Abstract
The present study detected distribution and expression of nerve growth factor and inducible nitric oxide synthase in the mesencephalon and diencephalon, as well as visual- and auditory-related nervous tissues, in a macaque model of type 2 diabetes using immunohistochemistry. Results showed that nerve growth factor expression decreased, but inducible nitric oxide synthase expression increased, in the mesencephalon and diencephalon, as well as visual- and auditory- related nervous tissues. These results suggested that nerve growth factor and inducible nitric oxide synthase play an important role in regulating the development of diabetic visual- and auditory-related diseases.
Keywords: diencephalon, immunohistochemistry, inducible nitric oxide synthase, mesencephalon, nerve growth factor, neural regeneration, optic nerve, type 2 diabetes
INTRODUCTION
Studies have shown that nerve growth factor (NGF) and inducible nitric oxide synthase (iNOS) are involved in the pathogenesis of diabetic neuropathy[1,2,3,4,5,6,7,8,9]. Neurotrophic factors exhibit dual biological functions that nourish neurons and promote neuronal growth during growth of the vertebrate nervous system[10]. In addition, NGF plays an important role in retinal development[11]. NGF has been shown to promote retinal ganglion cell survival following resection of the optic nerve in adult mice, as well as promote axonal regeneration of ganglion cells and nerve fiber growth[12]. Nitric oxide synthase is comprised of three types: endothelial nitric oxide synthase, neuronal nitric oxide synthase, and iNOS. Nitric oxide is involved in the development of ischemic brain injury and exhibits dual functions: nitric oxide produced by endothelial nitric oxide synthase has a neuroprotective effect, but nitric oxide generated by neuronal nitric oxide synthase and iNOS over-expression has toxic effects on nerves[13]. Cytotoxicity and cell inhibition, which is the result of excessive endogenous or exogenous nitric oxide release, results in nerve and retinal injury. iNOS is a major limiting factor in the process of nitric oxide generation. Under normal conditions, retinal and nervous system tissues express small amounts of iNOS, but expression is activated under a pathological state. A previous study[14] showed that high glucose levels results in increased iNOS expression in nervous tissues and retinal cells, leading to increased nitric oxide production.
The present study analyzed NGF and iNOS expression in multiple audio/visual-related nuclei. In addition, most diabetic animal models are induced by streptozocin, although streptozocin has been shown to influence NGF in vivo expression and lead to altered expression at diabetes onset, compared to a spontaneous diabetic animal, which affects study results[15]. Therefore, the present study utilized a spontaneous diabetic macaque model to better understand the disease process in humans.
The present study focused on distribution and expression of NGF and iNOS in the mesencephalon and diencephalon, as well as visual- and auditory-related nervous tissues, using immunohistochemistry. The relationship between NGF and iNOS during the development of diabetic neuropathy was determined to better understand the molecular mechanisms of iNOS and NGF in diabetic neuropathy.
RESULTS
Quantitative analysis of experimental animals
Three mature macaques with spontaneous diabetes were selected for the diabetes group, and three healthy, age-matched macaques with diabetes served as the control group. Six macaques were included in the final analysis.
Distribution of NGF and iNOS expression in mesencephalon and diencephalon, as well as visual- and auditory-related nervous tissues
NGF and iNOS expression was visible as brown and granular in the cytoplasm. Both factors were expressed in cytoplasm of photoreceptor cells (cone cells and rod cells), as well as optic nerve membrane cells and brain cells. NGF-positive reaction products were mainly located in the thalamic reticular nucleus, ventral posterolateral nucleus, ventral posteromedial nucleus (VPM), medial geniculate nucleus, lateral geniculate nucleus, retina, and the optic nerve. iNOS-positive reaction products were mainly distributed in the thalamic reticular nucleus, medial geniculate nucleus, lateral geniculate nucleus, superior colliculus, inferior colliculus, the retina, and the optic nerve.
Distribution of NGF expression in the mesencephalon, diencephalon, optic nerve, and retina
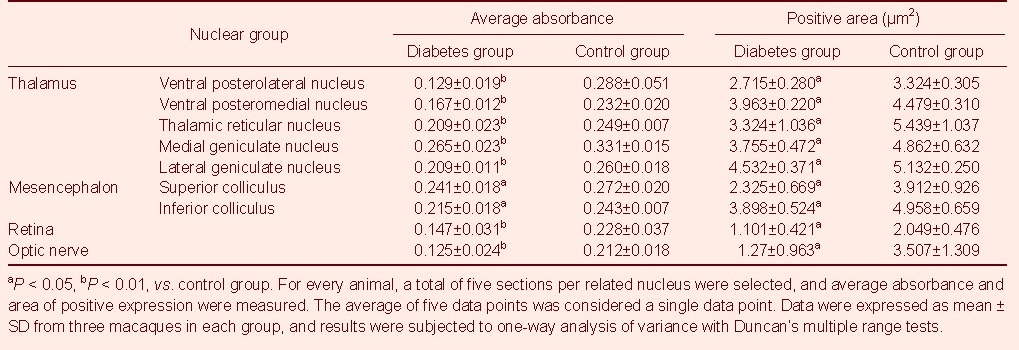
NGF expression is shown in Figure 1 and Table 1. Compared with the control group, NGF distribution and expression decreased in the mesencephalon and diencephalon of diabetic macaques (P < 0.05), as well as average absorbance in reticular nucleus, ventral posterolateral nucleus, ventral posteromedial nucleus, medial geniculate nucleus, and lateral geniculate nucleus nuclei, and the retina and optic nerve (P < 0.01). In addition, NGF expression significantly decreased in the superior colliculus and inferior colliculus nuclei (P < 0.05).
Figure 1.
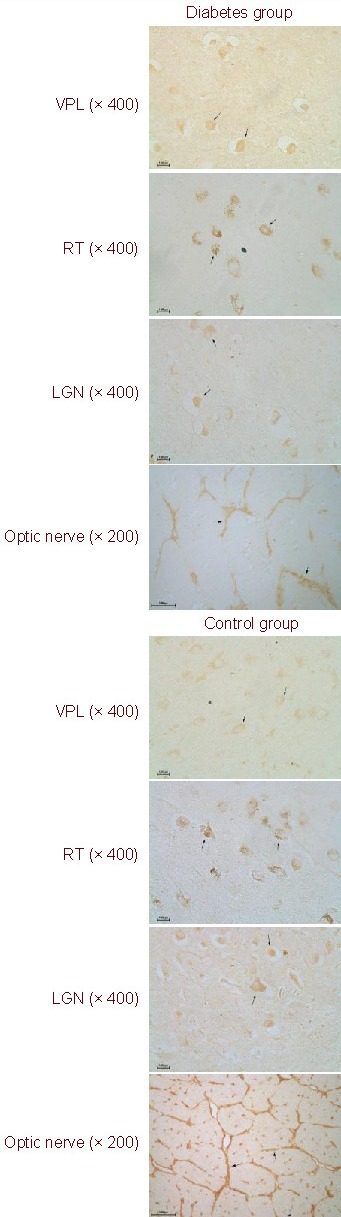
Nerve growth factor expression in the mesencephalon, diencephalon, optic nerve, and retina (immunohistochemical staining, fluorescent microscope).
All positive products (arrows) are extensively distributed in both groups. Nerve growth factor is primarily expressed in the cytoplasm of optic nerve membrane cells and brain cells. In the diabetes group, expression is decreased.
VPL: Ventral posterolateral nucleus; RT: reticular nucleus; LGN: lateral geniculate nucleus.
Table 1.
Expression of nerve growth factor in the mesencephalon, diencephalon, optic nerve, and retina
Distribution of iNOS expression in the mesencephalon, diencephalon, optic nerve, and retina
Immunohistochemistry results showed that compared with the control group, iNOS distribution and expression significantly increased in the mesencephalon and diencephalon of diabetic macaques (P < 0.05). Average absorbance was maximal in reticular nucleus, medial geniculate nucleus, lateral geniculate nucleus, superior colliculus, and inferior colliculus nuclei, as well as the retina and optic nerve (P < 0.01), followed by VPM and VPL nuclei (P < 0.05; Table 2 and Figure 2).
Table 2.
Inducible nitric oxide synthase expression in the mesencephalon, diencephalon, optic nerve, and retina
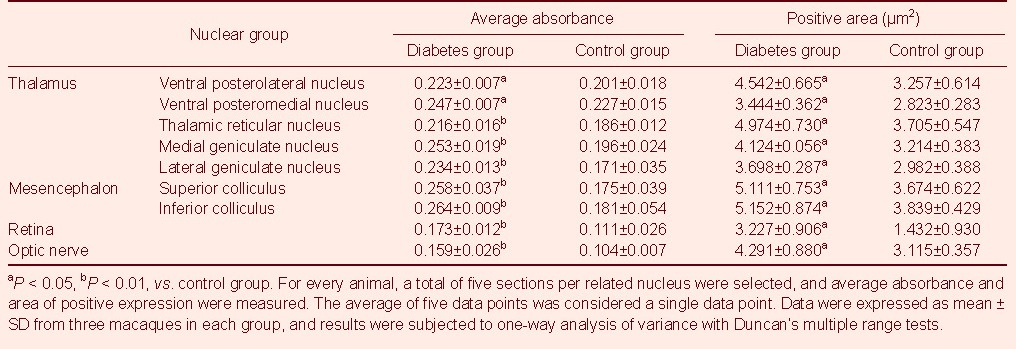
Figure 2.

Inducible nitric oxide synthase expression in the mesencephalon, diencephalon, optic nerve, and retina (immunohistochemical staining, fluorescent microscope, × 400).
All positive products are extensively distributed in both groups. inducible nitric oxide synthase expression is primarily within the cytoplasm of photoreceptor cells (cone cells and rod cells) and brain cells. In the diabetes group, expression is increased.
LGN: Lateral geniculate nucleus; COS: superior colliculus.
DISCUSSION
NGF and iNOS expression and distribution in the mesencephalon and diencephalon of type 2 diabetic macaques
In recent years, studies focused on the relationship between NGF and diabetic neuropathy have obtained some progress. In diabetic rats, the central and peripheral nervous systems exhibit decreased NGF expression[16]. NGF expression increases at an early stage of diabetes, but decreases with development of the disease in a rat model[17]. NOS-positive neurons exist in the rat hypothalamic paraventricular nucleus, supraoptic nucleus, periventricular nucleus, and lateral hypothalamic area[18]. In addition, high blood sugar leads to increased NOS expression[19]. Results from the present study showed that NGF and iNOS were widely distributed in the brain, but the distribution within the mesencephalon and diencephalon varied. Compared with the control group, NGF expression in the mesencephalon, diencephalon, and related visual and auditory nervous tissues decreased in the diabetes group, although iNOS expression increased. These results suggested that during the early stages of injury, NGF plays a role in neuronal nutrition and regeneration, which was consistent with previous findings[15]. NGF expression decreased when repair effect did not ameliorate development of diabetes. In addition, high iNOS expression could induce harm.
High blood sugar led to increased iNOS expression, thereby heightening nitric oxide production; excessive nitric oxide production and release led to neurotoxicity via free radicals and further aggravated neuronal damage. A previous study showed that endothelial nitric oxide synthase expression decreases in the hypothalamus and brain stem reticular neurons in a high blood sugar rat model at 6 weeks of age[20]. These results suggested in addition to disease progression, a short-term protective effect was generated by the endothelial nitric oxide synthase-mediated decline, and neurotoxic effects were mediated by neuronal nitric oxide synthase and iNOS. Nerve injury took place during the interim and late stages of disease. NGF played a role in nerve nutrition and regeneration. In addition, iNOS increased nerve injury mediated by nitric oxide, resulting in an indirect effect on diabetes neuropathy.
NGF expression significantly decreased in reticular nucleus, ventral posterolateral nucleus, ventral posteromedial nucleus, medial geniculate nucleus, and lateral geniculate nucleus nuclei. In addition, iNOS significantly increased in the geniculate nuclear group, as well as superior colliculus and inferior colliculus. The thalamic reticular nucleus is involved in vision and is connected with important nuclei related to visual pathways[21]. The reticular nucleus contains visual, somatosensory, and auditory areas, and each sensation has a representative area. The ventral posterior nucleus is the main relay station for body sensory functions. The geniculate nuclear group, which includes the medial geniculate nucleus and lateral geniculate nucleus, receives visual and auditory input from the brainstem, and these neurons are specific sense relay nucleus. Superior colliculus and inferior colliculus represent subcortical visual and auditory reflex centers, respectively, which receive input from visual and auditory pathways. These results suggested that abnormal NGF and iNOS expression in the reticular nucleus, ventral posterior nucleus, and geniculate nuclear groups, as well as other nuclei, correlated with occurrence and development of audio-visual sensory and consciousness disturbances in diabetes patients.
NGF and iNOS expression in the optic nerve and retina of type 2 diabetic macaques
Diabetic and optic neuropathies are frequently occurring complications of diabetes. The optic nerve is part of the central nervous system and is very sensitive to ischemia, hypoxia, and metabolic disorders[22]. A previous study confirmed that in diabetic rats, diabetes-induced ischemia and hypoxia of the optic nerve results in damage to intracellular aerobic respiration, as well as a series of compensatory responses[23]. Compared with the control group, NGF and iNOS expression significantly decreased and increased, respectively, in the optic nerve of the diabetic group and increased, suggesting that expressional changes played an important role in the development of optic nerve lesions. Results from a previous study[24] showed multi-fold increased NGF expression in rat retinal neurons at early stage. In addition, another study discussed iNOS expression in diabetic rat retinal tissue and its influence on cell apoptosis[25], concluding that iNOS expression increases in the retina of diabetic rats in accordance with duration of disease. These results demonstrated that iNOS expression significantly increased in the optic nerve and retina in diabetic groups. However, significantly decreased NGF expression suggested NGF-induced decreased nerve growth and regeneration, together with increased damage due to iNOS, were important factors in the development of diabetic retinopathy.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The study was performed at the Experimental Animal Engineering Center, College of Veterinary Medicine, Sichuan Agricultural University, China from October 2010 to March 2011.
Materials
Related indices (blood sugar, blood insulin, and glycated hemoglobin) were measured in three 8-year-old macaques with spontaneous diabetes and three healthy age-matched macaques, which were provided by the National (Sichuan) Experimental Rhesus Monkey Resources Base.
The experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[26]. The animals had not received medical or immunomodulatory treatments prior to experimentation.
Methods
Tissue section preparation
Under deep anesthesia, the animals were sacrificed. The mesencephalon, diencephalon, eyeball, and optic nerve were rapidly dissected. Tissue specimens were fixed in 4% paraformaldehyde in phosphate buffer (pH 7.4) for 18–24 hours and routinely processed for paraffin embedding and serial sectioning into 4-μm thick sections for subsequent hematoxylin-eosin staining and immunohistochemistry. Sections stained by hematoxylin-eosin were used for nuclei location.
NGF and iNOS immunohistochemistry
Serial sections were de-paraffinized and rehydrated in alcohol. Tissue sections were blocked with 3% hydrogen peroxide, then subjected to antigen retrieval in citric acid buffer (pH 6.0) by microwave for 4 × 6 minutes. The sections were incubated with rabbit anti-human NGF or iNOS polyclonal antibody (Boster, Wuhan, China; 1: 100 of dilution) overnight at 4°C. The sections were then washed three times with PBS (pH 7.4). The sections were incubated with goat anti-rabbit secondary antibody (1: 100; Boster) at 37°C for 20 minutes. Immunohistochemical staining was performed using a standard avidin-biotin-peroxidase complex technique (immunohistochemistry kit, Boster). The staining was visualized with diaminobenzidine (Boster), and the sections were dehydrated and mounted. Negative controls were incubated with PBS.
Microscopic observation and image analysis
Anatomical position of the nuclei was identified according to the Rat Brain Atlas (Paxinos and Watson, 2006)[27]. Tissue sections were observed by light microscopy (Nikon, Tokyo, Japan), and images were captured at 100 ×, 200 ×, and 400 × magnification using a Nikon50i-BF fluorescent biological digital microscope (Nikon). For each animal, a total of five sections per related nucleus were selected. Average absorbance and areas with positive expression were measured using the Jiangsu Jetta 801 morphological image analysis system (Jieda, Nanjing, Jiangsu, China).
Statistical analysis
Statistical analysis was performed using SPSS 11.5 software (SPSS, Chicago, IL, USA). Data were expressed as mean ± SD and subjected to one-way analysis of variance with Duncan's multiple range tests. P < 0.05 was considered statistically significant.
Footnotes
Conflicts of interest: None declared.
Funding: The study was supported by the Program for Changjiang Scholars and Innovative Research Teaming University, No. IRT0848; Sichuan Province International Technology Cooperation and Communication Research Programs, No. 2010HH0013; Sichuan Province Basic Research Program, No. 2011JY0054; the National Key Research Program of China, No. 2011ZX09301-001, 2011ZX09307-301-3; Science and Technology Support Programs of Sichuan Province, No. 2011JO0040, 2011ZO0034.
Ethical approval: This research was approved by the Ethics Committee of Laboratory Animal Management Committee of Sichuan Province, China.
(Edited by Gu P, Wang XD/Su LL/Song LP)
REFERENCES
- [1].Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [2].Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med. 2004;82(8):510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- [3].Ziegler D, Rathmann W, Meisinger C, et al. Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes. Eur J Pain. 2009;13(6):582–587. doi: 10.1016/j.ejpain.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [4].Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: Where are we now and where to go? J Diabetes. 2011;2(1):18–32. doi: 10.1111/j.2040-1124.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheng HT, Dauch JR, Hayes JM, et al. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68(11):1229–1243. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Semra YK, Sherif S, Lincoln J. NGF protects paravertebral but not prevertebral sympathetic neurons against exposure to high glucose in vitro. Brain Res. 2009;1285:164–173. doi: 10.1016/j.brainres.2009.05.089. [DOI] [PubMed] [Google Scholar]
- [7].Ieda M, Fukuda K. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles:the regulatory mechanisms of cardiac innervation and their critical roles in cardiac performance. J Pharmacol Sci. 2009;109(3):348–358. doi: 10.1254/jphs.08r25fm. [DOI] [PubMed] [Google Scholar]
- [8].Ferrari CKB, França EL, Honorio-França AC. Nitric oxide, health and disease. J Appl Biomed. 2009;7:163–173. [Google Scholar]
- [9].Nagareddy PR, Xia Z, McNeill JH, et al. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol. 2005;289:5, H2144–2152. doi: 10.1152/ajpheart.00591.2005. [DOI] [PubMed] [Google Scholar]
- [10].Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans. 2007;35(Pt 2):424–427. doi: 10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- [11].Sang YZ, Liu X, Liu L, et al. Effect of nerve growth factor on the ultrastructural changes of neuro sensory retina in diabetic rats. Guoji Yanke Zazhi. 2008;8(6):1117–1121. [Google Scholar]
- [12].Huang CG, Wei SJ, Jiang H, et al. Protective effect of nerve growth factor on optic nerve injury in rats. Zhongguo Linchuang Kangfu. 2004;8(25):5409–5411. [Google Scholar]
- [13].Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175–182. doi: 10.1136/thorax.58.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gui XC, Liu ZH, Liu JH, et al. Influence of glucose on expression of caveolin-1 and vascular endothelial growth factors in vascular endothelial cells. Zhongguo Dongmai Yinghua Zazhi. 2004;12(1):38–41. [Google Scholar]
- [15].Choi SZ, Son MW. A novel botanical drug for the treatment of diabetic neuropathy. Arch Pharm Res. 2011;34(6):865–867. doi: 10.1007/s12272-011-0621-2. [DOI] [PubMed] [Google Scholar]
- [16].Gao ZF, Feng Y, Ju H, et al. Expression of NGF in dorsal horn and dorsal root ganglion of diabetic rats. Zhongguo Dongmai Yinghua Zazhi. 2009;25(2):147–150. [Google Scholar]
- [17].Cheng TH, Dauch IR, Oh SS, et al. p38 mediates mechanical allodynia in a mouse model of type 2 diabetes. Mol Pain. 2010;6(28):1–14. doi: 10.1186/1744-8069-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu JX, Zhou MF, Zhang H, et al. Distribution of nitric oxide synthase positive neurons of the hypothalamus in rats. Henan Yike Daxue Xuebao. 1996;31(2):42–44. [PubMed] [Google Scholar]
- [19].Ste-Marie L, Hazell AS, Bémeur C, et al. Immunohistochemical detection of inducible nitric oxide synthase, nitrotyrosine and manganese superoxide dismutase following hyperglyce-mic focal cerebral ischemia. Brain Res. 2001;918(122):10–19. doi: 10.1016/s0006-8993(01)02903-1. [DOI] [PubMed] [Google Scholar]
- [20].Sun YJ, Zhou LN, Deng ZH, et al. Study on nitric oxide synthetase in hypothalamus and brain -stem reticular structure. Dangdai Kangfu. 2001;5(3):47–48. [Google Scholar]
- [21].Li ZK, Ren LS, Su GH. The connections of the visual portion of the thalamic reticular nucleus in golden hamsters. Jiepou Xuebao. 1984;15(3):274–279. [Google Scholar]
- [22].Su JM. Advances on diabetic optic neuropathy. Wujing Yixueyuan Xuebao. 2008;17(11):1026–1030. [Google Scholar]
- [23].Xie XJ, Li RQ, Liao PZ, et al. Pathological studies of diabetic rat optic nerve (light and electron- microscopic investigation) Yanke Zazhi. 1997;15(1):23–25. [Google Scholar]
- [24].Tayyeba K, Matragoon S, Bindu A, et al. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes. 2008;57(4):889–898. doi: 10.2337/db07-1669. [DOI] [PubMed] [Google Scholar]
- [25].Zhang L, Jiang S, Kemaier A, et al. Expression of iNOS of retina in diabetic rats and its relationship with apoptosis of retina. Guoji Yanke Zazhi. 2009;9(1):47–18. [Google Scholar]
- [26].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [27].Paxinos G, Watson C. 3rd ed. New York: Academic Press; 2006. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]


