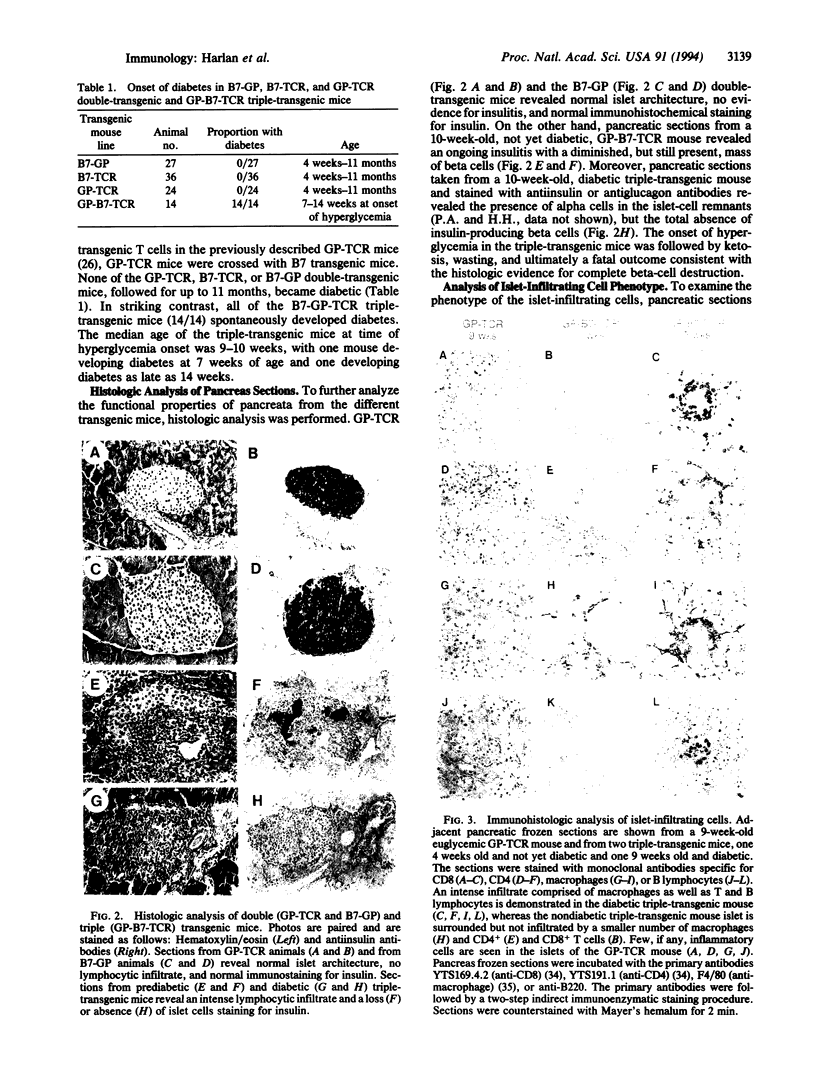
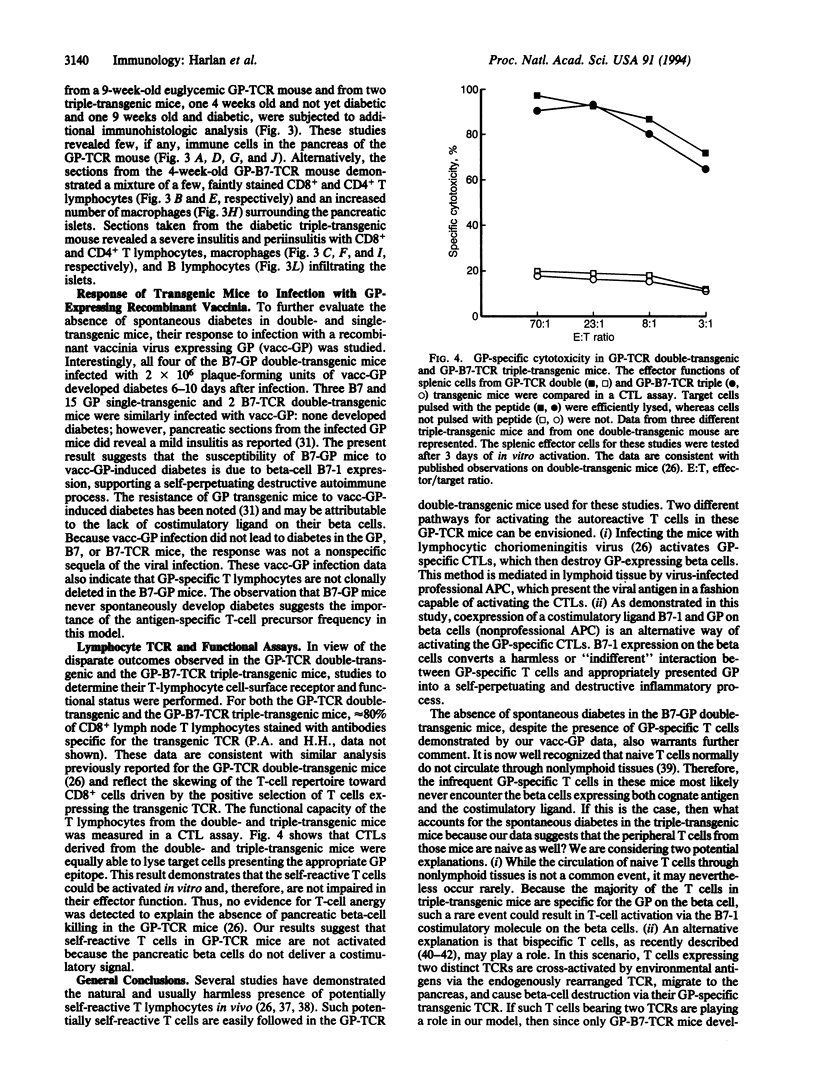
Abstract
T lymphocytes have been implicated in the onset of many autoimmune diseases; however, the mechanisms underlying T-cell activation toward self antigens are poorly understood. To study whether T-lymphocyte costimulation can overcome the immunologic unresponsiveness observed in an in vivo model, we have created transgenic mice expressing the costimulatory mouse molecule B7-1, a ligand for the CD28 receptor, on pancreatic beta cells. We now report that triple-transgenic mice expressing both B7-1 and a viral glycoprotein on their beta cells, along with T cells expressing the viral-glycoprotein-specific transgenic T-cell receptor, all develop insulitis (lymphocytic infiltration of the pancreatic islets) and diabetes. In striking contrast, the T cells in double-transgenic mice expressing the same viral glycoprotein (but no B7) on their pancreatic beta cells and the transgenic T-cell receptor on their T cells, reported earlier, remain indifferent to the glycoprotein-expressing beta cells. In fact, all three transgenes are required to initiate immune-mediated destruction of the beta cells. Mice expressing any of the transgenes alone, or any two in combination, maintain normal islet architecture and never spontaneously develop insulitis or diabetes. Our results show that aberrant B7 expression on peripheral tissues may play an important role in the activation of self-reactive T cells and further suggest that abnormal expression of costimulatory receptors may be involved in various T-cell-mediated autoimmune diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Borgulya P., Kishi H., Uematsu Y., von Boehmer H. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell. 1992 May 1;69(3):529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- Brändle D., Müller C., Rülicke T., Hengartner H., Pircher H. Engagement of the T-cell receptor during positive selection in the thymus down-regulates RAG-1 expression. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9529–9533. doi: 10.1073/pnas.89.20.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtles S. S., Trembleau S., Drexler K., Hurtenbach U. Absence of T cell tolerance to pancreatic islet cells. J Immunol. 1992 Sep 15;149(6):2185–2193. [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G. J., Rhynhart K., Nadler L. M. Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991 Oct 15;137(2):429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Hathcock K. S., Laszlo G., McKnight A. J., Kim J., Du L., Lombard D. B. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993 Nov 5;262(5135):907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Segil J. M., Lee G., Whitman J. F., Nadler L. M. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989 Oct 15;143(8):2714–2722. [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Bradshaw J., Linsley P., Hodes R. J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993 Nov 5;262(5135):905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Koulova L., Clark E. A., Shu G., Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991 Mar 1;173(3):759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake R. A., O'Hehir R. E., Verhoef A., Lamb J. R. CD28 mRNA rapidly decays when activated T cells are functionally anergized with specific peptide. Int Immunol. 1993 May;5(5):461–466. doi: 10.1093/intimm/5.5.461. [DOI] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D. J., Zeng Y., Thistlethwaite J. R., Montag A., Brady W., Gibson M. G., Linsley P. S., Bluestone J. A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992 Aug 7;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Linsley P. S. Costimulation of T-cell growth. Curr Opin Immunol. 1992 Jun;4(3):265–270. doi: 10.1016/0952-7915(92)90075-p. [DOI] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Mackay C. R. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993 Jun;5(3):423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Lee K., Turka L. A., Green J., Thompson C., Shimizu Y. Discordant expression of CD28 ligands, BB-1, and B7 on keratinocytes in vitro and psoriatic cells in vivo. Am J Pathol. 1993 Apr;142(4):1029–1040. [PMC free article] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Aichele P., Pircher H., Odermatt B., Herrera P., Higuchi Y., Buerki K., Hengartner H., Zinkernagel R. M. Induction of diabetes is influenced by the infectious virus and local expression of MHC class I and tumor necrosis factor-alpha. J Immunol. 1993 Jun 1;150(11):5185–5194. [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Padovan E., Casorati G., Dellabona P., Meyer S., Brockhaus M., Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993 Oct 15;262(5132):422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- Pircher H., Baenziger J., Schilham M., Sado T., Kamisaku H., Hengartner H., Zinkernagel R. M. Characterization of virus-specific cytotoxic T cell clones from allogeneic bone marrow chimeras. Eur J Immunol. 1987 Feb;17(2):159–166. doi: 10.1002/eji.1830170202. [DOI] [PubMed] [Google Scholar]
- Razi-Wolf Z., Freeman G. J., Galvin F., Benacerraf B., Nadler L., Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4210–4214. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Klöppel G., Madsen O. D., Storch M. J., Heitz P. U. Distribution patterns of proinsulin and insulin in human insulinomas: an immunohistochemical analysis in 76 tumors. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;63(1):51–61. doi: 10.1007/BF02899244. [DOI] [PubMed] [Google Scholar]
- Sansom D. M., Hall N. D. B7/BB1, the ligand for CD28, is expressed on repeatedly activated human T cells in vitro. Eur J Immunol. 1993 Jan;23(1):295–298. doi: 10.1002/eji.1830230148. [DOI] [PubMed] [Google Scholar]
- Schluesener H. J., Wekerle H. Autoaggressive T lymphocyte lines recognizing the encephalitogenic region of myelin basic protein: in vitro selection from unprimed rat T lymphocyte populations. J Immunol. 1985 Nov;135(5):3128–3133. [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992 Dec 24;71(7):1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- Shahinian A., Pfeffer K., Lee K. P., Kündig T. M., Kishihara K., Wakeham A., Kawai K., Ohashi P. S., Thompson C. B., Mak T. W. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993 Jul 30;261(5121):609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Storch M. J., Petersen K. G., Licht T., Kerp L. Recognition of human insulin and proinsulin by monoclonal antibodies. Diabetes. 1985 Aug;34(8):808–811. doi: 10.2337/diab.34.8.808. [DOI] [PubMed] [Google Scholar]
- Taketo M., Schroeder A. C., Mobraaten L. E., Gunning K. B., Hanten G., Fox R. R., Roderick T. H., Stewart C. L., Lilly F., Hansen C. T. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Lin H., Brady W., Leiden J. M., Wei R. Q., Gibson M. L., Zheng X. G., Myrdal S., Gordon D. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Paine R., 3rd, Schieven G. L., Thompson G. B., Ledbetter J. A. Signal transduction via CD4, CD8, and CD28 in mature and immature thymocytes. Implications for thymic selection. J Immunol. 1991 Mar 1;146(5):1428–1436. [PubMed] [Google Scholar]
- Vandenberghe P., Delabie J., de Boer M., De Wolf-Peeters C., Ceuppens J. L. In situ expression of B7/BB1 on antigen-presenting cells and activated B cells: an immunohistochemical study. Int Immunol. 1993 Mar;5(3):317–321. doi: 10.1093/intimm/5.3.317. [DOI] [PubMed] [Google Scholar]