Abstract
In the present study, Schwann cells were isolated from the sciatic nerve of neonatal mice and purified using dispase and collagenase. Results showed that after the first round of purification with dispase, most of the Schwann cells appeared round in shape and floated in culture solution after 15 minutes. In addition, cell yield and cell purity were higher when compared to the collagenase group. After the second round of purification, the final cell yield for the dispase group was higher than that for the collagenase group, but no significant difference was found in cell purity. Moreover, similar results in cell quantity and purity were observed in adult Sprague-Dawley rats. These findings indicate that purification with dispase can result in the rapid isolation of Schwann cells with a high yield and purity.
Keywords: dispase, differential detachment, purification, Schwann cell, neural regeneration
INTRODUCTION
Isolation and purification of Schwann cells (SCs) is an initial but crucial step for SC biological research and cell preparation of tissue engineered nerves[1]. However, SC purification remains a difficult process because primary cultures are easily contaminated with fibroblasts, which proliferate much faster than SCs and become a predominant cell type in in vitro cultures.
In the past decade, numerous purification methods have been reported for the isolation of SCs from a cell pool[2,3,4,5,6]. The details of these methods appear quite diverse, and all utilize different techniques to select for SCs from fibroblast populations. Antimitotic treatment[5] is based on the difference in proliferation rate between SCs and fibroblasts in vitro, whereas antibody-mediated cytolysis[7] is based on the specific expression of Thy1.1 by fibroblasts. Immunoselective methods[8,9,10] take advantage of the specific expression of P75NTR by SCs, and repeated explantation methods[10,11,12] are based on the different migration speeds between SCs and fibroblasts. Lastly, differential adhesion methods[13,14] utilize different adhesion capabilities between SCs and fibroblasts. The differential detachment method, first reported by Jin et al[2], utilized collagenase, which differentially cleaved the extracellular links between cells and the bottom of the culture vessel. Chernousov et al[15] found that digestion of the SC extracellular matrix with collagenase effectively disrupted most of the matrix including fibronectin fibrils, where collagenase removed collagen with no significant effect on fibronectin fibrils.
Theoretically, the more specific the enzymatic effect, the more efficient the purification should be. To increase cell yield during differential detachment, the present study replaced collagenase with various proteases (data not shown), and finally identified dispase (neutral protease), which can achieve high cell purity and high cell yield. The present study compared the results of SC purification between dispase and collagenase.
RESULTS
SC morphology after the first round of purification with dispase and collagenase
After 48 hours of primary culture, a purification procedure was conducted by incubating cells with enzymatic solution at 37°C. In the dispase group, most of the SCs appeared round in shape after 15 minutes, but only a few SCs in the collagenase group exhibited a similar morphological change. After 30 minutes, SCs in both groups appeared round in shape, while morphology of fibroblasts remained unchanged (Figures 1A and E). SCs were detached by shaking horizontally, based on different detachment window periods for the two types of cells. In the dispase group, most of the round-shaped cells were suspended (Figure 1C) and detached from the flask; however, in the collagenase group, a number of round-shaped cells remained adhered to the flask or the surface of fibroblasts (Figure 1G). Furthermore, in the collagenase group, high magnification microscopy revealed that many fibroblasts in the flasks appeared shrunken after enzymatic treatment, and some fibroblasts became detached after shaking (Figures 1F and H). In contrast, fibroblasts in the dispase group maintained their original morphology and remained adhered to the flask (Figures 1B and D).
Figure 1.
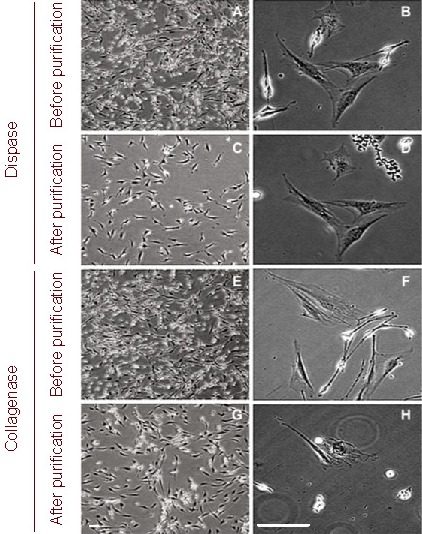
Morphology of Schwann cells after the first round of purification (light microscope). Scale bars: 50 μm.
After 30 minutes of enzymatic treatment, Schwann cells in both groups appeared round in shape, while fibroblasts did not have obvious morphological changes (A, B, E and F).
Schwann cells were detached by shaking the flask horizontally. In the dispase group, most of the rounded cells were suspended and few Schwann cells remained adhered to the flask (C); however, in the collagenase group, a few rounded cells remained attached to the flasks or the surface of fibroblasts (G).
In the collagenase group, under high magnification, many fibroblasts in flasks exhibited significant shrinkage after enzymatic treatment and some of these fibroblasts detached during shaking along with the round Schwann cells (H); in contrast, fibroblasts in the dispase group maintained their original morphology and adherence (D).
Influence of two rounds of purification on cell purity and cell yield with dispase and collagenase
Cells obtained from each flask were collected and quantified using a hemocytometer. Counts revealed that there were 111.7 ± 10.6 × 104 and 67.0 ± 3.6 × 104 cells per flask in the dispase and collagenase groups, respectively. After cell counting, cells were collected and reseeded onto new laminin-coated culture vessels for 48 hours. After the first round of purification, cultures contained 98.3 ± 1.3% pure SCs in the dispase group and 94.5 ± 2.2% in collagenase group as determined by quantification based on immunostaining of P75NTR at 72 hours (24 hours after the first purification procedure) (Figure 2). Both cell purity and cell yield were significantly higher in the dispase group when compared to the collagenase group (P < 0.05).
Figure 2.
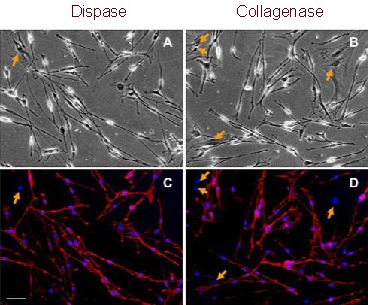
P75NTR expression in Schwann cells from the sciatic nerve of neonatal mice after the first round of purification (immunofluorescent staining). Scale bar: 40 μm.
All cells in a bipolar or tripolar shape were positive for P75NTR (red), while the flat-shaped fibroblasts (arrows) were negative. Nuclei were visualized by 4′-6-diamidino-2- phenylindole staining (blue).
(A, B) Light microscope; (C, D) fluorescent microscope.
To avoid the re-emergence and expansion of remnant fibroblasts, one more round of purification was performed at 96 hours (48 hours after the first round of purification). The total yield of purified SCs reached 196.0 ± 7.0 × 104 per flask in the dispase group and 108.7 ± 3.5 × 104 per flask in the collagenase group after two rounds of cell expansion and purification (P < 0.05; Figure 3). Cells obtained from each flask were collected and reseeded onto new laminin-coated dishes. At 120 hours (24 hours after the second round of purification), SC purity increased to 99.5 ± 0.5% in the dispase group and 98.3 ± 1.3% in the collagenase group (Figure 4). This difference was not significant (P > 0.05).
Figure 3.
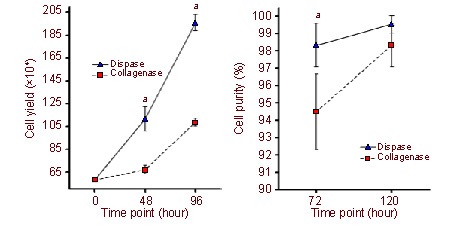
Schwann cell purity and yield after two rounds of purification. aP < 0.05, vs. collagenase group.
(A) The cell yield per flask was significantly higher in the dispase group when compared with the collagenase group at each time point after purification (P < 0.05). 48 hours: the first round of purification; 96 hours: the second round of purification.
(B)There was a significant difference in cell purity between the two groups after the first round of purification (at 72 hours; P < 0.05), but no significant difference was found after the second round of purification (at 120 hours; P > 0.05).
Figure 4.
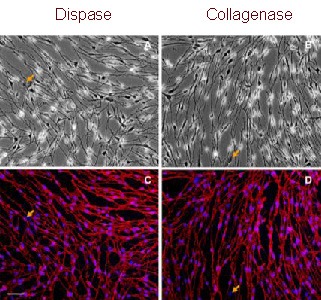
P75NTR expression in Schwann cells after the second round of purification (immunofluorescent staining). Scale bar: 40 μm.
All cells in a bipolar or tripolar shape were positive for P75NTR (red), while flat-shaped fibroblasts (arrows) were negative. Nuclei were visualized by 4′-6-diamidino-2- phenylindole staining (blue).
(A, B) Light microscope; (C, D) fluorescent microscope.
To confirm these results, the above experiments were performed in triplicate using mouse cultures (supplementary Table 1 online). Additionally, we also purified SCs from adult Sprague-Dawley rats using dispase, which was also performed in triplicate. Data are summarized in supplementary Table 1 online. Consistent results were observed with respect to cell purity and cell yield per flask among the six independent tests (supplementary Table 1 online). Interestingly, according to the immunostaining results, SCs derived from newborn mice did not express S100, a specific marker for SCs (data not shown), while SCs from adult rats were positive for S100 and P75NTR, but negative for Thy1.1 (Figure 5).
Figure 5.
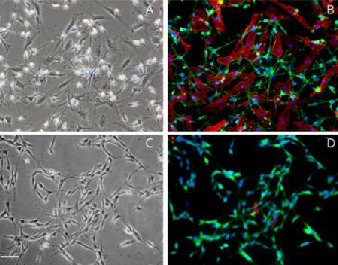
Double-immunofluorescent staining of Schwann cells with P75NTR/Thy1.1 and S100/Thy1.1 after the second round of purification. Scale bar: 40 μm.
All cells in a bipolar or tripolar shape were positive for both S100 (green) and P75NTR (green), while flat-shaped fibroblasts were Thy1-positive only (red). Nuclei were visualized by 4′-6-Diamidino-2-phenylindole staining (blue).
(A) Bright field of cells before purification (light microscope).
(B) Fluorescent view of P75NTR/Thy1.1 double staining before purification (fluorescent microscope).
(C) Bright field of cells after two rounds of purification with dispase (light microscope).
(D) Fluorescent view of S100/Thy1.1 double staining after two rounds of purification with dispase (fluorescent microscope).
DISCUSSION
A previous study reported a new method for obtaining highly purified (above 99%) in vitro cultures of SCs from newborn mice based on differential detachment time windows between SCs and fibroblasts after treatment with multiplex collagenase[2]. Later, Wu et al[3] applied this method for harvesting SCs from pre-degenerated sciatic nerves of adult rats and achieved highly pure yields. This method does not require special equipment[8,9] or cytotoxic agents[4,5]. However, nearly half of the harvested SCs were discarded during purification in order to obtain a purer culture[2]. Additionally, this method requires accurate judgment to determine the degree of digestion of tissue, which is a key step for purification.
In the present study, dispase treatment resulted in high SC purity (above 98%) using the differential detachment method. Even after one round of purification, the purity of cultures was significantly higher than that of the collagenase group. Moreover, the dispase group resulted in almost double the final cell yield when compared with the collagenase group, which was equal to the cell yield obtained using only half the original nerve tissue required for the method based on collagenase treatment in clinical applications. These improvements may be due to the enzymatic specificity of dispase, which has been proven to cleave fibronectin and type IV collagen but not laminin, type V collagen, serum albumin, or transferrin[16]. SCs mainly excrete fibronectin, type IV collagen and perlecan, while fibroblasts produce various types of collagens, glycosaminoglycans, reticular and elastic fibers, and glycoproteins. Interestingly, we observed that collagenase detached some fibroblasts along with SCs, while none were observed to detach in the dispase group. These mis-detached fibroblasts may decrease the cell purity after the first round of purification. Thus, we presume that the specific enzymatic activity of dispase makes it more effective for Schwann cell isolation than collagenase.
Furthermore, we speculate that this differential detachment method is appropriate for SCs derived from other species and may also be appropriate for other cell types, which excrete a similar extracellular matrix to SCs. This speculation was confirmed by isolating SCs from adult rats using the dispase method (supplementary Table 1 online). Based on previous work, which reveled differential detachment methods were appropriate for SC isolation from both newborn and adult animals[2,3], the dispase method may also be useful in isolating SCs in a more clinically relevant setting for the repair or regeneration of nerve injuries in adults.
In conclusion, dispase can tremendously improve the efficiency of the differential detachment method for SC purification, and, to the best of our knowledge, this method is the most effective approach for SC purification and expansion, as it is a safe, convenient, and rapid procedure that results in high cell yields.
MATERIALS AND METHODS
Design
A comparative cytological study.
Time and setting
Experiments were performed at the Central Laboratory of Shanghai, First People's Hospital, and the Shanghai Key Laboratory of Tissue Engineering, Shanghai Jiao Tong University School of Medicine, China, from January 2010 to May 2011.
Materials
A total of 42 healthy male and female, newborn C57Bl/6 mice, aged 6–7 days, regardless of body mass, and 24 adult Sprague-Dawley male and female rats, aged 4 weeks, regardless of body mass, were provided by Shanghai SLAC Laboratory Animal Co., Ltd. and housed in the Central Laboratory of Shanghai First People's Hospital on a 12 hour light/dark cycle at 22°C, with a humidity of 40–67%, under specific pathogen-free conditions. All procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[17].
Methods
Preparation of laminin coated flasks
Culture flasks (BD Falcon, Franklin Lakes, NY, USA) were coated with laminin (Roche, Basel, Switzerland) prior to primary cell cultures. A sterile coating solution of 400 ng/mL laminin in minimum essential medium alpha (α-MEM; Gibco, Carlsbad, CA, USA) was prepared, and the flasks and plates were coated with coating solution (100 μL/cm2) and incubated overnight at 37°C. After incubation, the coating solution was aspirated and the coated flasks or dishes were stored aseptically at 4°C and used within 1 week.
SC isolation and primary culture
For adult rats, pre-degeneration was performed for 10 days as described previously[3,18]. Newborn mice or adult rats with pre-degenerated nerves were sacrificed by decapitation or cervical dislocation and their sciatic nerves were harvested aseptically under a dissecting microscope (Olympus, Tokyo, Japan). An enzymatic minimum essential medium alpha solution was prepared with 0.03% (w/v) dispase II (neutral protease, grade II, Roche) and 0.1% (w/v) collagenase NB 4 (Serva, Heidelberg, Germany).
To obtain SCs, 12 sciatic nerve segments derived from newborn mice in one representative study were subjected to enzymatic digestion. The harvested nerve segments were centrifuged at 600 × g for 5 minutes at 4°C and the supernatant was discarded. The enzymatic solution (100 μL per segment) was added and incubated within a cell incubator (Thermo, Waltham, Massachusetts, USA) at 37°C for 20–30 minutes. With a Pasteur pipette, remnant nerve segments were mechanically disaggregated by vigorous pipetting for 3–5 minutes and the mixture was further centrifuged at 600 × g for 5 minutes at 4°C. After removal of the supernatant, the cell pellet was resuspended in SC culture medium composed of α-MEM medium supplemented with 10% (v/v) fetal bovine serum, 2 μM forskolin (Sigma, St. Louis, MO, USA) and 10 ng/mL heregulin-β-1 (Pepro Tech, London, UK)[4,16]. The isolated cells were then seeded evenly into six 25-cm2 laminin coated flasks (5.8 × 105 cells per flask) at a density of 2.0–2.6 × 104 cells/cm2 and stored in a cell incubator in a humidified atmosphere of 5% carbon dioxide at 37°C. These flasks were randomly divided into two groups (3 flasks in each group): dispase and collagenase groups.
SC purification
After 48 hours of culture, the culture medium was replaced with enzymatic solution containing 0.06% (w/v) dispase II or 0.05% (w/v) collagenase NB 4 diluted in minimum essential medium alpha at 0.1 mL/cm2, incubated at 37°C for 30 minutes. The flasks were shaken horizontally for 1–3 minutes to suspend detached cells. The suspended cells were then collected into a 15-mL conical tube (BD Falcon) and centrifuged at 600 × g at 4°C for 5 minutes. After removal of supernatant, the pellet was resuspended in SC culture medium and plated onto a laminin-coated culture flask.
Quantification of SC yield
Cell yield after the first round of purification was assessed at 48 hours following enzymatic treatment by quantifying cells with a hemocytometer. Final cell yield was determined by quantifying cells at the end of the second purification after protease treatment at 96 hours. To differentiate between viable (unstained) and dead (stained) cells, 0.2% (w/v) trypan blue was utilized during each course of cell quantification by light microscopy (Olympus, Tokyo, Japan).
Immunohistochemical staining for the identification of SC purity
SC purity at 24 hours after the first and second round of purifications (at 72 hours and at 120 hours) was analyzed using immunostaining. Suspended cells (10%) obtained from each group were seeded onto a new laminin-coated 3.5-cm dish after purification and quantified. After 24 hours of culture, cells were fixed at 72 hours and 120 hours with 4% (w/v) paraformaldehyde for 10 minutes followed by three washes with phosphate buffered saline (PBS; pH 7.2–7.4) and then blocked with 5% (w/v) bovine serum album (Sigma) in PBS for 30 minutes at room temperature. The cells were incubated with rabbit anti-P75NTR polyclonal antibody (1: 200; Abcam, Cambridge, UK), rabbit anti-S100 polyclonal antibody (1: 200; DAKO, California, USA) or rat anti-Thy1.1 monoclonal antibody (1: 200; eBioscience, California, USA) at room temperature for 2 hours. Cells without primary antibody incubation served as a blank control. After three washes with PBS, the cells were further incubated with Alexa Fluor® 488 or Alexa Fluor® 546 goat anti-rabbit IgG or goat anti-rat IgG (1: 1 000; Invitrogen, Carlsbad, CA, USA) for 60 minutes at room temperature. Cell nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI; 1: 1 000; diluted in PBS; DAKO) for 10 seconds. After a final wash in PBS, labeled cells were visualized under a fluorescence microscope (Olympus) and the images were digitally recorded and were processed with Image-Pro Plus (Media Cybernetics, Atlanta, GA, USA). P75NTR- or S100-positive cells were recognized as SCs and quantified in three photo-areas randomly selected from each 3.5-cm dish to obtain an average number. The total number of cells was calculated by counting the number of DAPI-stained cell nuclei. The percentage of SC purity was calculated using the following equation: (numbers of P75NTR- or S100-positive cells/total number of cells) × 100%[1].
Statistical analysis
Data were expressed as the mean ± SD. For quantitative comparison and analysis, the values were subjected to a paired t-test using Excel (Microsoft, Redmond, WA, USA). A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank Dr. Wanyao Xia, Shanghai Key Laboratory of Tissue Engineering, for his kind support on flow cytometric analysis.
Footnotes
Conflicts of interest: None declared.
Funding: This research was supported by the National Natural Science Foundation of China, No. 30872630, 81000522 and 30973050; and Shanghai Shenkang Laboratory Funding, No. SHDC12007706.
Ethical approval: All animal experimental protocols were approved by the Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 7, No. 4, 2012 after selecting the “NRR Current Issue” button on the page.
(Edited by Jiang XM, Li GF/Su LL/Song LP)
REFERENCES
- [1].Madduri S, Gander B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst. 2010;15(2):93–103. doi: 10.1111/j.1529-8027.2010.00257.x. [DOI] [PubMed] [Google Scholar]
- [2].Jin YQ, Liu W, Hong TH, et al. Efficient Schwann cell purification by differential cell detachment using multiplex collagenase treatment. J Neurosci Methods. 2008;170(1):140–148. doi: 10.1016/j.jneumeth.2008.01.003. [DOI] [PubMed] [Google Scholar]
- [3].Wu W, Jin YQ, Kretlow JD, et al. Purification of Schwann cells from adult rats by differential detachment. Biotechnol Lett. 2009;31(11):1703–1708. doi: 10.1007/s10529-009-0064-8. [DOI] [PubMed] [Google Scholar]
- [4].Wei Y, Zhou J, Zheng Z, et al. An improved method for isolating Schwann cells from postnatal rat sciatic nerves. Cell Tissue Res. 2009;337(3):361–369. doi: 10.1007/s00441-009-0836-4. [DOI] [PubMed] [Google Scholar]
- [5].Wood PM. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976;115(3):361–375. doi: 10.1016/0006-8993(76)90355-3. [DOI] [PubMed] [Google Scholar]
- [6].Niapour A, Karamali F, Karbalaie K, et al. Novel method to obtain highly enriched cultures of adult rat Schwann cells. Biotechnol Lett. 2010;32(6):781–786. doi: 10.1007/s10529-010-0230-z. [DOI] [PubMed] [Google Scholar]
- [7].Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- [8].Manent J, Oguievetskaia K, Bayer J, et al. Magnetic cell sorting for enriching Schwann cells from adult mouse peripheral nerves. J Neurosci Methods. 2003;123(2):167–173. doi: 10.1016/s0165-0270(02)00349-7. [DOI] [PubMed] [Google Scholar]
- [9].Vroemen M, Weidner N. Purification of Schwann cells by selection of p75 low affinity nerve growth factor receptor expressing cells from adult peripheral nerve. J Neurosci Methods. 2003;124(2):135–143. doi: 10.1016/s0165-0270(02)00382-5. [DOI] [PubMed] [Google Scholar]
- [10].Assouline JG, Bosch EP, Lim R. Purification of rat Schwann cells from cultures of peripheral nerve: an immunoselective method using surfaces coated with anti-immunoglobulin antibodies. Brain Res. 1983;277(2):389–392. doi: 10.1016/0006-8993(83)90953-8. [DOI] [PubMed] [Google Scholar]
- [11].Oda Y, Okada Y, Katsuda S, et al. A simple method for the Schwann cell preparation from newborn rat sciatic nerves. J Neurosci Methods. 1989;28(3):163–169. doi: 10.1016/0165-0270(89)90032-0. [DOI] [PubMed] [Google Scholar]
- [12].Wrathall JR, Rigamonti DD, Kao CC. Cultures enriched in Schwann-like cells from dissociated nerve segments of the adult cat. Brain Res. 1981;224(1):218–223. doi: 10.1016/0006-8993(81)91137-9. [DOI] [PubMed] [Google Scholar]
- [13].Pannunzio ME, Jou IM, Long A, et al. A new method of selecting Schwann cells from adult mouse sciatic nerve. J Neurosci Methods. 2005;149(1):74–81. doi: 10.1016/j.jneumeth.2005.05.004. [DOI] [PubMed] [Google Scholar]
- [14].Wrathall JR, Rigamonti DD, Braford MR, et al. Non-neuronal cell cultures from dorsal root ganglia of the adult cat: production of Schwann-like cell lines. Brain Res. 1981;229(1):163–181. doi: 10.1016/0006-8993(81)90753-8. [DOI] [PubMed] [Google Scholar]
- [15].Chernousov MA, Stahl RC, Carey DJ. Schwann cells use a novel collagen-dependent mechanism for fibronectin fibril assembly. J Cell Sci. 1998;111(Pt 18):2763–2777. doi: 10.1242/jcs.111.18.2763. [DOI] [PubMed] [Google Scholar]
- [16].Stenn KS, Link R, Moellmann G, et al. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93(2):287–290. doi: 10.1111/1523-1747.ep12277593. [DOI] [PubMed] [Google Scholar]
- [17].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [18].Tomita K, Hata Y, Kubo T, et al. Effects of the in vivo predegenerated nerve graft on early Schwann cell migration: quantitative analysis using S100-GFP mice. Neurosci Lett. 2009;461(1):36–40. doi: 10.1016/j.neulet.2009.05.075. [DOI] [PubMed] [Google Scholar]


