Abstract
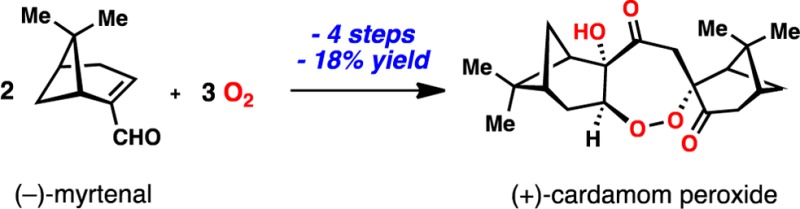
A four-step synthesis of the antimalarial terpene cardamom peroxide, a 1,2-dioxepane-containing natural product, is reported from (−)-myrtenal and molecular oxygen. This highly concise route was guided by biosynthetic logic and enabled by an unusual manganese-catalyzed, tandem hydroperoxidation reaction. The absolute configuration of the cardamom peroxide is reported, and its mode of fragmentation following Fe(II)-mediated endoperoxide reduction is established. These studies reveal the generation of reactive intermediates distinct from previously studied endoperoxide natural products.
Organic peroxides are pertinent to numerous facets of human health ranging from intermediates in lipid oxidation to potential treatments for cancer.1 Notwithstanding debate over their mechanism(s) of action,2 terpene-derived endoperoxides and derivatives thereof have proven exceptionally useful to combat malaria, a disease which affects several hundred million people annually (Figure 1a).3 Artemisinin combination therapy (ACT), which includes derivatives of the sesquiterpene lactone artemisinin (1), has remained a frontline treatment for chloroquine-resistant malaria for many years, yet reports of delayed parasite clearance in patients receiving ACTs are beginning to surface.4 Undoubtedly, the search for promising new antimalarials will continue, and organic peroxides, which can generate numerous and diverse cytotoxic intermediates,2c continue to receive much attention. However, the number of readily available peroxide-containing natural products, from which comprehensive medicinal chemistry efforts can be leveraged, remains quite limited and the economic constraints associated with small-molecule treatments for this disease requires the design and execution of exceedingly simple solutions if synthetic chemistry is to be employed.5,6 The cardamom peroxide (2), isolated by Clardy and co-workers7 from Amomum krervanh Pierre (Siam cardamom), displays potent activity against P. falciparum (EC50 = 170 nM) and possesses a 1,2-dioxepane motif distinct from the trioxane found in 1. While many peroxide-containing natural products have been isolated to date, only a very small subset contains these 7-membered peroxidic architectures.8,9 These attributes, combined with a complete lack of further biological investigation, unknown absolute configuration, and mysterious biosynthetic origins, make this terpene endoperoxide an attractive synthetic target and a valuable lead compound. We were drawn to hypothetical eq 1 (blue box, Figure 1b), wherein inexpensive pinene (∼US$0.02/gram) and multiple units of molecular oxygen serve as the sole building blocks of 2, as a blueprint for a simple synthesis of 2. Herein we report a four-step, enantiospecific synthesis of the cardamom peroxide adhering to this idea, determination of its absolute configuration, and elucidation of its mode of fragmentation following Fe(II)-mediated reductive activation.
Figure 1.
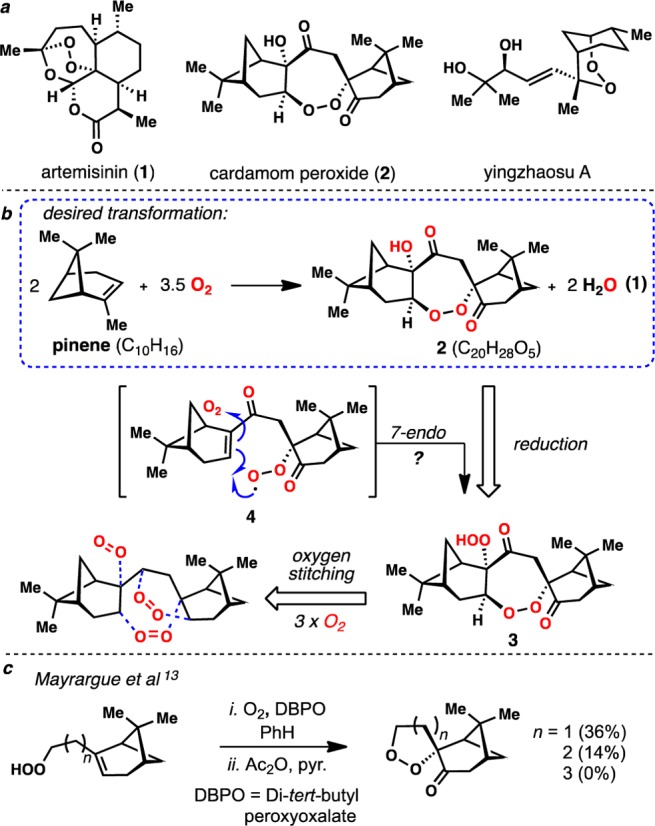
(a) Various terpene-derived endoperoxides with potent antimalarial activity. (b) Desired hypothetical transformation and oxygen stitching blueprint. (c) Previous attempts to forge the 1,2-dioxepane motif in 2 via peroxy radical cyclization have failed.
The cardamom peroxide (2) appears to be a dimeric monoterpene, and this recognition offers simplification to the construction of its carbocyclic ring system. The origin of the plethora of oxygen atoms, especially the bridging 7-membered endoperoxide, however, remains mysterious, as the mechanism by which endoperoxides are incorporated onto terpene frameworks is, in many cases, not well understood.10 We speculated that 2 might initially be formed in nature as diperoxide 3 (Figure 1b). This hypothesis hinted that: (i) two monoterpene units and three units of molecular oxygen are the building blocks of 2, (ii) the two carbonyl groups stem from the same molecule of oxygen, and (iii) the bridging endoperoxide is formed via a 7-endo cyclization of a peroxy radical (see 4→3).11,12 While attractive on paper, the known preference of peroxy radicals to cyclize in a 6-exo manner was disconcerting as was the inability of Mayrargue and co-workers to forge a 1,2-dioxepane ring system via peroxy radical cyclization en route to 2 (Figure 1c).13,14
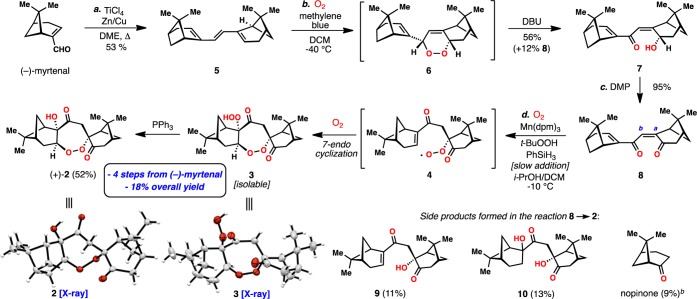
Seeking to corroborate these ideas through synthesis, we began by preparing the dimeric C-20 carbon skeleton of 2, and the venerable McMurray coupling afforded C2-symmetric triene 5 in one step from inexpensive (−)-myrtenal (Scheme 1). Subjecting this sensitive material to singlet oxygen installed the first unit of molecular oxygen via a [4 + 2] cycloaddition reaction, and following Kornblum–DeLaMare rearrangement15 of intermediate endoperoxide 6, dienone 7 and small amounts of 8, presumably formed via air oxidation, were furnished in good yield. A subsequent Dess-Martin periodinane oxidation smoothly converted the remainder of 7 into 8. Taking inspiration from the pioneering work of Mukaiyama,16 Isayama,17 and Magnus,18 on formal Co- and Mn-catalyzed olefin hydration and hydroperoxidation, in addition to several other powerful hydrofunctionalization processes,19,20 we envisioned that if a chemo- and regioselective hydrometalation of the a–b bond in 8 could be achieved, postulated biosynthetic intermediate 4 might be generated in the presence of oxygen. While the conditions employed for such hydrofunctionalization reactions are quite mild, substrate 8 presents a unique challenge to this chemistry with regard to chemo-, regio-, and diastereoselectivity. Nevertheless, after extensive experimentation it was discovered that when a solution of PhSiH3 was added slowly over 12 h to a cold (−10 °C) and vigorously oxygenated DCM/i-PrOH solution of 8, Mn(dpm)3 (20 mol %), and t-BuOOH (1.5 equiv), diperoxide 3 was formed, and following addition of PPh3, the cardamom peroxide could be isolated in 52% yield as a single isomer. The structures of both 2 and the intermediate diperoxide 3 were confirmed by X-ray crystallography. The selectivity of this tandem hydroperoxidation process is quite remarkable: (i) addition of the putative manganese hydride occurs preferentially across the a–b bond in a chemo- and regioselective manner, (ii) the presumed manganese enolate reacts with oxygen diastereoselectively, (iii) the resulting peroxy radical undergoes 7-endo cyclization, (iv) the resulting radical is stereoselectively quenched with an additional molecule of oxygen from the exo-face of the newly formed ring system, and (v) the terminal peroxide is chemoselectively reduced. The chiral pinane-type rings subtly orchestrate much of the selectivity imparted to this complex process. Nonetheless, achieving synthetically useful yields of 2 required the evaluation and fine-tuning of numerous catalytic systems (Table 1). While conditions employing Mn(dpm)318,19b,19d,19l quickly emerged as superior to other common metal hydride generating systems based on iron19h,19i,19k and cobalt,16,17 yields of 2 still remained modest (entries 1–5). Suspecting that premature reduction of peroxy radical 4 contributed to diminished yields of 2 and side product formation (i.e 9, 10, and nopinone), slow addition of the phenylsilane was employed and increased the yield of 2 to 41% (entry 6). Finally, the incorporation of t-BuOOH (1.5 equiv),19c,19e,19f,19l combined with slow PhSiH3 addition, furnished synthetically useful and reproducible quantities of 2 (52% isolated yield, entry 7). Utilizing this four-step synthetic sequence, over half a gram of cardamom peroxide has been prepared to date. Interestingly synthetic 2 constructed from (−)-myrtenal exhibited [α]D = +123.2° (c = 0.005 g/mL, hexanes), reported [α]D = +111.35° (concentration not reported). Thus the absolute configuration of the monoterpene units that comprise 2 appear to be opposite to what was previously assumed on the basis of simple monoterpenes (i.e., myrtenol) of known absolute configuration isolated alongside 2 from Amomum krervanh Pierre.7
Scheme 1. Four-Step Synthesis of (+)-Cardamom Peroxide from (−)-Myrtenal and Oxygen.
Reagents and conditions: a) TiCl4 (10 equiv), Zn/Cu (40 equiv), DME, 65 °C, 5 h, then add (−)-myrtenal (1.0 equiv), 65 °C, 48 h (53%); b) O2 (1 atm), methylene blue (2 mol %), DCM, −40 °C, 2 h, then add DBU (5 equiv), −40 °C → −20 °C, 4 h (56% 7 and 12% 8); c) DMP (1.3 equiv), DCM, rt, 1 h, (95%); d) Mn(dpm)3 (20 mol %), t-BuOOH (1.5 equiv), PhSiH3 (2.5 equiv) added over 12 h, O2 (1 atm), i-PrOH/DCM (6.5:1), −10 °C, 12 h, then add PPh3 (52% 2, 9% nopinone, 11% 9, 13% 10); DCM = dichloromethane, DME = 1,2-dimethoxyethane, THF = tetrahydrofuran, DBU = 1,8-Diazabicyclo[5.4.0]undec-7-ene, DMP = Dess-Martin Periodinane, dpm = Tris(dipivaloylmethanato). b Yield determined by GC.
Table 1. Metal-Catalyzed Synthesis of 2: Selected Optimizationa,b.
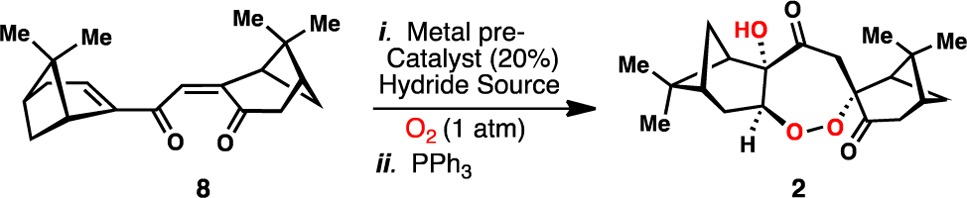
| entry | conditions | isolated yield (%)a |
|---|---|---|
| 1 | Fe2(ox)3·6H2O (5 equiv), NaBH4 (6.4 equiv), EtOH/H2O, 0 °C | 0 |
| 2 | Fe∥(Pc), NaBH4 (3.0 equiv), EtOH, 0 °C | 0 |
| 3 | Fe(acac)3, PhSiH3 (2.5 equiv), EtOH, 0 °C → rt | 0 |
| 4 | Co(acac)2, PhSiH3 (2.5 equiv), DCM/i-PrOH, –10 °C → rt | 6 |
| 5 | Mn(dpm)3, PhSiH3 (2.5 equiv), DCM/i-PrOH, –10 °C | 34 |
| 6 | Mn(dpm)3, PhSiH3 (2.5 equiv), DCM/i-PrOH, –10 °C | 41b |
| 7 | Mn(dpm)3, PhSiH3 (2.5 equiv), t-BuOOH (1.5 equiv), DCM/i-PrOH, –10 °C | 52b |
Reaction performed on a 0.1 mmol scale using 20 mol % of metal catalyst unless otherwise stated.
Phenylsilane added slowly over 12 h as a solution in DCM. Pc = Phthalocyanine. ox = oxalate, acac = acetylacetonate.
Fe(II)-mediated O–O bond reduction remains the hallmark of peroxide-based antimalarials, and a strong consensus exists that oxygen-centered radicals are initially formed.21 These fleeting intermediates are then believed to lead to a variety of toxic, downstream species, including carbon-centered radicals, carbocations, and epoxides, which vary in structure and reactivity depending on the starting endoperoxides employed.2c,21 With sufficient quantities of cardamom peroxide in hand, we sought to determine its mode of reductive cleavage (Figure 2). Subjecting 2 to stoichiometric FeCl2 in degassed MeCN/H2O at room temperature led to the formation of three isolable cleavage products identified as acids 11, 12, and 13 (the structures of 11 and 13 were verified by X-ray crystallography). Presumably the cardamom peroxide generates an acyl radical intermediate (15) via C–C cleavage of oxygen-centered radical 14. Oxidation of 15 by Fe(III) produces an acylium ion (16) which in this case is quenched by water to form 12.22 The remaining hydroxyl groups then engage the 1,3- dicarbonyl motif in a cyclodehydration process affording 11 and small amounts of 13.23
Figure 2.
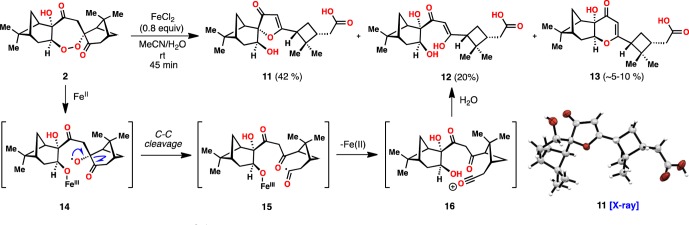
Reductive activation of the cardamom peroxide.
In conclusion, an enantiospecific synthesis of the complex cardamom peroxide (2), a 1,2-dioxepane-containing terpene and nanomolar inhibitor of P. falciparum, has been developed with a high level of efficiency. In addition to securing substantial quantities of 2, confirming its absolute configuration, and elucidating its mode of reductive cleavage, this work highlights an unusual example of large-ring endoperoxide synthesis via peroxyradical cyclization–a maneuver that could find use in the synthesis of other peroxide-containing natural products. Perhaps most importantly, however, by revealing the hidden symmetry elements present in 2, a blueprint for the synthesis of complex peroxide-containing compounds from simple, readily available building blocks and inexpensive reagents has been drawn. Efforts to expand on nature’s collection of biologically active endoperoxides are underway and will be reported in due course.
Acknowledgments
This work was supported by generous start-up funds from UC-Berkeley. We thank Dr. Chris Canlas and Daniel T. Bregante for NMR spectroscopic and technical assistance respectively. Dr. Antonio DiPasquale is acknowledged for X-ray crystallographic analysis and support from the NIH Shared Instrument Grant (S10-RR027172).
Supporting Information Available
Experimental details and spectroscopic data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Spiteller G. Free Radic. Biol. Med. 2006, 41, 362. [DOI] [PubMed] [Google Scholar]; b Dembitsky V. M.; Gloriozova T. A.; Poroikov V. V. Mini Rev. Med. Chem. 2007, 7, 571. [DOI] [PubMed] [Google Scholar]; c Chaturvedi D.; Goswami A.; Saikia P. P.; Barua N. C.; Rao P. G. Chem. Soc. Rev. 2010, 39, 435. [DOI] [PubMed] [Google Scholar]
- For recent discussions on this topic, see:; a Golenser J.; Waknine J. H.; Krugliak M.; Hunt N. H.; Grau G. E. Int. J. Parasitol. 2006, 36, 1427. [DOI] [PubMed] [Google Scholar]; b O’Neill P. M.; Barton V. E.; Ward S. A. Molecules 2010, 15, 1705. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Posner G. H.; O’Neill P. M. Acc. Chem. Res. 2004, 37, 397. [DOI] [PubMed] [Google Scholar]
- For recent reviews on synthetic O–O bond-containing antimalarials, see:; a Slack R. D.; Jacobine A. M.; Posner G. H. Med. Chem. Commun. 2012, 3, 281. [Google Scholar]; b Tang Y.; Dong Y.; Vennerstrom J. L. Med. Res. Rev. 2004, 24, 425. [DOI] [PubMed] [Google Scholar]; c Muraleedharan K. M.; Avery M. A. Drug Discov. Today 2009, 14, 793. [DOI] [PubMed] [Google Scholar]
- Dondorp A. M.; Nosten F.; Yi P.; Das D.; Phyo A. P.; Tarning J.; Lwin K. M.; Ariey F.; Hanpithakpong W.; Lee S. J.; Ringwald P.; Silamut K.; Imwong M.; Chotivanich K.; Lim P.; Herdman T.; An S. S.; Yeung S.; Singhasivanon S. P.; Day N. P.; Lindegardh N.; Socheat D.; White N. J. N. Engl. J. Med. 2009, 361, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a recent, highly concise, asymmetric total synthesis of 1, see:Zhu C.; Cook S. P. J. Am. Chem. Soc. 2012, 134, 13577. [DOI] [PubMed] [Google Scholar]
- For a combined synthetic biological/semisynthetic route to 1, see:Paddon C. J.; Westfall P. J.; Pitera D. J.; Benjamin K.; Fisher K.; McPhee D.; Leavell M. D.; Tai A.; Main A.; Eng D.; Polichuk D. R.; Teoh K. H.; Reed D. W.; Treynor T.; Lenihan J.; Fleck M.; Bajad S.; Dang G.; Dengrove D.; Diola D.; Dorin G.; Ellens K. W.; Fickes S.; Galazzo J.; Gaucher S. P.; Geistlinger T.; Henry R.; Hepp M.; Horning T.; Iqbal T.; Jiang H.; Kizer L.; Lieu B.; Melis D.; Moss N.; Regentin R.; Secrest S.; Tsuruta H.; Vazquez R.; Westblade L. F.; Xu L.; Yu M.; Zhang Y.; Zhao L.; Lievense J.; Covello P. S.; Keasling J. D.; Reiling K. K.; Renninger N. N.; Newman J. D. Nature 2013, 496, 528. [DOI] [PubMed] [Google Scholar]
- Kamchonwongpaisan S.; Nilanonta C.; Tarnchompoo B.; Thebtaranonth C.; Thebtaranonth Y.; Yuthavong Y.; Kongsaeree P.; Clardy J. Tetrahedron Lett. 1995, 36, 1821. [Google Scholar]
- a Casteel D. A. Nat. Prod. Rep. 1999, 15, 55. [DOI] [PubMed] [Google Scholar]; b Liu D.-Z.; Liu J.-K. Nat. Prod. Bioprospect. 2013, 3, 161. [Google Scholar]
- For an example of a simple, but potent antimalarial 7-membered ring endoperoxide, see:Posner G. H.; Wang D.; González L.; Tao X.; Cumming J. N.; Klinedinst D.; Shapiro T. A. Tetrahedron Lett. 1996, 37, 815. [Google Scholar]
- Reports suggest that, in certain cases, enzymatic assistance is not required. For a discussion on the peroxide forming step in the biosynthesis of 1 in Artemisia annua L., see:; a Covello P. S. Phytochemistry 2008, 69, 2881. [DOI] [PubMed] [Google Scholar]; b Brown G. D. Molecules 2010, 15, 7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroxyradical cyclizations strongly favor the 6-exo-trig pathway, see:; a Funk M. O.; Isaac R.; Porter N. A. J. Am. Chem. Soc. 1975, 97, 1281. [DOI] [PubMed] [Google Scholar]; b Porter N. A.; Funk M. O.; Gilmore D.; Isaac R.; Nixon J. J. Am. Chem. Soc. 1976, 98, 6000. [DOI] [PubMed] [Google Scholar]; c Porter N. A. Acc. Chem. Res. 1986, 19, 262. [Google Scholar]; d Boukouvalas J.; Pouliot R.; Fréchette Y. Tetrahedron Lett. 1995, 36, 4167. [Google Scholar]; e Takuyasu T.; Kunikawa S.; McCullough K. J.; Masuyama A.; Nojima M. J. Org. Chem. 2005, 70, 251. [DOI] [PubMed] [Google Scholar]; f Szpilman A. M.; Korshin E. E.; Rozenberg H.; Bachi M. D. J. Org. Chem. 2005, 70, 3618. [DOI] [PubMed] [Google Scholar]
- For insightful reviews on the synthesis of cyclic peroxides, see:; a Korshin E. E.; Bachi M. D.. Synthesis of Cyclic Peroxides, Patai’s Chemistry of Functional Groups, Online; Wiley: 2009; pp 1–117. [Google Scholar]; b Dussault P. Synlett 1995, 997. [Google Scholar]
- Cointeaux L.; Berrien J.-F.; Mayrargue J. Tetrahedron Lett. 2002, 43, 6275. [Google Scholar]
- For additional synthetic work related to 2, see:Cointeaux L.; Berrien J.-F.; Mahuteau J.; Trân Huu-Dâu M. E.; Cicéron L.; Danis M.; Mayrargue J. Bioorg. Med. Chem. 2003, 11, 3791. [DOI] [PubMed] [Google Scholar]
- Kornblum N.; DeLaMare H. E. J. Am. Chem. Soc. 1951, 73, 880. [Google Scholar]
- a Isayama S.; Mukaiyama T. Chem. Lett. 1989, 1071. [Google Scholar]; b Mukaiyama T.; Isayama S.; Inoki S.; Kato K.; Yamada T.; Takai T. Chem. Lett. 1989, 449. [Google Scholar]; c Inoki S.; Kato K.; Takai T.; Isayama S.; Yamada T.; Mukaiyama T. Chem. Lett. 1989, 515. [Google Scholar]
- Isayama S. Bull. Chem. Soc. Jpn. 1990, 63, 1305. [Google Scholar]
- a Magnus P.; Payne A. H.; Waring M. J.; Scott D. A.; Lynch V. Tetrahedron Lett. 2000, 41, 9725. [Google Scholar]; b Magnus P.; Waring M. J.; Scott D. A. Tetrahedron Lett. 2000, 41, 9731. [Google Scholar]; c Magnus P.; Scott D. A.; Fielding M. R. Tetrahedron Lett. 2001, 42, 4127. [Google Scholar]; d Magnus P.; Fielding M. R. Tetrahedron Lett. 2001, 42, 6633. [Google Scholar]
- a Waser J.; Carreira E. M. J. Am. Chem. Soc. 2004, 126, 5676. [DOI] [PubMed] [Google Scholar]; b Waser J.; Carreira E. M. Angew. Chem., Int. Ed. 2004, 43, 4099. [DOI] [PubMed] [Google Scholar]; c Waser J.; Nambu H.; Carreira E. M. J. Am. Chem. Soc. 2005, 127, 8294. [DOI] [PubMed] [Google Scholar]; d Waser J.; Gaspar B.; Nambu H.; Carreira E. M. J. Am. Chem. Soc. 2006, 128, 11693. [DOI] [PubMed] [Google Scholar]; e Gaspar B.; Carreira E. M. Angew. Chem., Int. Ed. 2007, 46, 4519. [DOI] [PubMed] [Google Scholar]; f Gaspar B.; Carreira E. M. Angew. Chem., Int. Ed. 2008, 120, 5842. [DOI] [PubMed] [Google Scholar]; g Gaspar B.; Carreira E. M. J. Am. Chem. Soc. 2009, 131, 13214. [DOI] [PubMed] [Google Scholar]; h Barker T. J.; Boger D. L. J. Am. Chem. Soc. 2012, 134, 13588. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Leggans E. K.; Barker T. J.; Duncan K. K.; Boger D. L. Org. Lett. 2012, 14, 1428. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Tokuyasu T.; Kunikawa S.; Masuyama A.; Nojima M. Org. Lett. 2002, 4, 3595. [DOI] [PubMed] [Google Scholar]; k Lo J. C.; Yabe Y.; Baran P. S. J. Am. Chem. Soc. 2014, 136, 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Iwasaki K.; Wan K. K.; Oppedisano A.; Crossley S. W. M.; Shenvi R. A. J. Am. Chem. Soc. 2014, 136, 1300. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Shigehisa H.; Aoki T.; Yamaguchi S.; Shimizu N.; Hiroya K. J. Am. Chem. Soc. 2013, 135, 10306. [DOI] [PubMed] [Google Scholar]; n Hashimoto T.; Hirose D.; Taniguchi T. Angew. Chem., Int. Ed. 2014, 53, 2730. [DOI] [PubMed] [Google Scholar]
- For the use of hydroperoxidation methodology in the synthesis of smaller-ring, peroxide-containing natural products and analogs, see:; a Xu X.-X.; Dong H.-Q. J. Org. Chem. 1995, 60, 3039. [Google Scholar]; b Tokuyasu T.; Masuyama A.; Nojima M.; McCullough K.; Kim H.-S.; Wataya Y. Tetrahedron 2001, 57, 5979. [Google Scholar]; c Tokuyasu T.; Kunikawa S.; Abe M.; Masuyama A.; Nojima M.; Kim H.-S.; Begum K.; Wataya Y. J. Org. Chem. 2003, 68, 7361. [DOI] [PubMed] [Google Scholar]
- For representative examples, see:; a Posner G. H.; Oh C. H. J. Am. Chem. Soc. 1992, 114, 8328. [Google Scholar]; b Posner G. H.; Oh C. H.; Wang D.; Gerena L.; Milhous W. K.; Meshnick S. R.; Asawamahasadka W. J. Med. Chem. 1994, 37, 1256. [DOI] [PubMed] [Google Scholar]; c Posner G. H.; Cumming J. N.; Ploypradith P.; Oh C. H. J. Am. Chem. Soc. 1995, 117, 5885. [Google Scholar]; d Jefford C. W.; Favarger F.; Vicente M. G. H.; Jacquier Y. Helv. Chim. Acta 1995, 78, 452. [Google Scholar]; e Jefford C. W.; Vicente M. G. H.; Jacquier Y.; Favarger F.; Mareda J.; Millasson-Schmidt P.; Brunner G.; Burger U. Helv. Chim. Acta 1996, 79, 1475. [Google Scholar]; f Bloodworth A. J.; Shah A. Tetrahedron Lett. 1996, 36, 7551. [Google Scholar]; g Wu W.-M.; Wu Y.; Wu Y.-L.; Yao Z.-J.; Zhou C.-M.; Li Y.; Shan F. J. Am. Chem. Soc. 1998, 120, 3316. [Google Scholar]; h Cazelles J.; Robert A.; Meunier B. J. Org. Chem. 1999, 64, 6776. [DOI] [PubMed] [Google Scholar]; i O’Neill P. M.; Bishop L. P. D.; Searle N. L.; Maggs J. L.; Storr R. C.; Ward S. A.; Park B. K.; Mabbs F. J. Org. Chem. 2000, 65, 1578. [DOI] [PubMed] [Google Scholar]; j Bachi M. D.; Korshin E. E.; Hoos R.; Szpilman A. M. J. Heterocycl. Chem. 2000, 37, 639. [Google Scholar]; k Szpilman A. M.; Korshin E. E.; Hoos R.; Posner G. H.; Bachi M. D. J. Org. Chem. 2001, 66, 6531. [DOI] [PubMed] [Google Scholar]; l O’Neill P. M.; Stocks P. A.; Pugh M. D.; Araujo N. C.; Korshin E. E.; Bickley J. F.; Ward S. A.; Bray P. G.; Pasini E.; Davies J.; Verissimo E.; Bachi M. D. Angew. Chem., Int. Ed. 2004, 43, 4193. [DOI] [PubMed] [Google Scholar]; m Taylor D. T.; Avery T. D.; Greatrex B. W.; Tiekink E. R. T.; Macreadie I. G.; Macreadie P. I.; Humphries A. D.; Kalkanidis M.; Fox E. N.; Klonis N.; Tilley L. J. Med. Chem. 2004, 47, 1833. [DOI] [PubMed] [Google Scholar]; n Tang Y.; Dong Y.; Wang X.; Sriraghavan K.; Wood J. K.; Vennerstrom J. L. J. Org. Chem. 2005, 70, 5103. [DOI] [PubMed] [Google Scholar]; o Schiaffo C. E.; Rottman M.; Wittlin S.; Dussault P. H. ACS Med. Chem. Lett. 2011, 2, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11–13 could also originate from the hydrolysis of a lactone intermediate formed following C–C cleavage.
- Upon standing in solution (CDCl3), 12 converts to these compounds.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.