, ether, and ester moieties are compatible with the reaction conditions. Cross-coupling of electronically dissimilar aminoquinoline benzamides proceeds with modest yields and selectivities.
Direct dehydrogenative arene coupling is the most efficient method for the formation of biaryls.1 However, most of the methods for arene homocoupling use polyfluorobenzene, five-membered- ring heterocycles, or phenol starting materials.2 Cross-coupling of heterocycles and acidic arenes with each other or simple benzene derivatives is also possible.3 In rare cases when simple arenes such as toluene are coupled, isomer mixtures of products are usually obtained.4 Furthermore, reactions often require either palladium or rhodium catalysts or several equivalents of silver salt additives. Few reports describe dimerization of directing-group-containing arenes. In a notable exception, Yu has described copper-promoted 2-arylpyridine dimerization.5 Methods for palladium- or ruthenium-catalyzed dimerization of 2-arylpyridines, phenylacetamides, and aryloxazolines have been reported by Sanford, Greaney, and Oi.6 Miura has recently reported a method for aminoquinoline-directed homocoupling of thiophenes mediated by copper.7 Thus, directed arene homocouplings are still rare. Furthermore, very few reports describe dehydrogenative arene couplings that do not require second-row transition metals as catalysts or additives.2f,3d,e,9g
We have reported that 8-aminoquinoline, picolinamide, and 2-pyridinylmethylamine auxiliaries can be used for palladium-, copper-, and cobalt-catalyzed C–H bond functionalization.8,10a–c Other research groups have extensively used these auxiliaries for palladium-, ruthenium-, iron-, and copper-catalyzed reactions.9 The aminoquinoline auxiliary can direct cobalt-catalyzed C–H activation/alkene, alkyne, and CO migratory insertion sequences, leading to ortho-functionalized benzoic acid derivatives.10a–c We report here cobalt-promoted, aminoquinoline-directed dimerization of arenes that affords biphenyldicarboxylic acid derivatives, uses oxygen as a terminal oxidant, and does not require second-row transition metal additives.
On the basis of previous results,10a–c we decided to use Co(acac)2 catalyst in an alcohol solvent. The reaction optimization was carried out with respect to solvent, oxidant, and base (Table 1). In contrast with previous cobalt-catalyzed reactions, trifluoroethanol is an inferior solvent compared to ethanol (entries 1 and 2). Sodium carbonate base afforded higher yield than sodium pivalate (entries 2 and 3). Reaction under oxygen gives higher yield than the reaction under air (entries 2 and 4). Manganese(III) acetate is an inferior co-oxidant compared with Mn(OAc)2 (entry 5). Use of 10 mol % of catalyst affords lower yield (entry 6), while the presence of cobalt is essential (entry 7). Temperatures less than 100 °C afford lowered yields (entry 8).
Table 1.
Optimization of Reaction Conditionsa

| ||||
|---|---|---|---|---|
| entry | solvent | oxidant | base | yield of 2, % |
| 1 | CF3CH2OH | Mn(OAc)2/O2 | Na2CO3 | 12 |
| 2 | EtOH | Mn(OAc)2/O2 | Na2CO3 | 97 |
| 3 | EtOH | Mn(OAc)2/O2 | NaOPiv | 53 |
| 4 | EtOH | Mn(OAc)2/air | Na2CO3 | 70 |
| 5 | EtOH | Mn(OAc)3·2H2O/air | Na2CO3 | 40 |
| 6b | EtOH | Mn(OAc)2/O2 | Na2CO3 | 49 |
| 7c | EtOH | Mn(OAc)2/O2 | Na2CO3 | NR |
| 8d | EtOH | Mn(OAc)2/O2 | Na2CO3 | 8 |
| 9e | EtOH | Mn(OAc)2 | Na2CO3 | NR |
Amide (0.1 mmol), oxidant (0.1 mmol), Co(acac)2 (0.05 mmol), base (0.1 mmol), solvent (1 mL), Q = quinolin-8-yl. Yields determined by NMR of crude reaction mixtures, with 1,1,2-trichloroethane as internal standard.
Co(acac)2 (10 mol %).
No Co(acac)2.
Reaction temperature = 60 °C.
Deoxygenated EtOH.
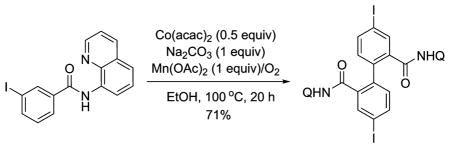
Reaction scope is presented in Table 2. Aminoquinoline benzamide (entry 1) affords dimerization product in 83% yield. Amides possessing electron-withdrawing (entries 4–8, 10, and 11) as well as electron-releasing substituents (entries 2, 3, 9, and 12) are reactive. While amides substituted at 3- and 4-positions afford products in high yields, substitution at the 2-position results in lower yields (entries 3 and 7). Similar to other high-valent cobalt-catalyzed C–H functionalization reactions,10a–c high functional group tolerance is observed. Iodo (entry 5), bromo (entries 6 and 7), nitro (entry 8), and ester (entry 11) substituents are tolerated. Heterocyclic substances, such as aminoquinoline thiophenecarboxylic acid amides, are reactive and afford bithiophenedicarboxylic acid derivatives in high yields (entries 13 and 14). In all cases, only one isomer of product was obtained.
Table 2.
Dimerization of Aminoquinoline Benzamidesa

| |||
|---|---|---|---|
| entry | Ar | product | yield, % |
| 1 | C6H5 |

|
83 |
| 2 | 4-MeC6H4 |

|
76 |
| 3 | 2-MeC6H4 |

|
37 |
| 4 | 4-CF3C6H4 |

|
80 |
| 5 | 3-IC6H4 |

|
71 |
| 6 | 4-BrC6H4 |

|
71 |
| 7 | 2-BrC6H4 |

|
54 |
| 8b | 4-NO2C6H4 |

|
83 |
| 9 | 4-MeOC6H4 |

|
91 |
| 10 | 4-CF3OC6H4 |
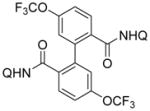
|
46 |
| 11c | 4-MeO2CC6H4 |

|
73 |
| 12 | 2-Naphthyl |

|
73 |
| 13 | 2-Thiophenyl |

|
71 |
| 14 | 3-Thiophenyl |

|
75 |
Amide (1 mmol), Co(acac)2 (0.5 mmol), Mn(OAc)2 (1 mmol), Na2CO3 (1 mmol), EtOH (5 mL). Yields are isolated yields. Please see Supporting Information for details.
Reaction under air.
Reaction was performed in MeOH (5 mL).
It would be useful to selectively cross-couple two different aminoquinoline benzamides. A 1/1 mixture if amides 3 and 4 was reacted in the presence of 1.5 equiv of Co(acac)2. The analysis of the reaction mixture showed that a 0.1/1/0.6 ratio of three possible coupling products 6, 7, and 8 was formed, with predominant formation of the cross-coupled product 7 (Scheme 1). Compound 7 was isolated in an acceptable 52% yield. Similarly, p-methoxybenzamide 3 was coupled with m-iodobenzamide 5 to afford a mixture of three coupling products 9, 10, and 8 in 0.2/1/0.5 ratio, with desired cross-coupling product 10 formed predominately. Compound 10 was isolated in 46% yield (Scheme 1). These experiments show that cross-coupling of electronically dissimilar aminoquinoline benzamides occurs with some selectivity, and synthetically viable yields of heterocoupling products can be obtained.
Scheme 1.
Cross-Coupling of Aminoquinoline Benzamides
The directing group can be removed by base-promoted hydrolysis of the amides (eq 1). Thus, dibromo amide 11 was heated in methanol with KOH to afford a 71% yield of 5,5′-dibromo-[1,1′-biphenyl]-2,2′-dicarboxylic acid.
 |
(1) |
In conclusion, we have developed a method for directed, dehydrogenative cobalt-promoted dimerization of aminoquinoline benzamides. Reactions proceed in ethanol solvent in the presence of Mn(OAc)2 cocatalyst and Na2CO3 base and use oxygen as a terminal oxidant. The reactions are functional-group-tolerant, with bromo, iodo, nitro, ether, and ester moieties compatible with the reaction conditions. Cross-coupling of aminoquinoline benzamides proceeds with modest yields and selectivities if electronically dissimilar amides are used. Future directions of the work involve mechanistic studies of the dimerization and isolation of reaction intermediates.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Grant E-1571, Chair E-0044), NIGMS (Grant No. R01GM077635), and University of Houston for supporting this research.
Footnotes
Notes
The authors declare no competing financial interest.
Detailed experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Reviews: Girard SA, Knauber T, Li CJ. Angew Chem, Int Ed. 2014;53:74. doi: 10.1002/anie.201304268.Wu Y, Wang J, Mao F, Kwong FY. Chem—Asian J. 2014;9:26. doi: 10.1002/asia.201300990.Hirano K, Miura M. Chem Commun. 2012;48:10704. doi: 10.1039/c2cc34659a.Cho SH, Kim JY, Kwak J, Chang S. Chem Soc Rev. 2011;40:5068. doi: 10.1039/c1cs15082k.Bugaut X, Glorius F. Angew Chem, Int Ed. 2011;50:7479. doi: 10.1002/anie.201103106.Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d.Hyster TK. Catal Lett. 2015;145:458.
- 2.(a) Do HQ, Daugulis O. J Am Chem Soc. 2009;131:17052. doi: 10.1021/ja907479j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Masui K, Ikegami H, Mori A. J Am Chem Soc. 2004;126:5074. doi: 10.1021/ja031855p. [DOI] [PubMed] [Google Scholar]; (c) Liang Z, Zhao J, Zhang Y. J Org Chem. 2010;75:170. doi: 10.1021/jo902265s. [DOI] [PubMed] [Google Scholar]; (d) Li Y, Wang WH, Yang SD, Li BJ, Feng C, Shi ZJ. Chem Commun. 2010;46:4553. doi: 10.1039/c0cc00486c. [DOI] [PubMed] [Google Scholar]; (e) Xia JB, Wang XQ, You SL. J Org Chem. 2009;74:456. doi: 10.1021/jo802227u. [DOI] [PubMed] [Google Scholar]; (f) Hewgley JB, Stahl SS, Kozlowski MC. J Am Chem Soc. 2008;130:12232. doi: 10.1021/ja804570b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Do HQ, Daugulis O. J Am Chem Soc. 2011;133:13577. doi: 10.1021/ja2047717. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kuhl N, Hopkinson MN, Glorius F. Angew Chem, Int Ed. 2012;51:8230. doi: 10.1002/anie.201203792. [DOI] [PubMed] [Google Scholar]; (c) Fan S, Chen Z, Zhang X. Org Lett. 2012;14:4950. doi: 10.1021/ol3023165. [DOI] [PubMed] [Google Scholar]; (d) Zou LH, Mottweiler J, Priebbenow DL, Wang J, Stubenrauch JA, Bolm C. Chem—Eur J. 2013;19:3302. doi: 10.1002/chem.201204502. [DOI] [PubMed] [Google Scholar]; (e) Mao Z, Wang Z, Xu Z, Huang F, Yu Z, Wang R. Org Lett. 2012;14:3854. doi: 10.1021/ol301517y. [DOI] [PubMed] [Google Scholar]; (f) Stuart DR, Fagnou K. Science. 2007;316:1172. doi: 10.1126/science.1141956. [DOI] [PubMed] [Google Scholar]; (g) Wei Y, Su W. J Am Chem Soc. 2010;132:16377. doi: 10.1021/ja109383e. [DOI] [PubMed] [Google Scholar]; (h) Itahara T. J Chem Soc, Chem Commun. 1981:254. [Google Scholar]; (i) Qin X, Feng B, Dong J, Li X, Xue Y, Lan J, You J. J Org Chem. 2012;77:7677. doi: 10.1021/jo301128y. [DOI] [PubMed] [Google Scholar]; (j) Potavathri S, Pereira KC, Gorelsky SI, Pike A, LeBris AP, DeBoef B. J Am Chem Soc. 2010;132:14676. doi: 10.1021/ja107159b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Han W, Mayer P, Ofial AR. Angew Chem, Int Ed. 2011;50:2178. doi: 10.1002/anie.201006208. [DOI] [PubMed] [Google Scholar]
- 4.Homocoupling: Ackerman LJ, Sadighi JP, Kurtz DM, Labinger JA, Bercaw JE. Organometallics. 2003;22:3884.Kar A, Mangu N, Kaiser HM, Beller M, Tse MK. Chem Commun. 2008:386. doi: 10.1039/b714928j.Yokota T, Sakaguchi S, Ishii Y. Adv Synth Catal. 2002;344:849.Izawa Y, Stahl SS. Adv Synth Catal. 2010;352:3223. doi: 10.1002/adsc.201000771.Rong Y, Li R, Lu W. Organometallics. 2007;26:4376.Cross-coupling: Wencel-Delord J, Nimphius C, Patureau FW, Glorius F. Angew Chem, Int Ed. 2012;51:2247. doi: 10.1002/anie.201107842.Hull KL, Sanford MS. J Am Chem Soc. 2007;129:11904. doi: 10.1021/ja074395z.Wang X, Leow D, Yu JQ. J Am Chem Soc. 2011;133:13864. doi: 10.1021/ja206572w.Campbell AM, Meyer EB, Stahl SS. Chem Commun. 2011;47:10257. doi: 10.1039/c1cc13632a.Shi Z, Li B, Wan X, Cheng J, Fang Z, Cao B, Qin C, Wang Y. Angew Chem, Int Ed. 2007;46:5554. doi: 10.1002/anie.200700590.Zhou L, Lu W. Organometallics. 2012;31:2124.Zhao X, Yeung CS, Dong VM. J Am Chem Soc. 2010;132:5837. doi: 10.1021/ja100783c.
- 5.Chen X, Dobereiner G, Hao XS, Giri R, Maugel N, Yu JQ. Tetrahedron. 2009;65:3085. [Google Scholar]
- 6.(a) Hull KL, Lanni EL, Sanford MS. J Am Chem Soc. 2006;128:14047. doi: 10.1021/ja065718e. [DOI] [PubMed] [Google Scholar]; (b) Pintori DG, Greaney MF. Org Lett. 2011;13:5713. doi: 10.1021/ol202212f. [DOI] [PubMed] [Google Scholar]; (c) Oi S, Sato H, Sugawara S, Inoue Y. Org Lett. 2008;10:1823. doi: 10.1021/ol800439e. [DOI] [PubMed] [Google Scholar]
- 7.Odani R, Nishino M, Hirano K, Satoh T, Miura M. Heterocycles. 2014;88:595. [Google Scholar]
- 8.(a) Zaitsev VG, Shabashov D, Daugulis O. J Am Chem Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; (b) Shabashov D, Daugulis O. J Am Chem Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tran LD, Popov I, Daugulis O. J Am Chem Soc. 2012;134:18237. doi: 10.1021/ja3092278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tran LD, Roane J, Daugulis O. Angew Chem, Int Ed. 2013;52:6043. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Truong T, Klimovica K, Daugulis O. J Am Chem Soc. 2013;135:9342. doi: 10.1021/ja4047125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Roane J, Daugulis O. Org Lett. 2013;15:5842. doi: 10.1021/ol402904d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selected examples. Ni: Aihara Y, Chatani N. J Am Chem Soc. 2014;136:898. doi: 10.1021/ja411715v.Song W, Lackner S, Ackermann L. Angew Chem, Int Ed. 2014;53:2477. doi: 10.1002/anie.201309584.Fe: Asako S, Ilies L, Nakamura E. J Am Chem Soc. 2013;135:17755. doi: 10.1021/ja4106368.Ru: Rouquet G, Chatani N. Chem Sci. 2013;4:2201. doi: 10.1002/anie.201301451.Pd: Zhang SY, He G, Nack WA, Zhao Y, Li Q, Chen G. J Am Chem Soc. 2013;135:2124. doi: 10.1021/ja312277g.He G, Chen G. Angew Chem, Int Ed. 2011;50:5192. doi: 10.1002/anie.201100984.Cu: Nishino M, Hirano K, Satoh T, Miura M. Angew Chem, Int Ed. 2013;52:4457. doi: 10.1002/anie.201300587.
- 10.Grigorjeva L, Daugulis O. Angew Chem, Int Ed. 2014;53:10209. doi: 10.1002/anie.201404579.Grigorjeva L, Daugulis O. Org Lett. 2014;16:4684. doi: 10.1021/ol502005g.Grigorjeva L, Daugulis O. Org Lett. 2014;16:4688. doi: 10.1021/ol502007t. Other recent examples of cobalt-catalyzed C–H bond functionalization: Ilies L, Chen Q, Zeng X, Nakamura E. J Am Chem Soc. 2011;133:5221. doi: 10.1021/ja200645w.Gao K, Yoshikai N. J Am Chem Soc. 2011;133:400. doi: 10.1021/ja108809u.Gao K, Yoshikai N. Angew Chem, Int Ed. 2011;50:6888. doi: 10.1002/anie.201101823.Yoshino T, Ikemoto H, Matsunaga S, Kanai M. Chem—Eur J. 2013;19:9142. doi: 10.1002/chem.201301505.Ikemoto H, Yoshino T, Sakata K, Matsunaga S, Kanai M. J Am Chem Soc. 2014;136:5424. doi: 10.1021/ja5008432.Hummel JR, Ellman JA. J Am Chem Soc. 2015;137:490. doi: 10.1021/ja5116452.Yu DG, Gensch T, de Azambuja F, Vasquez-Cespedes S, Glorius F. J Am Chem Soc. 2014;136:17722. doi: 10.1021/ja511011m.Li J, Ackermann L. Angew Chem, Int Ed. 2014 doi: 10.1002/anie.201409247.Zhang LB, Hao XQ, Zhang SK, Liu ZJ, Zheng XX, Gong JF, Niu JL, Song MP. Angew Chem, Int Ed. 2015;54:272. doi: 10.1002/anie.201409751.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



