Abstract
Gulf War Illness is associated with toxic exposure to cholinergic disruptive chemicals. The cholinergic system has been shown to mediate the central executive of working memory (WM). The current work proposes that impairment of the cholinergic system in Gulf War Illness patients (GWIPs) leads to behavioral and neural deficits of the central executive of WM. A large sample of GWIPs and matched controls (MCs) underwent functional magnetic resonance imaging during a varied-load working memory task. Compared to MCs, GWIPs showed a greater decline in performance as WM-demand increased. Functional imaging suggested that GWIPs evinced separate processing strategies, deferring prefrontal cortex activity from encoding to retrieval for high demand conditions. Greater activity during high-demand encoding predicted greater WM performance. Behavioral data suggest that WM executive strategies are impaired in GWIPs. Functional data further support this hypothesis and suggest that GWIPs utilize less effective strategies during high-demand WM.
Twenty-five percent of the 700,000 troops deployed to the Persian Gulf War during 1990–1991 returned with a chronic and often disabling constellation of symptoms (Research Advisory Committee on Gulf War Veterans’ Illnesses, 2008). This unique symptom cluster, known as Gulf War Illness (GWI), is the most prevalent health issue affecting veterans of this campaign and features: fatigue or sleep issues, widespread neuropathic pain, neurological/mood/cognitive changes (e.g., chronic headaches, cognitive difficulties, mood disturbances), gastrointestinal issues (e.g., chronic diarrhea, abdominal cramping), respiratory issues (e.g., wheezing, coughing), and/or unexplained rashes (Golomb, 2008; Research Advisory Committee on Gulf War Veterans’ Illnesses, 2008). Similar chronic symptomatology is exhibited by populations with either chronic (Ecobichon, 1994) or acute (Yokoyama et al., 1998) cholinergic toxicity. In accordance, strong associations have been found between GWI and exposure to cholinergic (Ch) disruptive chemicals, such as sarin nerve agents, organophosphate pesticides and pyridostigmine bromide (Chao et al., 2010; Golomb, 2008; Haley et al., 2009; Haley and Tuite, 2013; Henderson et al., 2002; Li et al., 2011; Research Advisory Committee on Gulf War Veterans’ Illnesses, 2008; Tuite and Haley, 2013; Haley et al., 2013). Although alternative etiologies of GWI have been proposed (e.g., vaccines, infectious disease, stress), the Ch toxicity hypothesis has been found to be the most consistent with results in both human and animal studies (e.g., Chao et al., 2010; Golomb, 2008; Haley et al., 2009; Haley and Tuite, 2013; Henderson et al., 2002; Li et al., 2011; Research Advisory Committee on Gulf War Veterans’ Illnesses, 2008; Tuite and Haley, 2013; Haley et al., 2013).
The etiology of GWI is thought to result from the delayed effects of toxic exposure to cholinesterase-inhibiting chemicals (Chao et al., 2010; Golomb, 2008; Haley et al., 2009; Henderson et al., 2002; Li et al., 2011; Haley et al., 2013). Toxic increases in acetylcholine availability leads to long-term Ch suppression and central and peripheral nervous system dysfunction in GWI (see Chao et al., 2010; Haley et al., 2013, 2009; Haley and Tuite, 2013; Henderson et al., 2002; Li et al., 2011). Repeated, low-level exposure to cholinesterase-inhibiting chemicals, like those experienced by veterans with GWI, also results in down-regulation of the muscarinic M1 and M3 receptor subtypes (e.g., Henderson et al., 2002). Muscarinic Ch transmission has been robustly linked to cognitive processes (see Bartus, 2000; Bentley et al., 2000; Hasselmo and Sarter, 2011). Specifically, selective action of acetylcholine on the M1 receptor has been shown to mediate working memory (WM) performance (Ragozzino et al., 2012).
WM is a cognitive process that permits moment-to-moment, short-term retention and manipulation of information. The amount of information this process can accommodate is known to have capacity limitations (Baddeley and Hitch, 1974; Cowan, 2001). When the volume of to-be-remembered information exceeds WM capacity (> 4 ± 1 units; Cowan, 2001), central executive strategies are required to reduce the volume of information so as to circumvent capacity constraints (Baddeley and Hitch, 1974). If such strategies are not utilized, item representations become degraded during maintenance due to temporal decay or an inability to keep supra-capacity items active through rehearsal. Thus, both performance speed and accuracy (i.e., efficiency) depend upon central executive strategies as task demand exceeds WM capacity (e.g., Baddeley and Hitch, 1974). The Ch system has been shown in associational (Baddeley et al., 1986, 1991) and experimental (Rusted, 1988; Rusted and Warburton, 1988) studies to be critical for central executive processes of WM.
In one set of studies, Baddeley et al. (1986, 1991) observed distinct central executive dysfunction in a patient population thought to have Ch aberrations (i.e., Alzheimer’s patients). Direct antagonism of the Ch system also has been shown to produce performance deficits on tasks that involve central executive function (Rusted, 1988; Rusted and Warburton, 1988). Rusted and Warburton noted that the underlying WM deficit associated with Ch blockade was an impairment of strategic executive processing in WM (1988). The few studies that have examined the effects of Ch deficits on neural systems during WM performance have reported functional activity differences within lateral prefrontal cortex (PFC) during Ch antagonism (Dumas et al., 2008; Voss et al., 2012). The central executive system has been shown to be mediated by lateral prefrontal cortex (D’Esposito et al., 1995; Rypma and D’Esposito, 1999; Rypma et al., 2002, 1999; Rypma, 2006).
Research has suggested that in high-demand conditions, when WM capacity is exceeded, executive strategies recruited by lateral prefrontal cortex (PFC) during the encoding of information, aid in performance by compressing high volume WM loads (Rypma et al., 1999; Rypma and D’Esposito, 2000). Conversely, lateral PFC activity delayed until individuals are attempting to reconstruct/retrieve this information, has been shown to be indicative of WM performance deficits (Rypma and D’Esposito, 1999, 2000). The tendency to bias lateral PFC activity toward encoding has similarly been found to be related to Ch augmentation and enhanced behavioral performance, where retrieval-based strategies have been associated with Ch blockade (see Bentley et al., 2011).
The present study examines for the first time the extent to which WM performance and lateral PFC systems are affected in GWI. Using a large sample of GWIPs we examined task-related blood-oxygen-level-dependent (BOLD) activity in dorsolateral PFC (DLPFC) and ventrolateral PFC (VLPFC) during delayed-response task performance (Sternberg working memory task [SWMT]; Sternberg, 1966). The literature reviewed above led to the prediction that, if the central executive system was affected in GWI, we would observe WM efficiency deficits with increases in task-demand (i.e., WM-load; Baddeley et al., 1986, 1991; Rusted, 1988; Rusted and Warburton, 1988). This literature also led to the prediction that group differences would emerge in lateral PFC activity associated with increased WM-load. Use of event-related fMRI methodology permitted us to test these hypotheses in encoding, maintenance, and retrieval phases of the SWMT. We hypothesized that if the Ch system was affecting the central executive system of WM, GWIPs would defer lateral PFC activation from the encoding period to the retrieval period for high-demand WM-loads (Bentley et al., 2011; Rypma and D’Esposito 1999, 2000; Rypma et al., 2002).
Materials and Methods
Participants and Procedure
Data were collected from 96 GWIPs and 44 MCs. Participants were screened for GWI using a factor analytic metric that identified unique GWI symptom clusters (Haley et al., 1997; Iannacchione et al., 2011). These unique symptom clusters consisted of 3 primary GWI classes, which were equally represented in the present study. All GWIP diagnoses were confirmed by a physician (RWH) via diagnostic interview. Syndrome 1 (n = 29) was characterized by problems with attention, memory, reasoning, and depression; syndrome 2 (n = 36), by chronic confusion, disorientation, balance disturbance, and impotence; and syndrome 3 (n = 31), by joint and muscle pain, fatigue, and extremity paresthesias (Haley et al., 1997; Iannacchione et al., 2011). No behavioral differences were found on the SWMT between these syndrome classes; thus they were combined for all subsequent analyses (all ps > .05; See Supplementary Table 1). MCs were Gulf War veterans without GWI who were age-, sex-, education-, handedness-, and rank-matched with GWIPs (Supplementary Table 2). GWIPs in the present study reported significantly greater exposure to chemical nerve gas alarms during deployment, as well as greater use of cholinesterase inhibitors (i.e., pyridostigmine bromide) as prophylaxis for sarin nerve agent exposures, compared to MCs (all ps < .001; See Supplementary Table 2). Evidence from a large-scale, epidemiological investigation has shown that such indicators significantly increase the risk of GWI (Haley and Tuite, 2013).
Participants were screened for fMRI contraindicators. All procedures were monitored by trained health professionals. Individuals deemed high-health risks or meeting the criteria for traumatic brain injury were excluded from the study. The current work was part of a multi-investigator, multi-university study. Two samples of Gulf War veterans (i.e., GW Sample; Seabees [35 GWIPs and 16 MCs] and National [61 GWIPs and 28 MCs] samples) were used in this study. Detailed descriptions of sampling procedures and clinical data for these samples can be found in Haley, Kurt, and Horn (Seabees sample [1997]) and Haley et al. (National sample [2013]). As our study groups were of unequal sizes, all distributions were scrutinized for violations of the homogeneity of variance assumption. Where necessary, degrees of freedom were adjusted to account for unequal variance between groups. All procedures were approved by the University of Texas Southwestern Medical Center and University of Texas at Dallas Institutional Review Boards. Participants consented before undergoing any procedure and received monetary compensation for their participation.
Behavioral Measurement
Three runs of the SWMT, each consisting of 54 trials and lasting 5 minutes per run, were administered during fMRI scanning. Each trial featured three task phases in which participants encoded letter stimuli (2 [low demand], 4 [medium demand], or 6 [high demand] letters; i.e., WM-load), maintained the stimuli while viewing a blank screen, and retrieved the stimuli in order to judge whether a letter on the decision screen was located within the to-be-remembered set. Each trial consisted of a 4 s encoding period, an 8 s maintenance period, and a 2 s retrieval period. Items were scored as correct or incorrect; accuracy was assessed as percent of correct trials. Reaction time (RT) was calculated as the average time it took to complete a correct trial. Trials exceeding 2 standard deviations of a participant’s average RT were not included in subsequent analyses. An overall WM efficiency measure was formulated to examine how expeditiously participants completed the SWMT and was calculated as SWMT accuracy scaled by the speed at which individuals completed the task (i.e., SWMT accuracy / SWMT RT). Working memory capacity (WMC) was also calculated on WM-load size 6 (WMC = [hit rate + correct rejection rate – 1] × 6; Cowan, 2001). GWIPs had a median WMC of 4 (Median Absolute Deviation = .83) and MCs had a median WMC of 4.67 (.67). Capacity calculations show that our high-demand condition (i.e., WM-load 6) was examining supra-capacity working memory performance.
Image Acquisition and Processing
Imaging data were acquired using a Siemens 3 Tesla magnet with a 12-channel head coil. High-resolution anatomical, magnetization-prepared rapid acquisition of gradient echo (MPRAGE; Brant-Zawadzki et al., 1992) scans were acquired using the following parameters: T1-weighted type, 1 mm isovoxel, 160 slices/volume, sagittal plane, 3.31 ms echo time, 12° flip angle, 256 × 256 matrix, left to right acquisition, 281 s scan duration. Functional scans during the SWMT were acquired using the following parameters: BOLD signal type, 3.5 mm isovoxel, 44 slices/volume, 197 volumes/run, transaxial plane, 20 ms echo time, 2000 ms repetition time, 90° flip angle, 64 × 64 matrix, foot to head acquisition, 394 s scan duration across 3 runs.
Anatomical data were discarded if they featured any artifact that would interfere with spatial localization (e.g., excessive motion issues, magnetic field inhomogeneities, interference caused by metallic implants). Functional data were discarded if they featured an irreconcilable artifact. This quality assurance protocol excluded a total of 10% from the MC sample and 15% from the GWIP sample. There was not a significant difference between groups in the numbers of excluded participants (p = .367).
Functional data were processed using Analysis of Functional NeuroImages (AFNI; Cox, 1996). After ramp time was removed, functional data were corrected for interleaved slice acquisition and motion effects, and were all aligned to the third functional volume of the first SWMT run. The MPRAGE image was also spatially aligned to the functional data. Data were spatially smoothed (6 mm FWHM Gaussian kernel) and high pass filtered at .015625 Hz. If motion correction parameters indicated presence of movements larger than 1 mm, visual inspection of functional and anatomical alignment was conducted for every time point to ensure that these data were correctly registered.
Task periods versus rest periods were modeled using regressors representing condition and load, for a total of 9 conditions across three runs. These 9 conditions represented encoding, maintenance, and retrieval at WM-load sizes 2, 4, and 6. Functional data were warped to Talairach space (Talairach and Tournaux, 1988). All three SWMT runs were concatenated. and task regressors derived from these three runs were convolved with a gamma-variate hemodynamic response function and were used as independent variables to predict the functional data using a generalized linear model. Four a priori ROIs were placed in standard space for left and right DLPFC (Brodmann’s Areas [BAs] 9 and 46) and VLPFC (BAs 44, 45, 47; Brodmann, 2006/1909). Functional data used in subsequent analyses represented average BOLD percent signal change, per condition and load, in the ROIs.
Results
Both the Seabees and the National samples showed equivalent SWMT performance (p > .05; Supplementary Table 3). Data were therefore combined for all subsequent analyses. However, GW Sample was found to have significant interaction effects with some of the functional repeated measures factors, likely due to age differences between the samples (p < .001). To ensure that GW Sample was not confounded with Group effects, these between-subjects effects were modeled holding GW Sample constant in our repeated measures analyses of the functional data (see below).
Behavioral Results
Group analyses revealed that GWIPs were significantly slower (MGWIP = 1626.03 ms [SEM = 36.96] vs. MMC = 1352.95 ms [54.31]) [t(138) = 4.15, p < .001], and less accurate (MGWIP = .87% [.01] vs. MMC = .93% [.006]) [t(137.44) = −5.36, p < .001] compared to MCs on the SWMT. GWIPs were also less efficient than MCs on the SWMT (MGWIP = 5.6 × 10−4 [1.5 × 10−5] vs. MMC = 7.5 × 10−4 [3.8 × 10−5]) [t(56.86) = −4.52, p < .001] (see also Supplementary Table 4). To test predicted GWIP deficits in WM efficiency as WM-load increases, we planned one-tailed t-tests to compare each group’s change in efficiency from WM-load 2 to 4 items and 2 to 6 items. Compared to MCs, GWIPs were significantly less efficient as WM-load increased from both 2 to 4 items (MGWIP = 1.0 × 10−4 [1.3 × 10−5] vs. MMC = 1.5 × 10−4 [1.9 × 10−5]) [t(83.51) = −2.31, p = .012] and 2 to 6 items (MGWIP = 2.2 × 10−4 [1.5 × 10−5] vs. MMC = 2.7 × 10−4 [2.1 × 10−5]) [t(85.93) = −1.95, p = .028]. A mixed model ANOVA of SWMT efficiency across WM-loads confirmed a significant difference in the slopes of the groups’ performance as load increased via a WM-load × Group interaction [Greenhouse-Geisser corrected F(1.74, 240.04) = 3.45, p = .040].
Regions of Interest Functional Analyses
Four ANOVA models were built to examine percent signal change in BOLD activity across ROIs--left and right DLPFC and left and right VLPFC--as a function of the independent variables, WM-load, and WM-phase. In all four ROIs, repeated measure ANOVAs showed WM-load, WM-phase, and WM-load × WM-phase interactions as significant predictors of change in BOLD signal (ps < .001). To investigate our hypothesis that compared to MCs, GWIPs deferred recruitment of lateral prefrontal cortex from encoding to retrieval at high-demand load sizes, Group was added to the model, holding Sample constant. This mixed-model procedure yielded a significant three-way (WM-load × WM-phase × Group) interaction for right DLPFC [Greenhouse-Geisser corrected F(3.31, 386.86) = 3.36, p = .016] and right VLPFC [Greenhouse-Geisser corrected F(3.47, 406.44) = 4.07, p = .005]. Levene’s tests of equality of error variances for Group showed a single group difference in error variance in right DLPFC at WM-load 2 during maintenance. This result did not affect subsequent analyses, which focused on high-demand load conditions. Three-way interactions were not significant for left DLPFC (p > .05) or left VLPFC (p > .05). Importantly, GW Sample did not affect these results (i.e., WM-load × WM-phase × Group × Sample; p > .05), indicating replicability across the two samples. Significant three-way interactions in right DLPFC and VLPFC showed that there were differences between GWIPs and MCs as WM-load increased and as WM-phase changed.
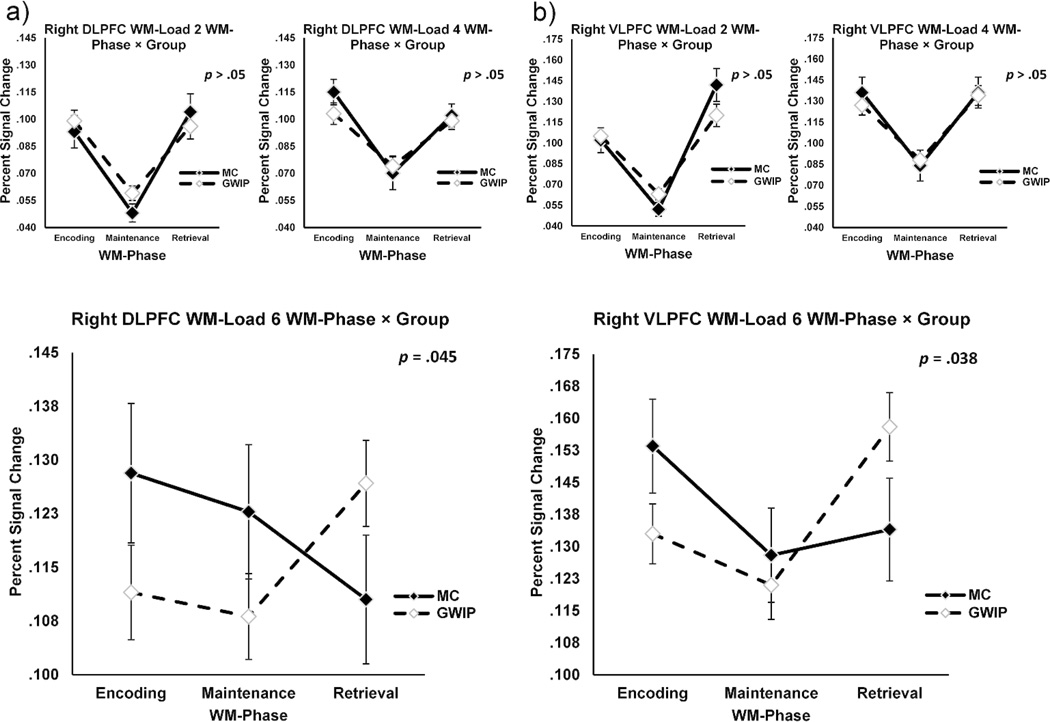
To further model these differences, mixed model ANOVAs were built for both ROIs, examining the interaction between WM-Phase × Group in each Load condition, holding Sample constant (Figure 1). No group differences were found for WM-loads 2 or 4 in BOLD activity across WM-phase for either ROI (p > .05). However, mixed models for WM-load 6 were significant for both right DLPFC and VLPFC activity (Figure 1). At high-demand WM-loads, MCs showed relatively high BOLD percent signal change during the encoding phase, but the BOLD response was attenuated during maintenance and retrieval. In contrast, GWIPs showed relatively depressed encoding and maintenance compared to MCs, but BOLD activity increased during retrieval.
Figure 1.
Repeated measures analyses of WM-phase by group interactions. (a) Right DLPFC percent signal change during encoding, maintenance, and retrieval, at WM-load 2 and 4 (top left) and at WM-load 6 (bottom left). (b) Right VLPFC percent signal change during encoding, maintenance, and retrieval, at WM-load 2 and 4 (top right), and at WM-load 6 (bottom right). p-values represent mixed-model, repeated measures ANOVAs. * = p < .05. Error bars represent standard error of the mean.
Right DLPFC and VLPFC WM-phase Contrasts
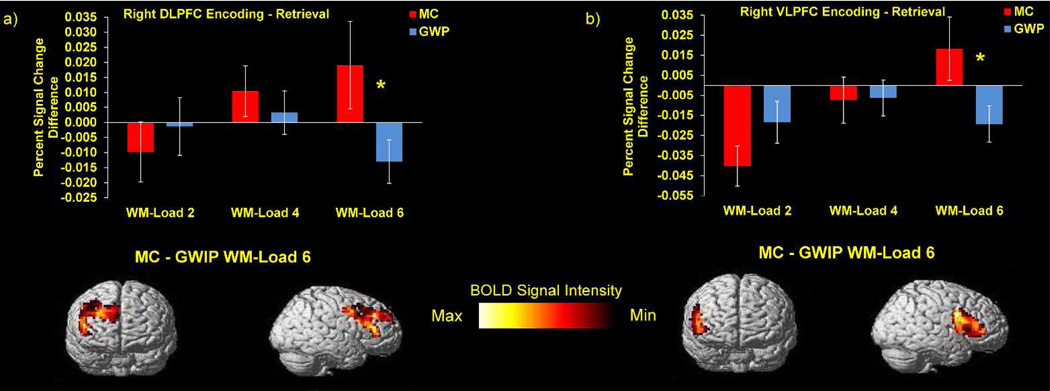
For both groups, peak BOLD activity was observed during high-demand WM-loads. However, this peak activity occurred during retrieval for GWIPs and during encoding for MCs. Encoding–retrieval contrasts were used in right DLPFC and VLPFC to examine the relative change in BOLD activity between these phases (Figure 2). These results showed that, for GWIPs, there was less BOLD activity at encoding than at retrieval (encoding – retrieval = negative) relative to MCs (encoding – retrieval = positive) during high-demand WM. These results illustrated that MCs emphasized encoding activity relative to retrieval activity, whereas GWIPs emphasized retrieval activity relative to encoding activity. Differential emphasis on encoding versus retrieval suggested the hypothesis that GWIPs and MCs use different processing strategies during high-demand WM performance.
Figure 2.
Group differences in encoding and retrieval processing strategies across WM-loads. (a) Bar graph of right DLPFC percent signal change difference between encoding and retrieval across WM-loads (top), MC – GWIP contrast of percent signal change difference in encoding and retrieval strategies at WM-load 6 (bottom). (b) Bar graph of right VLPFC percent signal change difference between encoding and retrieval across WM-loads (top), MC – GWIP contrast of percent signal change difference in encoding and retrieval strategies at WM-load 6 (bottom). * = p < .05. Error bars represent standard error of the mean.
Superior WM performance of MCs relative to GWIPs might occur because MCs implement a more efficient high-demand encoding-based strategy than GWIPs. In testing this hypothesis we found that greater high-demand BOLD activity in right DLPFC and VLPFC during encoding significantly predicted greater WM efficiency [β DLPFC = .067, p = .035, r2 = .037; β VLPFC = .105, p < .001, r2 = .106], whereas no substantive predictive relationship was found between high-demand BOLD activity during maintenance [β DLPFC = .030, p = .374, r2 = .007; β VLPFC = .048, p = .080, r2 = .026] or retrieval [β DLPFC = .040, p = .278, r2 = .010; β VLPFC = .032, p = .219, r2 = .012]. These results hold that greater lateral PFC BOLD activity during high-demand encoding predicted greater WM efficiency. As GWIPs shifted high-demand processing away from encoding and toward retrieval, it is likely that this retrieval-based processing strategy resulted in less efficient performance.
Discussion
In this study, we compared GWIPs to MCs on WM performance and lateral PFC activation to test hypotheses of reduced PFC-related WM function. To our knowledge, this is one of very few fMRI studies to evaluate GWIPs (see Calley et al., 2010; Gopinath et al., 2012; and Odegard et al., 2012). Consistent with our hypotheses, we observed WM performance deficits in GWIPs as well as group-differential BOLD activation in right DLPFC and VLPFC during WM. There were significant differences between groups in WM efficiency and PFC activity, as well as Load × Group interactions on WM efficiency and WM-load × WM-phase × Group on right lateral PFC activity. Further, the data suggested that GWIPs deferred neural processing of high demand WM loads from the encoding to the retrieval phase of the SWMT. Functional imaging and behavioral results supported the hypothesis of central executive dysfunction in GWIPs compared to MCs.
Behavioral results showed that as WM-load size increased from two items, GWIPs had significantly greater declines in efficiency relative to MCs. This and the observed WM-load × Group efficiency interaction implicated that GWIPs evinced a behavioral signature of central executive dysfunction. Taken together, the present results suggest that reduced WM efficiency with increasing WM-load results from an inability to strategically manipulate information for later retrieval, resulting in information degradation or loss (cf. Rypma and D’Esposito, 1999; Salthouse, 1996). Behavioral results also implicate overall WM deficits in GWIPs, possibly reflecting additional short-term storage deficits (Golomb, 2008; Horn et al., 1997). Lateral PFC BOLD activity further suggested a pattern of mediation of these executive processes by the Ch system.
In a review of 63 functional imaging studies of Ch modulation of cognition, Bentley et al. (2011) noted that within PFC, the Ch system aids in neural processing during high-demand conditions. Their review suggested that Ch augmentation increased activity within neural executive systems (particularly DLPFC) under high-demand conditions. This supports the hypothesis of a relationship between acetylcholine availability and additional recruitment of executive prefrontal processes when cognitive systems are at capacity (cf. Bentley et al., 2011). In the present study, group differences in BOLD activity across task phases were observed only at high-demand/supra-capacity conditions. Differences in allocation of BOLD resources occurred during encoding and retrieval phases in right DLPFC and VLPFC. Further, GWIPs showed a greater shift in BOLD activity away from encoding and toward retrieval processes in right DLPFC and VLPFC during supra-capacity WM. However, greater supra-capacity BOLD activity during encoding in right DLPFC and right VLPFC predicted greater WM efficiency, suggesting that GWIPs retrieval-based strategy might not facilitate supra-capacity WM performance. Indeed, encoding and retrieval strategies have also been shown to be mediated by Ch availability (see Bentley et al., 2011).
During episodic memory, Ch augmentation is associated with an increase in neural activity in medial temporal lobe while individuals are encoding information (e.g., Kukolja et al., 2009). Ch augmentation is also associated with a decrease in activity in medial temporal lobe during retrieval (e.g., Kukolja et al., 2009). Ch antagonism has been shown to have the opposite effect by attenuating encoding and facilitating retrieval processes (see Bentley et al., 2011). Accordingly, it has been postulated that the Ch system might mediate enhancement of incoming information by inhibiting interference from parallel internal (retrieval) processes (Hasselmo, 1995; Hasselmo and Giocomo, 2006; Hasselmo and McGaughy, 2004). To wit, animal and computational models reveal that high acetylcholine levels potentiate encoding by inhibiting feedback “noise” from internal processing (e.g., Hasselmo, 1995; Hasselmo and McGaughy, 2004). During supra-capacity WM conditions, it’s likely that Ch signals to and from lateral PFC follow a similar pattern as those in medial temporal lobe.
Lateral PFC is innervated by the Ch system via lateral ascending fibers from the basal forebrain (Selden et al., 1998). Moreover, basal forebrain is thought to be employed via descending fibers from PFC when executive control of mental resources is required (Sarter et al., 2006). Ch ascending fibers to lateral PFC and descending fibers to basal forebrain have been proposed as mediating executive functions in the cortex when increased effort is necessary (Sarter et al., 2006). Damage to this basal forebrain-lateral PFC circuit in GWI, due to exposure to Ch disruptive agents, would inhibit GWIPs’ ability to adequately recruit encoding processes during high-demand WM (i.e., supra-capacity). This failure would place inordinate demands upon retrieval processes to scan a larger and more degraded memory set, reducing accuracy and increasing RT (i.e., reducing efficiency).
The present results showed behavioral deficits and deferred activation of lateral prefrontal processing for high-demand (i.e., supra-capacity) WM-loads in GWIPs. Impairment of the Ch system in GWIPs is posited as contributing to the maladaptive central executive processing strategies observed in GWIPs. Impairments to the Ch system probably exert effects in brain regions outside of lateral PFC. Exploration of these effects awaits future research. The present results lend insight into the cognitive changes associated with GWI and suggests future directions examining the central executive sequelae of patient populations with Ch deficits (e.g., Alzheimer’ Disease [Terry and Buccafusco, 2003], Autism [Deutsch et al., 2010], and Schizophrenia [AhnAllen, 2012]).
Supplementary Material
Acknowledgments
This work was supported by the Friends of Brain Health research endowment (to NAH), the Department of Veterans Affairs (VA549-P-0027 to RWH), and the National Institutes of Health (1R01AG029523 to BR). The authors wish to thank Mr. Vamsi Daliparthi, Mr. Travis Weaver, and Ms. Angela Burke for their exceptional efforts on various aspects of data quality control and manuscript preparation. We would also like to thank Mr. Andrew Hillis, Ms. Lee Jordan, Dr. Mary Jo Maciejewski, and Ms. Traci Sandoval for their technical support.
Footnotes
Financial Disclosures
NAH, JLH, MAM, IJB, RMB, ES-K, and BR declare no biomedical financial interests or potential conflicts of interest. RWH received an honorarium from Targeted Medical Pharma, Inc. for a review of a Food and Drug Administration application for a nonpharmaceutical medication to treat fatigue, with possible benefit to Gulf War Illness patients.
References
- AhnAllen CG. The role of the alpha7 nicotinic receptor in cognitive processing of persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):103–108. doi: 10.1097/YCO.0b013e3283503637. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower G, editor. Recent Advances in Learning and Motivation. Vol. 8. Academic Press; New York: 1974. pp. 47–90. [Google Scholar]
- Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. Q J of Exp Psychol. 1986;32A:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s Disease. Brain. 1991;114:2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative disease, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: Insights from human pharmacological functional neuroimaging. Prog Neurobiol. 2011;94:360–388. doi: 10.1016/j.pneurobio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant-Zawadzki M, Gillan GD, Nitz WR. MPRAGE: A three dimensional, T1-weighted, gradient-echo sequence—initial experience in the brain. Radiology. 1992;182:769–775. doi: 10.1148/radiology.182.3.1535892. [DOI] [PubMed] [Google Scholar]
- Brodmann K. In: Brodmann’s localization in the cerebral cortex: The principles of comparative localization in the cerebral cortex based on cytoarchitectonics, trans. Garey LJ, editor. New York: Springer; 2006/1909. [Google Scholar]
- Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in U.S. veterans. Neurotoxicology. 2010;31(5):493–501. doi: 10.1016/j.neuro.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calley CS, Kraut MA, Spence JS, Briggs RW, et al. The neuroanatomic correlates of semantic memory deficits in patients with Gulf War Illness: a pilot study. Brain Imaging and Behavior. 2010;4(4):248–255. doi: 10.1007/s11682-010-9103-2. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Urbano MR, Neumann SA, Burket JA, Katz JA. Cholinergic abnormalities in autism: is there a rationale for selective nicotinic agonist interventions? Clin Neuropaharmacol. 2010;33(3):114–120. doi: 10.1097/WNF.0b013e3181d6f7ad. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Saykin AJ, McAllister TW, McDonald BC, et al. Nicotinic versus muscarinic blockade alters verbal working memory-related brain activity in older women. Am J Geriatr Psychiatry. 2008;16(4):272–282. doi: 10.1097/JGP.0b013e3181602a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecobichon DJ. Organophosphorus ester insecticides. In: Ecobichon DJ, Joy RM, editors. Pesticides and Neurological Diseases. 2nd ed. Boston, MA: CRC Press Inc; 1994. pp. 171–250. [Google Scholar]
- Gopinath K, Gandi P, Goyal A, Jiang L, et al. FMRI reveals abnormal central processing of sensory and pain stimuli in ill Gulf War veterans. Neurotoxicology. 2012;33(3):261–271. doi: 10.1016/j.neuro.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley RW, Kurt TL, Hom J. Is there a Gulf War Syndrome? Searching for syndromes by factor analysis of symptoms. JAMA. 1997;277:215–222. [PubMed] [Google Scholar]
- Haley RW, Spence JS, Carmack PS, Gunst RF, et al. Abnormal brain response to cholinergic challenge in chronic encephalopathy from the 1991 Gulf War. Psychiatry Res: Neuroimaging. 2009;171:207–220. doi: 10.1016/j.pscychresns.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Haley RW, Tuite JJ. Epidemiological evidence of health effects from long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemiology. 2013;40(3):178–189. doi: 10.1159/000345124. [DOI] [PubMed] [Google Scholar]
- Haley RW, Charuvastra E, Shell WE, Buhner DM, et al. Cholinergic autonomic dysfunction in veterans with Gulf War Illness: Confirmation in a population-based sample. JAMA Neurol. 2013;70(2):191–200. doi: 10.1001/jamaneurol.2013.596. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidations. Prog in Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM. Cholinergic modulation of cortical function. J Mol Neurosci. 2006;30(1–2):133–135. doi: 10.1385/JMN:30:1:133. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharn. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RF, Barr EB, Blackwell WB, Clark CR, et al. Response of rats to low levels of sarin. Tox and Applied Pharma. 2002;184:67–76. [PubMed] [Google Scholar]
- Horn J, Haley RW, Kurt TL. Neuropsychological correlates of Gulf War syndrome. Arch Clin Neuropsychol. 1997;12(6):531–544. [PubMed] [Google Scholar]
- Iannacchione VG, Dever JA, Bann CM, Considine KA, et al. Validation of a research case definition of Gulf War Illness in the 1991 US Military population. Neuroepidemiology. 2011;37:129–140. doi: 10.1159/000331478. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Fink GR. Cholinergic stimulation enhances neural activity with encoding but reduces neural activity associated with retrieval in humans. J Neurosci. 2009;29(5):8119–8128. doi: 10.1523/JNEUROSCI.0203-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Spence JS, Buhner DM, Hart J, Jr, et al. Hippocampal dysfunction in Gulf War veterans: Investigation with ASL perfusion MR imaging and physostimine challenge. Neuroradiology. 2011;261(1):218–225. doi: 10.1148/radiol.11101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard TN, Cooper CM, Farris EA, Arduengo J, et al. Memory impairment exhibited by veterans with Gulf War Illness. Neurocase. 2012 doi: 10.1080/13554794.2012.667126. advance online publication. Retrieved from: http://www.tandfonline.com/doi/abs/10.1080/13554794.2012.667126?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed&#.Ub0AN5zAxnQ. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Artis S, Singh A, Twose TM, et al. The selective M1 muscarinic cholinergic agonist CDD-0102A enhances working memory and cognitive flexibility. J Pharmacol Exp Ther. 2012;340(3):588–594. doi: 10.1124/jpet.111.187625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Advisory Committee on Gulf War Veterans’ Illnesses. Gulf War Illness and the health of Gulf War veterans. Washington: Department of Veterans Affairs; 2008. Retrieved from: http://www1.va.gov/rac-gwvi/. [Google Scholar]
- Rusted JM. Dissociative effects of scopolamine on working memory in healthy young volunteers. Psychopharmacology. 1988;96:487–492. doi: 10.1007/BF02180029. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Warburton DM. The effects of scopolamine on working memory in healthy young volunteers. Psychopharmocology. 1988;96:145–152. doi: 10.1007/BF00177553. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. The roles of prefrontal brain regions in components of working memory: Effects of memory load and individual differences. PNAS. 1999;96:6448–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrielli JD. Load dependent roles of frontal brain regions in the maintenance of working memory. NeuroImage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience. 2000;3(5):509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Rypma B, Gabrieli JDE. Functional neuroimaging of short-term memory: the neural mechanisms of mental storage. Behav Brain Sci. 2001;24:143. [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cog Neuroscience. 2002;14(5):721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Rypma B. Factors controlling neural activity during delayed-response task performance: Testing a memory organization hypothesis of prefrontal function. Neuroscience. 2006;139(1):223–225. doi: 10.1016/j.neuroscience.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: The neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam M-M. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournaux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s Disease-related cognitive deficits: Recent challenges and their implications for novel drug development. JPET. 2003;306(3):821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- Tuite JJ, Haley RW. Meterological and intelligence evidence of long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemilogy. 2013;40(3):160–177. doi: 10.1159/000345123. [DOI] [PubMed] [Google Scholar]
- Voss B, Thienel R, Reske M, Kellermann T, et al. Cholinergic blockade under working memory demands encountered by increase rehearsal strategies: evidence from fMRI in healthy subjects. Eur Arch Psychiatry Clin Neurosci. 2012;262(4):329–339. doi: 10.1007/s00406-011-0267-6. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Araki S, Murata K, Nishikitani M, et al. Chronic neurobehavioral and central and autonomic nervous system effects of Tokyo subway sarin poisoning. J Physiol Paris. 1998;92(3–4):317–323. doi: 10.1016/s0928-4257(98)80040-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.