Abstract
Antitumor immunity suppresses tumorigenesis, but we do not understand how transformed cells initiate those immune responses that are essential for effective tumor immunosurveillance. Using the 3-MCA fibrosarcoma model, we identified IL-17D as a tumor-expressed cytokine that recruits natural killer cells, leading to the polarization of M1 macrophages and tumor rejection.
Keywords: antitumor immunity, innate immunity, interleukin 17D, natural killer cells, tumor rejection
Host immunity can both prevent and promote tumor formation.1-3 Recent work has shown that antitumor immunity eliminates immunogenic cells of a heterogeneous, “unedited” tumor, thereby promoting the escape of less immunogenic, more homogenous, “edited” tumor cells1,2 in a process termed cancer immunoediting. Although it has been demonstrated that edited tumors can produce tumor-promoting cytokines such as granulocyte macrophage colony stimulating factor 2 (CSF2, better known as GM-CSF), transforming growth factor β (TGFβ), and tumor necrosis factor α (TNFα;see reviews4,5), little is known about cytokines that are expressed by unedited neoplastic cells. We hypothesized that tumor-expressed factors in unedited tumors can activate host tumor immunosurveillance and that these factors must be silenced in order for tumors to survive in immune competent individuals. Our group has identified interleukin-17D (IL-17D) as a tumor-expressed cytokine that can recruit natural killer (NK) cells by inducing tumor endothelial cells to express chemokine (C-C) motif ligand 2 (CCL2, better known asMCP-1, monocyte chemoattractant protein 1).6 Remarkably, we found that IL-17D is abundantly expressed in highly immunogenic, unedited tumors but at low levels in poorly immunogenic, edited tumors.
To study unedited and edited tumors, we used a model system whereby fibrosarcomas are induced by 3-methylcholanthrene in either immunodeficient recombination activating gene 2 null (RAG2−/-) or immunocompetent wild-type (WT) mice, leading to the generation of highly immunogenic, unedited “regressor” or poorly immunogenic, edited “progressors” tumor cell lines, respectively. The immune system can recognize, infiltrate, become activated, and destroy regressor but not progressor tumor cells.1-3 We performed a microarray analysis comparing regressor to progressor tumor cells to identify cytokines preferentially expressed in regressor tumor cells. Interestingly, we found that one cytokine, IL-17D, is expressed and secreted to a higher extent in regressor compared to progressor tumor cells. Publicly available NCBI Gene Expression Omnibus (GEO) data sets further demonstrated that IL-17D expression is suppressed in metastatic and high-grade human tumor samples compared to primary and low-grade tumors, respectively, suggesting that IL-17D expression in certain human cancers abrogates tumor progression. (6 refer to Fig. 1 in O'Sullivan et al.). IL-17D overexpression in various progressor tumor cell lines was sufficient to mediate tumor rejection or delay growth in syngenëic WT recipients (6 refer to Fig. 2 in O'Sullivan et al.). Importantly, we determined that IL-17D overexpression does not impact tumor cell growth rate in vitro or tumor development upon transplantation in immunodeficient mice, suggesting that the primary role for tumor-expressed IL-17D is to initiate antitumor immunity. Accordingly, we found that NK cells are present at higher percentages within progressor tumors expressing IL-17D as compared to controls, and that M1 tumor-associated macrophages (TAMs) preferentially accumulate in the presence of recruited tumor-infiltrating NK cells (6 refer to Figs. 3 and 4 in O'Sullivan et al.). We found that IL-17D is not responsible for directly recruiting NK cells and M1 TAMs, but, rather, functions to stimulate tumor endothelial cells to produce MCP-1 that in turn, recruits NK cells into the tumor microenvironment and, subsequently polarizes TAMs toward an M1 phenotype (6 refer to Fig. 5 in O'Sullivan et al.). We then tested whether MCP-1 production and resultant NK cell recruitment was required for IL-17D mediated antitumor immunity by depleting MCP-1 or NK cells in WT mice transplanted with IL-17D overexpressing progressor tumor cells. Overexpression of IL-17D in progressors failed to inhibit tumor growth upon blockade of either NK cells or MCP-1 (6 refer Figs. 3 and 5 in O'Sullivan et al.), demonstrating that NK cells and MCP-1 are essential for the antitumor effect of IL-17D (6 refer to Fig. 2 in O'Sullivan et al.).
Prior studies investigating the roles of other IL-17 cytokines in tumor progression have focused primarily on IL-17A and IL-17 producing T helper (Th17) cells and have identified both pro- and antitumor activities (see review 7). In part, the contradictory roles of IL-17A and Th17 cells observed in tumor progression can be attributed to the fact that a major effect of IL-17A is the recruitment of neutrophils, an innate immune population known to have diverse roles in tumor progression.8 In light of these observations, the effective use of IL-17A as an antitumor immunotherapeutic will most likely be context dependent. In contrast, on the basis of our observation that IL-17D stimulates NK cell infiltration into the tumor microenvironment, macrophage M1 polarization and tumor regression (Fig. 1), we predict that IL-17D therapy would more consistently exhibit an antitumor effect. Apart from their ability to directly kill tumor cells, NK cells in the tumor microenvironment have been shown to promote antitumor T cell9 and macrophage responses.1 However, as the forced overexpression of IL-17D can effect the rejection of some but not all edited tumors, IL-17D-based therapy will likely need to be used in combination with other immunotherapeutics, such as checkpoint blockade and (or) inhibitors of regulatory T cells.
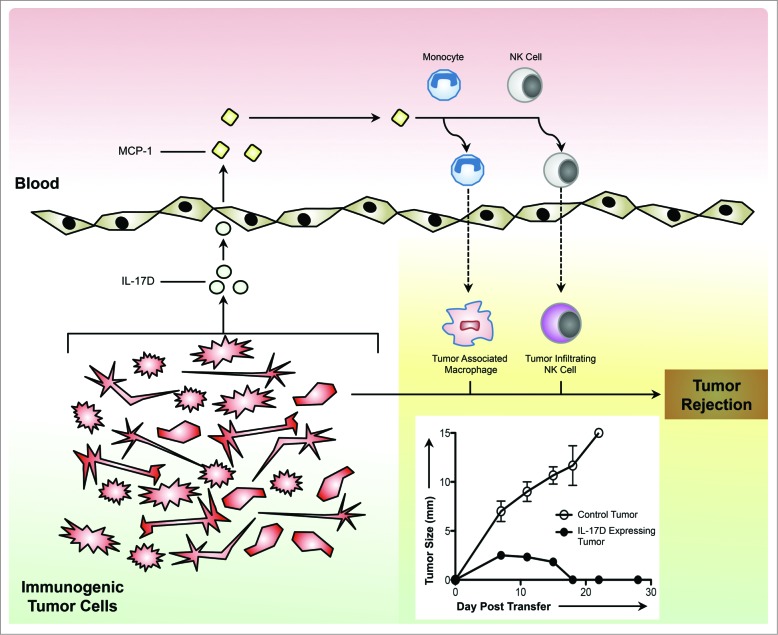
Figure 1.
Immunogenic tumor-derived IL-17D recruits NK cells to activate antitumor immunity and promote tumor regression. Secreted from immunogenic tumor cells, IL-17D stimulates the production of MCP-1 from tumor endothelial cells. In turn, MCP-1 attracts monocytes and NK cells into IL-17D-expressing tumors, leading to tumor rejection. Abbreviations: Interleukin-17D (IL-17D); Monocyte Chemotactic Protein 1 (MCP-1, aka CCL2).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Saddawi-Konefka R, O'Sullivan T, Vermi W, Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF, Teng MW, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med 2012; 209:1869-82; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: Integrating Immunity's Roles in Cancer Suppression and Promotion. Science 2011; 331:1565-70 ; PMID:; http://dx.doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 3. Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410:1107-11; PMID:; http://dx.doi.org/ 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- 4. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9:361-71; PMID:; http://dx.doi.org/ 10.1038/nrc2628 [DOI] [PubMed] [Google Scholar]
- 5. Lewis CE. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006; 66:605-12; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 6. O'Sullivan T, Saddawi-Konefka R, Gross E, Tran M, Mayfield SP, Ikeda H, Bui JD. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep 2014; 7:989-98; PMID:; http://dx.doi.org/ 10.1016/j.celrep.2014.03.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zou W, Restifo NP. TH17 cells in tumour immunity and immunotherapy. Nat Publishing Group 2010; 10:248-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-b: “N1” versus “N2” TAN. Cancer Cell 2009; 16:183-94; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diefenbach A, Jensen E, Jamieson A, Raulet D. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 2001; 413:165-71; PMID:; http://dx.doi.org/ 10.1038/35093109 [DOI] [PMC free article] [PubMed] [Google Scholar]