Abstract
Background & Aims:
Melatonin, a naturally occurring hormone in the human body, has been reported to cause preoperative anxiolysis and sedation without impairing orientation. The aim of the following study was to evaluate and to compare the effects of oral melatonin and oral midazolam on preoperative anxiety, sedation, psychomotor, and cognitive function.
Materials and Methods:
A study conducted on 120 patients aged 16-55 years, of American Society of Anesthesiologists Grade 1 and 2 posted for elective surgery, with each group of melatonin, midazolam, and placebo comprising 40 patients. Patients were given either 0.4 mg/kg oral melatonin or 0.2 mg/kg oral midazolam or a placebo 60-90 min before induction. Preoperative anxiety was studied before and 60-90 min after giving medications using visual analog scale (VAS) anxiety score, orientation score, and sedation score. Psychomotor and cognitive functions were studied using the digit symbol substitution test (DSST) and trail making test (TMT) tests. Data were analyzed using Chi-square test or Kruskal–Wallis analysis of variance and the value of P < 0.05 was considered as statistically significant.
Results:
Changes in VAS anxiety scores were significant when melatonin was compared with placebo (P = 0.0124) and when midazolam was compared with placebo (P = 0.0003). When melatonin was compared with midazolam, no significant difference (P = 0.49) in VAS anxiety scores was observed. Intergroup comparison of sedation scores showed melatonin (P = 0.0258) and midazolam (P = 0.0000) to be statistically significant when compared with placebo. No changes in orientation scores occurred in melatonin and placebo group. Change in DSST scores and TMT scores were seen to be significant only in midazolam group.
Conclusion:
Oral melatonin 0.4 mg/kg provides adequate anxiolysis comparable to that of oral midazolam. Unlike midazolam, oral melatonin 0.4 mg/kg does not impair the general cognitive and psychomotor function especially cognitive aspects such as working memory, memory retrieval, sustained attention, and flexibility of thinking.
Keywords: Cognition, melatonin, midazolam, preoperative anxiety, psychomotor performance
Introduction
Preoperative anxiety is described as an unpleasant state of uneasiness or tension that is secondary to a patient being concerned about a disease, hospitalization, anesthesia, and surgery, or the unknown.[1] Benzodiazepines, mainly midazolam are most commonly used as premedicants to decrease anxiety.[2] Midazolam though has several drawbacks.[3] Hence an alternative premedicant to midazolam will definitely have a widespread appeal.
Melatonin is a hormone secreted by the pineal gland. Melatonin is different from benzodiazepines and their derivatives in that it exerts a promoting effect on sleep by amplifying day/night differences in alertness and sleep quality and displaying a modest sleep inducing effect, quite mild as compared to that seen with benzodiazepines.[4] Melatonin has also been reported to cause preoperative anxiolysis and increase in levels of sedation without impairing orientation.[5,6] Hence, the aim of this study was to compare the effect of oral melatonin and oral midazolam on preoperative anxiety, cognitive, and psychomotor functions.
Materials and Methods
After obtaining the hospital ethical committee clearance and informed consent from all the patients, 120 patients of American Society of Anesthesiologists (ASA) I and II physical status aged between 16 and 55 years scheduled to undergo elective surgery requiring general anesthesia were randomly assigned to three Groups A, B and C (n = 40 patients/group) according to a computer generated list based on whether they will receive oral melatonin 0.4 mg/kg, oral midazolam 0.2 mg/kg or placebo respectively. The drugs used in our study were Tab. meloset 3 mg (melatonin) from Aristo pharmaceutical company and Tab. midazolam 7.5 mg (midazolam) from Neon Pharmaceutical Company. Low dose multi-vitamin tablets were used as placebo. Each patient received either of the drug based on the generated list in a thick opaque similar looking envelope by the preoperative nurse. Both patient and investigator were unaware of the type of drug the patient received. Patients with ASA III physical status or greater, age more than 55 years and <16 years, mentally impaired patients, those with a history of psychiatric disorders or on any anti-psychotic drugs intake, sleep disorders, low intelligence quotient and inability to read and write basic alphabets were excluded from the study.
All the patients were assessed the day before surgery. The visual analog scale (VAS) anxiety score (i.e., 0 = no anxiety, 10 = worst imaginable anxiety), and the objective tests for psychomotor and cognitive performance like digit symbol substitution test (DSST) and the trail making test (TMT) A and B were explained to them during that time and patients were asked to do it in a sample test.[5,7,8,9,10,11,12] A modification of VAS scale was used in our study which was 50-cm long and 10-cm high card, diagonally divided into a white and a bright red triangle. The centimeter scale was on the rear side of the card. The extremes were marked “no anxiety” at the white end and “anxiety as bad as ever can be” at the red end.[5] The assessment of sedation was done using sedation scale[5,6,7] where, 0 = alert; 1 = arouses to voice; 2 = arouses with gentle tactile stimulation; 3 = arouses with vigorous tactile stimulation and 4 = lack of responsiveness. Orientation was assessed using orientation score[5] (0 = none, 1 = orientation in either space or time, 2 = orientation in both space and time). Cognitive and psychomotor function were assessed using DSST and the TMT A and B tests. The DSST is a pencil and paper test of psychomotor performance in which the subject is given a key grid of numbers and matching symbols and a test section with numbers and empty boxes.[8] This test consists of a sheet of paper, with a key numbered 1 through 9 at the top, with each number being ascribed to a different symbol. Beneath the key are five rows of 25 randomly distributed numbers without their corresponding symbol [Figure 1]. The patient is asked to substitute as many symbols as possible in a 90 s period, starting with the first row and working from left to right. The test is scored by counting the number of correct symbols inserted.[9]
Figure 1.
Digit symbol substitution test
The TMT is a brief paper and pencil neuropsychological test often used for screening for cognitive impairment. It consists of two parts, Part A and B. In Part A, the circles are numbered 1-25, and the patient should draw lines to connect the numbers in ascending order. In Part B, the circles include both numbers (1-13) and letters (A-L); as in Part A, the patient draws lines to connect the circles in an ascending pattern, but with the added task of alternating between the numbers and letters (i.e., 1-A-2-B-3-C, etc.). The patient should be instructed to connect the circles as quickly as possible, without lifting the pen or pencil from the paper. Time is noted as the patient connects the “trail.” If the patient makes an error, it is immediately pointed out and the patient is allowed to correct it. Errors affect the patient's score only in if the corrections of errors are included in the completion time for the task.[10,11,12]
If there is psychomotor derangement and cognitive dysfunction, the DSST scores will decrease and the TMT time will increase.
Our study was designed to give the drug according to the body weight of the patient. As the drugs were available in the tablet form of fixed dosage, there was difficulty in accurately measuring and administering the drug according to the patient's body weight. Nevertheless, the pharmacist tried to approximate the dosage according to the body weight in kg.
The patient was shifted from the ward to a quiet room near the operation theater 2 h before surgery. The drug was given to the patient by the pharmacist/preoperative room nurse 60-90 min before induction time in a thick opaque similar looking envelope. Patient had to take the drug orally with few sips of water. Before giving the tablets, patient's anxiety, orientation and sedation levels were assessed using VAS anxiety score, orientation score and sedation score by the investigator. Cognitive and psychomotor functions were assessed using DSST and the TMT A and B. Patient was asked to relax and sleep following the intake of tablet. After 60-90 min patient was assessed again with the same above parameters and the tests were repeated again. Occurrence of side-effects of melatonin and midazolam were also noted if any.
The sample size was determined based on previous studies.[5,6,7] The assumption that placebo could have an effect in reducing anxiety in 20% of patients, whereas melatonin and midazolam in at least 50% of patients, and to provide 80% power with an error equal to 0.05, a sample size of 34/group was determined to be sufficient. To account for multiple outcomes and dropouts, we increased the sample size to 40 patients/group. All the relevant data were analyzed using SPSS version 14.0(SPSS Inc, Chicago, IL.). Chi-square test was used for analyzing categorical data such as age, gender, and ASA grade. Nonparametric data were analyzed using Kruskal-Wallis analysis of variance test, Mann-Whitney U-test, and Wilcoxon matched pairs test. P < 0.05 was taken as statistically significant.
Results
The three groups were comparable to each other in terms of age, sex, gender, weight, and ASA status [Table 1]. There were four drop outs in each of the two groups-melatonin and placebo, and three drop outs in midazolam group mainly because of patients surpassing the stipulated time for induction i.e., 60-90 min after giving premedication.
Table 1.
Demographic and other basic data of the patients
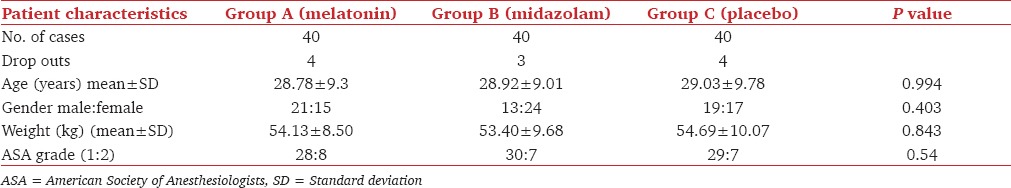
Table 2 shows the baseline VAS anxiety scores in each group before premedication and 60-90 min after premedication. The change in values before and 60-90 min after premedication were significant in all the three groups as the P < 0.05. However, when intergroup comparison was done, statistically significant difference in VAS anxiety scores was seen when melatonin was compared with placebo (P = 0.0124) and when midazolam was compared with placebo (P = 0.0003) for VAS anxiety scores after giving the premedication. When melatonin was compared to midazolam, there was no significant difference (P = 0.49) in VAS anxiety scores after giving the premedication thus showing that oral midazolam and oral melatonin were equally effective in producing anxiolysis.
Table 2.
VAS anxiety score; sedation score; orientation score
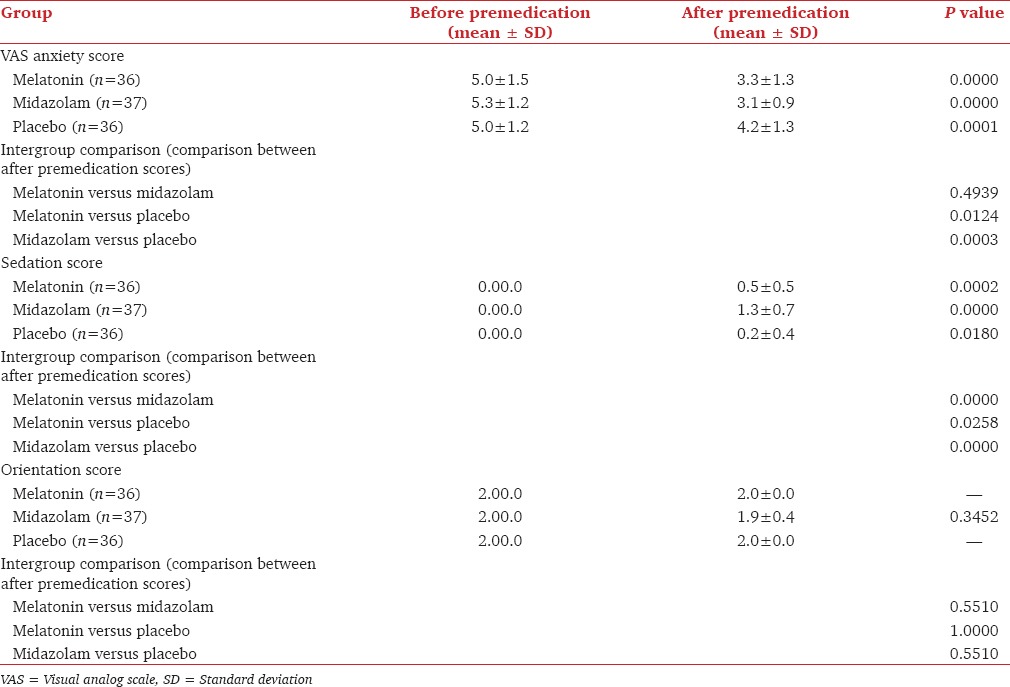
The sedation scores in all the three groups, i.e., melatonin, midazolam and placebo group before and after premedication were statistically significant [Table 2]. However, they were very highly significant in melatonin and midazolam group. During the intergroup comparison of sedation scores, melatonin showed a statistically significant (P = 0.0258) difference in sedation scores after giving the premedication when compared with placebo. Midazolam showed very highly significant difference (P = 0.0000) in sedation scores after giving the premedication when compared to both melatonin and placebo thus showing that midazolam produced the highest degree of sedation when compared with placebo and melatonin group.
There were no changes in orientation scores [Table 2] in melatonin and placebo group before and 60-90 min after giving premedication. Hence, P value was not applicable. There was a difference in midazolam group which was not statistically significant (P = 0.345). This showed that melatonin did not produce any change in orientation.
Table 3 shows the baseline DSST scores for before premedication and 60-90 min after premedication. The DSST scores were increased in melatonin and placebo group 60-90 min after premedication when compared to before premedication and they were decreased in midazolam group. Hence, the clinically important P value of statistical significance was seen only in midazolam group (P = 0.0000). The comparison of mean difference between the midazolam and placebo group for DSST scores were highly significant (P = 0.0001), but there was no difference between the melatonin and placebo group (P = 0.57). This showed that psychomotor and cognitive functions were not affected in melatonin group patients whereas they were significantly affected in midazolam group patients.
Table 3.
DSST score
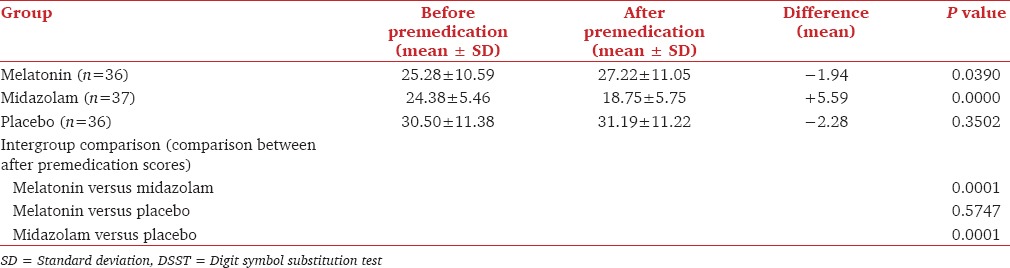
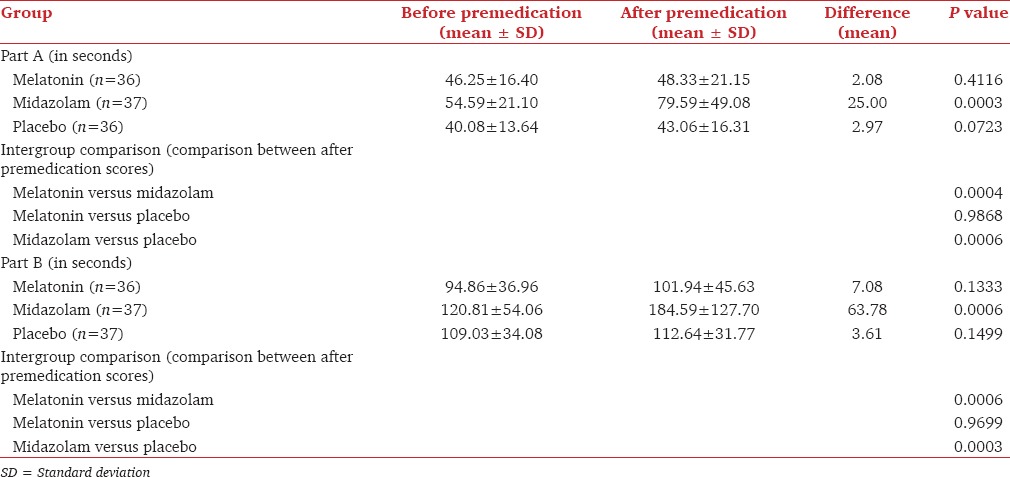
Table 4 shows the results of TMT including both Part A and B. The TMT scores were significant only in midazolam group (P = 0.0003 for Part A; P = 0.0006 for Part B) when before and after premedication scores were compared. During the intergroup comparison, significant difference was seen when midazolam was compared to either melatonin or placebo. There was no difference in TMT scores (both Part A and B) for after premedication when melatonin was compared with placebo. This showed that midazolam produced the maximum derangement in both psychomotor and cognitive functions after premedication and before surgery. It also showed that like placebo, melatonin did not produce any psychomotor or cognitive derangement.
Table 4.
Trail making test
Discussion
Melatonin has a hypnotic/sedative effect when administered orally. This may be due to its circadian rhythm regulation effect. The sedative effect of melatonin is due to modulation of gamma-aminobutyric acid (GABAA) receptors in the brain through its action on melatonin receptors (MT1 and MT2). Binding of melatonin to the MT1 receptor appears to affect the GABAA receptor through the G-coupled protein pathway. This enhances the binding of GABA to the GABAA receptor, which is similar to how other anesthetic drugs such as propofol and benzodiazepines exert their anesthetic effects.[13,14,15] Several studies have used oral melatonin dose ranging from 3 mg dual dose to 10 mg single dose per orally.[16] Kain et al. safely used oral melatonin in children with the maximum dose of 0.4 mg/kg without any major side-effects.[17] We selected the dose of oral melatonin as 0.4 mg/kg body weight because our study group included adult patients and we wanted the maximum effectiveness of oral melatonin with the safest maximum dose previously used by various authors. The peak effect of exogenous melatonin ranges from 60 to 150 min.[15] The peak action of oral midazolam is from 30 to 90 min. The benzodiazepine midazolam is a commonly used premedicant and its oral dosage ranges from 0.25 to 0.75 mg/kg. The equivalence of dose of the two drugs was chosen based on previous published literature and we chose to give both the drugs 60-90 min before induction.[5,6,7,16,17,18,19]
Studies done by Ionescu et al.,[7] Naguib and Samarkandi,[5,6] Samarkandi et al.[18] and Acil et al.[19] compared the effects of melatonin and midazolam with that of a placebo, where they found that anxiolysis in the melatonin group was comparable to that produced by midazolam group. Our study showed that oral midazolam and oral melatonin were equally effective in producing anxiolysis when compared with placebo. This was in contrast to the other studies like those of Sury and Fairweather,[20] Kain et al.,[17] Capuzzo et al.,[21] and Isik et al.[22] wherein, they did not find any significant anxiolytic effect of melatonin when compared to either midazolam or placebo. The above authors could not find the anxiolytic effect of oral melatonin probably because the dose of melatonin which they used (maximum 10 mg) was lesser than what we used (0.4 mg/kg). In one of the studies,[21] the study group patients were elderly, which may be a contributory factor towards the insignificant effect. Nevertheless, elderly population has been shown to be refractory to the hypnotic and anxiolytic effects of melatonin.[23] Hence, melatonin's sedative/anxiolytic properties diminish over age and in the elderly its effects may be negligible.
Five studies compared sedation levels after premedication with melatonin, midazolam, or placebo.[5,6,7,19,24] Increased levels of sedation in the melatonin and midazolam group versus placebo were evident at 60 and 90 min after premedication in two studies done by Naguib and Samarkandi.[5,6] In our study, we found that the sedation scores in all the three groups, i.e., for melatonin, midazolam and placebo group before and after premedication were statistically significant (P < 0.05). However, they were very highly significant in melatonin and midazolam group. The intergroup comparison of sedation scores showed that midazolam produced the highest degree of sedation when compared to melatonin and placebo. Melatonin also showed sedative properties when compared with placebo. This outcome was in contrast to studies by Sury and Fairweather[20] and Isik et al.[22] where they did not find any significant sedative effect of melatonin when compared with placebo. Our study also showed that though melatonin produced sedation, it was not at par with the midazolam group. Melatonin produced enough sedation which would calm the patient and induce a natural sleep which is very much desirable as against the deep sedation produced by midazolam group. Hence, patients sedated with melatonin would require less preoperative monitoring (mild sedation) than patients sedated with midazolam (moderate to deep sedation).
Cognition is one of the four domains of assessment of neurological and behavioral functions. Cognitive subdomains include sustained attention, executive functioning including working memory, explicit and implicit memory, intelligence, time orientation, registration, attention, and judgment.[25] Cognitive impairment is a decline in function in either one or multiple domains of cognitive function.[12]
Psychomotor processing involves the fundamental cognitive operations that enable sensation perception and motor actions.[12] Psychomotor impairment means a generalized slowing down of mental and physical activity.[26] There are several elaborative cognitive tests. One limitation of our study is that we did not include them. However, we have used the DSST and TMT which are simple and reliable tests for assessing cognitive and psychomotor function. The DSST tests a number of higher mental functions including sensory perception, vigilance, visual acuity, ability to alter eye fixation quickly, fine muscular coordination, and mental concentration.[9]
The TMT is a neuropsychological test of visual attention and task switching. It can provide information about visual search speed, scanning, speed of processing, mental flexibility, and executive functioning.[27] Part A depends on visual scanning and psychomotor speed. Part B requires executive control, flexibility of thinking, and greater demand for working memory.[28]
The mean difference in DSST scores for before and after premedication comparison in each group (i.e., melatonin, midazolam, and placebo) was −1.94, +5.59, and −2.28, respectively. This shows that the DSST scores were increased and patient performed the test better in melatonin and placebo group 60-90 min after premedication when compared with before premedication. The practice effect may be the reason for this observation wherein after subjects have completed a certain test, usually perform better on that same test the next time around simply because of increased level of familiarity with that test.[29]
In our study, using TMT for cognitive dysfunction, we found that midazolam produced the maximum derangement in both psychomotor and cognitive functions after premedication and before surgery. Furthermore, melatonin was similar to placebo and did not produce any derangement in both psychomotor and cognitive function. Needless to say, this profile of oral melatonin would definitely add to its advantage because any ideal premedicant would require only the anxiolytic and sedative properties rather than psychomotor or cognitive derangement.
The limitations of our study were that we did not assess the psychomotor and cognitive functions postoperatively, which would have provided some light on the usefulness of melatonin for ambulatory or day care surgeries wherein the postoperative impairment of cognitive and psychomotor functions should be detrimental to the patient.
In our study, we used 0.4 mg/kg oral melatonin in adults. There is no other published study using this dose in adults. Previous published studies have used doses smaller than this.[7,30] Doses equal to or larger than this have been used by some authors in children.[17,18,22] They however did not assess cognitive and psychomotor function in a similar manner to our study. Few authors have assessed cognitive and psychomotor function in adults with oral melatonin, but their drug doses were lesser than ours and all of them used sublingual route of administration.[4,5,6,19,23] Nevertheless, our study aimed at deriving maximum effect of melatonin with the safest dose used in previous studies.
Conclusion
Oral melatonin (0.4 mg/kg) given 60-90 min before surgery provides adequate anxiolysis comparable to that of oral midazolam (0.2 mg/kg) and provides sedation better than placebo, but not better than oral midazolam. Unlike midazolam, oral melatonin does not affect orientation. Furthermore, unlike midazolam, oral melatonin 0.4 mg/kg does not impair general cognitive and psychomotor performance especially cognitive aspects such as working memory, memory retrieval, sustained attention, and flexibility of thinking. Nevertheless, further studies on finer aspects of cognitive and psychomotor function with different doses of melatonin are warranted in the near future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ramsay MA. A survey of pre-operative fear. Anaesthesia. 1972;27:396–402. doi: 10.1111/j.1365-2044.1972.tb08244.x. [DOI] [PubMed] [Google Scholar]
- 2.Caumo W, Torres F, Moreira NL, Jr, Auzani JA, Monteiro CA, Londero G, et al. The clinical impact of preoperative melatonin on postoperative outcomes in patients undergoing abdominal hysterectomy. Anesth Analg. 2007;105:1263–71. doi: 10.1213/01.ane.0000282834.78456.90. [DOI] [PubMed] [Google Scholar]
- 3.McCann ME, Kain ZN. The management of preoperative anxiety in children: An update. Anesth Analg. 2001;93:98–105. doi: 10.1097/00000539-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed RA, Samarkandi A, Al-Mansouri SM, Al Obeidan SA. Sedation characteristics of melatonin and midazolam for premedication of adult patients undergoing cataract surgery under local anaesthesia. Saudi J Anaesth. 2007;1:6. [Google Scholar]
- 5.Naguib M, Samarkandi AH. The comparative dose-response effects of melatonin and midazolam for premedication of adult patients: A double-blinded, placebo-controlled study. Anesth Analg. 2000;91:473–9. doi: 10.1097/00000539-200008000-00046. [DOI] [PubMed] [Google Scholar]
- 6.Naguib M, Samarkandi AH. Premedication with melatonin: A double-blind, placebo-controlled comparison with midazolam. Br J Anaesth. 1999;82:875–80. doi: 10.1093/bja/82.6.875. [DOI] [PubMed] [Google Scholar]
- 7.Ionescu D, Badescu C, Ilie A, Miclutia I, Iancu C, Ion D, et al. Melatonin as pre medication for laparoscopic cholecystectomy: A double blind, placebo controlled study. South Afr J Anaesth Analg. 2008;14:8–11. [Google Scholar]
- 8.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–25. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim C, Hong S, Kim B, Cheon J, Lee Y, Koh H, et al. Comparison of various tests designed to assess the recovery of cognitive and psychomotor function after ambulatory anaesthesia. Korean J Anesthesiol. 2008;55:291–7. [Google Scholar]
- 10.Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the trail making test. J Clin Psychol. 1987;43:402–9. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Gaudino EA, Geisler MW, Squires NK. Construct validity in the trail making test: What makes Part B harder? J Clin Exp Neuropsychol. 1995;17:529–35. doi: 10.1080/01688639508405143. [DOI] [PubMed] [Google Scholar]
- 12.Lezak MD, Howieson DB, Loring DW. 4th ed. New York: Oxford University Press; 2004. Neuropsychological Assessment. [Google Scholar]
- 13.Naguib M, Gottumukkala V, Goldstein PA. Melatonin and anesthesia: A clinical perspective. J Pineal Res. 2007;42:12–21. doi: 10.1111/j.1600-079X.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I. Analgesic effects of melatonin: A review of current evidence from experimental and clinical studies. J Pineal Res. 2011;51:270–7. doi: 10.1111/j.1600-079X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 15.Melatonin Monograph. Altern Med Rev. 2005;10:326–36. [PubMed] [Google Scholar]
- 16.Yousaf F, Seet E, Venkatraghavan L, Abrishami A, Chung F. Efficacy and safety of melatonin as an anxiolytic and analgesic in the perioperative period: A qualitative systematic review of randomized trials. Anesthesiology. 2010;113:968–76. doi: 10.1097/ALN.0b013e3181e7d626. [DOI] [PubMed] [Google Scholar]
- 17.Kain ZN, MacLaren JE, Herrmann L, Mayes L, Rosenbaum A, Hata J, et al. Preoperative melatonin and its effects on induction and emergence in children undergoing anesthesia and surgery. Anesthesiology. 2009;111:44–9. doi: 10.1097/ALN.0b013e3181a91870. [DOI] [PubMed] [Google Scholar]
- 18.Samarkandi A, Naguib M, Riad W, Thalaj A, Alotibi W, Aldammas F, et al. Melatonin vs. midazolam premedication in children: A double-blind, placebo-controlled study. Eur J Anaesthesiol. 2005;22:189–96. doi: 10.1017/s0265021505000335. [DOI] [PubMed] [Google Scholar]
- 19.Acil M, Basgul E, Celiker V, Karagöz AH, Demir B, Aypar U. Perioperative effects of melatonin and midazolam premedication on sedation, orientation, anxiety scores and psychomotor performance. Eur J Anaesthesiol. 2004;21:553–7. doi: 10.1017/s0265021504007094. [DOI] [PubMed] [Google Scholar]
- 20.Sury MR, Fairweather K. The effect of melatonin on sedation of children undergoing magnetic resonance imaging. Br J Anaesth. 2006;97:220–5. doi: 10.1093/bja/ael144. [DOI] [PubMed] [Google Scholar]
- 21.Capuzzo M, Zanardi B, Schiffino E, Buccoliero C, Gragnaniello D, Bianchi S, et al. Melatonin does not reduce anxiety more than placebo in the elderly undergoing surgery. Anesth Analg. 2006;103:121–3. doi: 10.1213/01.ane.0000222476.62547.ed. [DOI] [PubMed] [Google Scholar]
- 22.Isik B, Baygin O, Bodur H. Premedication with melatonin vs midazolam in anxious children. Paediatr Anaesth. 2008;18:635–41.22. doi: 10.1111/j.1460-9592.2008.02608.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhdanova IV. Melatonin as a hypnotic: Pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Naguib M, Samarkandi AH, Moniem MA, Mansour Eel-D, Alshaer AA, Al-Ayyaf HA, et al. The effects of melatonin premedication on propofol and thiopental induction dose-response curves: A prospective, randomized, double-blind study. Anesth Analg. 2006;103:1448–52. doi: 10.1213/01.ane.0000244534.24216.3a. [DOI] [PubMed] [Google Scholar]
- 25.Han ES, Lee Y, Kim J. Association of cognitive impairment and frailty in community dwelling older adults. Int Psychogeriatr. 2013;23:1–9. doi: 10.1017/S1041610213001841. [DOI] [PubMed] [Google Scholar]
- 26.Dorlands W. Dorlands Medical Dictionary for Health Consumers. 31st ed. Philadelphia: Elsevier; 2007. [Google Scholar]
- 27.Arnett JA, Labovitz SS. Effect of physical layout in performance of the trail making test. Psychol Assess. 1995;7:220–1. [Google Scholar]
- 28.Tombaugh TN. Trail making test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 29.Hope At, Woolman PS, Gray WM, Asbury AJ, Millar K. A system for psychomotor evaluation; design, implementation and practice effects in volunteers. Anaesthesia. 1998;53:545–50. doi: 10.1046/j.1365-2044.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- 30.Mowafi HA, Ismail SA. Melatonin improves tourniquet tolerance and enhances postoperative analgesia in patients receiving intravenous regional anesthesia. Anesth Analg. 2008;107:1422–6. doi: 10.1213/ane.0b013e318181f689. [DOI] [PubMed] [Google Scholar]


