Abstract
In this study, we established a rat model of optic nerve crush to explore the effects of erythropoietin on retinal ganglion cell axonal regeneration. At 15 days after injury in erythropoietin treated rats, retinal ganglion cell densities in regions corresponding to the 1/6, 3/6 and 5/6 ratios of the retinal radius were significantly increased. In addition, the number of growth associated protein-43 positive axons was significantly increased at different distances (50, 250 and 500 μm) from the crush site after erythropoietin treatment. Erythropoietin significantly increased growth associated protein-43 protein levels in the retina after crush injury, as determined by western blot and immunofluorescence analysis. These results demonstrate that erythropoietin protects injured retinal ganglion cells and promotes axonal regeneration.
Keywords: erythropoietin, retinal ganglion cells, axonal regeneration, optic nerve crush, neural regeneration
INTRODUCTION
Injury to the optic nerve can lead to axonal degeneration and retinal ganglion cell (RGC) death, resulting in irreversible vision loss[1,2]. Therefore, therapies should focus on the protection of injured RGCs and the promotion of axonal regeneration[3,4,5,6,7].
Erythropoietin (EPO), a renal cytokine regulating hematopoiesis, is produced by different cell types within the central nervous system. EPO has been shown to be neuroprotective, and it promotes the regeneration of neurons in the central nervous system[8]. EPO may function via an anti-apoptotic mechanism, as well as through anti-inflammatory effects at the site of injury[9]. In vitro experiments show that EPO protects cultured neurons against glutamate toxicity[10,11], and it reduces ischemic neuronal damage and neurological dysfunction in rodent models of stroke[12,13,14,15]. EPO also prolongs the lifespan of cultured cortical neurons and promotes neuronal growth[16,17]. Moreover, the administration of EPO has a significant effect on axonal regrowth of fibers in the central nervous system[18]. In vivo studies show that EPO is neuroprotective in animal models of stroke, mechanical trauma and excitotoxic injury[19,20,21,22,23,24,25]. Recent evidence indicates that EPO can stimulate postnatal neovascularization by increasing endothelial progenitor cell mobilization from the bone marrow[26]. EPO in the retina has been demonstrated to have a neuroprotective effect on RGCs[27]. Following retinal ischemia, EPO prevents apoptotic cell death and improves functional recovery[28]. In addition, EPO promotes neuronal survival and axonal regrowth in the central nervous system. EPO has a neuroprotective effect on RGCs and significantly reduces the death of primary cultures of RGCs induced by glutamate and nitric oxide[29] in vitro. Furthermore, it stimulates the regeneration of injured RGC axons following optic nerve injury[30,31,32]. However, these studies only focused on the effects of EPO on axonal regeneration in vitro, and very little data is available on EPO's actions on axonal regeneration in vivo. To investigate the axonal regeneration-promoting effects of EPO after optic nerve crush, we examined the effects of EPO on RGCs using retrograde 1, 1-dioctadecyl-3, 3, 3′, 3′-tetramethyl-indocarbocyanine perchlorate (DiI) labeling of the superior colliculus, as well as by assessing the expression of growth associated protein-43 (GAP-43) in the retina and in the region distal to optic nerve crush, using immunofluorescence and western blot analysis.
RESULTS
Quantitative analysis of experimental animals
A total of 72 rats were equally and randomly assigned to three groups: sham-surgery (free of crush), phosphate buffered saline (PBS; optic nerve crush-PBS intravitreal injection) and EPO (optic nerve crush-EPO intravitreal injection) groups. All 72 rats were included in the final analysis.
Protective effects of EPO on RGCs after optic nerve crush
To determine RGC densities, retrograde labeling using the fluorescent tracer DiI was used[31]. A large number of DiI-labeled RGCs were present in the sham-surgery and EPO groups 15 days after optic nerve crush. However, many RGCs in the PBS group died, and the microglia were DiI-positive, having phagocytosed RGCs (Figure 1).
Figure 1.
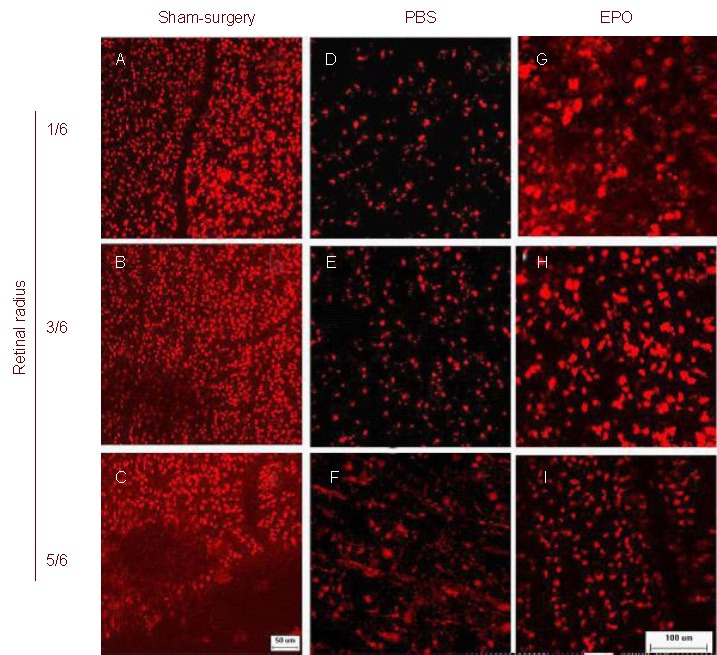
Morphology of RGCs after optic nerve crush (DiI staining; scale bar: 50 μm in A–C and 100 μm in D–I. Representative photographs of the flat-mounted retinas at the corresponding areas (at 1/6, 3/6 and 5/6 of the retinal radius) showed DiI-labeled RGCs in retinas on day 15 after injury.
Almost all RGCs were labeled and the morphology of the RGCs was round in the sham-surgery (A–C) and EPO (G–I) groups. In the PBS group (D–F), there were a few DiI-labeled RGCs and microglia were obviously labeled at 1/6 and 3/6 radius, and were more numerous at 5/6 retinal radius. RGCs: Retinal ganglion cells; Dil: 1, 1-dioctadecyl-3, 3, 3′, 3′-tetramethyl-indocarbocyanine perchlorate; PBS: phosphate buffered saline; EPO: erythropoietin.
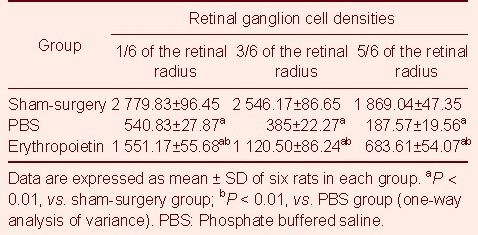
RGCs situated at the 1/6, 3/6 and 5/6 fractions of the retinal radius were quantified. Statistical analysis showed that compared with the sham-surgery group, the number of RGCs was significantly reduced in the PBS group (P < 0.01); and compared with the PBS group, RGC densities were significantly increased in the EPO group (P < 0.01; Table 1).
Table 1.
Quantification of surviving retinal ganglion cells (cells/mm2) in different groups
EPO promotes rat RGC axonal regeneration after optic nerve crush
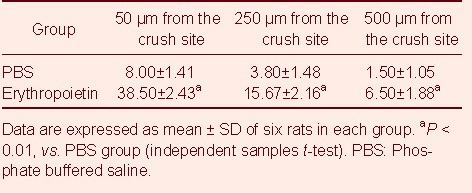
GAP-43 expression was examined to determine the effects of EPO on RGC axonal regeneration. At 15 days after optic nerve crush, animals were sacrificed and longitudinal optic nerve sections were immunostained for GAP-43, an axonal growth-associated protein which is commonly used as a marker of axonal regrowth[33], to identify regenerating axons (Figure 2). As expected, numerous GAP-43 positive axons passed through the crush site and the distal optic nerve segment in the EPO group. In contrast, the PBS group showed very few GAP-43 positive axons passing through the crush site. The sham-surgery group showed no staining. In addition, the number of GAP-43 positive axons at defined distances from the crush site (50, 250 and 500 µm) was quantified (Table 2). The data show that the number of axons in the EPO group was significantly more than in the PBS group (P < 0.01; Table 2), suggesting that EPO promotes RGC axonal regeneration after optic nerve crush.
Figure 2.
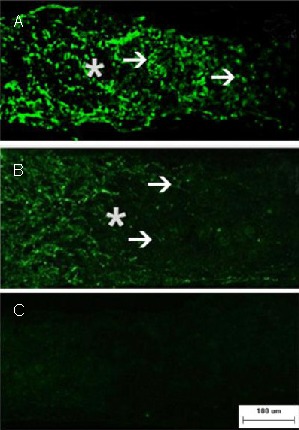
Axonal regeneration in retinal ganglion cells after optic nerve crush (immunofluorescence staining; fluorescein isothiocyanate-labeling; scale bar: 100 μm).
Representative photomicrographs show the crush site (asterisk) and regenerating axons with growth associated protein-43 immunofluorescence (arrowheads) on day 15 after injury.
Numerous growth associated protein-43 positive axons are seen passing through the crush site and they grew beyond the distal crush site in the EPO group (A). In contrast, very few axons traversed the crush site in the PBS group (B). In addition, the sham-surgery group (C) showed no staining. PBS: Phosphate buffered saline; EPO: erythropoietin.
Table 2.
Quantification of growth associated protein-43 positive axons (per mm) at the designated distances from the crush site in the different groups
EPO promotes GAP-43 expression in rat retina after optic nerve crush
In optic nerve fibers, GAP-43 levels are abundant during development, but expression is absent in adults[33]. GAP-43 expression in adult retina is significantly increased after optic nerve injury[34,35]. We investigated the effects of EPO on GAP-43 expression in the retina using western blot analysis and immunofluorescence labeling after optic nerve crush. EPO increased GAP-43 expression in the ganglion cell layer of the retina. In comparison, GAP-43 expression was low in the PBS group (Figure 3). Retinal GAP-43 levels in the PBS and EPO groups were higher than in the sham-surgery group, as determined by western blot analysis (P < 0.01; Figure 4). Moreover, the GAP-43 protein level in the EPO group was significantly higher than in the PBS group (P < 0.01; Figure 4), suggesting that EPO upregulates GAP-43 expression in the retina after optic nerve crush.
Figure 3.
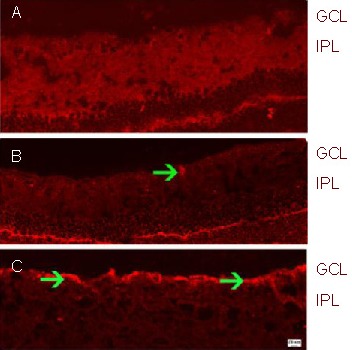
GAP-43 expression in the retina after optic nerve crush (immunofluorescence; Cy3-labeled; scale bars: 10 µm in (A–B) and 20 μm in (C).
(A) Sham-surgery group; (B) PBS group; (C) EPO group.
The retina in the sham-surgery group showed little GAP-43 expression. In contrast, GAP-43 expression was intense (arrowhead, red fluorescence) in the PBS group. The immunoreactivity of GAP-43 (arrowheads, red fluorescence) in the EPO group was greater than in the PBS group.
GAP-43: Growth associated protein-43; GCL: ganglion cell layer; IPL: inner plexiform layer; PBS: phosphate buffered saline; EPO: erythropoietin.
Figure 4.
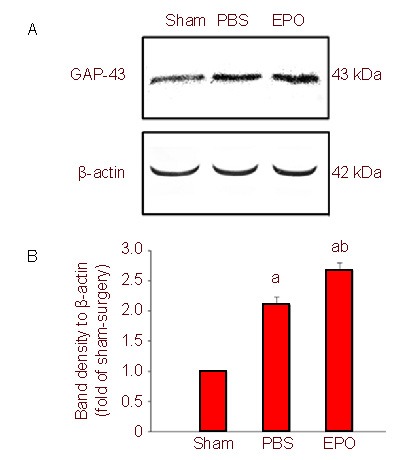
Growth associated protein-43 (GAP-43) expression in retina after optic nerve crush (western blot).
(A) Representative western blots for GAP-43 in the retina. Erythropoietin (EPO) treatment resulted in significantly increased GAP-43 expression.
(B) Quantification of western blot bands for GAP-43 protein levels from six independent experiments.
aP < 0.01, vs. sham-surgery (sham) group; bP < 0.01, vs. phosphate buffered saline (PBS) group (one-way analysis of variance).
DISCUSSION
The optic nerve crush model mimics the pathological changes of optic nerve degeneration, and it is suitable for research into optic nerve injury and regeneration. Our results demonstrate that EPO exerts a protective effect after optic nerve injury. Intravitreal injection of EPO enhanced RGC survival and axonal regeneration. The axonal growth-associated protein, GAP-43, is a marker of axonal outgrowth, and in optic nerve fibers, as in most axons of the central nervous system, it is abundantly expressed during development, but it is absent in adults. GAP-43 expression in adult retina is significantly increased after optic nerve injury[33,34,35]. GAP-43 expression in the PBS group was higher than in the sham-surgery group, but EPO increased GAP-43 protein levels compared with PBS. This suggests that EPO promotes RGC axonal regeneration after optic nerve crush by upregulating GAP-43 expression.
Optic nerve injury triggers RGC death[1,2], which is primarily apoptotic[36]. To date, no strategy has successfully delayed RGC death beyond 15 days post-lesion[37,38]. Our present study shows that all RGC axons are irreversibly damaged and that the neuroprotective effect manifests only as delayed apoptosis. PBS-treated cells died rapidly 15 days after optic nerve crush, whereas EPO protected the damaged RGCs 15 days after crush. EPO treatment delayed RGC death after optic nerve crush, consistent with previous results showing that EPO is an efficacious neuroprotective agent for other neurodegenerative diseases of the central nervous system[19,22,23,24,30,31,32]. This protection is accompanied by the EPO-mediated induction of anti-apoptotic factors (Bcl-2 and Bcl-XL)[39,40]. In the present study, EPO attenuated RGC death induced by optic nerve crush, thus confirming its antiapoptotic potential.
The effects of EPO might in part be mediated by macrophages and Müller cells[41], which may affect RGCs after EPO directly increases RGC survival and neurite extension. Activated macrophages secrete numerous factors that are neuroprotective for RGCs and which promote axonal regeneration[42,43]. In the rat visual system, neurotrophic factors promote the survival of RGCs and optic nerve regeneration, directly, or indirectly through retinal astrocyte/Müller cell activation. Co-treatment with epidermal growth factor and EPO suppresses epidermal growth factor-induced glial reactivity[44]. EPO and its receptor are known to be expressed in the brain and retinal tissue. EPO expression is induced upon central nervous system injury, suggesting an autocrine protective signaling mechanism[45,46,47]. Of clinical relevance is the observation that exogenous EPO can penetrate the intact blood-brain barrier[48], suggesting that exogenous EPO can easily reach the site of injury.
In summary, EPO acts as a neuroprotectant for RGCs after optic nerve crush and promotes axonal regeneration.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed in Shanghai Institute of Traumatology and Orthopedics, Shanghai, China, from June 2009 to July 2011.
Materials
Seventy-two adult male Sprague-Dawley rats, weighing 200–250 g, were provided by the Laboratory Animal Center of Shanghai Institute of Traumatology and Orthopedics, China (license No. SCXK (Hu) 2007-0005; SYXK (Hu) 2008-0049). All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research[49].
Methods
Retrograde DiI-labeling of RGCs from the superior colliculus
Deeply anesthetized rats were placed in a stereotaxic apparatus (Stoelting, Kiel, Germany), and the skin overlying the skull was cut open and retracted. The lambda and Bergman sutures served as landmarks for drilling six holes. 1.5 μL DiI (10% solution in DMSO; Molecular Probes, Eugene, OR, USA) was then applied to both superior colliculi, individually, using a micropipette[50]. To ensure adequate RGC labeling, animals were allowed 3 days for retrograde transport of DiI before optic nerve crush.
Establishment of the optic nerve crush model
Surgical procedures were performed as described previously[51]. Rats that had previously received DiI injections were used. All surgeries were performed aseptically and on the right eye alone. Animals were anesthetized by intraperitoneal injection of 10% chloral hydrate solution (420 mg/kg). A 1.0–1.5 cm incision was made in the skin above the right orbit. The optic nerve of the right eye was exposed under an operating microscope (Alcon, Fort Worth, TX, USA), and the sheath was opened longitudinally. Using special forceps (force at 40 g; Suzhou Medical Instrument Factory, Jiangsu, China), the optic nerve was crushed within the sheath, 1 mm behind the optic nerve head, for 9 seconds, avoiding injury to the ophthalmic artery. Nerve injury was verified by the appearance of a clearing at the crush site; the vascular integrity of the retina was verified by fundoscopic examination after dilating the pupil with atropine[31]. In the sham-surgery group, the optic nerve of the right eye was exposed, and the sheath was opened longitudinally, but the crush procedure was not performed. The skin was then closed with sutures, and bacitracin ointments were applied to the wound. Animals with a postoperative complication (e.g., cataract) were excluded from analysis. Using this optic nerve crush technique, all RGC axons were irreversibly damaged and neuroprotection could be measured by analyzing the delay in apoptosis.
Drug administration
All intravitreal injections were performed using a glass microelectrode (WPI, Florida, USA) connected to a Hamilton precision syringe (Hamilton, Reno, NV, USA), puncturing the eye at the cornea-sclera junction. The lens was not punctured. EPO (R&D Systems, Minneapolis, MN, USA) was diluted in PBS. Except for the sham-surgery group which had no intravitreal injection, the PBS and EPO groups received an intravitreal injection of 3.5 µL PBS or EPO [6 U EPO (8.3 µg = 1 000 U EPO)], respectively, for each time point. The intravitreal injections were performed four times: immediately after optic nerve crush (day 0), and at days 3, 6 and 9.
Quantity of DiI-labeled RGCs in the retina
At 15 days after optic nerve injury, deeply anesthetized animals were transcardially perfused with 4% paraformaldehyde. The eyes were rapidly enucleated, rinsed, punctured with a needle through the pupil, and then fixed for 1 hour in freshly prepared 4% paraformaldehyde. The retinas were dissected and washed with PBS. After a final wash, four cuts were made with fine iris scissors from the edge to the center of the retinas to flatten them. They were mounted with the RGCs facing up on glass slides in glycerol, and the coverslip was sealed with clear nail polish. The slides were stored in the dark at –20°C until analysis. Labeled RGCs were examined with a confocal microscope (Axiovert 35; Zeiss, Oberkochen, Germany) using a rhodamine filter (560 nm for DiI). RGC densities were determined by quantifying labeled RGCs in 12 distinct areas of 62 500 µm2 each (four areas per retinal quadrant at three different eccentricities of 1/6, 3/6 and 5/6 of the retinal radius). Cell number was quantified with a computerized image-analysis system (Image Pro plus Version 6.0; Media Cybernetics, Silver Spring, MD, USA) in duplicate by two independent investigators in a blind fashion. The average number of RGCs in 12 distinct areas was used to calculate the mean density of RGCs for each retina. Secondary DiI-stained activated microglia, after RGC phagocytosis, were separated using morphologic criteria and excluded from examination[52]. All averaged data are expressed as mean RGC densities (cells/mm2) ± SD.
Preparation of retina and optic nerve samples
After the rats were transcardially perfused with 4% paraformaldehyde, the eyes were enucleated with at least 5 mm of optic nerve attached, and bisected. The eyes were dissected as eye cups, without the cornea or lens, and the optic nerves were dissected free from connective tissue. The eye cups and optic nerves were immediately fixed overnight, and transferred to 30% sucrose solution and stored overnight at 4°C. The tissues were then embedded, frozen and cut. Longitudinal sections (16 µm for optic nerve) were obtained by cutting through the globe or optic nerve along the anterior-posterior axis. The sections were collected on gelatin-coated glass slides and stored at –80°C for subsequent use.
Immunofluorescence labeling for GAP-43 expression in the retina and optic nerve
Retinal/optic nerve sections were dehumidified at 37°C for 1 hour, and nonspecific binding was blocked by application of 10% normal goat serum. Primary antibody (mouse anti-rat GAP-43 monoclonal antibody, 1: 100, sc-17790; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were applied at 4°C overnight. Negative controls were treated with PBS or serum. Secondary antibodies (Cy3-labeled anti-mouse IgG or anti-mouse fluorescein isothiocyanate antibody, 1: 100; Invitrogen, Carlsbad, CA, USA) were applied for 45 minutes at room temperature. Cell nuclei were counterstained with 4, 6-diamidino-2- phenylindole (1: 1 000; Sigma-Aldrich, St. Louis, MO, USA). Immunoreactivity was examined with a confocal microscope (Axiovert 35; Zeiss). For evaluation of RGC axon regeneration, GAP-43 positive axons were quantified as previously described[45]. Briefly, the optic nerve sections processed for anti-GAP-43 immunoreactivity were photographed using a confocal microscope. Images of whole sections were assembled from single pictures taken with a 20 × objective. Using a calibrated ocular (Zeiss) to measure distance, the number of GAP-43 positive axons crossing a line at distance 50, 250, 500 µm from the end of the crush site was determined. By measuring the cross-sectional width of the nerve at the point of quantification, axon counts were converted into axon crossings per unit nerve width (axons per mm) and the average of these over the four sections was obtained. For all immunohistochemical staining, three sections per eye were examined and there were six rats in each group.
Western blot analysis for retinal GAP-43 expression
Eighteen rat retinas were used for western blot analysis. Total retinal protein was extracted from pulverized samples using modified radioimmunoprecipitation (modified RIPA) buffer with a Halt™ protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, Illinois, USA). Protein concentrations were determined using the Bradford protein assay (Bio-Rad, Hercules, CA, USA)[49]. Each retina served as an individual sample (n = 6 per group). Equal amounts of protein (20 μg/lane) were separated on polyacrylamide gels and then electrotransferred onto a nitrocellulose membrane. After blocking for 3 hours in Tris-buffered saline with 0.1% Tween-20 and 3% bovine serum albumin, membranes were incubated overnight at 4°C with primary antibodies (GAP-43, 1: 100, sc-17790) in Tris-buffered saline with 0.1% Tween-20 containing 3% bovine serum albumin. Membranes were then washed and incubated with alkaline phosphatase conjugated secondary antibodies in Tris-buffered saline with 0.1% Tween-20 (1: 500; Sigma-Aldrich) for 2 hours and developed using NBT/BCIP substrate (Promega, Madison, WI, USA). The densities of the bands on the membrane were scanned and analyzed with Image Pro Plus version 6.0 (Media Cybernetics).
Statistical analyses
Data are expressed as mean ± SD, unless otherwise stated. Statistical analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was used to compare data among three groups, followed by post-hoc tests. Independent samples t-test was applied to compare data between two groups. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank Jian Qing, Department of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, for assistance.
Footnotes
Conflicts of interest: None declared.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81070728.
Ethical approval: The study was approved by the Animal Ethical Committee of Institutional Review Board of Ruijin Hospital, Shanghai, China.
(Edited by Li YY, Bai J/Su LL/Song LP)
REFERENCES
- [1].Tang Z, Zhang S, Lee C, et al. An optic nerve crush injury murine model to study retinal ganglion cell survival. J Vis Exp. 2011;50:2685. doi: 10.3791/2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp Neurol. 1998;153(1):1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- [3].Levin LA. Axonal loss and neuroprotection in optic neuropathies. Can J Ophthalmol. 2007;42(3):403–408. [PubMed] [Google Scholar]
- [4].Zalish M, Lavie V, Duvdevani R, et al. Gangliosides attenuate axonal loss after optic nerve injury. Retina. 1993;13(2):145–147. doi: 10.1097/00006982-199313020-00010. [DOI] [PubMed] [Google Scholar]
- [5].Fitzgerald M, Payne SC, Bartlett CA, et al. Secondary retinal ganglion cell death and the neuroprotective effects of the calcium channel blocker lomerizine. Invest Ophthalmol Vis Sci. 2009;50(11):5456–5462. doi: 10.1167/iovs.09-3717. [DOI] [PubMed] [Google Scholar]
- [6].Thaler S, Fiedorowicz M, Rejdak R, et al. Neuroprotective effects of tempol on retinal ganglion cells in a partial optic nerve crush rat model with and without iron load. Exp Eye Res. 2010;90(2):254–260. doi: 10.1016/j.exer.2009.10.013. [DOI] [PubMed] [Google Scholar]
- [7].Tsai RK, Chang CH, Sheu MM, et al. Anti-apoptotic effects of human granulocyte colony-stimulating factor (G-CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT-dependent. Exp Eye Res. 2010;90(5):537–545. doi: 10.1016/j.exer.2010.01.004. [DOI] [PubMed] [Google Scholar]
- [8].Chatagner A, Hüppi PS, Ha-Vinh Leuchter R, et al. Erythropoietin and neuroprotection. Arch Pediatr. 2010;17(Suppl 3):S78–84. doi: 10.1016/S0929-693X(10)70905-2. [DOI] [PubMed] [Google Scholar]
- [9].Bernaudin M, Marti HH, Roussel S, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19(6):643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- [10].Sirén AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98(7):4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Morishita E, Masuda S, Nagao M, et al. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76(1):105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- [12].Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97(19):10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- [14].Belayev L, Khoutorova L, Zhao KL, et al. A novel neurotrophic therapeutic strategy for experimental stroke. Brain Res. 2009;1280:117–123. doi: 10.1016/j.brainres.2009.05.030. [DOI] [PubMed] [Google Scholar]
- [15].Cho GW, Koh SH, Kim MH, et al. The neuroprotective effect of erythropoietin-transduced human mesenchymal stromal cells in an animal model of ischemic stroke. Brain Res. 2010;1353:1–13. doi: 10.1016/j.brainres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- [16].Maggioni D, Nicolini G, Chiorazzi A, et al. Different effects of erythropoietin in cisplatin- and docetaxel-induced neurotoxicity: an in vitro study. J Neurosci Res. 2010;88(14):3171–3179. doi: 10.1002/jnr.22465. [DOI] [PubMed] [Google Scholar]
- [17].Sanchez PE, Navarro FP, Fares RP, et al. Erythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life span. J Comp Neurol. 2009;514(4):403–414. doi: 10.1002/cne.22020. [DOI] [PubMed] [Google Scholar]
- [18].Ransome MI, Turnley AM. Erythropoietin promotes axonal growth in a model of neuronal polarization. Mol Cell Neurosci. 2008;38(4):537–547. doi: 10.1016/j.mcn.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [19].Wakida K, Shimazawa M, Hozumi I, et al. Neuroprotective effect of erythropoietin, and role of metallothionein-1 and -2, in permanent focal cerebral ischemia. Neuroscience. 2007;148(1):105–114. doi: 10.1016/j.neuroscience.2007.04.063. [DOI] [PubMed] [Google Scholar]
- [20].van der Kooij MA, Groenendaal F, Kavelaars A, et al. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev. 2008;59(1):22–33. doi: 10.1016/j.brainresrev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [21].Yazihan N, Uzuner K, Salman B, et al. Erythropoietin improves oxidative stress following spinal cord trauma in rats. Injury. 2008;39(12):1408–1413. doi: 10.1016/j.injury.2008.03.010. [DOI] [PubMed] [Google Scholar]
- [22].Keller M, Yang J, Griesmaier E, et al. Erythropoietin is neuroprotective against NMDA-receptor-mediated excitotoxic brain injury in newborn mice. Neurobiol Dis. 2006;24(2):357–366. doi: 10.1016/j.nbd.2006.07.007. [DOI] [PubMed] [Google Scholar]
- [23].Taoufik E, Petit E, Divoux D, et al. TNF receptor I sensitizes neurons to erythropoietin- and VEGF-mediated neuroprotection after ischemic and excitotoxic injury. Proc Natl Acad Sci U S A. 2008;105(16):6185–6190. doi: 10.1073/pnas.0801447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yoo JY, Won YJ, Lee JH, et al. Neuroprotective effects of erythropoietin posttreatment against kainate-induced excitotoxicity in mixed spinal cultures. J Neurosci Res. 2009;87(1):150–163. doi: 10.1002/jnr.21832. [DOI] [PubMed] [Google Scholar]
- [25].Mengozzi M, Cervellini I, Bigini P, et al. Endogenous erythropoietin as part of the cytokine network in the pathogenesis of experimental autoimmune encephalomyelitis. Mol Med. 2008;14(11-12):682–688. doi: 10.2119/2008-00086.Mengozzi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heeschen C, Aicher A, Lehmann R, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102(4):1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- [27].Zhong L, Bradley J, Schubert W, et al. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci. 2007;48(3):1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]
- [28].Jehle T, Meschede W, Dersch R, et al. Erythropoietin protects retinal ganglion cells and visual function after ocular ischemia and optic nerve compression. Ophthalmologe. 2010;107(4):347–353. doi: 10.1007/s00347-009-2030-1. [DOI] [PubMed] [Google Scholar]
- [29].Yamasaki M, Mishima HK, Yamashita H, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050(1-2):15–26. doi: 10.1016/j.brainres.2005.05.037. [DOI] [PubMed] [Google Scholar]
- [30].Weishaupt JH, Rohde G, Pölking E, et al. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45(5):1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- [31].Kretz A, Happold CJ, Marticke JK, et al. Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell Neurosci. 2005;29(4):569–579. doi: 10.1016/j.mcn.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [32].King CE, Rodger J, Bartlett C, et al. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp Neurol. 2007;205(1):48–55. doi: 10.1016/j.expneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- [33].Reh TA, Tetzlaff W, Ertlmaier A, et al. Developmental study of the expression of B50/GAP-43 in rat retina. J Neurobiol. 1993;24(7):949–958. doi: 10.1002/neu.480240708. [DOI] [PubMed] [Google Scholar]
- [34].Doster SK, Lozano AM, Aguayo AJ, et al. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron. 1991;6(4):635–647. doi: 10.1016/0896-6273(91)90066-9. [DOI] [PubMed] [Google Scholar]
- [35].Meyer RL, Miotke JA, Benowitz LI. Injury induced expression of growth-associated protein-43 in adult mouse retinal ganglion cells in vitro. Neuroscience. 1994;63(2):591–602. doi: 10.1016/0306-4522(94)90552-5. [DOI] [PubMed] [Google Scholar]
- [36].Agudo M, Pérez-Marín MC, Lönngren U, et al. Time course profiling of the retinal transcriptome after optic nerve transection and optic nerve crush. Mol Vis. 2008;14:1050–1063. [PMC free article] [PubMed] [Google Scholar]
- [37].Cheng L, Sapieha P, Kittlerova P, et al. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22(10):3977–3986. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang CW, Lu Q, You SW, et al. CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury. Invest Ophthalmol Vis Sci. 2005;46(4):1497–1503. doi: 10.1167/iovs.04-0664. [DOI] [PubMed] [Google Scholar]
- [39].Silva M, Grillot D, Benito A, et al. Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through Bcl-XL and Bcl-2. Blood. 1996;88(5):1576–1582. [PubMed] [Google Scholar]
- [40].Silva M, Benito A, Sanz C, et al. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J Biol Chem. 1999;274(32):22165–22169. doi: 10.1074/jbc.274.32.22165. [DOI] [PubMed] [Google Scholar]
- [41].Fu QL, Wu W, Wang H, et al. Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue retinal ganglion cells after chronic ocular hypertension. Cell Mol Neurobiol. 2008;28(2):317–329. doi: 10.1007/s10571-007-9155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yin Y, Cui Q, Li Y, et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23(6):2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vereyken EJ, Heijnen PD, Baron W, et al. Classically and alternatively activated bone marrow derived macrophages differ in cytoskeletal functions and migration towards specific CNS cell types. J Neuroinflammation. 2011;8:58. doi: 10.1186/1742-2094-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nickerson PE, McLeod MC, Myers T, et al. Effects of epidermal growth factor and erythropoietin on Müller glial activation and phenotypic plasticity in the adult mammalian retina. J Neurosci Res. 2011;89(7):1018–1030. doi: 10.1002/jnr.22629. [DOI] [PubMed] [Google Scholar]
- [45].Böcker-Meffert S, Rosenstiel P, Röhl C, et al. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci. 2002;43(6):2021–2026. [PubMed] [Google Scholar]
- [46].Juul SE, Anderson DK, Li Y, et al. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43(1):40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- [47].Fu A, Hui EK, Lu JZ, et al. Neuroprotection in stroke in the mouse with intravenous erythropoietin-Trojan horse fusion protein. Brain Res. 2011;1369:203–207. doi: 10.1016/j.brainres.2010.10.097. [DOI] [PubMed] [Google Scholar]
- [48].Chi OZ, Hunter C, Liu X, et al. Effects of erythropoietin on blood-brain barrier disruption in focal cerebral ischemia. Pharmacology. 2008;82(1):38–42. doi: 10.1159/000127839. [DOI] [PubMed] [Google Scholar]
- [49].Fontainhas AM, Townes-Anderson E. RhoA and its role in synaptic structural plasticity of isolated salamander photoreceptors. Invest Ophthalmol Vis Sci. 2008;49(9):4177–4187. doi: 10.1167/iovs.07-1580. [DOI] [PubMed] [Google Scholar]
- [50].Vidal-Sanz M, Villegas-Perez MP, Bray GM, et al. Persistent retrograde labeling of adult rat retinal ganglion cells with the carbocyanine dye DiI. Exp Neurol. 1988;102(1):92–101. doi: 10.1016/0014-4886(88)90081-7. [DOI] [PubMed] [Google Scholar]
- [51].Leon S, Yin Y, Nguyen J, et al. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20(12):4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Swanson KI, Schlieve CR, Lieven CJ, et al. Neuroprotective effect of sulfhydryl reduction in a rat optic nerve crush model. Invest Ophthalmol Vis Sci. 2005;46(10):3737–3741. doi: 10.1167/iovs.05-0155. [DOI] [PubMed] [Google Scholar]


