Abstract
A rat model of Parkinson's disease was induced by injecting lactacystin stereotaxically into the left mesencephalic ventral tegmental area and substantia nigra pars compacta. After rats were intragastrically perfused with Anchanling, a Chinese medicine, mainly composed of magnolol, for 5 weeks, when compared with Parkinson's disease model rats, tyrosine hydroxylase expression was increased, α-synuclein and ubiquitin expression was decreased, substantia nigra cell apoptosis was reduced, and apomorphine-induced rotational behavior was improved. Results suggested that Anchanling can ameliorate Parkinson's disease pathology possibly by enhancing degradation activity of the ubiquitin-proteasome system.
Keywords: Parkinson's disease, Anchanling, ubiquitin-proteasome system, α-synuclein, tyrosine hydroxylase, cell apoptosis
INTRODUCTION
Loss of substantia nigra dopaminergic neurons and intracytoplasmic Lewy body formation are the main pathological changes in Parkinson's disease (PD)[1,2,3,4]. Lewy body formation is highly correlated with functional impairment of the ubiquitin-proteasome system (UPS)[5,6,7,8,9]. The UPS is an important pathway involving protein degradation in vivo, responsible for clearing mutated, damaged and misfolded proteins, and comprises ubiquitin, ubiquitin-related enzymes and the proteasome. Abnormal changes in ubiquitin or ubiquitin-related enzymes E1, E2, E3 or protease may influence UPS function[10,11,12]. Recent evidence indicates that intracellular α-synuclein protein is misfolded or overexpressed in response to α-synuclein gene mutations, which inhibits proteasome degradation and induces abnormal α-synuclein accumulation, resulting in neurotoxicity[13]. Overexpression of α-synuclein in nerve cells can induce ubiquitin-dependent proteasome dysfunction and cell apoptosis[14]. Furthermore, proteasome dysfunction can result in abnormal protein aggregation. Stereotaxic injection of the proteasome inhibitor lactacystin into the substantia nigra is toxic to dopaminergic neurons, and induces protein aggregation and inclusion body formation[15,16]. An in vitro experiment further demonstrated that lactacystin can induce ubiquitinated protein aggregation and promote intracytoplasmic ubiquitin-positive inclusion body formation[17].
Substitution therapy with levodopa has been commonly used to treat PD. It ameliorates PD symptoms, but induces severe toxicity and side effects and does not control PD progression. Recent studies demonstrated that Chinese medicines can delay PD progression, relieve adverse effects due to Western medicine, and control symptoms of non-motor disturbance in PD[5,6,7,8,9,10,11,12,13,14,15,16,17,18]. However, studies mainly focused on oxidative stress, cell apoptosis, excitatory amino acid toxicity and immune reactions to investigate the mechanism of Chinese medicines[19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
Anchanling (ACL), a Chinese medicine, is effective in treating early stage PD, and exerts positive effects in PD patients with advanced stage disease, or poor response to dopamine therapy. However, the pharmacodynamic mechanism remains poorly understood. In the present study, a rat model of PD was established using lactacystin, and the effects of ACL on cell apoptosis and UPS function were observed by immunohistochemistry and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) techniques.
RESULTS
Quantitative analysis of experimental animals
Following one-week adaptation, 60 of 80 Sprague-Dawley rats were selected according to their food intake, behavior and coat[36,37]. Lactacystin was stereotaxically injected in the left mesencephalic ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) of 50 rats to establish a PD model. A total of 49 rats survived after lesion, and 27 were selected following screening of apomorphine-induced behavior. Ten were used as the vehicle-treated (PD) group and 10 as the ACL group, respectively treated with distilled water and ACL by intragastric perfusion. Another 10 of 60 rats were used as the control group. Therefore, 30 rats were included in the final analysis.
Influence of ACL on substantia nigra tyrosine hydroxylase (TH) expression in lesioned rats
Immunofluorescent labeling showed that TH was expressed in cells of the substantia nigra of control group rats and the number of TH-positive cells was 293.8 ± 13.0 per field of view (× 200) (n = 6). After 5 weeks on vehicle treatment, the number of TH-positive cells was significantly reduced in the lesioned group (53.50 ± 14.05 per field of view (× 200); n = 6) compared with control group (P < 0.05). 5 weeks of ACL increased TH-positive cells in the substantia nigra of rats (130.33 ± 11.91 per field of view (× 200); n = 6) compared with the vehicle-treated group (P < 0.05), but this remained lower than the control group (P < 0.05; Figure 1).
Figure 1.
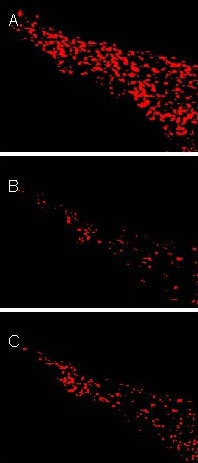
Substantia nigra tyrosine hydroxylase (TH) expression in rats (immunofluorescent staining, × 200).
TH-positive reaction was represented by red fluorescence. A large number of TH-positive cells were observed in the control group (A). Compared with the control group, the number of TH-positive cells was significantly decreased in the vehicle-treated (model) group (B). Anchanling (C) increased TH-positive cells in rats compared with the vehicle-treated group.
Influence of ACL on substantia nigra α-synuclein and ubiquitin expression in lesioned rats
Immunofluorescence and thioflavin S (a chromogenic marker of amyloid substance) labeling were used to examine protein aggregation. The rate of thioflavin S and α-synuclein double labeling, as well as thioflavin S and ubiquitin double labeling was significantly increased at 5 weeks in the vehicle-treated group compared with the control group. ACL significantly reduced thioflavin S and α-synuclein double labeling, as well as thioflavin S and ubiquitin double labeling (Figures 2, 3).
Figure 2.
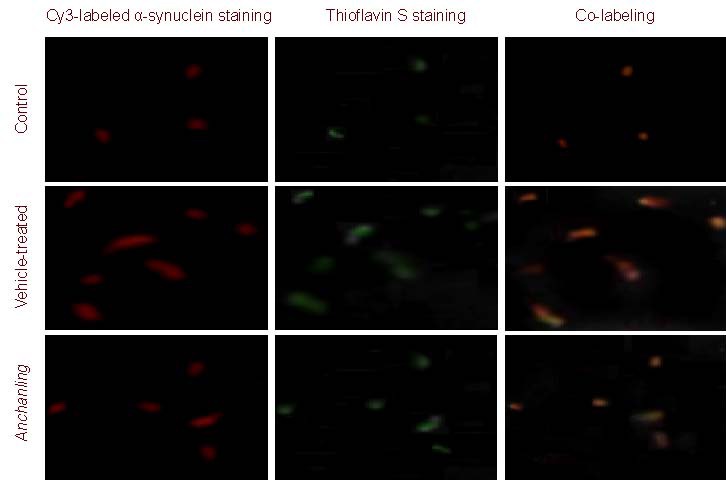
Alpha-synuclein protein expression in rat substantia nigra (immunofluorescence double-labeling staining, × 200).
Thioflavin S was used as a chromogenic marker of amyloid substance. Red fluorescence represents α-synuclein staining, and green represents thioflavin S staining. In the vehicle-treated (model) group, co-labeling of α-synuclein and thioflavin S was evident. In the Anchanling group, the co-labeling was reduced compared with the model group. Only weak red and green fluorescence was observed in the control group.
Figure 3.
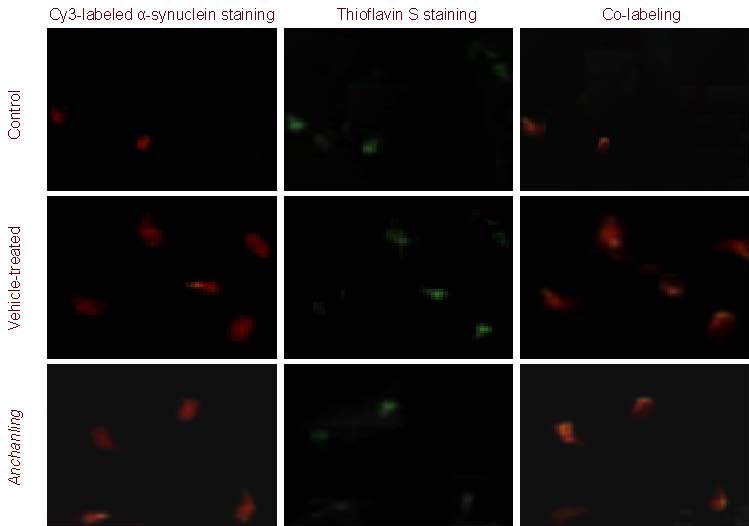
Ubiquitin protein expression in rat substantia nigra (immunofluorescence double-labeling staining, × 200).
Thioflavin S was used as chromogenic marker of amyloid substance. Red fluorescence represents ubiquitin staining, and green represents thioflavin S staining. In the vehicle-treated (model) group, co-labeling of ubiquitin and thioflavin S was evident. In the Anchanling group, the co-labeling was reduced compared with the model group. Only weak red and green fluorescence was observed in the control group.
Influence of ACL on nigral apoptosis in lesioned rats
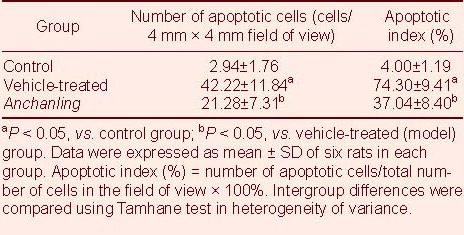
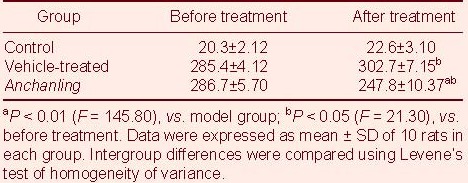
TUNEL showed that cell apoptosis was significantly increased in the substantia nigra of the vehicle treated group compared with the control group (P < 0.05). However, it was significantly decreased in the ACL group compared with the vehicle treated group (P < 0.05; Table 1).
Table 1.
Cell apoptosis in substantia nigra of rats
Influence of ACL on behavior in lesioned rats
Three weeks after lesion locomotion was significantly reduced. Crawling movements and escape responses to external stimuli were delayed. Spontaneous rotational behavior, sniffing and back bending appeared. At five weeks, all rats underwent apomorphine induction. Rotation toward the healthy side was significantly increased in the vehicle-treated group and ACL group (P < 0.05), but the frequency of apomorphine-induced rotation in the Anchanling group is less than vehicle-treated group (P < 0.01). The control group rats did not show aberrant rotational behavior (Table 2).
Table 2.
Frequency of apomorphine-induced rotation (rotations/30 minutes) before and after Anchanling treatment
DISCUSSION
In the present study, PD symptoms were induced in rats by injection of lactacystin into the SNc and VTA. As previously published[38], thioflavin S was used as a chromogenic marker of amyloid substance to observe abnormal protein aggregation in cells. Immunofluorescent double labeling showed stronger co-staining of α-synuclein and thioflavin S in the lesioned, vehicle-treated group compared with the control group, suggesting α-synuclein aggregation in the former. In addition, co-staining of ubiquitin and thioflavin S was evident in the vehicle-treated group, indicating ubiquitination of amyloid protein. TUNEL showed that cell apoptosis in the substantia nigra was significantly increased in the vehicle-treated group compared with the control group. Therefore, microinjection of lactacystin into the SNc and VTA can successfully induce pathological characteristics of PD. This is consistent with a correlation between proteasome dysfunction, α-synuclein aggregation and cell apoptosis in the substantia nigra. That is, functional disturbance of proteasomes can induce α-synuclein aggregation and cell apoptosis in the substantia nigra. Proteasome dysfunction can trigger cell cycle signals in dopaminergic neurons by upregulating cell cycle regulating factor expression, thereby inducing neuronal apoptosis and degeneration[36]. The proteasomal inhibitor lactacystin can activate caspase-3 and induce PC12 apoptosis[37]. Therefore, we conclude that lactacystin inhibits proteasome function in the UPS, thereby blocking α-synuclein degradation, inducing α-synuclein aggregation, cell structural damage, triggering apoptosis, and leading to cell degeneration and death.
In the present study, ACL reduced α-synuclein aggregation and cell apoptosis in the substantia nigra compared with the vehicle-treated group, possibly by improving proteasomal degradation of α-synuclein, reducing protein aggregation and inclusion body formation, and/or activating apoptosis-inhibiting signal pathways to protect nigral cells.
In conclusion, UPS dysfunction can induce α-synuclein and ubiquitin aggregation and inclusion body formation, resulting in neuronal apoptosis in the substantia nigra. ACL may ameliorate PD pathology by enhancing the function of the ubiquitin-proteasome system.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment was used.
Time and setting
The experiment was performed at the SPF Animal Laboratory, Shenzhen Institute for Biochemical Drug Control, and Central Laboratory of First Affiliated Hospital of Shenzhen University, China from June 2007 to February 2009.
Materials
Animals
A total of 80 healthy, adult, male Sprague Dawley rats, weighing 200–250 g, were provided by the Medical Animal Experimental Center of Guangdong Province (No. SCXK (Yue) 2003-0002; 2006A015). The rats were housed in humidity of 40–70% at 20–26°C. The experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of the People's Republic of China[39].
Drugs
ACL was provided by the Shenzhen Institute of Gerontology. The crude drugs were purchased from Shenzhen Accord Pharmaceutical Co., Ltd., China. ACL comprised magnolia bark, fourstamen stephania root and Chinese Magnolivine Fruit and prepared by routine water-ethanol method[40]. Suspension was prepared by mixing with distilled water and administrated intragastrically. The active component was magnolol (5, 5’-Diallyl-2, 2’-biphenyldiol, molecular weight 266.32) and honokiol (3’, 5-Diallyl-2-4’-dihydroxybiphenyl, molecular weight 266.33). They were isomeric[41]. Their chemical structural formulas are shown in Figure 4.
Figure 4.
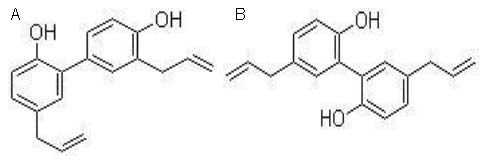
Chemical structures of honokiol (A) and magnolol (B).
Methods
Establishment of PD model
Following behavioral tests, animals without rotational behavior were included for subsequent experiments. The rats were anesthetized by intraperitoneal injection of 2% pentobarbital (45 mg/kg; Sigma, St. Louis, MO, USA), and placed at the stereotaxis instrument (RWD Life Science, Shenzhen, China). The head was routinely sterilized, and the scalp was cut open, and the periosteum was dissected. According to rat stereotaxic atlas (supplementary Figure 1 online), SNc and VTA location was identified[42]: SNc: 5.0 mm posterior to bregma, 1.7 mm left to stereotaxic atlas, 7.6 mm below cranium surface; VTA: 4.6 mm posterior to bregma, 0.9 mm left to stereotaxic atlas, 7.5 mm below cranium surface. According to body mass and cranium size, the coordinates were confirmed and marked using three edged needle. The cranium was drilled using dental drill, and a microsyringe (Hamilton, CH-7402 Bonaduz, GR, Switzerland) was slowly inserted according to coordinates to inject lactacystin (Sigma; 8 μg (4 μL) per point) in vehicle-treated and ACL groups, while the control group was injected with the same volume of normal saline. The injection was inserted to a depth of the center of pinhole slope (zero at cerebral dura mater), 1 μL/min. The needle was maintained for 10 minutes, and slowly removed. The wound was sutured, followed by intraperitoneal injection of penicillin (10 × 104 U/kg/d) for one week to prevent infection. In addition, the wound was disinfected with iodine tincture, once a day. The sutures were removed seven days later.
Evaluation of model
Behaviors were observed at 9: 00 am every seven days from one week postoperatively. The rats were intraperitoneally injected with 0.5 mg/kg apomorphine (Sigma). The rotational behaviors of rats were observed in a plastic tub (40 cm diameter) and rotations within five minutes after injection were recorded for three weeks. Successful lesioning was identified if the rats rotated towards the right within 30 minutes, with mean speed > 7 r/min. Rats with rotations towards the left or rotations towards the right at < 7 r/min or without rotation were excluded.
ACL administration
The ACL group rats were administrated ACL at a dose of 10 times of that for a 60 kg human, dissolved in normal saline, 1.5 mL/100 g[43], 9: 00-10: 00 a.m., once a day. Animals were weighed every week, and the dose was adjusted accordingly. The drugs were administrated for five consecutive weeks. The vehicle-treated and control groups were perfused with the same volume of normal saline. All rats were administrated immediately following model establishment.
Behavioral detection
The rats were induced with apomorphine at 1, 3, 5 weeks after ACL administration to observe rotations and other abnormal behaviors. Changes in rotation frequency before and after administration and among groups were compared.
Immunofluorescent staining for TH expression in the substantia nigra
Five weeks after administration, two rats were randomly selected from each group, anesthetized by intraperitoneal injection with 2% pentobarbital (45 mg/kg), followed by perfusion of 4% paraformaldehyde via left ventricular intubation. Brains were harvested, fixed in postfixation solution containing 20% sucrose at 4°C for 2 hours, followed by postfixation solution containing 30% sucrose at 4°C for 2 hours. Serial coronal sections were prepared originating from the substantia nigra using freezing microtome (Microm HN525, Therm, Walldrorf, Germany), 30 μm thick. Symmetrical sections from the substantia nigra were selected for TH immunohistochemistry. The sections were washed with 0.01 M phosphate buffered saline (PBS) (pH 7.4), blocked in 2% bovine serum albumin at room temperature for 30 minutes, incubated with mouse anti-TH polyclonal antibody (1: 200; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at 4°C overnight, washed, incubated with Cy3-labeled goat anti-rat secondary antibody dilution (1: 200; Santa Cruz Biotechnology Inc.) at 37°C for 1 hour, shaken, rinsed, mounted with 50% neutral resin, and observed by fluorescence microscope (Leica Inc.). The mean number of TH-positive cells in bilateral substantia nigra sections under 200 × magnification was calculated.
Immunofluorescent double labeling for α-synuclein and ubiquitin in the substantia nigra
The sections were washed with 0.01 M PBS (pH 7.4), blocked in 2% bovine serum albumin at room temperature for 30 minutes, incubated with mouse anti- α-synuclein (1: 500) and ubiquitin polyclonal antibodies (1: 400; Sigma) at 4°C overnight, washed, incubated with Cy3-labeled goat anti-mouse secondary antibody dilution (1: 200; Santa Cruz Biotechnology Inc.) at 37°C for 1 hour, followed by 0.1% thioflavin S (Sigma) for 10 minutes. The sections were differentiated with 80% ethanol for 5 minutes, shaken, rinsed, mounted with 50% neutral resin, and observed by fluorescence microscope.
TUNEL detection for cell apoptosis in the substantia nigra
After administration, two rats were randomly selected from each group, anesthetized, and the midbrain was harvested, fixed in 4% paraformaldehyde for 24 hours, dehydrated by gradient ethanol, paraffin embedded, followed by coronal sectioning, 5 μm thick. Five sections were selected from each block of brain tissues. TUNEL was performed according to TUNEL kit (R&D Systems, Minneapolis, MN, USA). Briefly, the sections were dewaxed, and washed with PBS, 3 × 5 minutes after antigen was retrieved by microwave, incubated with 20% normal goat serum at room temperature for 30 minutes. TUNEL reaction solution was added to the sections and incubated at 37°C for 90 minutes, washed with PBS, 3 × 5 minutes, followed by 3% H2O2 methanol solution at room temperature for 10 minutes and incubation at 37°C for 90 minutes. The sections were treated with POD transforming agent at 37 °C for 30 minutes, washed with PBS, 3 × 5 minutes, visualized with diaminobenzidine/H2O2, stained with hematoxylin, dehydrated, cleared, and mounted with neutral resin. Six sections were selected from each group, and three 4 mm × 4 mm fields of view were selected. The number of TUNEL-positive cells and apoptotic index were analyzed using Leica Qwin image analysis system software (Leica Microsystems Imaging Solutions Ltd., Software Version 3.0, Cambridge, UK).
Statistical analysis
Data were analyzed using SPSS version 10.0 (SPSS, Chicago, IL, USA) and are expressed as mean ± SD. In homogeneity of variance, data were analyzed using repeated measurement of analysis of variance, one-way analysis of variance or least significant difference-t test; while data were analyzed with Tamhane test in heterogeneity of variance.
Acknowledgments:
We thank the staff of Animal Laboratory, Shenzhen Institute for Biochemical Drug Control, and Clinical Institute of Chinese and Western Medicine, First Affiliated Hospital of Shenzhen University, China, for technical support.
Footnotes
Conflicts of interest: None declared.
Funding: This study was supported by the National Natural Science Foundation of China (Influence of Anchanling on endoplasmic reticulum stress and ubiquitin-proteasome system in PD model), No. 30772757.
Ethical approval: This study received permission from the Animal Ethnics Committee of First Affiliated Hospital of Shenzhen University, China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 7, No. 3, 2012 after selecting the “NRR Current Issue” button on the page.
(Edited by Zhao Y, Wang Z/Su LL/Wang L)
REFERENCES
- [1].Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- [2].Giasson BI, Lee VM. Are ubiquitination pathways central to Parkinson's disease? Cell. 2003;114(1):1–8. doi: 10.1016/s0092-8674(03)00509-9. [DOI] [PubMed] [Google Scholar]
- [3].Greenamyre JT, Hastings TG. Biomedicine. Parkinson's--divergent causes, convergent mechanisms. Science. 2004;304(5674):1120–1122. doi: 10.1126/science.1098966. [DOI] [PubMed] [Google Scholar]
- [4].Lansbury PT, Jr, Brice A. Genetics of Parkinson's disease and biochemical studies of implicated gene products. Curr Opin Cell Biol. 2002;14(5):653–660. doi: 10.1016/s0955-0674(02)00377-0. [DOI] [PubMed] [Google Scholar]
- [5].Bukhatwa S, Zeng BY, Rose S, et al. A comparison of changes in proteasomal subunit expression in the substantia nigra in Parkinson's disease, multiple system atrophy and progressive supranuclear palsy. Brain Res. 2010;1326:174–183. doi: 10.1016/j.brainres.2010.02.045. [DOI] [PubMed] [Google Scholar]
- [6].Lee FK, Wong AK, Lee YW, et al. The role of ubiquitin linkages on alpha-synuclein induced-toxicity in a Drosophila model of Parkinson's disease. J Neurochem. 2009;110(1):208–219. doi: 10.1111/j.1471-4159.2009.06124.x. [DOI] [PubMed] [Google Scholar]
- [7].Olanow CW, McNaught KS. Ubiquitin-proteasome system and Parkinson's disease. Mov Disord. 2006;21(11):1806–1823. doi: 10.1002/mds.21013. [DOI] [PubMed] [Google Scholar]
- [8].Betarbet R, Sherer TB, Greenamyre JT. Ubiquitin-proteasome system and Parkinson's diseases. Exp Neurol. 2005;191(Suppl 1):S17–27. doi: 10.1016/j.expneurol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- [9].Barrachina M, Castaño E, Dalfó E, et al. Reduced ubiquitin C-terminal hydrolase-1 expression levels in dementia with Lewy bodies. Neurobiol Dis. 2006;22(2):265–273. doi: 10.1016/j.nbd.2005.11.005. [DOI] [PubMed] [Google Scholar]
- [10].Heo JM, Rutter J. Ubiquitin-dependent mitochondrial protein degradation. Int J Biochem Cell Biol. 2011;43(10):1422–1426. doi: 10.1016/j.biocel.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Taylor EB, Rutter J. Mitochondrial quality control by the ubiquitin- proteasome system. Biochem Soc Trans. 2011;39(5):1509–1513. doi: 10.1042/BST0391509. [DOI] [PubMed] [Google Scholar]
- [12].Egeler EL, Urner LM, Rakhit R, et al. Ligand-switchable substrates for a ubiquitin-proteasome system. J Biol Chem. 2011;286(36):31328–31336. doi: 10.1074/jbc.M111.264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Forloni G, Bertani I, Calella AM, et al. Alpha-synuclein and Parkinson's disease: selective neurodegenerative effect of alpha- synuclein fragment on dopaminergic neurons in vitro and in vivo. Ann Neurol. 2000;47(5):632–640. [PubMed] [Google Scholar]
- [14].Stefanis L, Larsen KE, Rideout HJ, et al. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21(24):9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao J, Liu ZG, Chen SD, et al. The degeneration of dopaminergic neuron and inclusion-like formation induced by the inhibitor of proteasome. Zhongfeng yu Shenjing Jibing Zazhi. 2004;21(6):493–495. [Google Scholar]
- [16].Zhang KZ, Wang J, Ding ZT, et al. The roles of proteasome on nigral degeneration and Lewy body formation. Zhonghua Laonian Yixue Zazhi. 2004;23(4):259–262. [Google Scholar]
- [17].Yang H, Chen SD, Li BZ, et al. The block of ubiquitin-proteasome pathway induces cell death and the formation of ubiquitin- immunoreactive inclusions in PC12 cells. Zhonghua Shenjing Ke Zazhi. 2005;38(7):430–433. [Google Scholar]
- [18].Niu C, Zhang J, Mei J, et al. Does neurotrophic factor benefit to PD therapy via co-function with ubiquitin-proteasome system? Med Hypotheses. 2011;76(4):589–592. doi: 10.1016/j.mehy.2011.01.007. [DOI] [PubMed] [Google Scholar]
- [19].Wang YC, Xu HL, Fu Q, et al. Resveratrol derived from Rhizoma Et Radix Polygoni Cuspidati and its liposomal form protect nigral cells of Parkinsonian rats. Zhongguo Zhongyao Zazhi. 2011;56(8):1060–1066. [PubMed] [Google Scholar]
- [20].Niu JY, Xu F. The protective effect and mechanism of green tea polyphenols in substantia nigra dopaminergic neurons of Parkinson's disease. Shandong Yiyao. 2010;54(11):56–57. [Google Scholar]
- [21].He JC, Wang ZH, Yuan CX, et al. Effects of compound rehmannia prescription on the neuroethology and oxidative stress in rats with Parkinson's disease. Zhongguo Kangfu Yixue Zazhi. 2009;25(7):590–592. [Google Scholar]
- [22].Dong MX, Niu YC, Pan Z, et al. The protective effect of pueraria isoflavones on MPP+-induced oxidative stress in the PC12. Zhongyao Yaoli yu Linchuang. 2009;26(2):48–49. [Google Scholar]
- [23].Gao LX, Liu K. Summary of Chinese medicine treatment of Parkinson's disease. Shizhen Guoyi Guoyao. 2010;21(3):724–725. [Google Scholar]
- [24].Li HL, Shu Y, Hou RR, et al. Neuroprotective effects of gastrodin on PQ and MB Induced dopaminergic neurons damage in C_(57) BL mice. Chengdu Zhongyiyao Daxue Xuebao. 2010;53(1):57–59. [Google Scholar]
- [25].Zhang L, Liu SM. Acanthopanax prevention Parkinson's disease. Zhongguo Zhongyiyao Xinxi Zazhi. 2007;17(3):95–96. [Google Scholar]
- [26].Yuan HL, Wang X, Zhang LP, et al. Mechanism and research progress of Chinese traditional medicine in the prevention and treatment of Parkinson's disease. Zhongguo Yaoli Xue Tongbao. 2010;26(7):850–854. [Google Scholar]
- [27].Dong MJ, Qian HY, Zhou SF, et al. Effect of Liuwei Rehmanniae Pill on Mouse's Oxidation Stress Reaction with Parkinson. Zhejiang Zhongyiyao Daxue Xuebao. 2009;34(6):756–757. [Google Scholar]
- [28].Ding HJ, He JC, Wang WW. Effects of Ditan decoction on behavioristics and oxidative stress reaction of rats with parkinson's disease. Shanghai Zhongyiyao Zazhi. 2009;56(3):63–65. [Google Scholar]
- [29].Wang WW, He JC, Ding HJ. Effect of tianma gouteng drink on the behavioural and oxidation stress response of Parkinson's disease rat. Zhongguo Laonian Xue Zazhi. 2010;30(12):1657–1659. [Google Scholar]
- [30].Guo SH, Bezard E, Zhao BL. Protective effect of green tea polyphenols against 6-OHDA induced apoptosis in SH-SY5Y cells through ROS-NO pathway. Bopu Xue Zazhi. 2006;28(4):550–551. [Google Scholar]
- [31].An LJ, Li ZG, Jiang B. Parkinson's disease and cell apoptosis. Zhongguo Xiandai Yixue Zazhi. 2006;20(7):1032–1036. [Google Scholar]
- [32].Chen JZ, Huang C, Li XM, et al. Research clew and practice of treating Parkinsonism with method of tonifying liver and kidney. Zhongguo Yiyao Xuebao. 2004;19(11):688. [Google Scholar]
- [33].He JC, Yuan SX, Wei HC, et al. The effect of nourishing the liver and kidney meridians and detoxifying herbs on cell apoptosis in PD rats. Zhonguo Laonian Xue Zazhi. 2003;22(5):217. [Google Scholar]
- [34].Zhu WF, Luo RJ, Zhou LP, et al. Experimental studies on Zhenchan Ning for Parkinson's disease. Guangzhou Zhongyiyao Daxue Xuebao. 2004;21(4):284. [Google Scholar]
- [35].Cai DF, Chen XQ, Gao Y, et al. Effect of Bushen Yanggan recipe on nigrostriatal function in Parkinsonian model rats after long-term levodopa treatment. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22(1):43. [PubMed] [Google Scholar]
- [36].Zhang ZT, Cao XB, Sun SG, et al. The effect and mechanism of proteasome inhibitor induced cell cycle reentry on dopaminergic neurons. Zuzhong yu Shenjing Jibing. 2006;13(3):134–137. [Google Scholar]
- [37].Yang H, Chen SD, Lu GQ, et al. Proteasomal inhibitor lactacystin induces cell apoptosis and caspase 3 activation in PC12 cells. Zhonghua Yixue Zazhi. 2005;85(29):2058–2061. [PubMed] [Google Scholar]
- [38].Gao XW. Wuhan: Hubei University of Traditional Chinese Medicine; 2007. Anchangling's effection on ubiquitin prosbasome system of parkinson's disease rat models. [Google Scholar]
- [39].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [40].Guo Y, Liang XY, Nian WL. Preliminary studies on extracting aqueous depsides from Salvia Yunanensis with water-alchohol methods. Yunnan Zhongyi Xueyuan Xuebao. 2001;24(4):6–8. [Google Scholar]
- [41].Sun AL, Feng L, Liu RM. Preparative isolation and purification of honokiol and magnolol from Magnolia Officinali s Rehd. et Wils by high-speed countercurrent chromatography. Fenxi Huaxue. 2005;33(7):1016–1018. [Google Scholar]
- [42].Bao XM, Shu SY. Beijing: People's Medical Press; 1991. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [43].Shi XQ. Beijing: People's Medical Press; 1986. Experiments Methods of Medical Animals. [Google Scholar]


