Abstract
This study sought to investigate the effects of Purendan superfine powder comprised of Momordica charantia, Radix Ginseng, and Radix Salviae Miltiorrhiae on neuronal apoptosis and expression of bcl-2, bax, and caspase-3, which are retinal apoptosis-associated factors in rats with diabetes mellitus induced by continuous intraperitoneal injection of streptozotocin. The results showed that Purendan superfine powder could upregulate the expression of bcl-2 protein and mRNA, and downregulate the expression of bax and caspase-3 in the retina of diabetes mellitus rats. In addition, Purendan superfine powder was shown to reduce the number of apoptotic neurons. Our experimental findings indicate that Purendan superfine powder can inhibit neuronal apoptosis in the retina of diabetes mellitus rats and has protective effects on diabetic retinopathy.
Keywords: Purendan superfine powder, diabetic retinopathy, bcl-2, bax, caspase-3, cellular apoptosis
INTRODUCTION
Diabetic retinopathy (DR) is the leading cause of vision loss in patients with diabetes[1]. Previous studies regarding the pathogenesis of DR mainly focused on the changes in retinal microcirculation[2,3,4]. In recent years, a number of studies have shown that DR is not only present with retinal microvascular disease, but also exists early in the dysfunction of retinal nerve tissue[5,6,7,8]. This is evidence that DR is a neurodegenerative disease, as it causes retinal injury and dysfunctions. Cellular apoptosis results in the lesions affecting nerve tissue in diabetic retinopathy[9,10]. Thus, it may be a strategy for prevention and treatment of DR through regulation of apoptosis-related genes and inhibition of cellular apoptosis, therefore leading to a neuroprotective effect and improving the functional status of DR.
The apoptosis occurs as a result of several apoptosis-related gene modulations, especially of the bcl-2 and bax genes, which are closely associated with the regulation of apoptosis[11,12]. Bcl-2 antagonizes cellular apoptosis[13,14] while bax promotes apoptosis[15,16,17,18]. Bcl-2 competitively binds with bax, forming a stable bcl-2/bax heterodimer, which attenuates the effect of bax and thus inhibits apoptosis[19]. Therefore, the altered ratio of bcl-2 and bax determines the fate of cells[20]. However, cells may survive if bcl-2 is dominant, but cells die if bax is dominant[21,22]. Caspase-3 is also known as the cell death executor as the expression and activation of caspase-3 irreversibly activates apoptosis[23,24,25,26].
Purendan superfine powder (PRD) consists of Momordica charantia as the principal drug, Radix Ginseng and Radix Salviae Miltiorrhiae as the adjuvant drugs, Radix Puerariae, Radix Polygoni Multiflori and Hirudo as the adjuvant and conductant drugs. According to the theory of traditional Chinese medicine, PRD can benefit vital energy, nourish yin, regulate blood circulation, and disperse blood stasis[27]. Modern pharmacological studies have confirmed that PRD inhibits oxidative stress, suppresses apoptosis and aging, improves microcirculation, and promotes tissue repair and regeneration[28,29]. A number of preliminary studies confirmed that PRD can effectively repair damaged islet β-cells and reduce blood sugar in diabetic rats, and protect primary vessels and microvessels against diseased lesions[28,30,31]. This study aims to elucidate the protective effect of PRD on retinal nerve tissues in diabetic rats, through analysis of retinal neuronal apoptosis and expression of the apoptosis- related genes bcl-2, bax, and caspase-3.
RESULTS
Quantitative analysis of experimental animals
A total of 36 rats were randomly divided into three groups: normal control, diabetic model, and PRD treatment. Diabetes mellitus was induced in rats in the diabetic model group and PRD treatment group. In addition, PRD-treated rats were treated with PRD for 3 consecutive months via intragastric administration after the diabetic model rats were successfully established. All 36 rats were eventually analyzed in the results.
PRD inhibits apoptosis of retinal cells in diabetic rats
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis was used to assess apoptosis. TUNEL-positive cells in the retina were considered as apoptotic cells. TUNEL-positive staining was seen in the nuclei as brown granular shapes, and positive cells were mainly seen in the retinal ganglion cell layer and inner nuclear layer, and exhibited scattered arrangement.
The apoptosis index of nerve cells in normal control rats was significantly lower than that in model rats (0.023 6 ± 0.004 3 vs. 0.224 4 ± 0.026 3, P < 0.01). Compared with the model group, the apoptosis index of PRD-treated rats was significantly lower (0.117 1 ± 0.019 6, P < 0.01; Figure 1).
Figure 1.
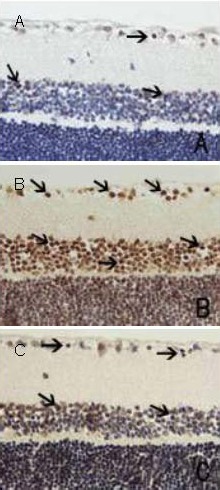
Neuronal apoptosis in retina by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (× 400). Arrows indicate apoptotic cells.
(A) Normal control group: a small number of brown-stained TUNEL-positive cells are seen in the retinal ganglion cell layer and in the inner nuclear layer.
(B) Diabetic model group: a large number of TUNEL-positive cells are seen in the retinal ganglion cell layer and in the inner nuclear layer.
(C) Purendan superfine powder treatment group: TUNEL-positive cells are seen in the retinal ganglion cell layer and in the inner nuclear layer, but the number was significantly less than the model group.
PRD affects Bcl-2, Bax, and caspase-3 protein expression in diabetic rats
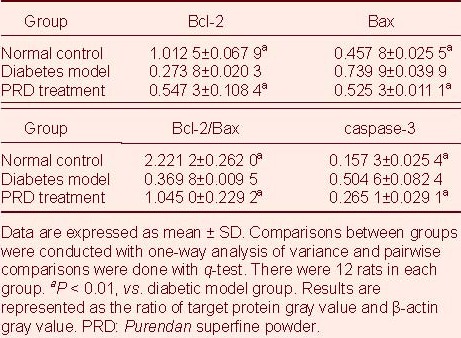
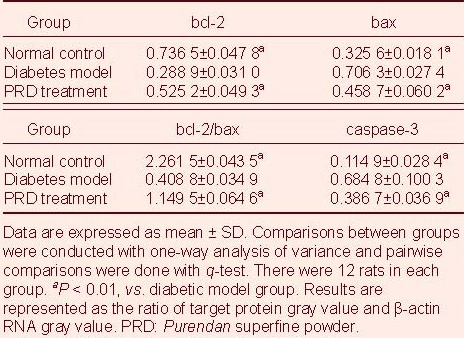
Western blot analysis showed that Bax and caspase-3 protein expression in retina of diabetic model rats was significantly increased (P < 0.01), while Bcl-2 protein expression and Bcl-2/Bax ratio representing activation of apoptosis was significantly decreased compared with the normal control group (P < 0.01). PRD treatment significantly reduced Bax and caspase-3 protein expression in diabetic rats, and significantly increased Bcl-2 protein expression and Bcl-2/Bax ratio (P < 0.01; Figure 2, Table 1).
Figure 2.
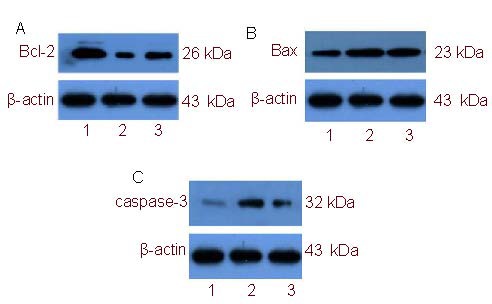
Western blot detection of Bcl-2 (A), Bax (B), and caspase-3 (C) protein expression in rat retinal tissue.
1–3: Normal control, diabetic model, Purendan superfine powder treatment groups, respectively.
Table 1.
Expression of Bcl-2, Bax, and caspase-3 protein in rat retina (western blot analysis)
PRD affects bcl-2, bax, and caspase-3 mRNA expression in diabetic rats
Reverse transcription PCR analysis showed that bax and caspase-3 mRNA expression was significantly increased (P < 0.01), while bcl-2 mRNA expression and bcl-2 mRNA/bax mRNA ratio were significantly lower in diabetic rats (P < 0.01) compared with normal control rats. PRD treatment significantly downregulated the expression of bax and caspase-3 mRNA (P < 0.01), but significantly increased bcl-2 mRNA expression and bcl-2 mRNA/bax mRNA ratio in diabetic rat retinas (P < 0.01; Figure 3, Table 2).
Figure 3.
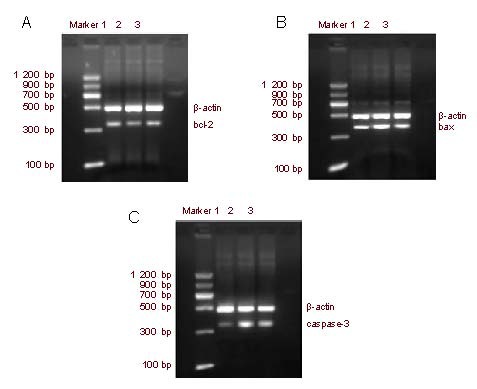
Reverse transcription-PCR detection of rat retinal tissue bcl-2 (A), bax (B), and caspase-3 (C) mRNA expression.
1–3: Normal control, diabetic model, Purendan superfine powder treatment groups, respectively.
Table 2.
Retinal bcl-2, bax, and caspase-3 mRNA expression in rats (reverse transcription-PCR)
DISCUSSION
Barber et al[32] initially reported that the number of apoptotic neurons in the retina of streptozotocin-induced diabetic rats was ten times as many as that of cells in their normal control group at 1 month after disease onset. Park et al[33] confirmed that retinal photoreceptors in diabetic rats had signs of apoptosis at 4 weeks, and the number of apoptotic cells also increased along with disease progression. The present study found similar results: that the apoptosis index in the model group was significantly higher than that in the normal control group. This is evidence that retinal nerve cells are involved in the development of DR.
This study showed that compared with the normal control rats, bcl-2, bax, and caspase-3 protein expression were significantly increased, while bcl-2 expression and bcl-2/bax ratio were significantly reduced in diabetic rats. This finding indicates that bcl-2, bax, and caspase-3 are key factors in inducing apoptosis in DR retinal nerve cells. Moreover, the apoptosis index and bax and caspase-3 expression were significantly decreased in retina of PRD-treated rats, while bcl-2 expression and bcl-2/bax ratio were significantly increased compared with the model group. This evidence suggests that PRD could inhibit retinal nerve cell apoptosis and play a protective role in the retinal tissue by upregulating the expression of the anti-apoptotic gene bcl-2, downregulating expression of the pro-apoptotic gene bax, and inhibiting caspase-3 activation.
In summary, apoptosis-related genes, including bcl-2, bax, and caspase-3 play a crucial role in the apoptosis of DR retinal nerve cells. PRD treatment can alter bcl-2, bax, and caspase-3 expression and inhibit neuronal apoptosis in diabetic rats, thus showing a neuroprotective effect on DR.
MATERIALS AND METHODS
Design
A randomized controlled animal study.
Time and setting
Experiments were performed from September 2009 to March 2011 in the Institute of Basic Medical Sciences, Chengde Medical College, China.
Materials
Animals
A total of 36, eight-week-old, male Wistar rats of clean grade, weighing 200–250 g, were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd., China (license number: SCXK (Beijing) 2006-0009). Rats were housed in clean cages at 23 ± 1°C and 12-hour light-dark cycle, allowing free access to food and water. All experimental treatments of animals were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[34].
Drugs
PRD consisted of Momordica charantia, Radix Ginseng, Radix Salviae Miltiorrhiae, Radix Puerariae, Radix Polygoni Multiflori and Hirudo at a proportion of 10: 1: 3: 1: 1: 0.3. Each 1 g of ultrafine powder is equivalent to 8.15 g crude drug. PRD was provided by the Institute of Chinese Minority Traditional Medicine, Central University for Nationalities, China.
Methods
Establishment of diabetes mellitus models
Diabetes in rats was induced with 2% streptozotocin (25 mg/kg; Sigma, St. Louis, MO, USA) via intraperitoneal injection for 3 days, and the model rats were considered successful upon the appearance of symptoms, such as blood glucose ≥ 16.7 mM[35].
PRD treatment
According to body surface area, the daily dose of PRD treatment was determined as 1.8 g/kg[36] administered orally for 3 months.
Specimen preparation
After PRD treatment was given, rats were intraperitoneally anesthetized with 10% of chloral hydrate (0.5 g/kg). Both eyeballs were quickly removed and one of the eyeballs was fixed with 4% paraformaldehyde for 24 hours for the TUNEL analysis after conventional dehydration, transparency, waxing, and embedding, while the other eyeball was dissected on ice under a microscope. The retinal tissue was stored in liquid nitrogen for subsequent western blot analysis and reverse transcription PCR analysis.
TUNEL in situ detection of retinal cell apoptosis
Retinal serial sections parallel to the sagittal axis of the optic nerve, at 4 μm thickness, were dewaxed and digested with Proteinase K solution (Shanghai Yangyu Company, Shanghai, China) for 15 minutes. Sections were then incubated with TUNEL reaction solution (Roche Company, Basel, Switzerland) at 37°C for 60 minutes, and with peroxidase transforming agent (Roche Company, Basel, Switzerland) at 37°C for an additional 30 minutes. Sections were finally stained with 3, 3’-diaminobenzidine, counterstained with hematoxylin, dehydrated, and mounted for detection. The nuclei of apoptotic cells were stained brown. Apoptotic index was determined as the ratio of the number of TUNEL-positive cells to total number of nerve cells in the same field, under 400 × magnification with a bright-field microscope (Olympus, Tokyo, Japan). Three slices per rat, five fields of vision in each, were randomly selected to calculate the average values.
Western blot analysis of retinal bcl-2, bax, and caspase-3 protein expression
Retinal tissues stored in liquid nitrogen were used to extract protein, and protein concentration was determined with a bicinchoninic acid protein kit (Beijing Solarbio Science & Technology Co., Ltd., China). Proteins (50 μg) were loaded onto 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and electrophoresed at 80–120 V for 2 hours, and then transferred to nitrocellulose membranes. Membranes were immunoblotted with mouse anti-rat bcl-2 (1: 200), bax (1: 200), caspase-3 (1: 200) and β-actin (1: 1 000) monoclonal antibodies (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at 4°C overnight after 5% skim milk blocking for 2 hours. Sections were incubated with horseradish peroxidase stained goat anti-mouse secondary antibody (1: 5 000; KPL, Gaithersburg, Maryland, USA) at room temperature for 1 hour after washing the membrane. The protein bands were visualized with Super ECL Plus ultra-sensitive light-emitting liquid. The scanned protein bands were analyzed with Quantity One-4.6.2 software (Bio-Rad, Hercules, CA, USA), with β-actin as an internal control. The gray-scale ratios of bcl-2, bax, caspase-3 bands to β-actin bands were calculated as the relative expression levels of the proteins of interest.
Reverse transcription PCR detection of retinal bcl-2, bax, and caspase-3 mRNA expression
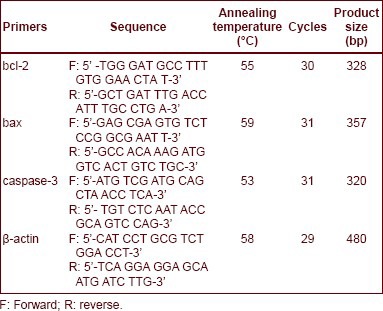
Total RNA of the retina stored in liquid nitrogen was extracted using a Trizol kit (Invitrogen, Carlsbad, CA, USA). RNA (5 μL) was electrophoresed in 1% agarose gel and three complete bands were clearly seen at 28, 18, and 5 seconds, indicating that the extracted total RNA was complete. Absorbance at 260 nm and 280 nm was read with a UV spectrophotometer (DU800; Beckman Coulter, Fullerton, CA, USA) and calculated as 1.9–2.1, indicating that RNA was not contaminated. Primer sequence is as follows:
3 μg total RNA as a template was reversely transcribed into cDNA. Reverse transcription reactions were as follows: 30°C for 10 minutes; 55°C for 30 minutes; 99°C for 5 minutes; 5°C for 5 minutes. PCR reactions were as follows: 94°C for 2 minutes; 94°C for 30 seconds; 50–65°C for 30 seconds; 72°C for 1 minute, cycling from step 2. PCR products were electrophoresed with 2% agarose gel, photographed with a type ZF transmission and reflection analyzer (Shanghai Jiapeng Technology, Shanghai, China) and analyzed with Quantity One-4.6.2 software. The ratio of the target band absorbance to the β-actin band absorbance was measured as the relative expression level of the mRNA of interest.
Statistical analysis
Data were expressed as mean ± SD and analyzed with SPSS 13.0 statistical software package (SPSS, Chicago, IL, USA). Multiple groups were compared using one-way analysis of variance, and pairwise comparisons were done with SNK-q test. A level of P < 0.05 was considered a statistically significant difference.
Acknowledgements:
We wish to thank all staff from the Institute of Basic Medical Sciences, Chengde Medical College, China for providing the experimental equipment and the use of facilities.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study was approved by Animal Ethics Committee of Hebei Province in China.
(Edited by Wang RT, Li YY/Yang Y/Wang L)
REFERENCES
- [1].Zhang W, Liu H, Al-Shabrawey M, et al. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2(2):96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Iacono P, Battaglia Parodi M, Bandello F. Antivascular endothelial growth factor in diabetic retinopathy. Dev Ophthalmol. 2010;46:39–53. doi: 10.1159/000320008. [DOI] [PubMed] [Google Scholar]
- [3].Mahdy RA, Nada WM. Evaluation of the role of vascular endothelial growth factor in diabetic retinopathy. Ophthalmic Res. 2011;45(2):87–91. doi: 10.1159/000317062. [DOI] [PubMed] [Google Scholar]
- [4].Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- [5].Park JW, Park SJ, Park SH, et al. Up-regulated expression of neuronal nitric oxide synthase in experimental diabetic retina. Neurobiol Dis. 2006;21(1):43–49. doi: 10.1016/j.nbd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [6].Carrasco E, Hernández C, Miralles A, et al. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care. 2007;30(11):2902–2908. doi: 10.2337/dc07-0332. [DOI] [PubMed] [Google Scholar]
- [7].Fletcher EL, Phipps JA, Ward MM, et al. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007;13(26):2699–2712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- [8].Neckell A. Adaptometry in diabetic patients. Oftalmologia. 2007;51(3):95–97. [PubMed] [Google Scholar]
- [9].Martin PM, Roon P, Van Ells TK, et al. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45(9):3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- [10].Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(2):1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195(2):158–167. doi: 10.1002/jcp.10254. [DOI] [PubMed] [Google Scholar]
- [12].Kutuk O, Basaga H. Bcl-2 protein family: implications in vascular apoptosis and atherosclerosis. Apoptosis. 2006;11(10):1661–1675. doi: 10.1007/s10495-006-9402-7. [DOI] [PubMed] [Google Scholar]
- [13].Safaeian L, Jafarian A, Rabbani M, et al. The role of strain variation in BAX and BCL-2 expression in murine bleomycin- induced pulmonary fibrosis. Pak J Biol Sci. 2008;11(23):2606–2612. doi: 10.3923/pjbs.2008.2606.2612. [DOI] [PubMed] [Google Scholar]
- [14].Lai WW, Yang JS, Lai KC, et al. Rhein induced apoptosis through the endoplasmic reticulum stress, caspase- and mitochondria-dependent pathways in SCC-4 human tongue squamous cancer cells. In Vivo. 2009;23(2):309–316. [PubMed] [Google Scholar]
- [15].Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe, et al. Inhalation of low-level formaldehyde increases the Bcl-2/Bax expression ratio in the hippocampus of immunologically sensitized mice. Neuroimmunomodulation. 2006;13(2):63–68. doi: 10.1159/000094829. [DOI] [PubMed] [Google Scholar]
- [16].Tan KO, Fu NY, Sukumaran SK, et al. MAP-1 is a mitochondrial effector of Bax. Proc Natl Acad Sci U S A. 2005;102(41):14623–14628. doi: 10.1073/pnas.0503524102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oshitari T, Yamamoto S, Hata N, et al. Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. Br J Ophthalmol. 2008;92(4):552–556. doi: 10.1136/bjo.2007.132308. [DOI] [PubMed] [Google Scholar]
- [18].Scorrano L. Opening the doors to cytochrome c: changes in mitochondrial shape and apoptosis. Int J Biochem Cell Biol. 2009;41(10):1875–1883. doi: 10.1016/j.biocel.2009.04.016. [DOI] [PubMed] [Google Scholar]
- [19].Alves NL, van Lier RA, Eldering E. Withdrawal symptoms on display: Bcl-2 members under investigation. Trends Immunol. 2007;28(1):26–32. doi: 10.1016/j.it.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [20].Burguillos MA, Hajji N, Englund E, et al. Apoptosis-inducing factor mediates dopaminergic cell death in response to LPS-induced inflammatory stimulus: evidence in Parkinson's disease patients. Neurobiol Dis. 2011;41(1):177–188. doi: 10.1016/j.nbd.2010.09.005. [DOI] [PubMed] [Google Scholar]
- [21].Tomasin R, Gomes-Marcondes MC. Oral administration of Aloe vera and honey reduces Walker tumour growth by decreasing cell proliferation and increasing apoptosis in tumour tissue. Phytother Res. 2011;25(4):619–623. doi: 10.1002/ptr.3293. [DOI] [PubMed] [Google Scholar]
- [22].Walensky LD. BCL-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ. 2006;13(8):1339–1350. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- [23].Yu L, Miao H, Hou Y, et al. Neuroprotective effect of A20 on TNF-induced postischemic apoptosis. Neurochem Res. 2006;31(1):21–32. doi: 10.1007/s11064-005-9004-8. [DOI] [PubMed] [Google Scholar]
- [24].Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35(1):24–27. doi: 10.5483/bmbrep.2002.35.1.024. [DOI] [PubMed] [Google Scholar]
- [25].Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:5, e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- [26].Choi EJ, Ahn WS, Bae SM. Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem Biol Interact. 2009;177(1):7–11. doi: 10.1016/j.cbi.2008.09.031. [DOI] [PubMed] [Google Scholar]
- [27].Dong ZJ, Tao XY, Wang HB, et al. Effects of purendan superfine powder on expression of retinal vascular endothelial growth factor and pigment epithelium-derived factor in diabetic rats. Jiepou Xuebao. 2011;42(4):498–502. [Google Scholar]
- [28].Dong ZJ, Wang HB, Tao XY, et al. Effects of PRD on retinal VEGF expression in diabetic rat. Zhongguo Zhongyi Jichu Yixue Zazhi. 2011;17(3):329–331. [Google Scholar]
- [29].Pang ZR, Zhao YT, Li JH, et al. Effect of PRD ultramicro-powder on the indexes correlated with glycometabolism in obese type 2 diabetes mellitus rats. Zhongguo Shiyan Fangji Xue Zazhi. 2010;16(5):107–110. [Google Scholar]
- [30].Jia CH, Liu BS, Pang ZR, et al. Study on the PRD on the glucose-decreasing effect of diabetic rats. Zhongguo Zhongyi Jichu Yixue Zazhi. 2002;8(9):669–671. [Google Scholar]
- [31].Pang ZR, Liu ZH, Su XH, et al. Effects of Pu Ren Dan submicron powder on ET-1, NO, FFAs in large blood vessel injury rats with obesity T2DM. Zhongguo Laonian Xue Zazhi. 2011;31(10):1779–1780. [Google Scholar]
- [32].Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- [33].Park SH, Park JW, Park SJ, et al. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46(9):1260–1268. doi: 10.1007/s00125-003-1177-6. [DOI] [PubMed] [Google Scholar]
- [34].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [35].Xiang X, Wang Z, Zhu Y, et al. Dosage of streptozocin in inducing rat model of type 2 diabetes mellitus. Wei Sheng Yan Jiu. 2010;39(2):138–142. [PubMed] [Google Scholar]
- [36].Zhang YP. Beijing: People's Medical Press; 2008. Pharmacological Experiment. [Google Scholar]


