Abstract
Background and Aims:
Effect of intrathecal dexmedetomidine as an adjuvant to isobaric ropivacaine in spinal anesthesia for abdominal hysterectomy is not much investigated. The objective was to assess the dose dependent effect of dexmedetomidine (3 mcg vs 5 mcg) as an adjunct to isobaric ropivacaine in spinal anesthesia.
Materials and Methods:
Forty selected female patients were randomized to receive intrathecal 0.5% isobaric ropivacaine (15 mg) with dexmedetomidine 3 mcg (Group D3) or 5 mcg (Group D5) in spinal anesthesia for abdominal hysterectomy. Block characteristics, hemodynamic changes, postoperative analgesia, and adverse effects were compared.
Results:
Both groups were comparable regarding sensory-motor block characteristics and postoperative analgesia (P > 0.05). Four (10%) patients of Group D5 and 5 (12.5%) of Group D3 could not achieve desired T6 sensory level and Bromage score of 3(complete motor block) hence were converted to general anesthesia at the outset. Nine (22.5%) patients each in both groups required ketamine supplementation (0.5 mg/kg) for intraoperative pain at the time of uterine manipulation. Incidence of hypotension was comparable (55.56% in Group D5 and 37.14% in Group D3, P = 0.11), but this occurred significantly earlier in Group D5, P < 0.001. Sedation was also significantly more in Group D5 as compared with Group D3, P < 0.01.
Conclusion:
We conclude that spinal anesthesia with isobaric ropivacaine (15 mg) with dexmedetomidine (3 mcg or 5 mcg) did not show much promise for abdominal hysterectomy as one third cases required analgesic supplementation. Both doses of dexmedetomidine produced a similar effect on block characteristic and postoperative analgesia; however, a dose of 5 mcg dose was associated with more hypotension and sedation.
Keywords: Abdominal hysterectomy, dexmedetomidine, isobaric ropivacaine
Introduction
Ropivacaine a newer amide local anesthetic with a high pKa and low lipid solubility has gained popularity as an intrathecal agent. It may be a suitable alternative as long acting local anesthetic because it is considered to be less cardiotoxic and has a significantly higher threshold for Central Nervous System (CNS) toxicity on a milligram basis than bupivacaine.[1]
Intrathecal use of ropivacaine has been reported relatively sparingly.[2,4] It has been reported that intrathecal injection of plain (glucose-free) ropivacaine in the dose of 15 and 22.5 mg produced a sensory block of very variable extent with a proportion of patients requiring general anesthesia because of inadequate distribution of block.[5,6] Therefore, studies were conducted to elucidate the efficacy of hyperbaric ropivacaine,[7,8] using adjuvants like clonidine,[9,10] fentanyl[11] or dexmedetomidine[12] along with isobaric ropivacaine. Intrathecal dexmedetomidine has been used in the dose of 3 mcg,[13,14] 5 mcg[15,16,17] and 10 mcg[15,18] 15 mcg[18] along with bupivacaine, and in the dose of 5 mcg as an adjuvant to plain ropivacaine.[12]
After an extensive literature, we were unable to locate any study in which intrathecal isobaric ropivacaine had been used for abdominal hysterectomy. Therefore, we planned this study to test the hypothesis whether there is any dose dependent effect of intrathecal administration of 3 or 5 mcg of dexmedetomidine when administered with isobaric ropivacaine on characteristics of sensory and motor block as well as hemodynamic changes, level of sedation and side effects in patients undergoing abdominal hysterectomy under spinal anesthesia.
Materials and Methods
After approval from Institutional Ethics Committee a prospective randomized double blind study was conducted at a tertiary center attached to a medical college in India. Subjects included 80 female patients of American Society of Anesthesiologists physical status I/II, age 30-80 yr, weight 40-80 kg and height >140 cm, posted for elective abdominal hysterectomy under subarachnoid block (SAB).
All patients were subjected to a detailed preanesthetic examination and investigations (hemogram, complete blood count, blood urea, creatinine, fasting blood sugar, chest X-ray, electrocardiography [ECG]) during this evaluation. Exclusion criteria comprised of patients on antihypertensive treatment, history of arrhythmias, uncontrolled, labile hypertension, and known hypersensitivity to the study drugs and general contraindications to spinal anesthesia.
Power and sample size were calculated using Epi Info 6. The success rate with intrathecal plain ropivacaine (15 mg) was reported to be 64% at the level of T10.[3] We hypothesized that by adding dexemedetoidine 3 or 5 mcg to intrathecal ropivacaine (15 mg), success rate at T6 would reach to 65% and 95% in Groups D3 and D5 respectively. For comparison of these two drug regimen for unmatched case: Control study with a confidence interval of 95% power of 80%, odds ratio 10.23, proposed sample size is 33 in each group. We enrolled 40 cases in each group to compensate for drop outs.
Primary outcome variable was achievement of adequate block (T6-peak sensory level, Bromage score = 3)[7,13] to allow start of surgery (success). Secondary outcome variables were hemodynamic changes, level of sedation and side effects.
Patients were randomly divided using sealed envelope technique into two groups of 40 patients each:
Group D5: received 3 mL of 0.5% isobaric ropivacaine (15 mg) with 5 μg dexmedetomidine intrathecally.
Group D3: received 3 mL of 0.5% isobaric ropivacaine (15 mg) with 3 μg dexmedetomidine intrathecally.
We have not taken any control group using isobaric ropivacaine alone because in the previous studies[5,6] intrathecal injection of plain ropivacaine produced a sensory block of very variable extent and considerable number of patients required general anesthesia to accomplish surgery.
To provide double-blindness, three anesthesiologists were involved in the study. One anesthesiologist prepared the drug, another gave spinal anesthesia and data were recorded by an independent third anesthesiologist who was unaware of group allocation, patients were also unaware of the drug regimen received.
Drug preparation
A volume of 3 mL of 0.5% isobaric ropivacaine (15 mg) in 5 mL dispovan syringe; dexmedetomidine was drawn in a standard 1 mL BD syringe (100 parts = 100 μg) with 3 parts for 3 μg and 5 parts for 5 μg, which was added to the ropivacaine syringe. Thus, intrathecal volume was 3.03 mL in Gr D3 and 3.05 mL in Gr D5 making no apparently significant volume difference.
Anesthesia technique
Before the commencement of anesthesia, patients were explained about the methods of sensory and motor assessments. All patients received 5 mg of diazepam as oral premedication night before surgery. Standard monitoring of heart rate (HR), noninvasive blood pressure (NIBP), ECG and pulseoximetry (SpO2) was done with a multipara monitor.
After preloading with an infusion of 500 mL ringer lactate through an 18 G peripheral intravenous (i.v.) cannula, patients were given spinal anesthesia in the lateral decubitus position under all aseptic precautions. Lumbar puncture was performed at L2-L3 intervertebral space through a midline approach using 25 G quincke spinal needle.3 mL (15 mg) of 0.5% isobaric ropivacaine with dexmedetomidine (3 or 5 μg) was injected in the subarachnoid space After intrathecal injection patient was placed supine and oxygen 3 L/min was given by venti mask.
Standard monitoring was done throughout the operation. ECG and pulse-oximetry was monitored continuously while NIBP was monitored every 3 min for first 15 min thereafter every 5 min until completion of surgery. Hypotension (decrease in systolic blood pressure of >20% of baseline or <100 mm of Hg) was treated with i.v. bolus of 6 mg ephedrine hydrochloride. Bradycardia (<60 beat/min) was treated with i.v. bolus of 0.3-0.5 mg of atropine sulfate.
Data recording
Demographic data such as age, weight, height, body mass index, diagnosis, and duration of surgery were noted. The height of sensory block was assessed by pinprick method (24G hypodermic needle) in mid-clavicular line bilaterally, loss of sensation to pin prick was considered as sensory block. Motor block was assessed according to the modified Bromage scale[7,13] (0: Patient able to move hip, knee, ankle, 1: Unable to move hip, able to move knee and ankle, 2: Unable to move hip and knee, able to move ankle, 3: Unable to move hip, knee and ankle). Onset of block was assessed by noting time to reach T10 dermatome sensory block, peak sensory level and Bromage 3 motor block. All time durations were calculated considering the time of end of spinal injection as time zero. Sensory and motor block level were recorded every 2 min for 20 min. Intraoperative sedation[19] was measured every 15 min using sedation score as: 0-No sedation (alert), 1-mild sedation (occasionally drowsy, easy to arouse), 2-moderate sedation (frequently drowsy, easy to arouse), 3-severe sedation (somnolent, difficult to arouse).
After 15 min of spinal injection, outcome of SAB was assessed. If patient had no sensory or motor block due to some technical reason, it was defined as “technical failure” and the case was excluded from the study. If peak sensory level could not reach to T6, and Bromage score was <3 then it was defined as “failed case” and converted to general anesthesia at the outset, to accomplish surgery and further data were not recorded.
If peak sensory level reached to T6 or above with a Bromage score of 3, surgery was allowed to start and the case was defined as “successful.” If surgery could be completed without any supplementation, the case was defined as “completely successful.” If patient complained of intraoperative pain, supplemental analgesia with ketamine (0.5 mg/kg) and midazolam (1 mg) were given and case was defined as “partially successful.” Intraoperative sedation and analgesia was given for supplementation during spinal anesthesia as described by many authors.[6,7]
Data related to intraoperative sedation and postoperative rescue analgesia were recorded only in completely successful cases, in which no supplementation was given.
After 45 min of SAB, sensory block was assessed every 10 min to know the time to two dermatomal regression. Intraoperative nausea, vomiting, bradycardia, sedation, other side effects and requirement of additional analgesics were recorded. In the postoperative period, whenever patient complained of pain (visual analog scale >3) intravascular infusion of diclofenac 75 mg was given as rescue analgesic. Time to first rescue analgesic and total analgesic consumption during first 24 h were also noted in terms of the number of doses and dose in milligram. At the end of surgery satisfaction score of the surgeon and patient were recorded as excellent/satisfactory/not satisfactory.
Statistical analysis
Categorical data such as patient distribution according to indication for surgery, peak sensory level, maximum Bromage score, complications, etc., were presented as number proportion and compared with Chi-square test. Continuous variables like age, weight, height, duration of surgery, sensory block characteristics (onset, peak sensory level, duration), motor block characteristics (onset), hemodynamic variables, etc., were presented as mean ±standard deviation and compared with Student's t-test (unpaired) using SPSS version 15, P < 0.05 was considered as statistically significant.
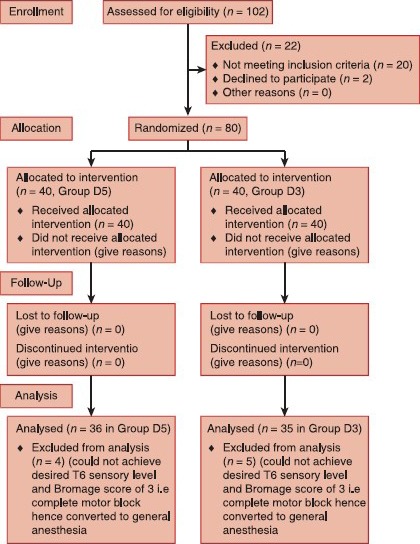
Consort flow diagram
Results
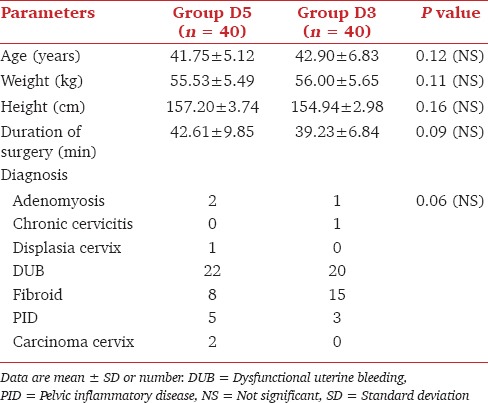
Both groups were comparable regarding mean values of age, weight, height, diagnosis, and duration of surgery (P > 0.05) [Table 1].
Table 1.
Comparison of demographic data, duration of surgery, and diagnosis
In our study, all patients had sensory and motor blockade after spinal anesthesia stating no “technical failure.” Hence, success rate (primary outcome) could be calculated for all 40 patients in each group. 4 (10%) patients of Group D5 and 5 (12.5%) patients of Group D3 peak sensory level was below T6 and Bromage score was <3, so they were categorized as “failed cases,” hence given general anesthesia with intubation at the outset to accomplish surgery and further data were not recorded. 36 (90%) patients of Group D5 and 35 (87.5%) of Group D3 achieved peak sensory level T6 or above and Bromage score of 3, were defined as “successful cases,” surgery was started in spinal anesthesia in these cases [Figure 1]. Thus, success rate in two groups was also comparable (90% in Group D5 and 87.5% in Group D3), P = 0.64.
Figure 1.
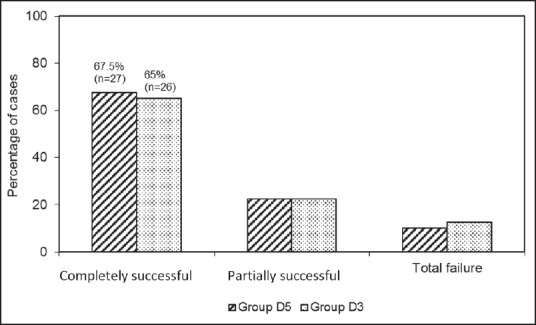
Overall failure rate (total + partial) in two groups
Surgery could be completed in spinal anesthesia without any supplementation in 27 (67.5%) cases in Group D5 and 26 (65%) cases in Group D3, which were defined as “completely successful cases,” 9 (22.5%) patients in each group complained of pain during uterine manipulation and required supplemental sedation and analgesia; single dose of ketamine (30 mg) and midazolam (1 mg), which were defined as “partial successful” cases. Success and failure rate were comparable in two groups (P = 0.64).
Time to reach T10 level was significantly more in Group D3 (5.14 ± 1.63 min) as compared with Group D5 (4.16 ± 1.59 min), P = 0.01. Time to reach maximum sensory level (min) was comparable in two groups, which was 12.17 ± 2.80 min in Group D5 and 12.76 ± 3.23 min in Group D3, (P = 0.413). Mean value of peak sensory level achieved was comparable in two groups (T 5.35 ± 1.01 in Group D5 and T 5.51 ± 0.61 in Group D3), P = 0.423. Time to two dermatomal regression was significantly more in Group D5 (98.75 ± 6.59 min) than in Group D3 (94.00 ± 5.40 min), P = 0.001 [Table 2].
Table 2.
Sensory-motor block characteristics
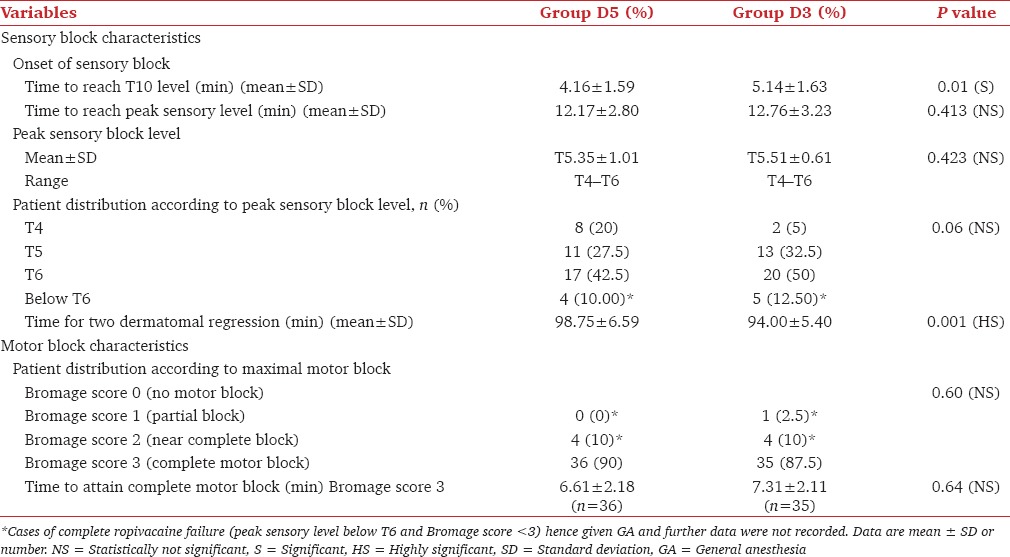
36 (90%) patients of Group D5 and 35 (87.5%) patients of Group D3 achieved complete motor block (Bromage score 3), P = 0.60. Time to attain complete motor block was slightly less in Group D5 (6.61 ± 2.18 min) than in Group D3 (7.31 ± 2.11 min), P = 0.64, which was clinically not significant and statistically not significant [Table 2].
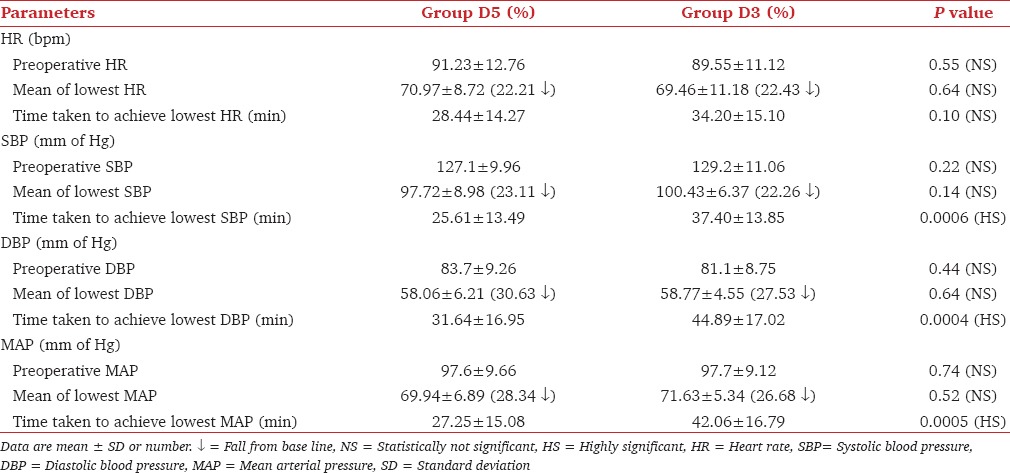
Both groups showed a significant fall from baseline in HR, SBP, diastolic blood pressure (DBP) and mean arterial pressure (MAP) (P < 0.05). The maximal fall in SBP, DBP and MAP occurred significantly earlier in Group D5 as compared with Group D3, P < 0.001 though the extent of fall was comparable in two groups, P > 0.05 [Table 3].
Table 3.
Comparison of hemodynamic parameters
Most common intraoperative adverse effect was hypotension with incidence of 55.56% (n = 20) in Group D5 and 37.14% (n = 13) in Group D3, P = 0.11. Incidence of hypotension, bradycardia, nausea, and vomiting were comparable in two groups [Figure 2]. Requirement of ephedrine in terms of the number of doses and total dose in milligram was comparable in two groups (P = 0.19 and 0.20, respectively). Similarly, there was no significant difference in requirement of atropine in terms of the number of doses (P = 0.89) and total doses in milligram (P = 0.60) in the two groups.
Figure 2.
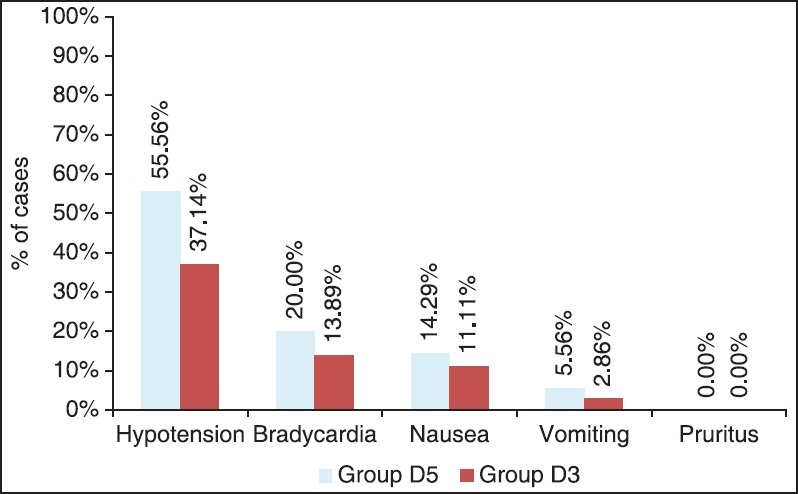
Adverse effects
Sedation was analyzed only in “completely successful” cases who did not receive any sedative analgesic supplementation intraoperatively to see the sedative effect of dexmedetomidine. Thus 27 patients in Group D5 and 26 patients in Group D3 were assessed for sedation score (0-3) at every 15 min intraoperatively Mean sedation score was significantly more in Group D5 (0.75 ± 0.16), as compared with Group D3 (0.32 ± 0.12), P = 0.01. Maximum sedation score was 1 (mild sedation) intraoperatively in both groups. Data related to intraoperative sedation and postoperative analgesia were analyzed only in “completely successful” cases who did not receive any sedation.
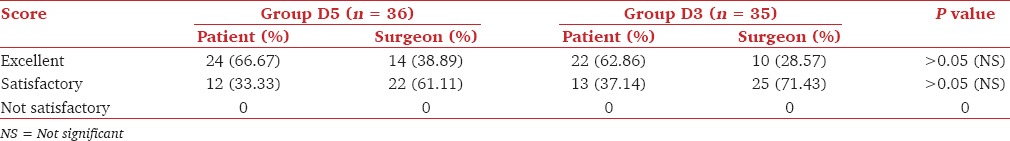
Data related to postoperative analgesia were also recorded in completely successful cases in whom no supplementation was given as it could affect analgesic level. Time of requirement of the first rescue analgesic was in Group D5 (180.83 ± 19.25 min) and Group D3 (174 ± 15.80 min) were comparable, (P = 0.10). Postoperative analgesic requirement in terms of the number of doses and total dose in milligram was also comparable in both groups (P = 0.29, P = 0.56, respectively). Both patients and surgeons were equally satisfied in both groups (P > 0.05), [Table 4].
Table 4.
Comparison of satisfaction score of patient and surgeon in both groups
Discussion
Ropivacaine is a newer amide local anesthetic, which is less toxic to the central nervous system and cardiovascular system and shows rapid recovery of motor function, which is now heralded as an alternative to bupivacaine. In the previous studies,[5,6] intrathecal injection of plain ropivacaine produced a sensory block of variable extent and considerable number of patients required general anesthesia to accomplish surgery.
Hyperbaric ropivacaine produces more predictable and reliable sensory and motor block, with faster onset than a plain solution.[7,8] Since commercial preparations of hyperbaric ropivacaine are not yet available; therefore, adjuvants added to isobaric solution are being investigated to overcome the disadvantage of plain ropivacaine. Addition of fentanyl,[11] clonidine[9,10] and dexmedetomidine[12] have been studied to see the effect on sensory and motor block characteristics of plain ropivacaine.
Plain ropivacaine in spinal anesthesia for abdominal hysterectomy is not much studied. Fettes et al. (2005)[20] evaluated the efficacy of plain ropivacaine for perineal surgery under spinal anesthesia and concluded that plain solutions of ropivacaine are less reliable for surgery above a dermatomal level of L1. Therefore, we did not include a control group with plain ropivacaine in our study. This study focused on observing the efficacy of intrathecal ropivacaine (15 mg) with two different dose of dexmedetomidine (3 and 5 mcg) in providing spinal anesthesia to accomplish abdominal hysterectomies.
Our total failure rate (10% in Group D5, 12.5% in Group D3) was similar to other studies using plain ropivacaine in the dose of 12 mg (13.33%[4] 16%[10]) or 15 mg (10.71%).[3] In contrast when intrathecal plain ropivacaine was used in the dose of 22.5 mg alone or with 5 mcg dexmedetomidine no failure rate was reported.[12] Earlier studies[5,6] on plain ropivcaine also mentioned that the failure rate is less with 22.5 mg ropivacaine than with 15 mg. All these studies have evaluated the efficacy of intrathecal plain ropivacaine in lower limb surgeries,[5,12] cesarean sections,[4,9] none of these included abdominal hysterectomies.
Incidence of failure is more frequent with intrathecal plain ropivacaine than with plain bupivacaine.[4] Ropivacaine is a levoisomer of bupivacaine and has propyl group in place of butyl group. These structural differences make it less lipid soluble resulting in less potency and less cardiotoxicity and also renders difficulty in penetration of large myelinated motor fibers.[21,22]
When dose-dependent effect of intrathecal dexmedetomidine using 5 or 10 mcg on isobaric bupivacaine was investigated, it was found that by increasing the dose of dexmedetomidine, onset of sensory and motor block was faster and block regression time was significantly more.[15] We also observed that mean time taken to reach T10 level was significantly less in patients receiving 5 mcg dexmedetomidine as compared with 3 mcg dose, but the difference of 1-min does not make clinical significance. Time to reach peak sensory level and to attain maximum motor block were comparable in the two groups. Time to two-dermatomal regression was also significantly more with 5 mcg dexmedetomidine though the difference of 4 min had no clinical significance.
Intrathecal alpha-2 adrenergic agonists have a well-established synergistic effect with local anesthetics because both have different mechanism of action. The local anesthetics act by blocking sodium channels, whereas the α2 adrenergic agonists act by binding to presynaptic C-fibers and postsynaptic dorsal horn neurons.[23,24] Intrathecal dexmedetomidine when combined with spinal local anesthetics prolongs the sensory block by depressing the release of C-fibers transmitters and by hyperpolarization of postsynaptic dorsal horn neurons. Motor block prolongation by α2 adrenergic agonist may result from binding of α2 agonists to motor neuron in the dorsal horn of the spinal cord.[25] Intrathecal α2 agonists have antinociceptive action for both somatic and visceral pain;[16] therefore, use of dexmedetomidine as an adjuvant to isobaric ropivacaine causes significant prolongation in duration of analgesia.[12,26] However, when we compared 3 and 5 mcg dose of dexmedetomidine along with ropivacaine postoperative pain scores and analgesic requirement was comparable.
It is a well-known fact that intrathecal local anesthetics block the sympathetic outflow and reduce the blood pressure and HR.[27] We also observed that HR and blood pressure showed a significant fall from baseline values intraoperatively in both groups (P < 0.05). The sympathetic block is usually near-maximal with the local anesthetic doses used for spinal anesthesia. The addition of a low dose of α2 agonist to a high dose of local anesthetic does not further affect the near-maximal sympatholysis.[28,29] Therefore, on intergroup comparison there was no significant difference in mean values of HR, SBP, DBP, and MAP in our study as documented by others.[13]
In our study, most common adverse effect was hypotension (55.56% in D5 and 37.14% in D3; P = 0.11). α2 agonists have shown to decrease intra and post-operative stress response effectively.[26] These agents also have substantial hemodynamic effect in causing hypotension and bradycardia.[29,30]
We observed that incidence of mild sedation was significantly more in Group D5 (30.56%) as compared to Group D3 (8.57%), P = 0.01. Dexmedetomidine is a partial agonist of the α2 adrenoceptors that are found densely in the pontine locus ceruleus, which is an important source of sympathetic nervous system innervations of the forebrain and a vital modulator of vigilance. The sedative effects evoked by α2 agonists most likely reflect inhibition of this nucleus.[32] Tan J O[31] compared the dose-dependent effect of dexmedetomidine (5 mcg and 10 mcg) and found that all patients had mild sedation.
The limitation of our study was that it was not sufficiently powered to evaluate any difference in adverse effects of two doses of dexmedetomidine to reach a conclusion. Further, we have not taken control group using intrathecal isobaric ropivacaine without dexmedetomidine, because previous studies[5,6] have mentioned unreliable anesthesia with plain ropivacaine. We also conducted a pilot study in 10 patients and had a very high failure rate which prevented us from planning a control group for our study.
Conclusion
We conclude that intrathecal dexmedetomidine in dose of 5 and 3 μg as an adjuvant to isobaric ropivacaine in spinal anesthesia was comparable regarding sensory and motor block characteristics and success rate. However, overall incidence of supplemental analgesic requirement with intrathecal isobaric ropivacaine 3 mL of 0.5% (15 mg) with 5 or 3 μg dexmedetomidine was disconcerting in both groups (32.5% vs. 35%; respectively; P > 0.05). In addition, the incidence of hypotension and sedation were higher with dose of 5 μg. Our study does not favor the use of isobaric ropivacaine (15 mg) with dexmedetomidine (3 or 5 mcg) in spinal anesthesia for abdominal hysterectomies.
We suggest that a larger study should be carried out in the future in which a comparison of varying doses of intrathecal isobaric ropivacaine with various adjuvants is evaluated, so that advantages and disadvantages of one regimen over another can be more clearly delineated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.David JS, Ferreti C, Amour J, Vivien B, Eve O, Petit P, et al. Effects of bupivacaine, levobupivacaine and ropivacaine on myocardial relaxation. Can J Anaesth. 2007;54:208–17. doi: 10.1007/BF03022642. [DOI] [PubMed] [Google Scholar]
- 2.Chung CJ, Choi SR, Yeo KH, Park HS, Lee SI, Chin YJ. Hyperbaric spinal ropivacaine for cesarean delivery: A comparison to hyperbaric bupivacaine. Anesth Analg. 2001;93:157–61. doi: 10.1097/00000539-200107000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Kallio H, Snäll EV, Tuomas CA, Rosenberg PH. Comparison of hyperbaric and plain ropivacaine 15 mg in spinal anaesthesia for lower limb surgery. Br J Anaesth. 2004;93:664–9. doi: 10.1093/bja/aeh257. [DOI] [PubMed] [Google Scholar]
- 4.Gautier P, De Kock M, Huberty L, Demir T, Izydorczic M, Vanderick B. Comparison of the effects of intrathecal ropivacaine, levobupivacaine, and bupivacaine for Caesarean section. Br J Anaesth. 2003;91:684–9. doi: 10.1093/bja/aeg251. [DOI] [PubMed] [Google Scholar]
- 5.van Kleef JW, Veering BT, Burm AG. Spinal anesthesia with ropivacaine: A double-blind study on the efficacy and safety of 0.5% and 0.75% solutions in patients undergoing minor lower limb surgery. Anesth Analg. 1994;78:1125–30. [PubMed] [Google Scholar]
- 6.Wahedi W, Nolte H, Klein P. Ropivacaine for spinal anesthesia. A dose-finding study. Anaesthesist. 1996;45:737–44. doi: 10.1007/s001010050306. [DOI] [PubMed] [Google Scholar]
- 7.Whiteside JB, Burke D, Wildsmith JA. Spinal anaesthesia with ropivacaine 5 mg ml(-1) in glucose 10 mg ml(-1) or 50 mg ml(-1) Br J Anaesth. 2001;86:241–4. doi: 10.1093/bja/86.2.241. [DOI] [PubMed] [Google Scholar]
- 8.Whiteside JB, Burke D, Wildsmith JA. Comparison of ropivacaine 0.5% (in glucose 5%) with bupivacaine 0.5% (in glucose 8%) for spinal anaesthesia for elective surgery. Br J Anaesth. 2003;90:304–8. doi: 10.1093/bja/aeg077. [DOI] [PubMed] [Google Scholar]
- 9.Öğün CO, Kirgiz EN, Duman A, Kara I, Ökesli S. The comparison of intrathecal isobaric ropivacaine and isobaric ropivacaine-clonidine for caesarean delivery. Internet J Anesthesiol. 2007;15:904–9. [Google Scholar]
- 10.Sagiroglu G, Sagiroglu T, Meydan B. The effects of adding various doses of clonidine to ropivacaine in spinal anesthesia. Eur J Med. 2009;41:149–53. [PMC free article] [PubMed] [Google Scholar]
- 11.Förster JG, Rosenberg PH. Small dose of clonidine mixed with low-dose ropivacaine and fentanyl for epidural analgesia after total knee arthroplasty. Br J Anaesth. 2004;93:670–7. doi: 10.1093/bja/aeh259. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Bogra J, Verma R, Kohli M, Kushwaha JK, Kumar S. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth. 2011;55:347–51. doi: 10.4103/0019-5049.84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim JE, Kim NY, Lee HS, Kil HK. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. Biol Pharm Bull. 2013;36:959–65. doi: 10.1248/bpb.b12-01067. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J. 2009;30:365–70. [PubMed] [Google Scholar]
- 16.Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind controlled study. Am J Appl Sci. 2009;6:882–7. [Google Scholar]
- 17.Mahendru V, Tewari A, Katyal S, Grewal A, Singh MR, Katyal R. A comparison of intrathecal dexmedetomidine, clonidine, and fentanyl as adjuvants to hyperbaric bupivacaine for lower limb surgery: A double blind controlled study. J Anaesthesiol Clin Pharmacol. 2013;29:496–502. doi: 10.4103/0970-9185.119151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eid HE, Mohamed AS, Youssef H. Dose-related prolongation of hyperbaric bupivacaine spinal anesthesia by dexmedetomidine. Ain Shams J Anesthesiol. 2011;2:83–95. [Google Scholar]
- 19.Burbano NH, Otero AV, Berry DE, Orr RA, Munoz RA. Discontinuation of prolonged infusions of dexmedetomidine in critically ill children with heart disease. Intensive Care Med. 2012;38:300–7. doi: 10.1007/s00134-011-2441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fettes PD, Hocking G, Peterson MK, Luck JF, Wildsmith JA. Comparison of plain and hyperbaric solutions of ropivacaine for spinal anaesthesia. Br J Anaesth. 2005;94:107–11. doi: 10.1093/bja/aei008. [DOI] [PubMed] [Google Scholar]
- 21.McClellan KJ, Faulds D. Ropivacaine: An update of its use in regional anaesthesia. Drugs. 2000;60:1065–93. doi: 10.2165/00003495-200060050-00007. [DOI] [PubMed] [Google Scholar]
- 22.Kuthiala G, Chaudhary G. Ropivacaine: A review of its pharmacology and clinical use. Indian J Anaesth. 2011;55:104–10. doi: 10.4103/0019-5049.79875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strebel S, Gurzeler JA, Schneider MC, Aeschbach A, Kindler CH. Small-dose intrathecal clonidine and isobaric bupivacaine for orthopedic surgery: A dose-response study. Anesth Analg. 2004;99:1231–8. doi: 10.1213/01.ANE.0000133580.54026.65. [DOI] [PubMed] [Google Scholar]
- 24.Dobrydnjov I, Axelsson K, Thörn SE, Matthiesen P, Klockhoff H, Holmström B, et al. Clonidine combined with small-dose bupivacaine during spinal anesthesia for inguinal herniorrhaphy: A randomized double-blinded study. Anesth Analg. 2003;96:1496–503. doi: 10.1213/01.ANE.0000061110.62841.E9. [DOI] [PubMed] [Google Scholar]
- 25.Harada Y, Nishioka K, Kitahata LM, Kishikawa K, Collins JG. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50,488H. Anesthesiology. 1995;83:344–52. doi: 10.1097/00000542-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Abdelhamid SA, El-Lakany MH. Intrathecal dexmedetomidine: Useful or not? J Anesth Clin Res. 2013;4:9. [Google Scholar]
- 27.Mantouvalou M, Ralli S, Arnaoutoglou H, Tziris G, Papadopoulos G. Spinal anesthesia: Comparison of plain ropivacaine, bupivacaine and levobupivacaine for lower abdominal surgery. Acta Anaesthesiol Belg. 2008;59:65–71. [PubMed] [Google Scholar]
- 28.Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 30.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 31.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: A meta-analysis. Eur J Anaesthesiol. 2011;28:3–6. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]
- 32.Jones GM, Murphy CV, Gerlach AT, Goodman EM, Pell LJ. High-dose dexmedetomidine for sedation in the intensive care unit: An evaluation of clinical efficacy and safety. Ann Pharmacother. 2011;45:740–7. doi: 10.1345/aph.1P726. [DOI] [PubMed] [Google Scholar]


